Abstract
Previously we reported that follicle stimulating hormone (FSH) affects bone degradation in human cells and in FSH-R null mice. Here we describe a FSH-R knockout bone formation phenotype. We used mesenchymal stem cells (MSCs), osteoblast precursors that express follicle stimulating hormone receptor (FSH-R), to determine whether FSH regulates bone formation. FSH stimulates MSC cell adhesion 1–3 h and proliferation at 24 h after addition. On the basis of phylogenetic and clinical precedents, we also examined effects of pregnant levels of human chorionic gonadotropin (hCG) on MSCs. We found effects similar to those of FSH, and RNAi knockdown of FSH-R abrogated both FSH and hCG effects on MSCs. In contrast to effects on MSCs, neither FSH nor hCG had significant effects on osteoblast maturation. Also in MSCs, short term treatment by FSH and hCG altered signaling pathways for proliferation, including Erk1/2 phosphorylation. Our results show augmentation of MSC proliferation by either FSH at menopausal levels or hCG at normal pregnant levels. We conclude that FSH-R participates in regulation of MSC precursor pools in response to either FSH or hCG, integrating the effects of these two glycoprotein hormones.
Keywords: FSH, hCG, Erk1/2, osteoporosis, osteoblast
Introduction
We previously characterized the expression of thyroid stimulating hormone receptor (TSH-R)1 and follicle stimulating hormone receptor (FSH-R),2 among others proteins, in bone. These studies were prompted by the unexpected occurrence of a bone phenotype in TSH-R knockout mice,3 indicating the importance of TSH-R in mesenchymal stem cells (MSCs), and the finding of FSH-R expression in cRNA gene screens of human or murine osteoclasts. The findings have been confirmed; and because the skeleton plays major roles in metabolism and reproduction, it is widely accepted that the primary glycoprotein hormones are active in the skeleton. There are, however, a number of unusual findings that remain difficult to explain. A major form of FSH-R in human monocytes and osteoclasts is a splice variant missing exon 9; and the absolute amount of FSH-R in peripheral cells is quite small.4
FSH is a member of a group of related hormones that includes luteinizing hormone (LH), thyroid stimulating hormone (TSH), and chorionic gonadotropin (hCG; the placental LH analog that maintains LH-R stimulation). Heterodimeric proteins sharing the α-chain peptide, these hormones and their receptors were discovered as pituitary (or placental) hormones that regulate endocrine glands in the human. Hormonal specificity is determined by the β-chains, which are products of gene duplication and divergence that retain homology. The TSHβ chain is the most divergent. Each hormone has a specific receptor, except for hCG and LH, which share the LH-R. In simpler phyla, this family of hormones has distributed functions. For example, in coelenterates, which have a primitive nervous system but no endocrine glands, a readily identifiable TSH gene is widely expressed and has a similar intron–exon structure to that found in mammals.5
This hormone family and its receptors have been extensively studied in lower vertebrates, where interesting differences relative to human hormonal response occur. In bony fishes, TSH-R is abundant in the thyroid and ovaries, and is detectable in other tissues, including heart, muscle, and brain.6 Also in fish, LH-R and FSH-R are expressed in the gonads; all higher orders retain this specialization. Curiously, multiple mRNA processing forms of the FSH-R occur in fish,7 which might reflect receptors with different functions, as discussed below. Further, the FSH-R in fishes binds both FSH and LH, whereas the LH-R recognizes only LH.8 In general, several groups have found strong FSH-R expression only in gonads, although low expression of FSH-R occurs in spleen of fish.9
Here we explore the role of FSH-R in human MSCs and osteoblasts differentiated from MSCs. We address the hypothesis that the FSH-R, possibly including isoforms with varying specificity, might integrate hCG and FSH effects, similar to its role in fishes.8 While this hypothesis might seem odd, in the 1970s radio-receptor competition assays showed an FSH-like activity in pregnant serum,10 although there is, for practical purposes, no FSH in pregnant serum. This activity largely was attributed to artifacts of the biological assays that demonstrated the effect,11 and this issue has been dormant since about 1990.
Our work supports a role for FSH-R in regulating MSC proliferation and maintenance of a non-differentiated state. However, there is no direct effect on osteoblast differentiation or bone formation. Our work also suggests that MSC-expressed FSH-R is less specific for FSH than is ovarian-expressed FSH-R. This might reflect that FSH-R type 2 (splicing variant missing exon 9) is a much larger percentage of receptor in peripheral cells than in ovary; this is not certain, however. Response of FSH-R to hCG might have created selective pressure for the very high expression of hCG that occurs during pregnancy. In any case, FSH-R in MSC does respond to hCG at concentrations that occur during pregnancy.
Materials and methods
Animal procedures were approved by the institutional animal care and use committee. FSH-R null (Fshr−/−) mice and wild-type controls were females, four months old. Fshr−/− mice with an exon 1 deletion were used,12 carried in 129T2svEmsJ mice.2 Dynamic histomorphometry used dual calcein labeled mice and was performed as described.13 Calcein was from Sigma, St. Louis, MO.
Human mesenchymal stem cells (MSCs), a population of rare progenitor cells from bone marrow capable of replication as undifferentiated cells or differentiating into bone, cartilage, fat, muscle, tendon and marrow stroma, were from Lonza (Walkersville, MD). Cells from one aliquot were used in all MSC experiments. MSCs were cultured in growth medium (Dulbecco's modified Eagle's medium with 10% fetal bovine serum). The MSCs were expanded twice with passage at 60% confluence and frozen in aliquots to allow experiments to be performed using cells from the same source at the same passage. For bone differentiation studies, MSCs at confluence in the fifth passage were placed in growth medium supplemented with 10 mM 2-glycerol phosphate, 30 µg/ml ascorbic acid, and 1 nM 1,25 dihydroxyvitamin D3 (differentiation medium). Vitamin D supports consistent mineralization; glucocorticoids such as dexamethasone were not used to avoid effects due to suppression of cell division.14 Media were replaced at three day intervals unless indicated. Alkaline phosphatase activity were performed as described.13
We characterized three endogenous FSH preparations from Fitzgerald Industries International (Acton, MA) in our previous publication,4 and analyzed recombinant FSH (R&D), for the FSH-dependent TNF-α mRNA production in human peripheral blood monocytes,15 as the screening assay for activity in peripheral cells. Fitzgerald highly pure FSH, >98% by SDS PAGE, from pituitary glands and R&D >95% recombinant FSH made in Chinese Hamster Ovary cell line (CHO-derived) showed the most consistent responses, and, at 25 ng/ml, the highly pure Fitzgerald FSH gave twice the response of the recombinant protein. The assay and a result using this highly pure pituitary FSH are shown in previous work.4 The physiological FSH has a longer half life, includes more isoforms, and is physiologically glycosylated.16 Since response to isoforms might vary, and the physiological hormone works best with the novel low-level receptor, we used the Fitzgerald highly pure FSH (specific activity > 5,000 IU/mg) in subsequent work. There are few clear differences in recombinant and purified hCG, so highly pure hCG, >98% by SDS PAGE (specific activity > 5,000 IU/mg), from urine of pregnant women, was used consistent with the FSH selection. For purified human proteins, potential cross-contamination is a concern. Manufacturer's lot assays from Fitzgerald included assays of LH, TSH, and prolactin which were all less than 1 part in 10,000; hCG was undetectable. Purity of hCG from urine less of an issue because it is present in overwhelming concentrations. However, we also ran sensitive clinical assays of both hCG and FSH for cross contamination using Beckman Access immunoassays. These showed that the hFSH has no hCG activity, and vice versa, to one part in 10,000. This effectively excludes artifacts due to FSH in the hCG or vice versa. The clinical assay mean values were 11 mIU/ng for FSH and 7 mIU/ng for hCG.
For cell adhesion or proliferation studies, cells were plated at 50% confluence at the start of the experiment. A 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay was used for calculating the number of attached cells. This assay involves the conversion of the water soluble MTT to water insoluble formazan. The formazan is then solubilized in DMSO, and the concentration was determined by optical density at 570 nm.
Live cell imaging was performed with a Cytoworks 100 instrument (Kairos Instruments, Pittsburgh, PA). Phase contrast images were taking at 1 hour intervals with a 20× objective.17 Mitoses numbers were counted from the phase images by blinded observers.
Expression of all isoforms of FSH-R in MSCs was reduced by transfecting with RNAi (Integrated DNA Technologies, Coralville, IA) using lipofectamine 2000 (Life Technologies, Grand Island, NY) according to the manufacturer's recommendations for transfection of RNAi in human cells. Briefly, 1000 pMol of RNAi in 250 µl of medium (Opti-MEM, Life Technologies) and 25 µl of lipofectamine in 250 µl of medium were preincubated for 15 min at 20 °C, mixed together, incubated for 15 min more, and then added to each well of a 6-well plate containing 70% confluent cells in 2 ml of medium. Assays for knockdown were done at 24 hours for the mRNA measurements and at 48 hours for the protein measurements. Assays using knockdown cells were started at 48 hours. The dicer substrate RNAis (RNAis) consisted of three FSH-R specific duplex constructs, as shown in Table 1. Controls were duplex sequences, including the scrambled control RNAi. FSH-R primer sequences, for the pair spanning exon 9, yielding 140 bp in the absence of exon 9 and 320 bp in its presence, were from our previous work.4 Osteoblast product mRNAs were studied by quantitative PCR as described,18 using primers in Table 1. All annealing temperatures were 55 °C. For each quantitative PCR primer pair agarose gels were run to document amplification to product of the expected size (not shown). Quantities were normalized relative to GAPDH using the constant 2.0 for a one cycle difference.18 Gene screening of transcripts in cell cultures was by cRNA production and hybridization using Affymetrix arrays as described.4
Table 1.
Duplex sequences for siRNA inhibition of FSH-R and PCR primers for osteoblast products
 |
Lysates of cells were produced and immunoblots were performed as described.4 FSH-R antibody was raised in goats to a 20 amino acid peptide from the N-terminus of human FSH-R, sc7798, Santa Cruz (Santa Cruz CA), and was used at 1:200. Mouse monoclonal anti-actin (clone AC-15, ascites fluid) was from Sigma and was used at 1:10,000. Phospho-p44/42 MAPK (Erk1/2) (Thr202/Tyr204) rabbit monoclonal antibodies (clone D13.14.4E) (1:2,000) and purified p44/42 MAPK (Erk1/2) rabbit antibodies (1:1,000) were from Cell Signaling (Danvers, MA). Anti-c-Myc mouse monoclonal antibodies (clone 9E10) (1:200) were from Santa Cruz. Purified mouse anti-p27 (Kip1) antibodies (1:500) were from BD Pharmingen (Franklin Lakes, NJ). Labeled proteins were detected with peroxidase-linked secondary anti-rabbit IgG and anti-mouse IgG (1:40,000) antibodies (Jackson ImmunoResearch Laboratories, West Grove, PA) by enhanced chemiluminescence (SuperSignal Substrate, ThermoScientific). Blots were re-probed for actin after the membrane was stripped for 20 min in Restore Plus stripping buffer (ThermoScientific).
Results
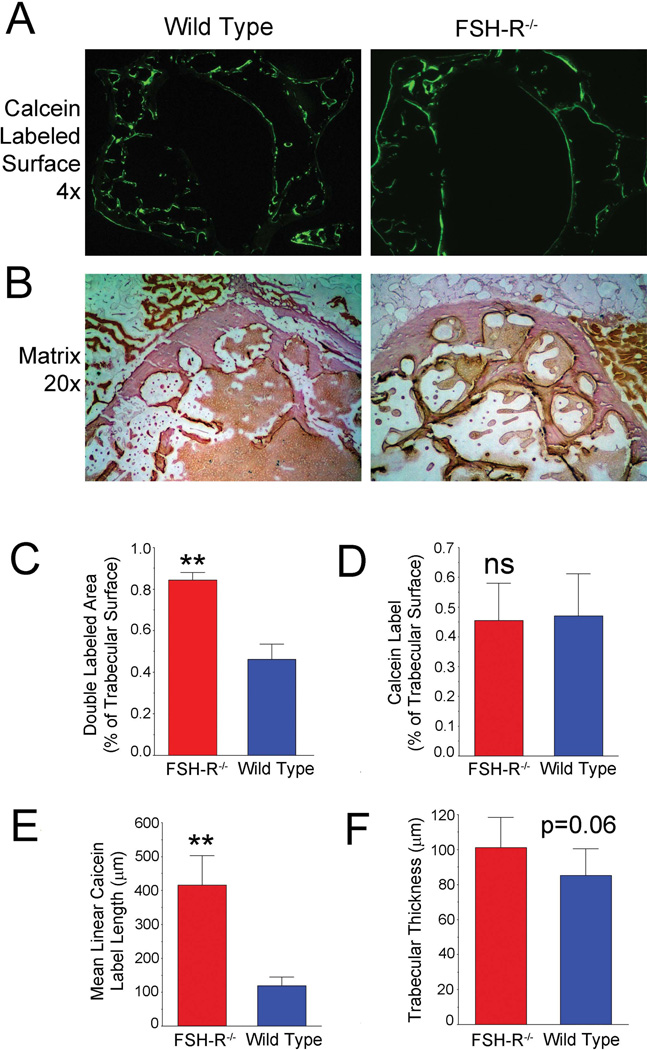
We conducted dynamic histomorphometry analysis of bone formation in four month-old female FSH-R KO mice and wild-type controls, in response to our finding that the animal had an unusually large percentage of double calcein labels.19 Double labels indicate that the formation of bone, in a given osteon, is active from five to one days before sacrifice, when labeling was done. Specifically, in low power cross sections of entire mouse vertebrae labeled with calcein (Fig. 1A) it is clear that the labels are less broken in the Fshr−/− animal (right) than in the wild type mouse (left). Hematoxylin-stained cross sections (Fig. 1B) showed a more uniform and smooth pattern of trabecular bone in the Fshr−/− animal. Consistent with these findings, a greater proportion of double labels was present in the Fshr−/− mice (p < 0.01) (Fig. 1C), even though total calcein label did not differ (Fig. 1D). Further, the linear cross sections of calcein labels, reflecting the size of bone forming units (Fig. 1E) differed by a large amount (p < 0.01). There was a trend, not reaching significance, in trabecular thickness (Fig. 1F), which was consistent with the reported increase of osteoclast activity in the Fshr−/− mice.2 Since the osteoclast FSH effect on bone resorption is known, we chose to investigate the contribution of FSH effect on MSC.
Figure 1. FSH-R–mediated effects on bone formation and bone degradation.
(A). Whole vertebral cross section of the Fshr−/− mouse calcein labeled 5 and 1 d before sacrifice. In these 3 mm fields, the wild-type mouse (left) has typical short regions of labeling, while the knockout (right) shows a unique pattern with long regions of active bone formation. (B). Hematoxylin-stained cross sections at 20× (fields are 600 µm across) show typical variability in the wild-type (left), but uniform and smooth swiss cheese–like trabecular bone in the Fshr−/−. (C). A much greater proportion of bone was double labeled in the FSH-R−/− animal (p < 0.01). (D). Total calcein label was unchanged. (E). Linear cross-sectional length of calcein labeling was increased in the FSH-R−/− animal (p < 0.01), reflecting the size of bone forming units. (F). Trabecular thickness was increased in the Fshr−/− consistent with previous work,2 but had marginal statistical difference (p = 0.06) in this case.
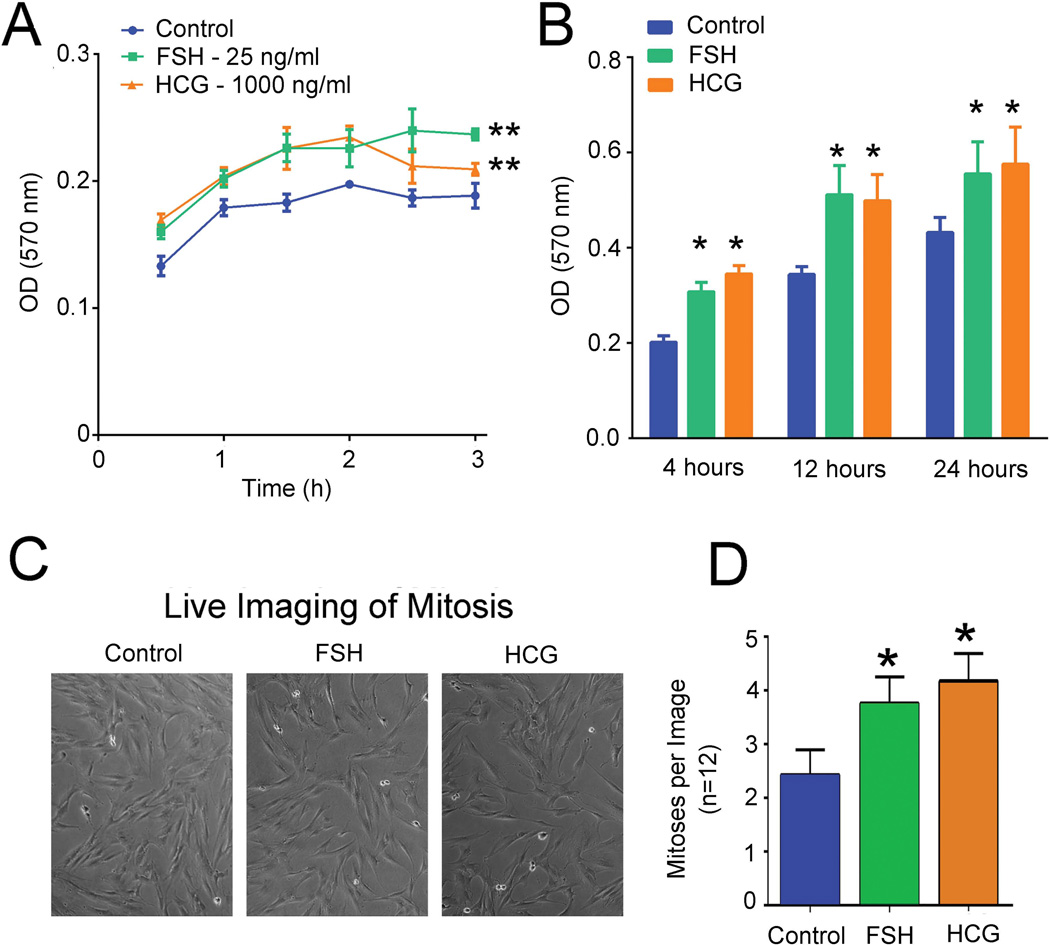
In MSCs there was a consistent increase in adherent cells using either 25 ng/ml of FSH or 1000 ng/ml of hCG (Fig. 2A). Clinical measurements of these ligands showed 11 mIU/ng for FSH and 7 mIU/ng for hCG. The concentrations used are in the physiological range for menopausal women or in pregnancy, respectively; lower concentrations were tested but had smaller and less consistent effects (not shown). Highly purified human hormones were used based on assay comparisons, and the glycoprotein hormone reagents were tested to avoid artifacts due to cross-contaminations (see Materials and Methods). Overall the increase in adherent cells was significant by analysis of variance, with p < 0.01 using either FSH or hCG. Additional cells were plated for 4, 12, and 24 h for cell proliferation assay using MTT labeling (Fig. 2B). Both FSH and hCG increased proliferation by 25% with p < 0.05 at all time points for either stimulus. To confirm that FSH and HCG increase proliferation of MSCs, we performed live cell imaging to track mitoses and found similar results (Fig. 2C and 2D).
Figure 2. Effects of FSH (25 ng/ml) and hCG (1000 ng/ml) on human MSCs adhesion and proliferation.
(A). Adhesion of MSCs in MTT assays at 30 min - 3h. At each time point, mean ± SD is shown, n = 4; ** indicates p < 0.01. (B). Effects on proliferation. After plating MSCs for 4, 12, or 24 h cell proliferation was measured using MTT. Mean ± SD, n = 4; ** indicates p < 0.01. (C). Live images of control and treated MSCs at 24 h time point. (D). Mitotic events in MSCs treated with FSH and hCG. Events were counted from phase images by observers blinded to conditions studied. Mean ±SD, n = 12; * p < 0.05 relative to the control.
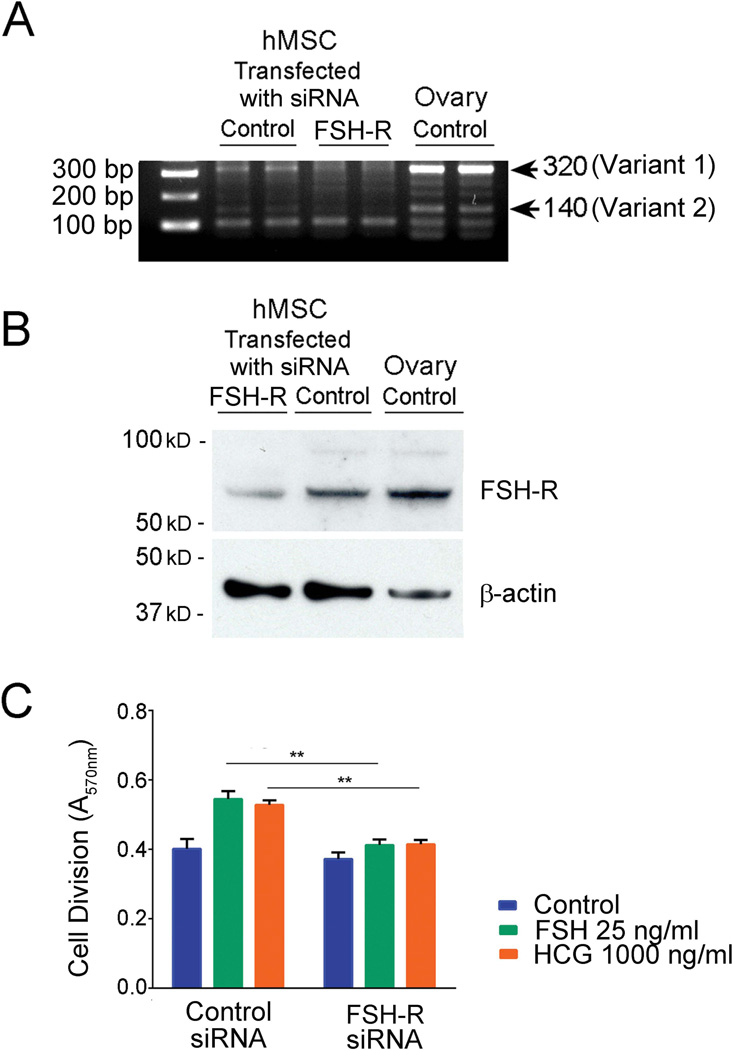
To verify the role of FSH-R, we evaluated the MSC proliferation response of FSH and hCG in the presence or absence of the FSH-R. We transfected MSCs with a mixture of three RNAis (Table 1). The control was scrambled RNAi. After RNAi treatment, FSH-R mRNA and protein were reduced by 80%, p < 0.01 relative to scrambled control RNAi (Fig. 3A and 3B). FSH and hCG increased proliferation of MSCs transfected with scrambled RNAi similarly to non-transfected cells. In contrast, FSH-R RNAi transfected MSCs did not respond to either FSH or hCG (Fig. 3C). Figure 3C presents proliferation at 12 h of MSC treatment with hormones; results at 4 and 24 h were similar (not shown).
Figure 3. Effects of MSCs transfection with FSH-R RNAi on FSH-R mRNA expression, protein expression, and on cell proliferation.
(A). FSH-R mRNA in MSCs after 24 h of transfection. The band at 320 bp is the type 1 isoform; the band at 140 bp is the type 2 isoform. (B). FSH-R protein in MSCs. Immunoblotting of lysates after 48 h of transfection. (C). Proliferative response of MSCs to FSH or hCG after transfection with FSH-R RNAi. MSCs were transfected with FSH-R RNAi or scrambled control RNAi for 48 h followed by 12 h treatment with FSH or hCG. Proliferation was measured by MTT assay (** p < 0.01 relative to scrambled control RNAi; n = 4, mean ± SD).
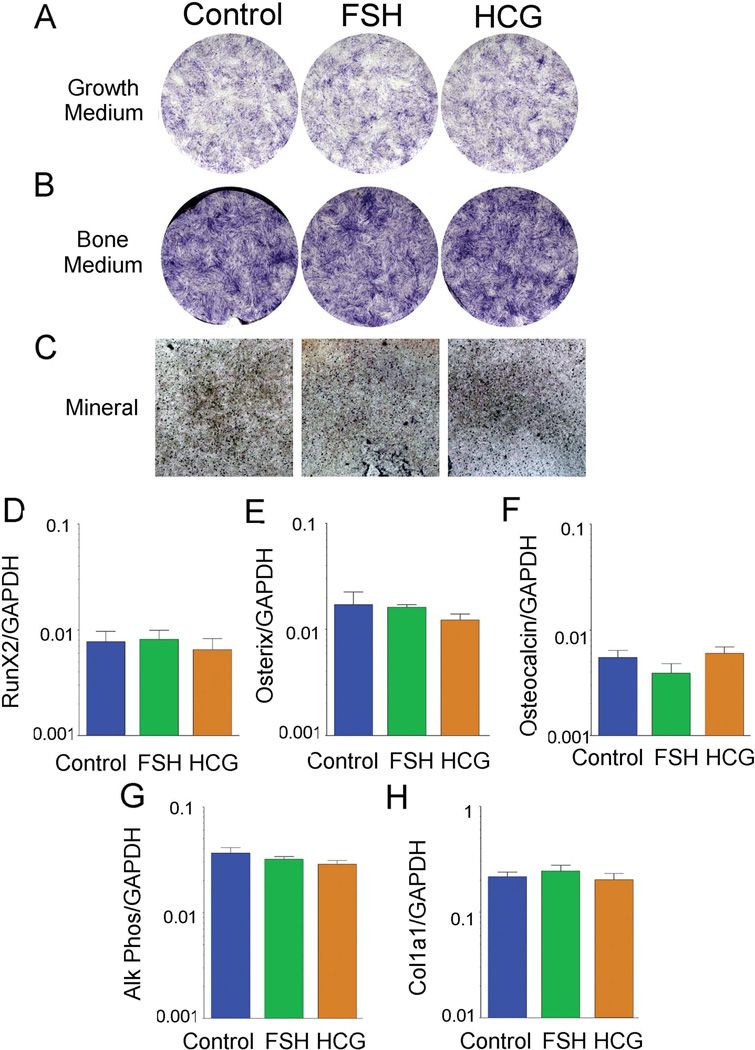
Next we evaluated the effects of FSH and hCG during MSC differentiation into osteoblasts. Alkaline phosphatase staining showed no difference after 21 days of treatment in either growth or differentiation (bone) medium (Fig. 4A and 4B). In differentiation medium, there were also no consistent differences in mineral density as determined by optical density (Fig. 4C). In addition, we performed quantitative PCR for osteoblast specific developmental and structural gene expression to detect any small effects of FSH or hCG. All assays were normalized to GAPDH. RNA expression of the osteoblastic transcription factors Runx2 and osterix and of the bone structural genes collagen type I α 1 (COL1A1), alkaline phosphatase, and osteocalcin were not significantly different among the treatments.
Figure 4. Osteoblast differentiation with and without FSH or hCG.
(A–B). Alkaline phosphatase activity in MSCs cultured in bone differentiation medium, B, relative to growth medium, A. (C). Mineral deposition (black) demonstrated by optical density in transmitted light. One of two duplicate cultures with similar results is shown. Fields are 0.5 cm across. (D–H). QPCR analysis for osteoblast specific gene expression in MSCs treated with FSH or hCG. All assays were normalized to GAPDH. One of three experiments with similar findings is shown.
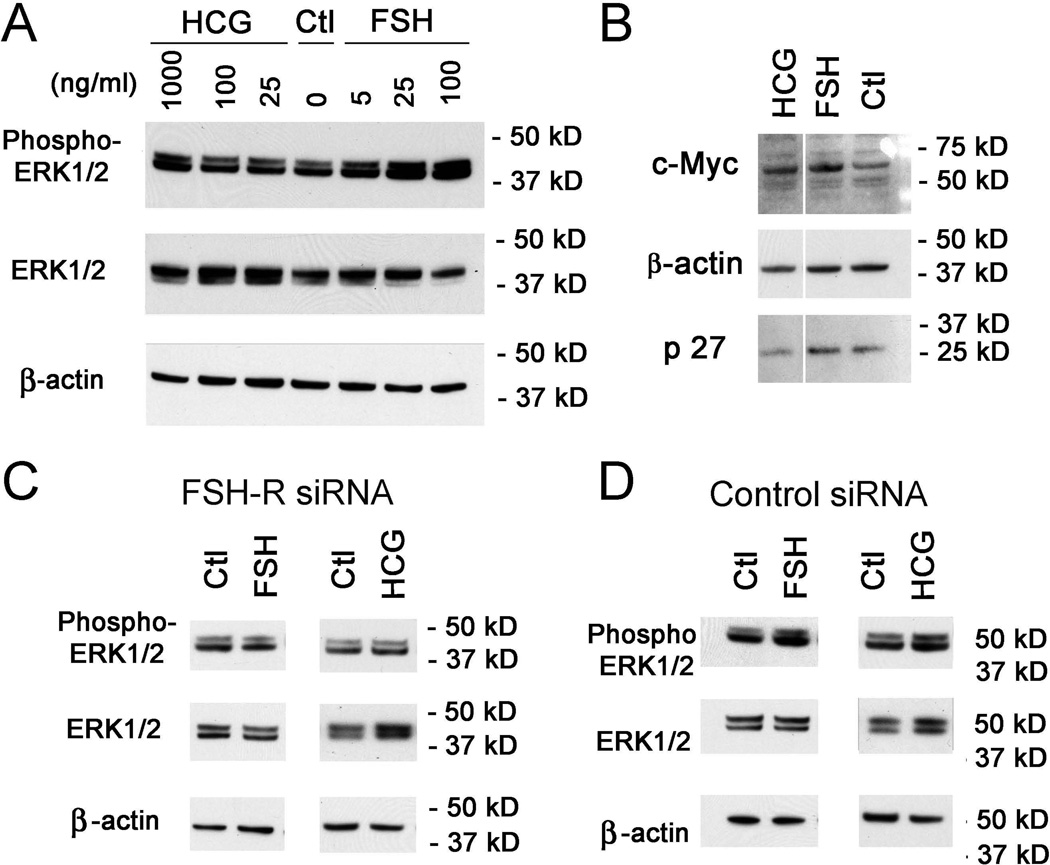
We then determined whether intracellular signaling pathways associated with MSC adhesion and proliferation but not differentiation20 were changed. The major finding, in lysates of MSCs treated with FSH or hCG for 10 min, was an increase in phosphorylation of Erk1/2 (Fig. 5A). Downstream ERK1/2 pathway components c-Myc and p2721 were studied (Fig. 5B). While c-Myc protein was increased at 24 h, there was no consistent alteration of p27.
Figure 5. Erk1/2 phosphorylation with and without FSH or hCG treatment in growing MSCs.
(A). Immunoblotting of lysates of MSCs treated with FSH or hCG for 10 min. (B). c-Myc and p27 protein expression in MSCs after addition of FSH or hCG for 24 h. One of three blots with similar findings is shown. (C–D). Effects of MSCs transfection with FSH-R RNAi on Erk1/2 phosphorylation. MSCs were transfected with FSH-R RNAi or control scrambled RNAi for 48 h followed by 10 min treatment with FSH or hCG. One of two blots with similar findings is shown.
Further, to verify that Erk1/2 signaling derives from FSH-R/ligand interactions, we repeated the 10 min FSH or HCG treatment of MSCs with FSH-R knock down. With FSH-R reduced greatly, Erk1/2 phosphorylation did not differ from control (Fig. 5C), while after treatment with scrambled RNAi Erk1/2 phosphorylation was increased similar to non-transfected cells (Fig 5D).
Discussion
Our results show that FSH-R modulates bone formation by increasing MSC proliferation and potentially delaying entry of MSCs into differentiation pathways. In mice, FSH-R loss results in larger bone forming units than occur in wild-type animals (Fig 1). The changes in bone formation pattern in FSH-R KO mice suggested that FSH affected osteoblasts or their precursors, MSCs. Differentiation of MSCs maintains the activity of bone formation by adding new layers of osteoblasts during bone formation.22 For subsequent studies, we used human MSCs to focus on FSH-R function in human biology. By working with MSCs in vitro, we separated the contribution of FSH effects on osteoblast precursors from those on bone degrading cells.2 Because we had previously found that FSH-R was expressed in human MSCs but not in mature osteoblasts,23 we first queried MSC adhesion and proliferation in response to FSH. FSH significantly increased both MSC proliferation and adhesion (Fig. 2). When we reduced FSH-R expression in MSCs (Fig. 3A and 3B), the proliferation response to FSH was attenuated (Fig. 3C). In contrast, we found no direct effect of FSH on osteoblast differentiation (Fig. 4). It is likely that FSH-R with its ligands enhances MSC proliferation, potentially preventing differentiation; over time this may reduce bone formation or promote MSC differentiation into other lineages, such as chondrocytes or adipocytes.
FSH and hCG are members of the same glycoprotein hormone family, and FSH-like activity was reported in serum with very high levels of hCG,10 but there is no measurable FSH in serum. Our hypothesis was that the expression of FSH-R isoforms in tissues including MSCs, osteoclasts, and their precursors might help integrate responses to glycoprotein hormones during pregnancy. We examined effects of pregnant levels of hCG on MSCs and found effects similar to those of FSH. Furthermore, RNAi knockdown of FSH-R negated both FSH and hCG effects on MSCs (Fig. 3C). Short term MSC treatment with FSH or hCG activated Erk1/2 phosphorylation, one of the signaling pathways for proliferation, which was also altered by FSH-R RNAi knockdown in MSCs, suggesting that the FSH-R participates in regulation of MSCs by either FSH or hCG, integrating effects of these two glycoprotein hormones. Several studies reported the presence of FSH-R in unexpected sites, including vascular endothelium in tumors,24 and in a variety of adrenal tumors.25 Since FSH-R is expressed on multiple types of cells, depending on conditions, FSH might either support or oppose26 bone formation. Thus, it is entirely possible that many other tissues may express FSH-R variably, and that it may function as a secondary regulator of many other tissues. On the other hand, the seven-transmembrane receptors, including the glycoprotein hormone receptors, have the property that very small numbers of receptors can, under favorable circumstances, make significant changes in cellular signaling, such as in the well known cholera toxin effect on adenylate cyclase in the gut, or photons for rhodopsin in the retina.27
After obtaining the results on adhesion and proliferation of MSCs with hCG and FSH, we did a gene screen comparing MSCs treated with either hCG 1000 ng/ml or FSH 25 ng/ml to untreated cells. An initial analysis showed that key targets related to stem cell proliferation and stemness were similarly increased, two-fold or more, by hCG and FSH, including the intermediate filament binding protein plectin 1, and the integrin subunit α 5, in both cases with all p values relative to the control of less than 0.0001 and no change between hCG and FSH (not illustrated).28,29 On the other hand, there were FSH and hCG signaling differences. The matrix protein perlecan (heparin sulfate proteoglycan 2) increased 2.5 fold, p = 0.002, with FSH, but it did not change with hCG. HCG is implicated in other FSH signaling studies and it also may regulate stemness and expansion,30,31 which might reflect that some FSH-R with the normal complement of exons is expressed along with the truncated FSH-R missing exon 9. Other mechanisms are possible, including that the full-length receptor in peripheral cells, including MSCs, might have a different spectrum of responses than the full-length receptor in the ovary.
In conclusion, low quantities of FSH-R found in human MSCs respond to physiological levels of FSH or hCG to regulate MSC proliferation without differentiation.
Acknowledgments
Experimental work was carried out by all of the authors, with the order cited reflecting the relative contribution. Harry C. Blair, Irina L. Tourkova and Lisa J. Robinson planned experiments, analyzed data, and wrote this report. We thank Stephanie Mihalik, University of Pittsburgh, Department of Pathology for editorial assistance. We thank Professor Johnny Huard, University of Pittsburgh, for assistance with live cell imaging. Supported by the Department of Veteran's Affairs (HCB) and by National Institutes of Health (USA) awards AR055208 (HCB), AR065407 (LJR and HCB) and AR0535 (LJR). Opinions expressed are not those of the Department of Veteran's Affairs.
Footnotes
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Abe E, Marians RC, Yu W, et al. TSH Is a Negative Regulator of Skeletal Remodeling. Cell. 2003;115:151–162. doi: 10.1016/s0092-8674(03)00771-2. [DOI] [PubMed] [Google Scholar]
- 2.Sun L, Peng Y, Sharrow AC AC, et al. FSH Directly Regulates Bone Mass. Cell. 2006;125:247–260. doi: 10.1016/j.cell.2006.01.051. [DOI] [PubMed] [Google Scholar]
- 3.Marians RC, Ng L, Blair HC, et al. Defining Thyrotropin-dependent and -independent Steps of Thyroid Hormone Synthesis by Using Thyrotropin Receptor-null Mice. Proc. Natl. Acad. Sci. U.S.A. 2002;99:15776–15781. doi: 10.1073/pnas.242322099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robinson LJ, Tourkova I, Wang Y, et al. FSH-Receptor Isoforms and FSH-dependent Gene Transcription in Human Monocytes and Osteoclasts. Biochem. Biophys. Res. Commun. 2010;394:12–17. doi: 10.1016/j.bbrc.2010.02.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vibede N, Hauser M, Williamson CJ, Grimmelikhuijzen F. Genomic Organization of a Receptor from Sea Anemones, Structurally and Evolutionarily Related to Glycoprotein Hormone Receptors from Mammals. Biochem. Biophys. Res. Commun. 1998;252:497–501. doi: 10.1006/bbrc.1998.9661. [DOI] [PubMed] [Google Scholar]
- 6.Kumar RS, Ijiri S, Kight K, et al. Cloning and Functional Expression of a Thyrotropin Receptor from the Gonads of a Vertebrate Bony Fish: potential thyroid-independent role for thyrotropin in reproduction. Mol. Cell. Endocrinol. 2000;167:1–9. doi: 10.1016/s0303-7207(00)00304-x. [DOI] [PubMed] [Google Scholar]
- 7.Kobayashi T, Andersen O. The Gonadotropin Receptors FSH-R and LH-R of Atlantic Halibut (Hippoglossus hippoglossus): isolation of multiple transcripts encoding full-length and truncated variants of FSH-R. Gen. Comp. Endocrinol. 2008;156:584–594. doi: 10.1016/j.ygcen.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 8.Bogerd J, Granneman JC, Schulz RW, Vischer HF. Fish FSH Receptors Bind LH: how to make the human FSH receptor to be more fishy? Gen. Comp. Endocrinol. 2005;142:34–43. doi: 10.1016/j.ygcen.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 9.Kumar RS, Ijiri S, Trant JM. Molecular Biology of the Channel Catfish Gonadotropin Receptors: 2. complementary DNA cloning, functional expression, and seasonal gene expression of the follicle-stimulating hormone receptor. Biol. Reprod. 2001;65:710–717. doi: 10.1095/biolreprod65.3.710. [DOI] [PubMed] [Google Scholar]
- 10.Stewart F, Allen WR, Moor RM. Pregnant Mare Serum Gonadotrophin: ratio of follicle-stimulating hormone and luteinizing hormone activities measured by radioreceptor assay. J Endocrinol. 1976;71:471–482. doi: 10.1677/joe.0.0710371. [DOI] [PubMed] [Google Scholar]
- 11.Simoni M, Khan SA, Nieschlag E. Serum Bioactive Follicle-stimulating Hormone-like Activity in Human Pregnancy is a Methodological Artifact. J Clin. Endocrinol. Metab. 1991;73:1118–1122. doi: 10.1210/jcem-73-5-1118. 1991. [DOI] [PubMed] [Google Scholar]
- 12.Dierich A, Sairam MR, Monaco L, et al. Impairing follicle-stimulating hormone (FSH) signaling in vivo: targeted disruption of the FSH receptor leads to aberrant gametogenesis and hormonal imbalance. Proc. Natl. Acad. Sci. U. S. A. 1998;95:13612–13617. doi: 10.1073/pnas.95.23.13612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Robinson LJ, Mancarella S, Songsawad D, et al. Gene Disruption of the Calcium Channel Orai1 Results In Inhibition of Osteoclast and Osteoblast Differentiation and Impairs Skeletal Development. Lab. Invest. 2012;92:1071–1083. doi: 10.1038/labinvest.2012.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang PL, Blair HC, Zhao X, et al. Comparison of fetal and adult marrow stromal cells in osteogenesis with and without glucocorticoids. Connect. Tissue. Res. 2006;47:67–76. doi: 10.1080/03008200600584074. [DOI] [PubMed] [Google Scholar]
- 15.Iqbal JL, Sun L, Kumar TR, et al. Follicle-stimulating hormone stimulates TNF production from immune cells to enhance osteoblast and osteoclast formation. Proc. Natl. Acad. Sci. U. S. A. 2006;103:14925–14930. doi: 10.1073/pnas.0606805103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gurgan T, Montjean D, Demirol A, Menezo YJ. Sequential (hFSH + recFSH) vs homogenous (hFSH or recFSH alone) stimulation: clinical and biochemical (cumulus cell gene expression) aspects. J Assist Reprod. Genet. 2014 Mar 18; doi: 10.1007/s10815-014-0208-1. 2014 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qu-Petersen Z, Deasy B, Jankowski BR, et al. Identification of a Novel Population of Muscle Stem Cells in Mice: potential for muscle regeneration. J Cell Biol. 2002;157:851–864. doi: 10.1083/jcb.200108150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Robinson LJ, Yaroslavskiy BB, Griswold RD, et al. Estrogen Inhibits RANKL-stimulated Osteoclastic Differentiation of Human Monocytes Through Estrogen and RANKL-regulated Interaction of Estrogen Receptor- α with BCAR1 and Traf6. Exp. Cell Res. 2009;315:1287–1301. doi: 10.1016/j.yexcr.2009.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhu LL, Blair H, Cao J, et al. Blocking Antibody to the β-subunit of FSH Prevents Bone Loss by Inhibiting Bone Resorption and Stimulating Bone Synthesis. Proc. Natl. Acad. Sci. U.S.A. 2012;109:14574–14579. doi: 10.1073/pnas.1212806109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pricola KL, Kuhn NZ, Haleem-Smith H, et al. Interleukin-6 Maintains Bone Marrow-derived Mesenchymal Stem Cell Stemness by an ERK1/2-dependent Mechanism. J Cell Biochem. 2009;108:577–588. doi: 10.1002/jcb.22289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhu LL, Tourkova I, Yuen T, et al. Blocking FSH Action Attenuates Osteoclastogenesis. Biochem. Biophys. Res. Commun. 2012;422:54–58. doi: 10.1016/j.bbrc.2012.04.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blair HC, Zaidi M, Huang CL, Sun L. The Developmental Basis of Skeletal Cell Differentiation and the Molecular Basis of Major Skeletal Defects. Biol. Rev. Camb. Philos. Soc. 2008;83:401–415. doi: 10.1111/j.1469-185X.2008.00048.x. [DOI] [PubMed] [Google Scholar]
- 23.Lefevre G, Calipel A, Mouriaux F, et al. Opposite Long-term Regulation of c-Myc and p27Kip1 Through Overactivation of Raf-1 and the MEK/ERK Module in Proliferating Human Choroidal Melanoma Cells. Oncogene. 2003;22:8813–8822. doi: 10.1038/sj.onc.1207099. [DOI] [PubMed] [Google Scholar]
- 24.Siraj A, Desestret V, Antoine M, et al. Expression of Follicle-stimulating Hormone Receptor by the Vascular Endothelium in Tumor Metastases. BMC Cancer. 2013;13:246. doi: 10.1186/1471-2407-13-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pawlikowski M, Winczyk K, Stępień H. Immunohistochemical Detection of Follicle Stimulating Hormone Receptor in Neuroendocrine Tumours. Endokrynol. Pol. 2013;64:268–271. doi: 10.5603/ep.2013.0004. [DOI] [PubMed] [Google Scholar]
- 26.Allan CM, Kalak R, Dunstan CR, et al. Follicle-stimulating Hormone Increases Bone Mass in Female Mice. Proc. Natl. Acad. Sci. U.S.A. 2010;107:22629–22634. doi: 10.1073/pnas.1012141108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blair HC, Wells A, Isales CM CM. Pituitary Glycoprotein Hormone Receptors in Non-endocrine Organs. Trends Endocrinol. Metab. 2007;18:227–233. doi: 10.1016/j.tem.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 28.Jeon JH, Suh NH, Kim MO, Han HJ. Glucosamine-induced Reduction of Integrin β4 and Plectin Complex Stimulates Migration and Proliferation in Mouse Embryonic Stem Cells. Stem Cells Dev. 1013;22:2975–2989. doi: 10.1089/scd.2013.0158. [DOI] [PubMed] [Google Scholar]
- 29.Rowland TJ, Miller LM, Blaschke AJ, et al. Roles of Integrins in Human Induced Pluripotent Stem Cell Growth on Matrigel and Vitronectin. Stem Cells Dev. 2010;19:1231–1240. doi: 10.1089/scd.2009.0328. [DOI] [PubMed] [Google Scholar]
- 30.Vasconcelos GL, Saraiva MV, Costa JJ, et al. Effects of GDF-9 and FSH on In Vitro Development, Viability and mRNA Expression in Bovine Preantral Follicles. Reprod. Fertil. Dev. 2013;25:1194–1203. doi: 10.1071/RD12173. [DOI] [PubMed] [Google Scholar]
- 31.Chen XD, Dusevich V, Feng JQ, et al. Extracellular Matrix Made by Bone Marrow Cells Facilitates Expansion of Marrow-derived Mesenchymal Progenitor Cells and Prevents Their Differentiation into Osteoblasts. JBone Miner. Res. 2007;22:1943–1956. doi: 10.1359/jbmr.070725. [DOI] [PubMed] [Google Scholar]