Abstract
The S-cone system is closely linked to the perception of blue/yellow. The trichromatic system of Old World monkeys and humans has relatively few S-cones in the fovea. In this study we investigated the distribution of putative S-cone afferents in macaques primary visual cortex (V1) which form a characteristic honeycomb arrangement in layer 4A. It was hypothesized that if there were a low number of S-cone opponent projecting neurons in central vision then this would be seen as a reduction in afferents in foveal layer 4A. Recent studies have shown that the vesicular glutamate transporter 2 (VGlut2) is a marker for thalamic afferent terminals in cortex. The distribution of VGlut2-immunoreactive (-ir) terminals was studied in the foveal and perifoveal representation of V1. It was found that there was a substantial reduction in the terminal density in the foveal representation: the density was 5 – 6 times lower in the foveal V1 than in regions representing perifoveal eccentricities of 1–2 degrees and beyond. These findings may provide the cortical substrate of foveal tritanopia, the reduced blue perceptual ability for small fields in the center of gaze.
Keywords: thalamic afferents, visual cortex, S-cones, vesicular glutamate transporter, macaque
Introduction
Color vision in trichromatic primates begins by transduction of the light signals by the three cone photoreceptors the L- (long), M-(medium), and S-(short) wavelength-sensitive cones. Following transduction the chromatic opponency in retinal ganglion cells (RGC’s), conferred by differential L-, M- and S-cone inputs to receptive field subregions (Lee 2011), is a crucial stage of color representation in many primates (Jacobs 2012). The perception of color is dependent on the chromatic pathways, with red/green perception closely associated with the L/M pathway and blue/yellow perception associated with the S/(L+M) pathway. In Great Apes and Old World monkeys there is diminished blue perceptual ability for small fields in the center of gaze, foveal tritanopia (Willmer 1944; Williams et al. 1981a, b).
The opponent cone inputs are evident in two main classes of RGC’s, L/M opponent and S/(L+M) opponent, with this latter one being further segregated into SON/(L+M) and SOFF/(L+M) (Dacey et al. 2013). These pathways leave the retina where the L/M opponent afferents terminate principally in the parvocellular (P) layers of the lateral geniculate nucleus (LGN), while the SON/(L+M) are thought to terminate principally in the koniocellular (K) layers or leafs of the LGN. The relative importance and distribution of the SOFF/(L+M) component is not well established but some indirect arguments suggest the involvement of the LGN P layers (Hendry and Yoshioka, 1994; see Chatterjee and Callaway, 2003). The geniculate afferents are thought to remain segregated in primary visual cortex (V1), with the L/M component of the P-layer LGN afferents terminating mainly in layer 4Cβ and putative SOFF/(L+M) P input projecting to lower 4A, while the SON/(L+M) opponent input from the K-layers terminates in upper layer 4A and the adjacent lower layer 3 (Hendry and Yoshioka 1994; Chatterjee and Callaway 2003; Casagrande et al. 2007) (Fig. 1).
Fig. 1.
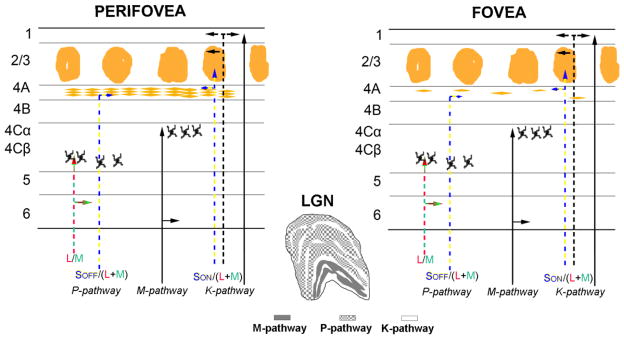
Schematic diagram of the visual retino-geniculo-striate pathway in fovea and perifovea of macaques. The upper half shows the main pathways from the LGN layers into the recipient layers of primary visual cortex (V1). The arrangement of afferent terminals in layer 4A is sparse in the foveal region whereas in the perifoveal region there is a rich pattern of afferent termination. The Scone opponent pathway from the K layers of the LGN projects preferentially to layer 4A and lower layer 3.
The distribution of the three cone photoreceptors classes across the retina is not uniform. The high density of receptors in the fovea is dominated by L- and M-cones in both macaques (de Monasterio et al. 1985; Wikler and Rakic 1990; Martin and Grunert 1999) and in human (Curcio et al. 1991). The density of S-cones is about 1% of the total cones central retina gradually rising to a peak of 6–8% at around two degrees eccentric (de Monasterio et al. 1985). In the primary visual cortex of macaques the central two degrees of visual space occupies about 100 mm2 of cortical area (Dow et al. 1981; Van Essen et al. 1984, Dow et al. 1985). We sought to determine how the separate S-cone dominated input to layer 4A (Fig. 1) is distributed compared to the input from the P-cell layers of LGN to layer 4C in the foveal and perifoveal regions of visual cortex.
The afferents from LGN are arranged in a characteristic honeycomb manner in layer 4A of macaques (Carroll and Wong-Riley 1984; Horton, 1984; Fitzpatrick et al. 1985). In the current study we took advantage of the observation that a selective isoform of the vesicular glutamate transporter 2 (VGlut2) is expressed in LGN afferent terminals but not in intracortical terminals (Nahmani and Erisir 2005; Coleman et al. 2010; Lei et al. 2013) leading to a distinct pattern of immunoreactivity in layer 4 of V1 in macaques (Garcia-Marin et al. 2013). We hypothesized that if there is a reduction in S-cone density in foveal retina and that this is manifested in a lower proportion of chromatically opponent retinal ganglion cells with S-cone subfields that persisted into the LGN and cortex, then one consequence would be a low density of inputs to layer 4A, the initial cortical recipient zone for the S-cone pathway (Hendry and Yoshioka 1994; Hendry and Reid 2000; Chatterjee and Callaway 2003). The density of the VGlut2-immunoreactive (-ir) puncta in layer 4A was measured at different positions in V1 corresponding to different regions of the visual field representation. In macaques the visual field representation of the fovea is lateral on the occipital operculum. It was found that the density of VGlut2-ir puncta – the putative LGN afferent terminals – in the foveal region was around 5–6 times lower than in the region of cortex representing the perifoveal visual field.
Methods and materials
Macaque brain tissue
Four, young adult, male Macaca fascicularis and one M. nemestrina macaque monkeys, previously used for anesthetized electrophysiological recordings were used in this study. Animals were prepared for recording as described elsewhere (Xing et al. 2004; Solomon et al. 2004). After 4–5 days of recordings, experiments were terminated by i.v. injection of a lethal dose of pentobarbital (60 mg/kg), and brain death was determined by a flat electroencephalogram.
Subsequently, animals were transcardially perfused with heparinized 0.01 M phosphate-buffered saline (PBS; pH 7.4) followed by 4 L of chilled 4% paraformaldehyde (PFA) in 0.1 M phosphate buffer (PB; pH 7.4). Fixative was run for at least 40 minutes. Some blocks of V1 were removed for track reconstruction of the recording locations of electrophysiologically characterized neurons, and the remaining V1 tissue was cut into small blocks and postfixed in the same fixative for 24–48 hours at 4 °C in 4% PFA. After fixation, serial tangential (30 μm), coronal (50 μm) or sagittal (50 μm) sections of V1 were prepared using a vibratome. Subsequently, sections were batch-processed for VGlut2 immunohistochemistry. The remaining sections and blocks were immersed in graded sucrose solutions, and were stored in a cryoprotectant solution at −20C.
The layers of area V1 were identified according to Brodmann’s (1909) nomenclature, modified by Lund (1973). This system distinguishes four subdivisions of layer 4, namely, 4A, 4B, 4Cα, and 4Cβ. Alternatively, Hassler’s (1967) nomenclature could be also used, and would be particularly useful when relating the homology of layers across taxa; in this terminology layer 4α and 4β correspond to layer 4Cα and 4Cβ in Brodmann’s nomenclature, whereas layer III is further subdivided into IIIA, IIIBα, IIIBβ and IIIC, corresponding to Brodmann’s upper part and lower part of layer III, 4A and 4B, respectively. A recent study from our laboratory described in detail the laminar distribution of VGlut2 in macaques (Garcia-Marin et al. 2013) following Brodmann’s nomenclature. Layer 4A showed the characteristic honeycomb pattern described earlier with cytochrome oxidase histochemistry (Horton and Hubel 1981; Carroll and Wong-Riley 1984; Horton, 1984).
All experimental procedures were approved by the New York University Institutional Animal Use and Care Committee and were conducted in strict compliance with the National Institutes of Health (NIH) guidelines for the care and experimental use of animals in research.
Alignment of sections with the visuotopic map
A detailed description of the visuotopic organization of the Old World monkeys primary visual cortex has been reported using different species, such as: baboons (Papio), cynomolgus (Macaca fascicularis), vervet (Cercopithecus aethiops pygerythrus), and rhesus monkeys (Macaca mulatta) (Talbot and Marshall 1941; Daniel and Whitteridge 1961; Guld and Bertulis 1976; Tootell et al. 1982; Dow et al. 1981; 1985). Dow et al (1985) provided a high resolution map of the foveal and perifoveal visual cortex with iso-eccentricity contours of 10 min of visual angle. The current study adopted Dow et al’s map to approximate eccentricity using their complex logarithmic equation, w = 7.7 * ln (x + iy + 0.33), where w is expressed in millimeters, x and y are expressed in degrees and i is the imaginary number. It should be noted that Dow et al’s results were obtained from recordings in rhesus monkeys so provides a relative guide for cynomolgus and pig-tailed macaques (Macaca nemestrina) used in the current study.
We superimposed this visuotopic map into surface of V1 and blocked the brain using the map as a guide. The three different cutting planes were approximately-coronal and -sagittal, and -tangential (Fig. 2A–C). In the ‘approximately-coronal plane’, the cutting plane was parallel to the horizontal meridian (HM); a series of around 300 sections, numbered from posterior to anterior (Fig. 2A, #1→#N) was obtained from a lateral block of V1 in each animal. Sections in the middle of the series, that had regions close to the apex of the most lateral portions of V1, were considered to contain regions closest to the foveal representation (sections #140–160 in fig. 2A). In the ‘approximately- sagittal plane’ the cutting plane was perpendicular to the presumed representation of the horizontal meridian; a series of around 240 sections, numbered from lateral to medial (Fig. 2B, #1→#N) was obtained from one animal. The foveal representation was in the most lateral sections that contain V1 (section #20). In the ‘tangential plane’ the block was trimmed in order to have the surface of the block parallel to the cortex between the inferior occipital sulcus (IOS) and ectocalcarine sulcus (ECS). Series of around 120 sections, numbered from dorsal to ventral (Fig. 2C, #1→#N) were obtained from a lateral block of V1 in two animals. Throughout the rest of the text we use coronal and sagittal for approximately-coronal and approximately-sagittal, respectively.
Fig. 2.
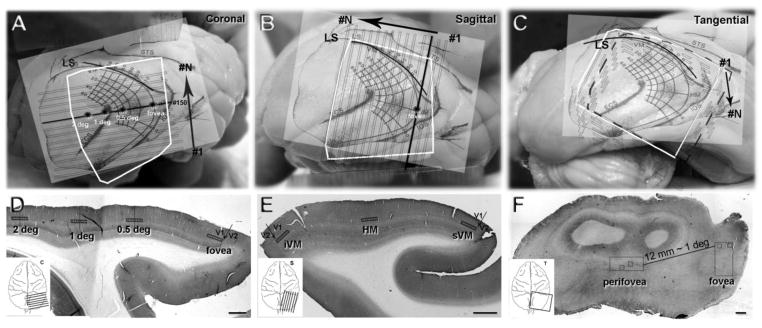
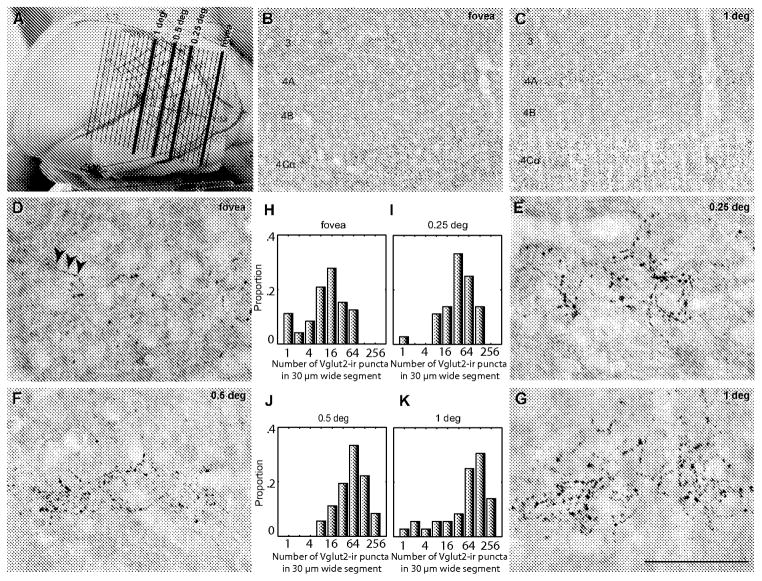
Eccentricity of foveal and perifoveal regions of cortex were estimated by the visual field map transformed by Dow et al. 1985. The map is shown schematically projected onto the cortex. A: the orientation of the coronal sections to the map placed on the V1 surface. The orientation of sequential sections is shown by the solid black horizontal line marked at the right-hand end labeled #150 running approximately mediolateral. The different eccentricities are marked with asterisks. The cutting sequence is depicted by the black arrow, the sequence was from posterior (#1) to anterior (#N) cutting direction. B: the orientation of the sagittal sections is shown, the solid black line running posterior to anterior shows the plane of the sections. The cutting sequence shown by the black arrow was from lateral (#1) to medial (#N). C: sections were cut tangential to the pial surface of the most lateral part of V1; in this series the sequence is indicated by the black arrow, starting with most dorsal sections (#1) and moving deeper into the cortex (#N). D–F, single sections in the coronal, sagittal and tangential planes that were immunoreacted for VGlut2. The V1/V2 border can be seen in the D and E as the abrupt transition between VGlut2-ir in layer 4C, and is marked by an arrow. The quantification of VGlut2-ir puncta was obtained from the sampling regions shown by the small boxes in the rectangles (D,E) along the length of layer 4A. In F, the sampling regions are shown as small squares within the two larger foveal and perifoveal areas (rectangles). White squares (A–C) represent the actual shape of the blocks. Scale bars (D–F) 1 mm.
Immunohistochemistry (IHC)
Free-floating sections were pre-treated using an antigen retrieval (AR) protocol that breaks the methylene bridges, and exposes the antigenic sites in order to allow the antibodies to bind. The AR protocol has been used to enhance the VGlut2 immunoreaction in rats (Varoqui et al. 2002), mice (Nakamura et al. 2005, 2007), and monkeys (Garcia-Marin et al. 2013). For AR, the buffer solution −0.01 M sodium citrate (pH 8.0) – was heated to 80°C and individual floating sections were incubated at this temperature for 15 minutes. Later, sections were cooled down to room temperature for 20 minutes and were subsequently rinsed 3 times in 0.01 M PBS. Sections were then pre-treated in 1.66% hydrogen peroxide (H2O2) for 15 min to remove endogenous peroxidase activity, and subsequently blocked for 1 h in 0.01 M PBS with 0.25% Triton-X and 3% normal goat serum. The sections were then incubated overnight at 4°C with mouse anti-VGlut2 antibody (1:4000, MAB5504, Chemicon International, Temecula, CA, USA) in 0.01 M PBS with 0.25% Triton-X and 3% normal goat serum. Sections were rinsed and incubated for 2 h with biotinylated goat anti-mouse (1:500, BA-9200: Vector Laboratories, Burlingame, CA). The sections were then incubated for 30 minutes in an avidin – biotin peroxidase complex (Vectastain ABC Elite PK6100, Vector Laboratories, Burlingame, CA), and finally reacted with Vector® VIP Peroxidase Substrate Kit (SK 4600, Vector Laboratories, Burlingame, CA) that produced an intense violet-colored precipitate. After staining, the sections were dehydrated, cleared with xylene, and coverslipped. Controls for the secondary antibodies were administered, either by replacing the primary antibody with preimmune goat serum in some sections, by omitting the secondary antibody, or by replacing it with an incompatible secondary antibody. No significant staining was detected under these control conditions.
The mouse anti-VGlut2 antibody is a recombinant protein from rat VGlut2. In Western blot analysis, it appears as an individual band of ~5 kDa. The staining pattern was identical to the data published for macaques in previous studies that used the same monoclonal VGlut2 antibody (Marion et al. 2013; Garcia-Marin et al. 2013; Balaram et al. 2013).
Quantification of VGlut2-ir puncta density in layer 4A
To estimate whether there was any variation in number of VGlut2-ir terminals in layer 4A consistent with the reduction of S-cones in the foveal region of the retina, we quantified the number of VGlut2-ir puncta in layer 4A in the foveal representation and at locations representing different perifoveal eccentricities in coronal and sagittal sections.
In both cases – for the coronal and sagittal sections – (Fig. 2D–E) 9 non-overlapping 100X objective images were acquired at different locations within each section for each of the series from each animal. For the sagittal sections the most lateral section with an unambiguous layer 4C was selected and designated the first V1 section and the section with the foveal representation. Nine images were obtained from this section. For eccentricities away from the foveal representation we estimated the cortical distance (in μm) from the section representing the fovea using the non-linear transformation of Dow et al (1985). Then we selected the corresponding section and obtained nine images. This was repeated for each sampling location. For the coronal sections, the region along the representation of the horizontal meridian was first estimated from a low power reconstruction of the full series of sections from posterior cortex to the lunate sulcus. In the aligned low power sections, beginning in the posterior sections there is a continuous lateral progression of the V1/V2 border, which reverses to a medial progression for the more anterior sections as they approach the lunate sulcus. Using the medial to lateral and then lateral to medial progression we selected the sections that had the most lateral V1/V2 border. The transition could be estimated within about 10 sections, or 0.5 mm of cortex. According to the transformation of Dow et al this was accurate to within about 0.1–0.2 deg. Having selected the section close to the representation of the horizontal meridian nine images at and near the V1/V2 border were obtained representing the fovea. For eccentricities away from the foveal representation we estimated the cortical distance (in μm) using the non-linear transformation of Dow et al (1985). Then we selected the corresponding distance from the V1/V2 border in the section that had been designated as close to the representation of to the horizontal meridian and obtained nine images. This was repeated for each sampling location.
Color images (1600 × 1200 pixels, 120 × 90 μm), were converted into black and white using Photoshop and loaded into MatLab (Fig. 3A). To estimate the background intensity in each image the distribution of pixel intensity was measured in three regions using a MatLab (MathWorks) image processing script (Fig. 3A, white squares). The user defined the size of a square in each image (usually 200 × 200 pixels, 15 × 15 μm) to be used for measuring the intensity of the background. Three squares of the same size were used in different locations within each image to estimate the background intensity. The user selected image regions in which there was no dense VGlut2 staining and that were clear of any artifactual staining (Fig. 3A). Histograms of the pixel intensity distribution (from 0–255) were obtained for the entire image and the three background squares (Fig. 3). The distributions for the three selected background regions were combined. For each image a threshold was selected, usually around 3 standard deviations below the mean of the combined background distribution, and was used as the threshold to generate a new binary image of the objects or particles (Fig. 3B) in which the background was removed. By performing the thresholding in each individual image based on the background of that image any large variations in intensity between images (across the same section or between sections) were normalized.
Fig. 3.
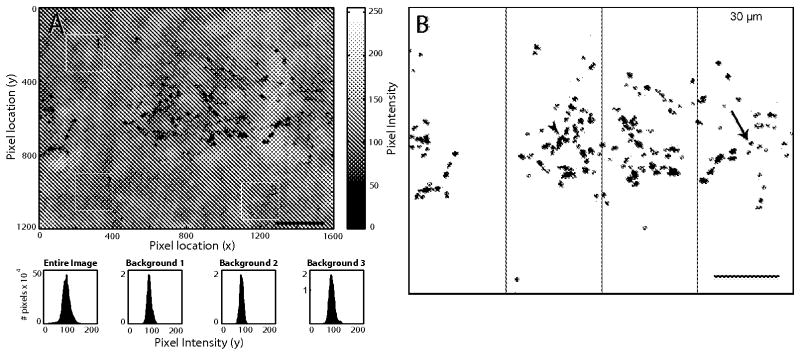
Quantification of VGlut2-ir Puncta in Layer 4A. A: High resolution micrograph of layer 4A stained with VGlut2. The background in each image was measured using a MatLab image processing script. B: In each image the intensity was thresholded to give an image of the puncta. In the each thresholded image, four non-overlapping segments that extended 30 μm in the horizontal plane (parallel to the layers) and the whole vertical extent of 4A were drawn in the images. ImageJ was used to quantify the number of VGlut2-ir puncta in each counting frame; only particles larger than 15 pix2 were included in the quantification (particles outlined with red). Scale bars: 20 μm
The binary images were analyzed with Image J (Rasband, 1997–2012). First, small particles (<15 pixel2) were excluded from the quantification. As the puncta appear as dark objects in the thresholded images (Fig. 3B; arrowhead puncta cluster, arrow single puncta), each separate object could include a single puncta or at times a cluster of puncta that could not be clearly separated by the imaging routine and were displayed as a single object. In a few regions there was a small blood vessel that was dense and included as a thresholded object, these were excluded from the analysis. Next, four non-overlapping counting regions that extended 30 μm parallel to layer 4A and the whole thickness of 4A were drawn in the images (Fig. 3B). Within each of these counting regions ImageJ returned the total area occupied by each object. Finally, to obtain an estimate of the number of individual puncta we divided the total area occupied by each object by the average single particle size. The average single particle size (50 pix2, 0.28 μm2) was estimated from a histogram of all objects across many sections with VGlut2-ir puncta in layer 4A. Using this approximation we obtained an estimate of the number of puncta in each 30 μm wide counting region, capturing the entire region that was likely to be 4A.
For the coronal plane, the region along the representation of the HM close to the lateral pole of V1 corresponds to the foveal retinal representation and progressively more medial regions are more eccentric, 0.25 degree (deg), 0.5 deg and 1 deg (Fig. 2A, stars along the dark horizontal line). In sections away from the HM representation, the eccentricities were re-calculated taking into account the distance between sections and the magnification factor of the cortex (Fig. 2A,D). In the sagittal plane, we determined which was the most lateral V1 section where layer 4C first appeared, this was taken as the section corresponding to the foveal representation in the cortex. Images were acquired along the representations of the HM, inferior and superior vertical meridians (iVM, sVM) corresponding to different eccentricities: fovea, 0.25 deg, 0.5 deg and 1 deg (Fig. 2B,E).
Thickness of VGlut2-ir label in layer 4A
The same coronal sections used for estimating the VGlut2 density were also used to measure the thickness of layer 4A labeled with VGlut2 Using a 40X objective and camera lucida we drew the upper and the lower limit of 4A label at eccentricities larger than 1 deg. For the foveal region, where the honeycomb arrangement was not clear, the vertical extent of 4A was calculated by measuring the width of the puncta distributed from what appeared to be single fibers running parallel to the V1 layers (Fig. 6B, arrowheads).
Fig. 6.
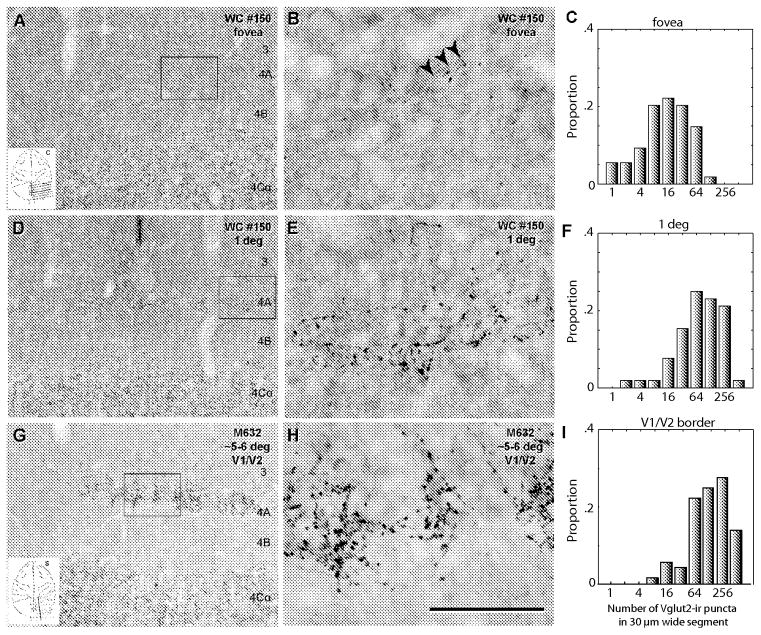
Comparison of VGlut2-ir puncta distribution at different cortical locations in coronal sections (A–F). In single sections (e.g. #150, Fig. 2D), images were collected at locations outlined by the squares. A: foveal region, the low power image (20x) that shows layer 4C with a high density of VGlut2-ir puncta, a region that is puncta free (layer 4B) and a region with few puncta above layer 4B corresponding to layer 4A. B: foveal region, high power (100x objective) image of a frame within the box. The arrows point to a presumptive LGN afferent. Note the low density of puncta. C: foveal region, histogram of the proportion of counting frames with puncta in each of the bins.. D–F, shows the same sequence as for A–C but for a region estimated to represent around 1 degree eccentric. Note the higher density of puncta and the greater vertical extent of their distribution. G–I shows the sequence in a sagittal section from a region at the V1/V2 border in a region estimated to represent an eccentricity of 5–6 degrees. Note the high puncta density at the border in 4A, the histogram (I) showed a similar distribution to the distribution at 1 degree (in F). Each histogram summarizes the results from 4 counting frames in each of 9 sampling images from two animals: Y-axis is the proportion, from the total of 72 counting frames, that had counts in each of the bins. Scale bar: A, D, G: 250 μm; B, E, H, 50 μm
Morphological arrangement of the honeycomb in tangential sections
The arrangement of the honeycomb is most clearly viewed in tangential sections. To ascertain whether the two dimensional organization of foveal and perifoveal representation in layer 4A differed, tangential sections stained for VGlut2 were acquired with a 20X objective on an Olympus VS120-SL Virtual Slide System. The Extended Focal Images (EFI) function of the VS120 allows the user to automatically acquire images at different focal points, and combine the in-focus parts of each image together to produce a single image with a greater depth of field than any of the individual images. Two large regions, corresponding to the foveal and perifoveal representation in V1 from two animals where acquired. The regions were approximately 1.5 × 5 mm (M632, Fig. 2C, F) and 2.9 × 2.6 mm (M629), with stacks of 40 and 30 sections (z = 30 μm) (M632 and M629) respectively. Within a single section, the representations of the foveal and perifoveal regions were separated by around 12 mm, corresponding to about 1 deg (Dow et al, 1985). Selected regions of these images (small boxes in Fig. 2F), in which the background staining was similar, were aligned in neighboring sections using the blood vessels as reference points. The serial images were cropped (400 × 400 μm), aligned, added to a stack and a composite EFI was obtained with ImageJ. In these composite images the background was removed, they were transformed to binary and the area occupied by VGlut2 puncta was obtained with ImageJ.
Calculation of shrinkage
The degree of tissue shrinkage after VGlut2 immunohistochemistry was measured by comparing sections in the x and y dimensions before and after tissue preparation with the aid of Neurolucida software (MicroBrightField). The average linear shrinkage was calculated by using the following formula: S=(A-0)/A, where A is the absolute value before processing and 0 is the observed value after processing. This yielded an average linear shrinkage of 12.15% (range 12.6% to 11.7%).
Statistics
Statistical comparisons between the densities of VGlut2-ir puncta in layer 4A at different eccentricities were performed by using either a parametric (one-way ANOVA and t-test) or a nonparametric test (Kruskal-Wallis or Mann-Whitney) depending on whether the data sets were approximately normally distributed and passed the test for homogeneity of variances, followed by suitable post-hoc tests. The data are presented as the mean ± SEM (VGlut2-ir puncta). All statistical studies were performed with the aid of the GraphPad Prism 5.1 statistical package (GraphPad, San Diego, CA).
Results
Inputs from the S-cones form an integral part of the blue/yellow opponent color pathway in primates (Fig. 1). The density of S-cones is lower in the fovea than perifovea (de Monasterio et al. 1985; Wikler and Rakic 1990; Martin and Grunert 1999). In the current study it was hypothesized that there would be a reduction in the cortical representation of blue/yellow pathway in foveal V1 compared to the peri- and parafoveal representation. In Old-World monkeys the main input to the LGN from the L/M opponent retinal ganglion cells is to the P-layers. While the S/(L+M) opponent input is segregated: the majority of the SON/(L+M) retinal ganglion cells project to the koniocellular layers of the LGN, while it has been proposed that the SOFF/(L+M) cells project to the P layers. The pathways remain segregated in V1, where the L/M component of the P-layer LGN afferents terminate mainly in layer 4Cβ. The combined S-cone opponent input (SON and SOFF) to V1 is mainly to layer 4A and lower layer 3. There may be segregation of the SOFF/(L+M) P input to lower 4A, while the K-layer inputs that are functionally SON/(L+M) opponent terminate in upper layer 4A and the adjacent lower layer 3 (Hendry and Yoshioka 1994; Chatterjee and Callaway 2003, Casagrande et al. 2007).
The vesicular glutamate transporter VGlut2 is preferentially located in the terminals of geniculate afferents (Nahmani and Erisir 2005), including those in 4C and 4A (Garcia-Marin et al. 2013). To determine whether there was a reduction in the density of LGN terminals in foveal layer 4A the distribution of VGlut2-ir puncta was assayed in the foveal and perifoveal regions of V1. Three different series of sections were prepared to determine the arrangement of puncta distribution: coronal (Fig. 2A, D), sagittal (Fig. 2B, E), and tangential (Figs. 2C, F). The density of terminals was estimated in sections that correspond to different retinal eccentricities in the coronal and sagittal sections. The total area occupied by the dense VGlut2-ir regions was estimated in the tangential sections.
Tangential sections: honeycomb arrangement
To obtain a picture of the three-dimensional arrangement of the VGlut2-ir puncta in layer 4A a series of tangential sections was obtained. Within the tangential sections the arrangement of the honeycomb is clearly seen, with the ‘walls’ of the honeycomb being positive for VGlut2 and the ‘holes’ of the honeycomb devoid of VGlut2 (Figs. 4 & 5). Qualitatively the honeycomb arrangement in the foveal region was weak when viewed in the tangential plane (Fig. 4A–E). In contrast the honeycomb arrangement in the perifoveal region was distinct (Fig. 5A–E). In addition, the puncta density appears higher in perifoveal region than the foveal region.
Fig. 4.
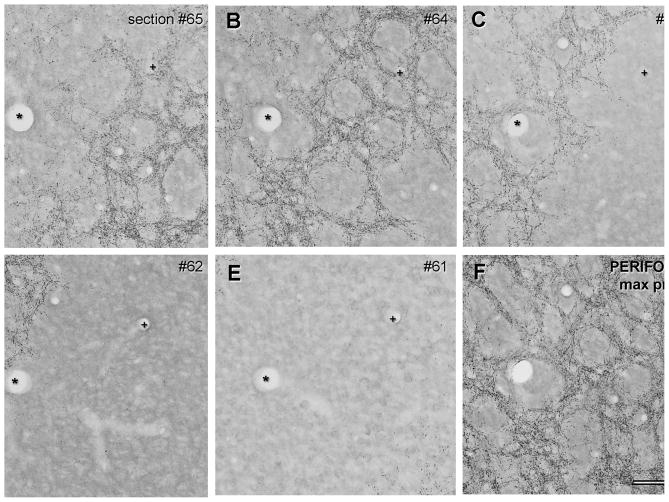
Tangential sections from the foveal representation in V1. A–E shows a sequential series of 30 μm thick sections through the depth of layer 4A. F: shows the maximum projection of the series, giving the two dimensional layout of the honeycomb in layer 4A. In the foveal region there is an arrangement of puncta that resembles the honeycomb pattern in the projection, although there are regions where the walls of the honeycomb appear to be one or two fibers wide. This was the method used to obtain the large field reconstruction shown in Fig. 8. Blood vessels (+,*) were used to align adjacent sections. Scale bar: 100 μm
Fig. 5.
Tangential sections from a region of V1 representing the perifovea. A–E shows a sequential series of 30 μm thick sections through the depth of layer 4A. F: shows the maximum projection of the series, giving the two dimensional layout of the honeycomb in layer 4A. In the perifovea the arrangement of puncta gives rise to a clear honeycomb pattern in the projection (F). The walls of the honeycomb have a relatively high density of puncta and are thicker than the corresponding walls in the foveal region (compared with Fig. 4F). Blood vessels (+,*) were used to align adjacent sections. Scale bar: 100 μm
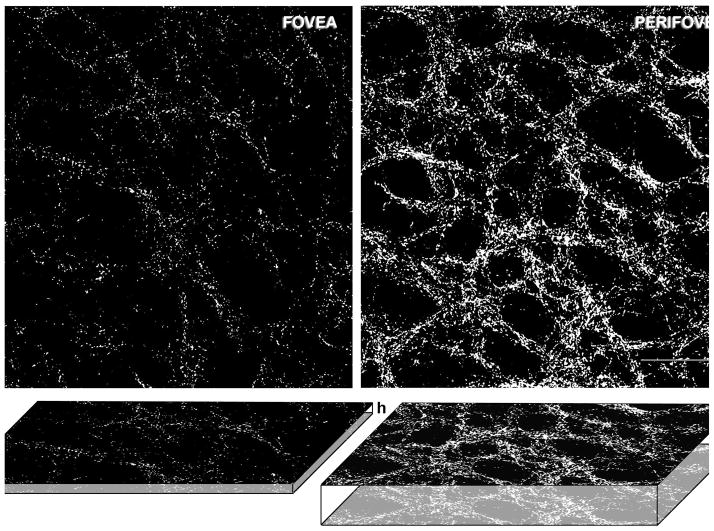
The percentage area occupied by VGlut2-ir puncta was measured in 6 regions corresponding to the fovea and 7 regions of the perifoveal representation in two animals (Fig. 2F). In each case five adjacent sections were merged to obtain the maximum projection of the fovea (Fig. 4F) and perifoveal representations (Fig. 5F). The percentage of the area occupied by VGlut2 puncta in the foveal region was 0.59±0.05, whereas in the perifoveal region the percentage of the of area occupied by VGlut2 was 4.51±1.60. Thus, the percentage of area occupied by VGlut2 in the foveal region was 7.7 times lower than in the perifoveal region. An estimate of the number of puncta was obtained from coronal and sagittal sections.
Puncta density changes with eccentricity
Coronal plane
Single coronal sections through the apex of lateral V1 near to the representation of the horizontal meridian were analyzed for puncta density from V1 cortex obtained from 2 animals. A series of around 300, coronal sections (50 μm), numbered from posterior to anterior (Fig. 2A, #1→#N) was obtained from a lateral block of V1 in each animal. The sections were referenced to the visual field map to get an approximate estimate of eccentricity (Fig. 2A). Sections in the middle of the series, close to the apex of most lateral V1, were considered to contain regions closest to the foveal representation (sections #140–160 in Fig. 2A). Locations on each section are marked with their corresponding approximate eccentricity: the fovea is at the lateral V1/V2 border (Fig. 2D, fovea), and more perifoveal eccentricities are shown as marks moving medially from the foveal representation (Fig. 2D, 0.5, 1 and 2 deg.). To compare fovea and perifovea, regions in layer 4A on each section corresponding to fovea and 1 degree eccentricity were analyzed (Fig. 6A, D).
Puncta counts were obtained from 9 images with 4 counting regions in each image that were 30 μm wide parallel to the layers and extended vertically to include the whole thickness of layer 4A in each of the two animals at each eccentricity. The counts are shown in the histograms in Fig. 6C, F and I. In the foveal representation 98% of the counting segments had fewer than 100 puncta counts (Fig. 6C), whereas at 1 degree eccentricity around 54% of the counting segments had 100 or fewer counts (Fig. 6F). In the foveal region there were no counting segments with more than 200 puncta. Whereas at 1 deg around 22% of the counting frames had more than 200 puncta (compare Fig. 6C, F), as would be expected from a honeycomb arrangement there were some regions with high density of terminals and others with a low density.
Quantitatively the density of VGlut2-ir puncta in layer 4A near the V1/V2 border at the lateral pole of V1 was considerably lower than the density at 1 deg on the horizontal meridian, (24.2±3.8 (range 0–138) vs 120.9±14.1 (range 2–423); P<0.0001, Mann Whitney test). There were 5 times fewer particles in the foveal representation than in the perifoveal representation (Fig. 6). To test if there was a reduction in puncta density near the V1/V2 border irrespective of eccentricity a similar analysis was made in sections at 5–6 degrees eccentricity near the representation of the vertical meridian. The density of puncta did not depend on whether the counting was on the horizontal or vertical meridian. The histogram (Fig. 6I) shows a similar distribution to the one observed at 1 deg, but there were more segments with a higher puncta count. The mean puncta count for 5–6 degs representation was 203.6 ± 17.4 (range 10 – 708) which was significantly greater than in the region representing 1 deg eccentricity (P<0.003, Mann Whitney test). At this eccentricity (5–6 deg) 84% of the counting segments had more than 200 VGlut2-ir puncta.
The thickness of the region of layer 4A with VGlut2-ir puncta was also considerably reduced in the region of the foveal representation. The vertical extent was measured in the same coronal sections used for estimating the VGlut2-ir puncta density. The average thickness of VGlut2-ir puncta in layer 4A at eccentricities greater than 0.5 degree (measured between 0.5 and 1 degrees) was 27.4 μm ± 0.6 μm (mean ± SEM, n= 161). In the foveal region there was a weak honeycomb arrangement. In most cases the extent of 4A was the width of the puncta arising from what appeared to be a single fiber running parallel to the layers (Fig. 6B, arrowheads), although in some cases individual puncta were observed sparsely distributed in this layer. In the foveal region the estimated vertical extent of 4A was 2.4 μm ± 0.10 μm (mean ± SEM, n= 135). The difference between the extent in the perifoveal and foveal regions was significant (P<0.0001, Mann Whitney test).
Sagittal plane
To verify the findings from coronal series a new series of sections in the sagittal plane was analyzed, where the visual field representation runs orthogonally on the section to the coronal representation. In particular the sagittal series allowed us to determine the first appearance of layer 4C – in the most lateral sections – therefore giving a relatively precise determination of the foveal representation.
In the sagittal plane the transition between V2 and V1 was marked by the appearance of a dense band of VGlut2-ir puncta in layer 4C at both the inferior (iVM) and superior (sVM) representation of the vertical meridian (Fig. 2E). In the first sections where layer 4C was apparent the band was less than a millimeter in width (posterior to anterior) but as the series progressed more medially the width increased and the inferior and superior vertical meridian representations were easily viewed as the characteristic transition be between V1 and V2 (Fig. 2E). We designated as the foveal representation the first section in which layer 4C was unambiguously observed. For eccentricities away from the foveal representation (0.25, 0.5 and 1 deg) we estimated the cortical distance (in μm) from the section representing the fovea using the non-linear transformation of Dow et al (1985) and selected the corresponding section (Fig. 2B, 7A).
Fig. 7.
Comparison of VGlut2-ir puncta distribution at different cortical locations in sagittal sections. A: shows the visual field map overlaid on V1, the orientation of the sections is shown by the solid lines with the position of each section on the presumptive map of the visual field and the corresponding eccentricity. B–C: low power images of the region corresponding to the foveal region (B) and to 1 degree eccentric (C). Upper layer 4C (4Cα) is clearly seen in the lower part of each image, with a puncta free region above corresponding to layer 4B. In C there is a region that is running parallel to 4C that corresponds to layer 4A and above this there are sparse puncta that belong to the CO rich patches of layer 2/3. There are almost no puncta in the region corresponding to layer 4A in B, but the sparse distribution in lower layer 3 that belongs to a CO-rich patch is present. D–G: high power (100x objective) images in sections that were estimated to be in the representation of the foveal region (D), and at 0.25 (E), 0.5 (F) and 1 (G) degree eccentric. Arrows in (D) appear to be puncta belonging to a single afferent fiber running parallel to the layers. The histograms in H–K show the proportion of counting frames (y-axis) with puncta in each of the bins for each eccentricity. The counts are from a single animal, the number of frames as boxes was the same as described in Fig. 6. Scale bar: A: 1.6 cm; B–C: 250 μm; D–G, 50 μm.
Qualitatively there was a very low density of puncta in the region of layer 4A in the most lateral sections, corresponding to the foveal representation (Fig. 7B, D). As in the coronal sections, the foveal region had few puncta, although often what appeared to be a single afferent fiber could be observed running parallel to the layers (Fig. 7D arrowheads). Because the sagittal plane provided sections more closely parallel to the isoeccentricity contours on the cortical surface, we made density estimates at a finer spacing than in the coronal plane. The distribution of counts was made in the foveal representation and at 0.25, 0.5 and 1 degree (Fig. 7).
Quantitative puncta counts were obtained in images as described for the coronal series. The proportion of counting frames with fewer than 100 puncta decreases from the foveal to 1 deg representation (100%, 91.7%, 69.4%, and 61.1%, fovea, 0.25, 0.5 and 1 degree, respectively). There is a systematic increase in the average number of puncta per counting frame with increasing eccentricity within the central one degree of visual field representation (Fig. 7D–G: 19.7±2.2 (range 0 – 81), 49.7±6.6 (range 0 – 154), 78.6±9.7 (range 8 – 228), 95.1±13.8 (range 0 – 358) counts for fovea, 0.25 deg, 0.5 deg, and 1 deg, respectively; significant differences between: fovea and 0.25 deg (p < 0.01), fovea and 0.5 deg (p < 0.0001), fovea and 1 deg (p < 0.0001), and 0.25 and 1 deg (p < 0.001) 1 way Anova). When comparing the density ofVGlut2-ir puncta in the fovea and at 1 deg the ratio was 4.8, similar to the ratio of 5 obtained in the coronal sections for the same eccentricities.
In contrast to the findings in layer 4A, in layer 4C the density of VGlut2-ir puncta does not appear to change with eccentricity (Suppl. Fig 1). Images were acquired in layer 4C underlying layer 4A (in the same vertical column) at the representation corresponding to foveal and perifoveal eccentricities. The images show the same density of puncta in layer 4C at foveal and perifoveal representations. A quantitative estimation of the number of puncta in 4Cα and 4Cβ at different eccentricities would necessitate the use of confocal images, because of the high density of puncta. If quantification is undertaken using brightfield images, when there is a high puncta density as in layer 4C, segmentation is a problem because many of the puncta end up merged into large particles. This is not the case for layer 4A where the particle density is sparser than in layer 4C.
Discussion
The results indicate that the strength of the putative S-cone input from LGN to visual cortex is highly correlated with the peripheral distribution of S-cones in the retina. The foveola of the retina has a low density of S-cone photoreceptors in many primate species, including baboons (Marc and Sperling 1977), Old World monkeys (de Monasterio et al. 1985; Martin and Grunert 1999) and humans (Curcio et al. 1991). In the one of the most studied species, macaque monkeys, the density of S-cones is about 1% of the total foveal cone population rising to peak at 6–8% of the total cone population at 1–2 degrees eccentricity (de Monasterio et al. 1985). The cortical representation of the central visual field is at the lateral pole of the posterior opercular cortex; this is where the lowest density of VGlut2-ir puncta in layer 4A was found in the current study (results summarized in Fig. 8).
Fig. 8.
Extended focus images from tangential sections of cortex were generated from the foveal and the perifoveal representation (Fig 2F; rectangles). From within these extended images two smaller regions of interest were cropped to obtain the regions shown in A–B (These are the small squares within the rectangular area in Fig 2F. The maximum projection was obtained using the method shown in Figs. 4 and 5. There is a clear intense honeycomb in the perifovea compared to the fovea. When the total number of particles was estimated using density and total extent in both the tangential and vertical (thickness) directions it was estimated that there was a 6–7 fold difference in the total number of presumptive terminals in the perifoveal compared to the foveal representations. Scale bar: 100 μm
In humans psychophysically there is a reduced discrimination for targets that depend on S-cone sensitivity in central vision that is not dependent on the absorption of the macular pigment and is thought to be related to the paucity of S-cones in the fovea (Konig 1894; Willmer 1944; Williams et al. 1981a,b). Normal trichomats performance in experiments that demonstrate foveal tritanopia is similar to the performance of tritanopic observers (lacking retinal S-cones).
One question that has not been addressed until now is how the cortical representation in the pathway that subserves the perception of hues evoked by S-cone opponent mechanisms, is organized in foveal cortex. Two main schemes come to mind. 1. The cortical circuits for “blue perception” in the fovea could stay be intact and receive the normal density of input. The photoreceptor input to this pathway could be could derived from the few cones in the foveal region and from additional S-cone input from non-corresponding regions of visual space that have a denser S-cone representation. This could be thought of as a system akin to the filling in of cortical territory after peripheral deafferentation (Merzenich and Jenkins 1993). A system like this would give rise to activity in the cortical S-cone circuits but only when derived from retinal inputs outside the fovea. 2. The cortical circuits for “blue perception” could be effectively missing in the foveal representation, whereby they retain their cortical organization but are effectively deafferented or have substantially reduced input. The current results strongly support the second alternative. The circuits are relatively faithful to the density of the peripheral representation. There are a number of important implications that arise from these conclusions. One is that during development there is a precise visuotopic map formed in the cortex, and that if there is reduced thalamic input to one cortical layer of the map it does not reorganize to maintain the expected density of inputs within its normal pathway (the K-pathway) nor does it get inputs from other afferent pathways (M or P-pathways) or even from the component of the K-pathway that is supplying the CO rich areas of layer 2/3. There is evidence that the afferents in the upper regions of the CO rich patches are from a different set of thalamic neurons that those that supply layer 4A and the neighboring regions in lower layer 3 (Casagrande et al. 2007).
Many aspects of the trichromatic visual systems of Old World monkeys and great apes, including humans are very similar. However there are subtle differences, one of these is in the arrangement of layer 4A in visual cortex. In Old World monkeys there is a distinct honeycomb arrangement of layer 4A in CO density (Carroll and Wong-Riley 1984; Horton, 1984) and in VGlut2 immunoreactivity (Garcia-Marin et al. 2013): this is thought to be the anatomical substrate for the input from the S-cone opponent neurons from the LGN (Chatterjee and Callaway 2003). While the organization of human primary visual cortex matches the organization of Old World monkey V1 in most ways it lacks the characteristic honeycomb organization of layer 4A revealed by CO staining (Horton 1984; Yoshioka and Hendry 1995; Preuss and Coleman 2002; Pruess et al. 1999) or VGlut2 immnumoreactivity (Garcia-Marin et al. 2013). Therefore how the S-cone opponent pathway is represented in human visual cortex is currently unknown. However, it would be predicted based on the results in the present study that there would be a reduced input to the Scone selective circuits in cortex representing foveal retina, maybe with a more extensive differential strength between layer 4C input from L and M cone opponent and non-opponent pathways and those carrying S-cone opponent signals because the relative paucity of S-cones in the foveal region of humans (Curcio et al. 1991) is greater than in macaques (de Monasterio et al. 1985).
Supplementary Material
Supplementary Fig. 1: High magnification images from 4Cα 4Cβ at the foveal and perifoveal representations. Note that there is no qualitative difference in the density of VGlut2-ir puncta in layer 4C at the foveal and perifoveal representations whereas layer 4A has a marked reduction of puncta density at the same foveal representation (Fig. 2). Scale bar: 20 μm
Acknowledgments
This work was supported by NIH grants EY17945 and P30EY013079. V.G-M was supported by the Spanish Ministry of Education, Science and Innovation (National R&D&I Plan 2008-2011, National Human Resources Mobility Programme, Postdoctoral Mobility in Foreign Centers, grant EX2009-0636). We thank Jenna Kelly for helpful suggestions and Claudia Farb, Yasmeen Azfal and Tunazzina Ahmed for their technical support.
Footnotes
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Balaram P, Hackett TA, Kaas JH. Differential expression of vesicular glutamate transporters 1 and 2 may identify distinct modes of glutamatergic transmission in the macaque visual system. J Chem Neuroanat. 2013;50–51:21–38. doi: 10.1016/j.jchemneu.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodmann K. In: Localisation in the cerebral cortex. Garey LJ, translator. Smith-Gordon; London: 1909. [Google Scholar]
- Carroll EW, Wong-Riley MT. Quantitative light and electron microscopic analysis of cytochrome oxidase-rich zones in the striate cortex of the squirrel monkey. J Comp Neurol. 1984;222:1–17. doi: 10.1002/cne.902220102. [DOI] [PubMed] [Google Scholar]
- Casagrande VA, Yazar F, Jones KD, Ding Y. The morphology of the koniocellular axon pathway in the macaque monkey. Cereb Cortex. 2007;17:2334–2345. doi: 10.1093/cercor/bhl142. [DOI] [PubMed] [Google Scholar]
- Chatterjee S, Callaway EM. Parallel colour-opponent pathways to primary visual cortex. Nature. 2003;426:668–671. doi: 10.1038/nature02167. [DOI] [PubMed] [Google Scholar]
- Coleman JE, Nahmani M, Gavornik JP, Haslinger R, Heynen AJ, Erisir A, Bear MF. Rapid structural remodeling of thalamocortical synapses parallels experience-dependent functional plasticity in mouse primary visual cortex. J Neurosci. 2010;30:9670–9682. doi: 10.1523/JNEUROSCI.1248-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curcio CA, Allen KA, Sloan KR, Lerea CL, Hurley JB, Klock IB, Milam AH. Distribution and morphology of human cone photoreceptors stained with anti-blue opsin. J Comp Neurol. 1991;312:610–624. doi: 10.1002/cne.903120411. [DOI] [PubMed] [Google Scholar]
- Dacey DM, Crook JD, Packer OS. Distinct synaptic mechanisms create parallel S-ON and S-OFF color opponent pathways in the primate retina. Vis Neurosci. 2013:1–13. doi: 10.1017/S0952523813000230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel PM, Whitteridge D. The representation of the visual field on the cerebral cortex in monkeys. J Physiol. 1961;159:203–221. doi: 10.1113/jphysiol.1961.sp006803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Monasterio FM, McCrane EP, Newlander JK, Schein SJ. Density profile of blue-sensitive cones along the horizontal meridian of macaque retina. Invest Ophthalmol Vis Sci. 1985;26:289–302. [PubMed] [Google Scholar]
- Dow BM, Snyder AZ, Vautin RG, Bauer R. Magnification factor and receptive field size in foveal striate cortex of the monkey. Exp Brain Res. 1981;44:213–228. doi: 10.1007/BF00237343. [DOI] [PubMed] [Google Scholar]
- Dow BM, Vautin RG, Bauer R. The mapping of visual space onto foveal striate cortex in the macaque monkey. J Neurosci. 1985;5:890–902. doi: 10.1523/JNEUROSCI.05-04-00890.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick D, Lund JS, Blasdel GG. Intrinsic connections of macaque striate cortex: afferent and efferent connections of lamina 4C. J Neurosci. 1985;5:3329–3349. doi: 10.1523/JNEUROSCI.05-12-03329.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Marin V, Ahmed TH, Afzal YC, Hawken MJ. Distribution of vesicular glutamate transporter 2 (VGlut2) in the primary visual cortex of the macaque and human. J Comp Neurol. 2013;521:130–151. doi: 10.1002/cne.23165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guld C, Bertulis A. Representation of fovea in the striate cortex of vervet monkey, Cercopithecus aethiops pygerythrus. Vision Res. 1976;16:629–631. doi: 10.1016/0042-6989(76)90010-9. [DOI] [PubMed] [Google Scholar]
- Hässler R. Comparative anatomy of central visual systems in day-and night-active primates. In: Hassler R, Stephan H, editors. Evolution of the Forebrain. New York: Plenum Press; 1967. pp. 419–434. [Google Scholar]
- Hendry SH, Reid RC. The koniocellular pathway in primate vision. Annu Rev Neurosci. 2000;23:127–153. doi: 10.1146/annurev.neuro.23.1.127. [DOI] [PubMed] [Google Scholar]
- Hendry SH, Yoshioka T. A neurochemically distinct third channel in the macaque dorsal lateral geniculate nucleus. Science. 1994;264:575–577. doi: 10.1126/science.8160015. [DOI] [PubMed] [Google Scholar]
- Horton JC. Cytochrome oxidase patches: a new cytoarchitectonic feature of monkey visual cortex. Phil Trans R Soc Lond B. 1984;304:199–253. doi: 10.1098/rstb.1984.0021. [DOI] [PubMed] [Google Scholar]
- Horton JC, Hubel DH. Regular patchy distribution of cytochrome oxidase staining in primary visual cortex of macaque monkey. Nature. 1981;292:762–764. doi: 10.1038/292762a0. [DOI] [PubMed] [Google Scholar]
- Jacobs GH. The evolution of vertebrate color vision. Adv Exp Med Biol. 2012;739:156–172. doi: 10.1007/978-1-4614-1704-0_10. [DOI] [PubMed] [Google Scholar]
- König A. Ueber den menschlichen Sehpurpur und seine Bedeutung fur das Sehen. Sitz Akad Wiss (Berlin) 1894:577–598. [Google Scholar]
- Lee BB. Visual pathways and psychophysical channels in the primate. J Physiol. 2011;589:41–47. doi: 10.1113/jphysiol.2010.192658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei W, Deng Y, Liu B, Mu S, Guley NM, Wong T, Reiner A. Confocal laser scanning microscopy and ultrastructural study of VGLUT2 thalamic input to striatal projection neurons in rats. J Comp Neurol. 2013;521:1354–1377. doi: 10.1002/cne.23235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund JS. Organization of neurons in the visual cortex, area 17, of the monkey (Macaca mulatta) J Comp Neurol. 1973;147:455–496. doi: 10.1002/cne.901470404. [DOI] [PubMed] [Google Scholar]
- Marc RE, Sperling HG. Chromatic organization of primate cones. Science. 1977;196:454–456. doi: 10.1126/science.403607. [DOI] [PubMed] [Google Scholar]
- Marion R, Li K, Purushothaman G, Jiang Y, Casagrande VA. Morphological and neurochemical comparisons between pulvinar and V1 projections to V2. J Comp Neurol. 2013;521:813–832. doi: 10.1002/cne.23203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin PR, Grunert U. Analysis of the short wavelength-sensitive (“blue”) cone mosaic in the primate retina: comparison of New World and Old World monkeys. J Comp Neurol. 1999;406:1–14. doi: 10.1002/(sici)1096-9861(19990329)406:1<1::aid-cne1>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Merzenich MM, Jenkins WM. Reorganization of cortical representations of the hand following alterations of skin inputs induced by nerve injury, skin island transfers, and experience. J Hand Ther. 1993;6:89–104. doi: 10.1016/s0894-1130(12)80290-0. [DOI] [PubMed] [Google Scholar]
- Nahmani M, Erisir A. VGlut2 immunochemistry identifies thalamocortical terminals in layer 4 of adult and developing visual cortex. J Comp Neurol. 2005;484:458–473. doi: 10.1002/cne.20505. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Hioki H, Fujiyama F, Kaneko T. Postnatal changes of vesicular glutamate transporter (VGlut)1 and VGlut2 immunoreactivities and their colocalization in the mouse forebrain. J Comp Neurol. 2005;492:263–288. doi: 10.1002/cne.20705. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Watakabe A, Hioki H, Fujiyama F, Tanaka Y, Yamamori T, Kaneko T. Transiently increased colocalization of vesicular glutamate transporters 1 and 2 at single axon terminals during postnatal development of mouse neocortex: a quantitative analysis with correlation coefficient. Eur J Neurosci. 2007;26:3054–3067. doi: 10.1111/j.1460-9568.2007.05868.x. [DOI] [PubMed] [Google Scholar]
- Preuss TM, Coleman GQ. Human-specific organization of primary visual cortex: alternating compartments of dense Cat-301 and calbindin immunoreactivity in layer 4A. Cereb Cortex. 2002;12:671–691. doi: 10.1093/cercor/12.7.671. [DOI] [PubMed] [Google Scholar]
- Preuss TM, Qi H, Kaas JH. Distinctive compartmental organization of human primary visual cortex. Proc Natl Acad Sci U S A. 1999;96:11601–11606. doi: 10.1073/pnas.96.20.11601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasband W. Image J. US National Institutes of Health; Bethesda, Maryland, USA: 1997–2012. http://imagej.nih.gob/ij/ [Google Scholar]
- Solomon SG, Peirce JW, Lennie P. The impact of suppressive surrounds on chromatic properties of cortical neurons. J Neurosci. 2004;24:148–160. doi: 10.1523/JNEUROSCI.3036-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbot SM, Marshall WH. Physiological studies on neural mechanisms of localization and discrimination. Am J Ophthalmol. 1941;24:1255–1264. [Google Scholar]
- Tootell RB, Silverman MS, Switkes E, De Valois RL. Deoxyglucose analysis of retinotopic organization in primate striate cortex. Science. 1982;218:902–904. doi: 10.1126/science.7134981. [DOI] [PubMed] [Google Scholar]
- Van Essen DC, Newsome WT, Maunsell JH. The visual field representation in striate cortex of the macaque monkey: asymmetries, anisotropies, and individual variability. Vision Res. 1984;24:429–448. doi: 10.1016/0042-6989(84)90041-5. [DOI] [PubMed] [Google Scholar]
- Varoqui H, Schafer MK, Zhu H, Weihe E, Erickson JD. Identification of the differentiation-associated Na+/PI transporter as a novel vesicular glutamate transporter expressed in a distinct set of glutamatergic synapses. J Neurosci. 2002;22:142–155. doi: 10.1523/JNEUROSCI.22-01-00142.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikler KC, Rakic P. Distribution of photoreceptor subtypes in the retina of diurnal and nocturnal primates. J Neurosci. 1990;10:3390–3401. doi: 10.1523/JNEUROSCI.10-10-03390.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DR, MacLeod DI, Hayhoe MM. Foveal tritanopia. Vision Res. 1981a;21:1341–1356. doi: 10.1016/0042-6989(81)90241-8. [DOI] [PubMed] [Google Scholar]
- Williams DR, MacLeod DI, Hayhoe MM. Punctate sensitivity of the blue-sensitive mechanism. Vision Res. 1981b;21:1357–1375. doi: 10.1016/0042-6989(81)90242-x. [DOI] [PubMed] [Google Scholar]
- Willmer E. Colour of small objects. Nature. 1944;153:774–776. [Google Scholar]
- Xing D, Ringach DL, Shapley R, Hawken MJ. Correlation of local and global orientation and spatial frequency tuning in macaque V1. J Physiol. 2004;557:923–933. doi: 10.1113/jphysiol.2004.062026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshioka T, Hendry SH. Compartmental organization of layer IVA in human primary visual cortex. J Comp Neurol. 1995;359:213–220. doi: 10.1002/cne.903590203. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Fig. 1: High magnification images from 4Cα 4Cβ at the foveal and perifoveal representations. Note that there is no qualitative difference in the density of VGlut2-ir puncta in layer 4C at the foveal and perifoveal representations whereas layer 4A has a marked reduction of puncta density at the same foveal representation (Fig. 2). Scale bar: 20 μm