Abstract
It has become axiomatic that critical windows of susceptibility to genotoxins exist and that genetic damage in utero may be a trigger for later life cancers. Data supporting this critical window hypothesis are remarkably few. This study provides a quantitative bridge between DNA damage by the liver carcinogen aflatoxin B1 (AFB1) during prenatal development and the risk of later life genetic disease. AFB1 was given to pregnant C57BL/6J mice, carrying F1 gestation day 14 (GD14) embryos of the B6C3F1 genotype. Ultra-high performance liquid chromatography and mass spectrometry (UPLC-MS) using aflatoxin-15N5-guanine adduct standards afforded measurement of the AFB1-N7-Gua and AFB1-FAPY adducts six hours post dosing in liver DNA of mothers and embryos. A parallel cohort gave birth and the livers of the F1 were analyzed for mutations in the gpt gene at three and ten weeks of age. The data revealed mutational spectra dominated by G:C to T:A mutations in both the mother and offspring that are characteristic of AFB1 and distinct from background. It was shown that adducts in GD14 embryos were 20-fold more potent inducers of mutagenesis than adducts in parallel-dosed adults. This sensitivity enhancement correlated with Ki67 staining of the liver, reflecting the proliferative potential of the tissue. Taken together, these data provide insight into the relative genetic risks of prenatal and adult exposures to AFB1. Early life exposure, especially during the embryonic period, is strikingly more mutagenic than treatment later in life. Moreover the data provide a baseline against which risk prevention strategies can be evaluated.
Keywords: Aflatoxin B1, prenatal exposure, mutagenesis, DNA adducts
Introduction
Factors including rapid cell division and unique developmental changes during the prenatal period create a remarkable window of susceptibility to the numerous toxic effects of environmental agents. Accordingly, maternal exposures to environmental factors during pregnancy influence the risk of many chronic adult-onset diseases in the offspring, including cancer.1–3 Early life including in utero exposures can also result in growth impairment, behavioral defects and teratogenesis.4–5
AFB1 is a toxin produced by fungi that contaminate food supplies especially in the developing world. It is a potent genotoxin that induces hepatocellular carcinoma (HCC) in most animal species, including humans.6 In sub-Saharan Africa and many parts of Asia where 80% of HCC cases occur, there is growing evidence that AFB1 exposure disproportionately affects childhood health in these regions.7
The toxic properties of AFB1 require metabolic activation in the liver to the reactive exo-8,9-epoxide by cytochrome P450s. The epoxide reacts at the N7 atom of guanine in DNA to form an initial AFB1-N7-Gua adduct,8–12 which can later be converted by hydrolysis of the imidazole ring to the chemically and biologically more stable AFB1-FAPY adducts, or by hydrolysis of the glycosidic bond to yield an abasic site.6 One of the FAPY adducts is thought to be the principal driver of AFB1 mutations13, which is primarily the G to T transversion. G:C to T:A is also the dominant mutation type observed in the liver tumors of people exposed to AFB1.14 A biochemical strategy that blocks AFB1-DNA damage involves AFB1-glutathione conjugation by alpha class glutathione-S-transferases and other Phase II enzymes that effectively shunt the AFB1-8,9-epoxide from DNA,15–16 rendering the toxin harmless in vivo. While mice start life very sensitive to AFB1, they become increasingly refractory over the first two weeks of life as the levels rise of the enzymes that destroy the epoxide,17 and this biochemical reprogramming is likely to be one factor that renders the adult mouse completely insensitive to carcinogenesis by AFB1 when treated during late adolescence or adulthood.17
Studies by Vesselinovitch and Wogan showed that a single exposure of the B6C3F1 mouse to AFB1 during the neonatal period, days 1 to 7 after birth, results in a high incidence of HCC in adulthood.18 By contrast adult mice, post day 28, exposed to the same dose do not develop HCC at any age. The high initial sensitivity to AFB1 that tapers off over the first two weeks of life makes the B6C3F1 mouse a good model to understand how specific host variables, such as toxin metabolism and DNA adduct formation, relate to later life biological outcomes, including growth stunting, cognitive defects and genetic diseases including cancer. This model also provides an opportunity to probe the basis for the selective sensitivity of males to carcinogenesis. Newborn B6C3F1 mice treated with single or multiple doses of AFB1 develop HCC by 82 weeks of age in >90% of the males, compared to <10% of identically treated females.18
The high sensitivity of newborn mice to AFB1 prompts the question as to whether and to what degree AFB1 also poses a risk to the fetus during gestation. In utero risk is suggested by the detection of AFB1-DNA adducts in human cord blood and placental tissue.19–20 AFB1 has been shown to act as a transplacental carcinogen in Sprague Dawley (SD) and Wistar rats 21–22 and in hepatitis B transgenic mice.23
Epidemiological and animal studies have established AFB1-DNA adducts as valuable predictors of carcinogenic risk in experimental animals and in humans.24 Adducts are widely used biomarkers because they are believed to be the chemical precursors to the genetic changes that initiate or facilitate the conversion of normal cells to cancer cells.14 To date there have been no studies quantitatively linking DNA adducts and mutagenic endpoints in mice treated in utero. Recent work has shown that the AFB1-N7-Gua and AFB1-FAPY adducts are formed in the liver DNA of B6C3F1 mice treated with AFB1 shortly after birth, at days 1–4 of life. Adduct levels in the neonates correlate with the frequency, types and distributions mutations in liver tissues of 3-week and 10-week old mice.25 The predominant mutation in these animals is the G:C to T:A transversion, which is a signature of genetic damage produced by aflatoxin exposure.25
To quantify the risk of even earlier life exposure to AFB1, in the present study we measured AFB1-DNA adduct levels, and characterized the subsequent frequency and spectrum of mutations arising in fetus-exposed livers at 3 and 10 weeks of age. Although several studies have examined mutations in mice treated with chemicals in utero, the quantitative relationships between chemical damage to DNA during fetal development and future health risks are largely unexplored. B6C3F1 mice were used, because they are known to be sensitive to AFB1-induced HCC and, of great importance, they are widely used in regulatory analyses as the principal murine model for assessment of chemicals for carcinogenic activity. The strain of B6C3F1 mice used was molecularly equipped with a gpt transgene that can use as reporter gene to detect base pair substitutions and deletions. The present study builds on earlier work relating DNA adducts and human HCC susceptibility26; it reveals, using an animal model, a strikingly strong early-life vulnerability to a naturally occurring carcinogen, using DNA adducts and gpt mutations as predictive metrics of later-life susceptibility.
Materials and Methods
Chemicals
AFB1 was purchased from Sigma Chemical (St. Louis, MO). Unless otherwise specified, all reagents were at least of ACS reagent grade.
Animals
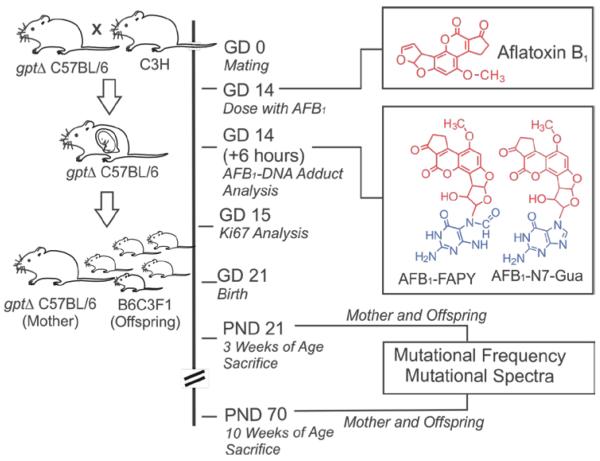
C57BL/6J gpt delta transgenic mice were obtained from Dr. Takehiko Nohmi.27 The gpt delta B6C3F1 mice used in our experiment were generated by breeding female gpt delta C57BL/6J mice, which harbor an estimated 80 copies of the gpt gene on chromosome 17, with male C3H/HeJ mice that were purchased from the Jackson Laboratories (Bar Harbor, Maine). Pregnant gpt delta C57BL/6J mice were administered AFB1 (6 mg/kg dissolved in 100 μL of DMSO for mutation analysis and 5 mg/kg for mutagenesis) via intraperitoneal (i.p.) injection or oral gavage (5 mg/kg for both adduct analysis and mutagenesis) on gestation day 14 (GD14); before being dosed with AFB1, the mothers used in this study had all given birth to at least one litter prior to inclusion in the study. Control animals received 100 μL DMSO only. At the indicated times (Figure 1), mice were euthanized and liver tissue was collected for isolation of DNA. All experiments were conducted in accordance with protocols approved by the MIT Committee on Animal Care.
Figure 1.
Experimental scheme for treatment of pregnant gpt delta C57BL/6J mice at gestation day 14 (GD14) with 6 mg/kg of AFB1, analysis of AFB1-DNA adducts at 6 hr and analysis of mutations in the gpt transgene at postnatal day 21 (3 weeks) and 70 (10 weeks) after birth.
DNA adducts analysis in liver tissues
DNA for AFB1 adduct analysis was isolated from nuclei using previously described methods.28 AFB1-DNA adducts were hydrolyzed by treatment with 0.1 N HCl at 95 °C for 15 min.9 After hydrolysis to liberate AFB1-DNA adducts, internal 15N5-guanine-derived standards were added to permit quantitative analysis by isotope dilution mass spectrometry for both AFB1-N7-Gua and AFB1- FAPY. Adducts were separated by UPLC-MS. The protonated parent ion of the AFB1-N7-Gua adduct (m/z 480.1) was selected and subjected to collision-induced fragmentation producing a m/z 152 product ion that was monitored to quantify adduct levels. The AFB1-FAPY adduct was measured by selection of the m/z 498 parent ion and monitoring the collision-induced product ion m/z 452.29
DNA isolation and in vitro packaging
Liver tissue was pulverized in a mortar and pestle under liquid nitrogen and stored at −80°C. Genomic DNA was extracted from approximately 25 mg of tissue using the RecoverEase DNA Isolation Kit (Agilent Technologies, Santa Clara, CA). λ-EG10 phages were packaged in vitro from genomic DNA using Transpack Packaging Extract (Agilent Technologies) following the manufacturer's instructions.
Gpt mutation assay and sequencing analysis
Mutations in the gpt gene were identified by selection with 6-thioguanine (6-TG), as previously described.27 Briefly, λ-EG10 phages rescued from genomic DNA were transfected into E. coli YG6020 expressing Cre recombinase and cultured on selective media containing chloramphenicol (Cm) and 6-TG or Cm alone for 72 hr until the appearance of colonies. 6-TG resistance was confirmed by regrowth of colonies on plates containing Cm and 6-TG. For sequencing, plasmid DNA was isolated using a Qiagen Miniprep Kit (Qiagen, Valencia, CA) according to the manufacturer's instructions. Sequencing of the gpt gene was performed by the Biopolymers Facility at Harvard Medical School (Boston, MA) on a 3730xL DNA Analyzer (Applied Biosystems, Foster City, CA) using the forward primer: 5' TCTCGCGCAACCTATTTTCCC -3'. Sequencing data were aligned with the E. coli gpt gene (GenBank M13422.1) using NCBI Nucleotide Blast. Mutation frequencies (MFs) were calculated as the number of confirmed 6-TG mutant colonies divided by the total number of Cm-resistant colonies.
Immunohistochemical staining of mouse liver for Ki67
Liver specimens were fixed in formalin, embedded in paraffin and cut into 4 μm sections. Following deparaffinization, rehydration and antigen retrieval with citrate buffer, endogenous peroxidase activity was blocked (DAKO 3950, Agilent Technologies). Sections were incubated for 60 min with a Ki67 antibody (Santa Cruz Biotechnology, Inc. USA; sc-15402) followed by a secondary biotinylated antibody (Rabbit anti-mouse IgA-B, Santa Cruz Biotechnology, Inc. USA; sc-358961). Immune complexes were visualized by addition of streptavidin conjugated horseradish peroxidase and diaminobenzidine (DAB) reagent. Tissues were counterstained with hematoxylin. A proliferation index was determined as the number of Ki67 positive cells in 10 high power fields (40X) divided by the total number of observed cells.
Statistical analysis
Student two-tailed t tests were used to analyze DNA adduct and mutation frequency data. Analyses were done with GraphPad Prism software and a value of less P < 0.05 was considered statistically significant.26, 30 The Mann-Whitney nonparametric test was used to determine the statistical significance of differences in MF.
Results
Mutagenic AFB1-DNA adducts form in both fetal and maternal liver
DNA adducts formed by AFB1 are potent inducers of G:C to T:A mutations,14, 31 which is among the most frequent mutations found in human HCC.14 To determine whether the developing fetus is at risk of genetic damage from maternal exposures to AFB1, pregnant gpt delta C57BL/6J mice at gestation day 14 were treated with the toxin and the levels of AFB1-DNA adducts in both maternal and B6C3F1 fetal livers were quantified six hr after administration of AFB1 via i.p. injection, providing a snapshot of adduct density at a time when it is reasonable to assume that carcinogenesis is initiated. The experimental scheme is shown in Figure 1. The amounts of AFB1-N7-Gua and AFB1-FAPY adducts were analyzed by quantitative isotope dilution mass spectrometry (Table 1).29 The highest levels of both adduct types were found in the C57BL/6J maternal DNA where there was approximately 2-fold greater amounts of the persistent AFB1-FAPY compared to AFB1-N7-Gua. These observations are consistent with the rapid removal of the latter adduct as well as its conversion to the more chemically stable AFB1-FAPY by chemical hydrolysis of the imidazole ring.28 Lower but measurable levels of AFB1 adducts were found in B6C3F1 fetal liver DNAs. A total of 12 fetal livers; six from each of two litters were analyzed. Within each litter there was a good correlation of adduct levels among individuals with equal amounts of the AFB1-N7-Gua and AFB1-FAPY adducts. Overall, fetal liver adduct levels (0.62 adducts/106 nucleotides) were 100-fold lower than in maternal liver (63.5 adducts/106 nucleotides). These results indicate that the toxin reaches the liver, and that the developing liver in utero is capable of metabolizing AFB1 to its reactive exo-8,9-epoxide, which has the ability to form covalent DNA adducts with potentially mutagenic properties.13,31
Table 1.
Level of AFB1-DNA adducts in maternal (C57BL/6J) and fetal (B6C3F1) liver six hr after treatment with 5 mg/kg AFB1 administered via i.p. injection or oral gavage.
| AFB1 adducts/106 nucleotides | ||||
|---|---|---|---|---|
| i.p. injection | Oral gavage | |||
| Fetus (n=12) | Mother (n=2) | Fetus (n=10) | Mother (n=2) | |
| AFB1-N7-Gua | 0.31 ± 0.25 | 18.5 ± 2.5 | 0.07 ± 0.04 | 6.2 ± 0.8 |
| AFB1-FAPY | 0.30 ± 0.19 | 45 ± 6 | <LOQ* | 19.1 ± 0.4 |
| Total | 0.62 ± 0.31 | 63.5 ± 8 | N/A | 25.3 ± 1.2 |
Mean ± SD
Limit of Quantitation
One potential complication of the experiment described above is that compounds administered to pregnant animals via i.p. injection could directly access the uterine compartment without entering the maternal circulation. To understand how this possibility might influence adduct levels, in a separate experiment, pregnant C57BL/6J mice were treated with AFB1 by gavage and once again DNA adducts were measured after six hr. After oral administration we found that the AFB1-N7-Gua and AFB1-FAPY adducts were present in maternal liver in the same proportion as found after i.p. injection; however, total adduct levels by this route, were about 2.5-fold lower than levels after i.p. administration. Both of the AFB1-DNA adduct types were also observed in the fetal DNA liver samples of gavage-treated animals, but only the AFB1-N7-Gua was present at amounts above the limit at which confident quantitation was possible. The levels of this adduct were about four-fold lower than those found in fetal DNA after i.p. dosing. Differences in the rates of absorption, distribution and metabolism of AFB1 are likely responsible for the lower levels of AFB1-DNA adducts following gavage administration. Based on this experiment, in which AFB1-DNA adducts were observed in fetal liver after treatment via either i.p. injection or by oral administration it is clear that the developing liver is at risk for genetic changes or toxicity that could predispose animals to develop later life diseases.
Aflatoxin is mutagenic to GD14-embryonic mice, as detected ten weeks after birth
The experiment above showed that DNA adducts form in the fetus, but at only about 1% the absolute level that occurs in the mother. We next probed whether the low but finite level of adduct formed in the fetus posed a significant threat for mutations that could be detected in adolescence or adulthood (at three or ten weeks of age). The gpt transgenic locus in the gpt delta C57BL/6J mouse was used to determine the impact of AFB1-DNA damage in utero on the mutant frequency in the livers of hybrid B6C3F1 mice. Pregnant gpt delta C56BL/6J mice were treated on GD14 with 6 mg/kg AFB1 via i.p. injection; control animals received the DMSO vehicle only. The E. coli gpt reporter gene was rescued from genomic DNA, and 6-TG resistant mutants were enumerated. This assay is sensitive to point mutations, such as base substitutions, and frameshifts resulting from small deletions and insertions, which are characteristic of the AFB1-induced mutational spectrum.14, 31
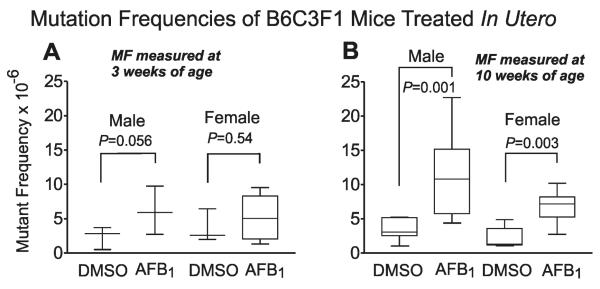
Mothers gave birth to B6C3F1 pups at around GD21. There were no observable phenotypic differences between AFB1-treated and control litters. In an initial experiment, mothers and pups were sacrificed at weaning, 3 weeks of age. Genomic DNA was isolated from liver tissue and mutational analysis was performed on the gpt transgene. At three weeks of age (Figure 2A), we did not observe a statistically significant increase of AFB1-induced mutations in the GD14 B6C3F1 mice above the baseline level in control (DMSO-treated) animals (both males and females).
Figure 2.
Gpt mutant fraction (MF) in gpt delta B6C3F1 liver at PND 21 (A) or PND 70 (B). Pregnant mice at gestation day 14 (GD14) were administrated 6 mg/kg of AFB1 or DMSO vehicle via i.p. injection. Plotted data; mean ± SD; n ≥ 5.
At ten weeks of age (Figure 2B), however, both males and females treated at GD14 showed a significantly elevated MF in AFB1-treated over control animals. We found that exposure to AFB1 on GD14 statistically increased the gpt MF by 3-fold in 10 week old male mice (P=0.001) and 2.5-fold in 10 week old females (P=0.003) compared with DMSO-treated controls. Overall, males and females, when treated in utero, appear to be equally sensitive to the mutagenic properties of AFB1 at the ten-week time point.
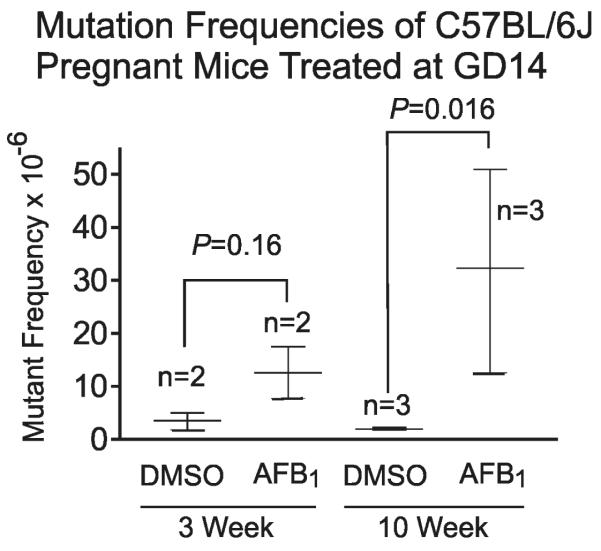
Figure 3 examines the gpt MFs of the C57BL/6J mothers of the B6C3F1 mice described above. The mothers followed the same pattern as their offspring, with significant enhancement of AFB1-induced mutations seen at 10 weeks after parturition (P=0.016). As indicated above, the mothers had 100x higher levels of DNA damage than the GD14 fetus, yet comparison of Figures 2B and 3 reveals only a 4.6 fold difference in the absolute MF between the mothers and fetuses (based upon MF measurements at 10 weeks post birth). The reasons for the apparently enhanced mutagenic potency of AFB1 lesions in the fetus are discussed below.
Figure 3.
Gpt mutant fraction (MF) in maternal gpt delta C57BL/6J liver at 3 weeks or 10 weeks after parturition. Pregnant mice at gestation day 14 (GD14) were administrated 6 mg/kg AFB1 or DMSO vehicle via i.p. injection. Plotted data; mean ± SD.
Aflatoxin induces distinctive mutational types and mutational spectra in the gpt gene of mice treated at GD14
To determine whether the types of mutations in the gpt gene were related to AFB1 treatment and distinct from controls, gpt mutants were sequenced from both control and AFB1-treated mice (the controls had fewer mutants, as expected). The types of mutations are enumerated in Supplementary Table 1 and included base substitutions, insertions and deletions in both AFB1-treated and control mice. The predominant mutations found in control liver were G:C to A:T transitions and deletions, whereas G:C to T:A transversions were most common in AFB1-treated animals. For example, at ten weeks 42% of total mutants were G:C to T:A. Interestingly, while the absolute MFs of three week old AFB1-treated and control mice were not statistically different (Figure 2A), the signature G:C to T:A mutation of AFB1 is clearly evident at three weeks in Supplementary Table 1, where this mutation accounts for 49% of all mutants. Overall, at three and ten weeks post birth, the B6C3F1 mice treated in utero showed a mutational pattern that is consistent with that known to be produced by the AFB1-N7-Gua and AFB1-FAPY adducts.14, 31
Supplementary figures 1 and 2, which plot the frequency distribution of types of mutations across the gpt gene, provide further granularity regarding the mutagenic properties of AFB1 following in utero exposure. As expected, this mutational spectrum is strongly influenced by the selective conditions used to isolate 6-TG resistant mutants. Nonsense and missense mutations plus insertions and deletions producing frameshifts that inactivate gpt determine the resistant phenotype. The mutational spectrum observed for B6C3F1 mice treated in utero looks identical to that determined previously by Woo et al.25 for animals treated at four days post parturition. As in the experiment done by Woo et al., we observed that the MF in liver seems to elevate by 2.5 to 3-fold between three and ten weeks of age (e.g., comparison of Figures 2A and 2B). Trying to understand the basis for this consistently observed elevation in absolute MF over time, a program based on the Adams-Skopek algorithm for comparison of mutational spectra32 was used to test the possibility that the distribution of mutations (and not simply the absolute MF) in the gpt gene may change between three-week and ten-weeks (Supplementary figures 1 and 2). The results of the Monte Carlo based analysis indicated that the two mutational spectra were not significantly different (P= 0.0689). Below, we speculate on other possible reasons why the MF rises between three and ten weeks of age.
Developmental stage and cell proliferation in the liver
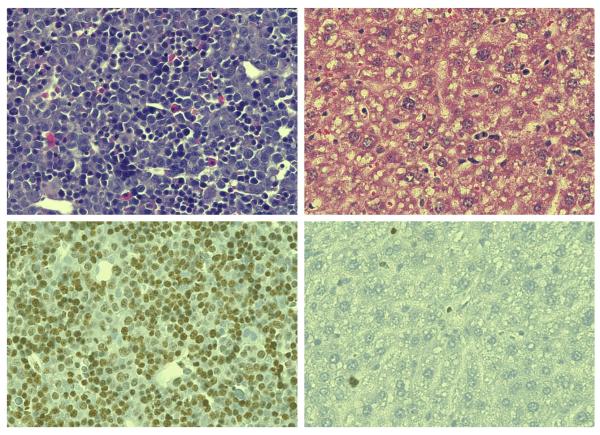
It is likely that replicating cells in the developing liver are more vulnerable than quiescent ones to genetic damage from environmental chemicals. If this sensitivity differential indeed exists, then AFB1-DNA adducts formed in the fetal liver would have a greater quantitative potential (on a mutation per-adduct basis) to cause mutations than AFB1 exposure later in life. To investigate the differences in growth rate between fetal and adult livers, liver tissues were harvested and assayed for the numbers of proliferating cells by immunohistochemical staining with an antibody to Ki67.33 Figure 4 shows that the proportion of cells expressing the Ki67 antigen and staining intensity varied between the developmental stages. In the fetal liver ~80% of cells were Ki67 positive. In contrast, the liver specimen obtained from an adult C57BL/6J mouse had <1% of cells that stained positive for Ki67. These results provide an important proliferation metric against which to compare mutation rate and adduct density over the mouse life course. In principle, the high rate of proliferation of the fetal liver could present a substantial liability.
Figure 4.
Immunochemical analysis of mouse liver for Ki67 cell proliferation marker in fetal (GD 15) and maternal (Adult). Hemotoxalin/Eosin stain, Top Panels; Ki67 and Hematoxaline counterstain, Bottom Panels.
Discussion
Neonatal and infant mice are highly sensitive to hepatocarcinogenesis during a relatively short period following birth.34 Intrauterine exposures to chemical carcinogens have also been demonstrated to induce liver tumors if exposures occur following organogenesis.35 These early life periods of sensitivity are dependent upon both the metabolic capability of the liver to activate carcinogens and the rapid cell division required for growth and development.17 In this study a quantitative association is made between AFB1 exposures, measured as covalent DNA adducts, in adult and embryonic mice and increased mutations in offspring. The presence of AFB1-DNA adducts in fetal liver following administration of AFB1 by either i.p. injection or gavage indicated that the fetal liver is able to produce the AFB1-exo-8,9-epoxide, the aflatoxin metabolite that best correlates with carcinogenic, toxic and mutagenic outcomes.
Evidence is provided that AFB1-DNA adducts formed in fetal liver DNA are much more likely to produce mutations than the same amount of DNA damage would if it occurred in the liver of an adult animal. For example, administration of AFB1 to pregnant mice results in only about 1% the level of adducts in the fetus at six hours, as compared to the level in the mother. All things being equal, one would expect a mutation rate in the fetus that was similarly 1% that of the mother. The mutation rate, by contrast, was much higher than expected (Table 2), with the fetus and mother showing only a 4.6 fold difference in MFs, rather than the anticipated 100-fold difference. Taken together, our observations on longitudinal mutagenic sensitivity of mice, normalized to the level of DNA damage, indicate that AFB1-DNA adducts have a greater likelihood of causing mutations in the developing fetus than they do in adults.
Table 2.
Influence of developmental stage on the relative risk of gpt mutations in liver from AFB1-DNA adducts after treatment with 6 mg/kg of AFB1 administered via i.p. injection.
| Fetus | Adult | |
|---|---|---|
| AFB1-DNA adducts (adducts/106 bases) | 0.62 | 63.5 |
| 10 wk gpt MF (×10−6) | 6.5 | 30 |
| gpt MF/DNA adducts | 10 | 0.5 |
| Relative risk of mutation1 | 20 | 1 |
| Mitotic Index (liver)2 | 80 | <1 |
Mutation risk relative to adult liver = [gpt MF/DNA adducts]/[gpt MF/DNA adducts]adult
Determined by Ki67 staining
It has long been assumed that the frequency of DNA replication correlates with the frequency of both spontaneous and DNA damage-induced mutations. We calculated an estimate of the ability of an AFB1 adduct to produce a mutation in the gpt transgene by dividing the gpt MF by the number of adducts per megabase in liver DNA (Table 2). The relative risk of a gpt mutation per AFB1 adduct is 20 fold greater in the fetal liver as compared to the adult. The mitotic index of the fetal liver was at least 80 fold higher than the adult liver, which leads us to believe that the high mutation per adduct result observed is owed to the high rate of fetal liver cell division. The present data give direct experimental support to the longstanding view that the high rate of cell division of young animals helps explain their enhanced risk to DNA damage mediated disease.
In addition to a higher than expected mutagenic potency of DNA adducts in the pre-natal liver, we observed that the MF of AFB1 appears to increase, albeit slightly, as the animal matures. This observation extends a conclusion made by Woo et al.25 where we saw that animals dosed at four days of age showed a slightly lower mutation frequency at three weeks of life compared to that at ten weeks of life. In the present experiment, the MF during this seven-week interval (between three and ten weeks of life) for mice treated in utero increased by 1.8-fold in males and 1.3-fold in females (after subtraction of the MF contribution from the DMSO controls). The increased MFs were correlated with increases in liver weight from 0.8 ± 0.07 to 1.4 ± 0.37 g in males (1.75-fold) and from 0.72 ± .05 to 0.97 ± 0.34 g in females (1.3-fold). This correlation is consistent with the rise in mutations being a function of increased cell number occurring as the liver gains weight during this growth period. It is not immediately obvious why an increase in cell number would cause a selective increase in MF at time points so distant from the time at which the toxin was administered. One explanation for the increase in MF is the selective expansion of mutant cells. The gpt transgene present in the recoverable phage lambda vector is not expressed in the mouse and is assumed to be genetically neutral, providing no advantage or disadvantage to cell growth.36 Consequently, it would be expected that once mutations are fixed by DNA replication, the average MF in the liver would remain constant. However, liver cell organization and function are continuously being remodeled and reprogrammed, respectively, during the late fetal and early neonatal periods resulting in unequal growth of liver cell types that could increase or decrease the observed MF. Zonal differences in cell proliferation in the liver occur during fetal development, postnatal growth and homeostasis in the adult organ.37–38 If tissue progenitor cells are at greater risk of mutation during fetal development, mutant cells emerging from these populations would be expected to increase the MF in the tissue, which could account for the observed increase in MF at the ten-week time point.
A second factor that could contribute to the enhanced MF between three and ten weeks is the possibility that “jackpot” mutations could have occurred early after dosing with AFB1. Statistically, however, it is unlikely that we would have observed jackpots in our analyses since the samples for mutational analysis were prepared from the entire liver. Consequently, only a small fraction of cells from a clone would be expected to be included in our analysis. Consistent with these expectations, we only rarely observed an identical gpt mutation in the same animal.
A third explanation for the increased MF during the seven-week period is the generation of new mutations arising from continued replication past AFB1-damage. AFB1-FAPY adducts, which represent 50% of AFB1-DNA adducts found six hr after dosing are very persistent. In a previous study in neonatal B6C3F1 mice the highly mutagenic AFB1-FAPY adduct was readily detectable in liver DNA three weeks after animals were dosed at four days of age.31 The continued predominance of G:C to T:A transversions in the mutational spectrum at ten weeks of age is consistent with the persistence of AFB1-FAPY in liver DNA after birth following transplacental exposures to AFB1.
One interesting observation in the present study was a higher than expected level of G:C to A:T mutations in the AFB1-dosed fetal liver than we observed in earlier work in an experiment in which animals of the same genotype were treated with the toxin shortly after birth. The fetal liver contains a different array of cell populations than is present in the mature liver; it is colonized by hematopoietic stem cells (HSCs) along with fetal macrophages until the end of prenatal life when HSCs migrate to the spleen and bone marrow.39–41 This developmental juxtaposition places proliferating hepatoblasts in the vicinity of immune system cells capable of producing reactive oxygen species. The latter may contribute to DNA damage that is reflected in the gpt mutational spectra observed following in utero AFB1 exposure. The majority of base substitution mutations from in utero AFB1 exposure were G:C to T:A transversions (40–50% of gpt mutants). The next most prevalent mutation type was the G:C to A:T transition (27% of gpt mutants), which is considered an indication of base damage by oxygen radicals.42–43 In our previous study of neonatal mice treated with AFB1 on day four, G:C to A:T transitions represented only 16% of total mutants.25 This higher relative amount of G:C to A:T in the present study (27% vs. 16%) suggests the possibility that the fetus is more prone to oxidative stress.
The proximity of HSCs to hepatoblasts and other cells in the fetal liver that are capable of metabolic activation of AFB1 could also place those cells at risk for mutation, increasing the chance of developing blood malignancies and myeloid diseases. This hypothesis is supported by the observed increase in leukemias among the F1 generation of rats after transplacental exposures to AFB1.21
Implications for human disease
Our data suggest that transplacental exposures to AFB1, on an adduct-normalized basis, present a much greater risk of genetic change than exposures that take place later in life. One conclusion is that estimates of risk of HCC in the general population may vastly underestimate the risk of adverse effects following prenatal exposures. Risk estimates typically are based on albumin- or urinary DNA-adduct levels, which correlate with genomic damage in people. From our study, we suggest that an additional factor that takes into account the rapid cell division associated with early life is an important amplifier of risk. Because of the elevated risk of adverse health effects to offspring it is especially important to prevent maternal exposures to aflatoxins. In addition, to mitigate the potential of adverse effects due to unavoidable exposures to aflatoxins in contaminated foods, it would be useful to explore preventive measures that enhance maternal pathways of metabolism and detoxification.
Supplementary Material
Novelty statement.
Young animals are typically more sensitive to carcinogens than older ones. The reason(s) for this differential sensitivity are not fully understood. This work shows that gestation day 14 mice experiencing in utero exposure to aflatoxin B1 are approximately 20-fold more sensitive to mutagenesis than an adult. This enhanced sensitivity to genetic change correlates with elevated expression of a marker of proliferation in the prenatal animal.
Acknowledgments
This work was supported by National Institutes of Health grants ES016313, P30-ES002109; P01 ES006052; P30 ES003819; P30 CA006973; The Center of Excellence on Environmental Health, Toxicology and Management of Chemicals, Thailand (S.C.). S.C. gratefully acknowledges a Schlumberger Foundation Faculty for the Future grant.
Grant sponsors: United States National Institutes of Health grants ES016313, P30-ES002109; P01 ES006052; P30 ES003819; P30 CA006973; The Center of Excellence on Environmental Health, Toxicology and Management of Chemicals, Thailand (S.C.). S.C. is supported by a Schlumberger Foundation Faculty for the Future grant.
List of abbreviations
- AFB1
aflatoxin B1
- AFB1-FAPY
formamidopyrimidine-AFB1
- AFB1-N7-Gua
8, 9-dihydro-8-(N7-guanyl)-9-hydroxy AFB1
- GD
gestation day
- Cm
chloramphenicol
- DAB
diaminobenzidine
- DMSO
dimethyl sulfoxide
- gpt
guanine phosphoribosyltransferase
- HBV
hepatitis virus B
- HCC
hepatocellular carcinoma
- HSCs
hematopoietic stem cells
- i.p
intraperitoneal
- UPLC-MS
Ultra-high performance liquid chromatography and mass spectrometry
- MF
mutation frequencies
- 6-TG
6-thioguanine
Footnotes
Financial disclosure, conflict of interest: The authors declare no financial or other conflicts of interest in the conception, conduct of the research or preparation of the results for publication.
References
- 1.Anderson LM, Diwan BA, Fear NT, et al. Critical windows of exposure for children's health: cancer in human epidemiological studies and neoplasms in experimental animal models. Environ Health Perspect. 2000;108:573–94. doi: 10.1289/ehp.00108s3573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gluckman PD, Hanson MA, Cooper C, et al. Effect of in utero and early-life conditions on adult health and disease. N Engl J Med. 2008;359:61–73. doi: 10.1056/NEJMra0708473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pallapies D. Trends in childhood disease. Mutat Res. 2006;608:100–11. doi: 10.1016/j.mrgentox.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 4.Ford AM, Ridge SA, Cabrera ME, et al. In utero rearrangements in the trithorax-related oncogene in infant leukaemias. Nature. 1993;363:358–60. doi: 10.1038/363358a0. [DOI] [PubMed] [Google Scholar]
- 5.Gilman EA, Wilson LM, Kneale GW, et al. Childhood cancers and their association with pregnancy drugs and illnesses. Paediatr Perinat Epidemiol. 1989;3:66–94. doi: 10.1111/j.1365-3016.1989.tb00371.x. [DOI] [PubMed] [Google Scholar]
- 6.Busby WF, Wogan GN. Aflatoxins; Chemical Carcinogens. In: Searle CD, editor. American Chemical Society. Washington, DC: 1984. pp. 945–1136. [Google Scholar]
- 7.International Agency for Research on Cancer IARC Cancer in Africa: epidemiology and prevention. IARC Sci Publ. 2003;8:1–414. [PubMed] [Google Scholar]
- 8.Essigmann JM, Croy RG, Nadzan AM, et al. Structural identification of the major DNA adduct formed by aflatoxin B1 in vitro. Proc Natl Acad Sci U S A. 1977;74:1870–4. doi: 10.1073/pnas.74.5.1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martin CN, Garner RC. Aflatoxin B -oxide generated by chemical or enzymic oxidation of aflatoxin B1 causes guanine substitution in nucleic acids. Nature. 1977;267:863–5. doi: 10.1038/267863a0. [DOI] [PubMed] [Google Scholar]
- 10.Lin JK, Miller JA, Miller EC. 2,3-Dihydro-2-(guan-7-yl)-3-hydroxy-aflatoxin B1, a major acid hydrolysis product of aflatoxin B1-DNA or -ribosomal RNA adducts formed in hepatic microsome-mediated reactions and in rat liver in vivo. Cancer Res. 1977;37:4430–8. [PubMed] [Google Scholar]
- 11.Groopman JD, Croy RG, Wogan GN. In vitro reactions of aflatoxin B1-adducted DNA. Proc Natl Acad Sci U S A. 1981;78:5445–9. doi: 10.1073/pnas.78.9.5445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Croy RG, Wogan GN. Quantitative comparison of covalent aflatoxin-DNA adducts formed in rat and mouse livers and kidneys. J Natl Cancer Inst. 1981;66:761–8. [PubMed] [Google Scholar]
- 13.Smela ME, Hamm ML, Henderson PT, et al. The aflatoxin B1 formamidopyrimidine adduct plays a major role in causing the types of mutations observed in human hepatocellular carcinoma. Proc Natl Acad Sci U S A. 2002;99:6655–60. doi: 10.1073/pnas.102167699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hussain SP, Schwank J, Staib F, et al. TP53 mutations and hepatocellular carcinoma: insights into the etiology and pathogenesis of liver cancer. Oncogene. 2007;26:2166–76. doi: 10.1038/sj.onc.1210279. [DOI] [PubMed] [Google Scholar]
- 15.Hayes JD, Judah DJ, Neal GE, et al. Molecular cloning and heterologous expression of a cDNA encoding a mouse glutathione S-transferase Yc subunit possessing high catalytic activity for aflatoxin B1-8,9-epoxide. Biochem J. 1992;285:173–80. doi: 10.1042/bj2850173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eaton DL, Gallagher EP. Mechanisms of aflatoxin carcinogenesis. Annu Rev Pharmacol Toxicol. 1994;34:135–72. doi: 10.1146/annurev.pa.34.040194.001031. [DOI] [PubMed] [Google Scholar]
- 17.Shupe T, Sell S. Low hepatic glutathione S-transferase and increased hepatic DNA adduction contribute to increased tumorigenicity of aflatoxin B1 in newborn and partially hepatectomized mice. Toxicol Lett. 2004;148:1–9. doi: 10.1016/j.toxlet.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 18.Vesselinovitch SD, Mihailovich N, Wogan GN, et al. Aflatoxin B1, a hepatocarcinogen in the infant mouse. Cancer Res. 1972;32:2289–91. [PubMed] [Google Scholar]
- 19.Hsieh LL, Hsieh TT. Detection of aflatoxin B1-DNA adducts in human placenta and cord blood. Cancer Res. 1993;53:1278–80. [PubMed] [Google Scholar]
- 20.Denning DW, Allen R, Wilkinson AP, et al. Transplacental transfer of aflatoxin in humans. Carcinogenesis. 1990;11:1033–5. doi: 10.1093/carcin/11.6.1033. [DOI] [PubMed] [Google Scholar]
- 21.Goerttler K, Lohrke H, Schweizer HJ, et al. Effects of aflatoxin B1 on pregnant inbred Sprague-Dawley rats and their F1 generation. A contribution to transplacental carcinogenesis. J Natl Cancer Inst. 1980;64:1349–54. doi: 10.1093/jnci/64.6.1349. [DOI] [PubMed] [Google Scholar]
- 22.Grice HC, Moodie CA, Smith DC. The carcinogenic potential of aflatoxin or its metabolites in rats from dams fed aflatoxin pre- and postpartum. Cancer Res. 1973;33:262–8. [PubMed] [Google Scholar]
- 23.Kaplanski C, Chisari FV, Wild CP. Minisatellite rearrangements are increased in liver tumours induced by transplacental aflatoxin B1 treatment of hepatitis B virus transgenic mice, but not in spontaneously arising tumours. Carcinogenesis. 1997;18:633–9. doi: 10.1093/carcin/18.4.633. [DOI] [PubMed] [Google Scholar]
- 24.Groopman JD, Kensler TW, Wild CP. Protective interventions to prevent aflatoxin-induced carcinogenesis in developing countries. Annu Rev Public Health. 2008;29:187–203. doi: 10.1146/annurev.publhealth.29.020907.090859. [DOI] [PubMed] [Google Scholar]
- 25.Woo LL, Egner PA, Belanger CL, et al. Aflatoxin B1-DNA adduct formation and mutagenicity in livers of neonatal male and female B6C3F1 mice. Toxicol Sci. 2011;122:38–44. doi: 10.1093/toxsci/kfr087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Groopman JD, Wild CP, Hasler J, et al. Molecular epidemiology of aflatoxin exposures: validation of aflatoxin-N7-guanine levels in urine as a biomarker in experimental rat models and humans. Environ Health Perspect. 1993;99:107–13. doi: 10.1289/ehp.9399107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nohmi T, Katoh M, Suzuki H, et al. A new transgenic mouse mutagenesis test system using Spi- and 6-thioguanine selections. Environ Mol Mutagen. 1996;28:465–70. doi: 10.1002/(SICI)1098-2280(1996)28:4<465::AID-EM24>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 28.Croy RG, Wogan GN. Temporal patterns of covalent DNA adducts in rat liver after single and multiple doses of aflatoxin B1. Cancer Res. 1981;41:197–203. [PubMed] [Google Scholar]
- 29.Egner PA, Groopman JD, Wang JS, et al. Quantification of aflatoxin-B1-N7-Guanine in human urine by high-performance liquid chromatography and isotope dilution tandem mass spectrometry. Chem Res Toxicol. 2006;19:1191–5. doi: 10.1021/tx060108d. [DOI] [PubMed] [Google Scholar]
- 30.Wattanawaraporn R, Woo LL, Belanger C, et al. A single neonatal exposure to aflatoxin B1 induces prolonged genetic damage in two loci of mouse liver. Toxicol Sci. 2012;128:326–33. doi: 10.1093/toxsci/kfs151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bailey EA, Iyer RS, Stone MP, et al. Mutational properties of the primary aflatoxin B1-DNA adduct. Proc Natl Acad Sci U S A. 1996;93:1535–9. doi: 10.1073/pnas.93.4.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Adams WT, Skopek TR. Statistical test for the comparison of samples from mutational spectra. J Mol Biol. 1987;194:391–6. doi: 10.1016/0022-2836(87)90669-3. [DOI] [PubMed] [Google Scholar]
- 33.Schluter C, Duchrow M, Wohlenberg C, et al. The cell proliferation-associated antigen of antibody Ki-67: a very large, ubiquitous nuclear protein with numerous repeated elements, representing a new kind of cell cycle-maintaining proteins. J Cell Biol. 1993;123:513–22. doi: 10.1083/jcb.123.3.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vesselinovitch SD. Perinatal mouse liver carcinogenesis as a sensitive carcinogenesis model and the role of the sex hormonal environment in tumor development. Prog Clin Biol. 1990:53–68. [PubMed] [Google Scholar]
- 35.Vesselinovitch SD, Koka M, Rao KV, et al. Prenatal multicarcinogenesis by ethylnitrosourea in mice. Cancer Res. 1977;37:1822–8. [PubMed] [Google Scholar]
- 36.Masumura K, Nohmi T. Spontaneous mutagenesis in rodents: Spontaneous gene mutations identified by neutral reporter genes in gpt delta transgenic mice and rats. J Health Sci. 2009;55:10. [Google Scholar]
- 37.Furuyama K, Kawaguchi Y, Akiyama H, et al. Continuous cell supply from a Sox9-expressing progenitor zone in adult liver, exocrine pancreas and intestine. Nat Genet. 2011;43:34–41. doi: 10.1038/ng.722. [DOI] [PubMed] [Google Scholar]
- 38.Burkhardt S, Bahnemann R, Failing K, et al. Zonal distribution of cell proliferation in the liver of untreated B6C3F1 and C57BL mice. Toxicol Pathol. 2004;32:100–5. doi: 10.1080/01926230490266000. [DOI] [PubMed] [Google Scholar]
- 39.Ciriza J, Thompson H, Petrosian R, et al. The migration of hematopoietic progenitors from the fetal liver to the fetal bone marrow: lessons learned and possible clinical applications. Exp Hematol. 2013;41:411–23. doi: 10.1016/j.exphem.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 40.Naito M, Hasegawa G, Takahashi K. Development, differentiation, and maturation of Kupffer cells. Microsc Res Tech. 1997;39:350–64. doi: 10.1002/(SICI)1097-0029(19971115)39:4<350::AID-JEMT5>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 41.Sasaki K, Sonoda Y. Histometrical and three-dimensional analyses of liver hematopoiesis in the mouse embryo. Arch Histol Cytol. 2000;63:137–46. doi: 10.1679/aohc.63.137. [DOI] [PubMed] [Google Scholar]
- 42.Reid TM, Fry M, Loeb LA. Endogenous mutations and cancer. Princess Takamatsu Symp. 1991;22:221–9. [PubMed] [Google Scholar]
- 43.Kreutzer DA, Essigmann JM. Oxidized, deaminated cytosines are a source of C -> T transitions in vivo. Proc Natl Acad Sci U S A. 1998;95:3578–82. doi: 10.1073/pnas.95.7.3578. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.