Abstract
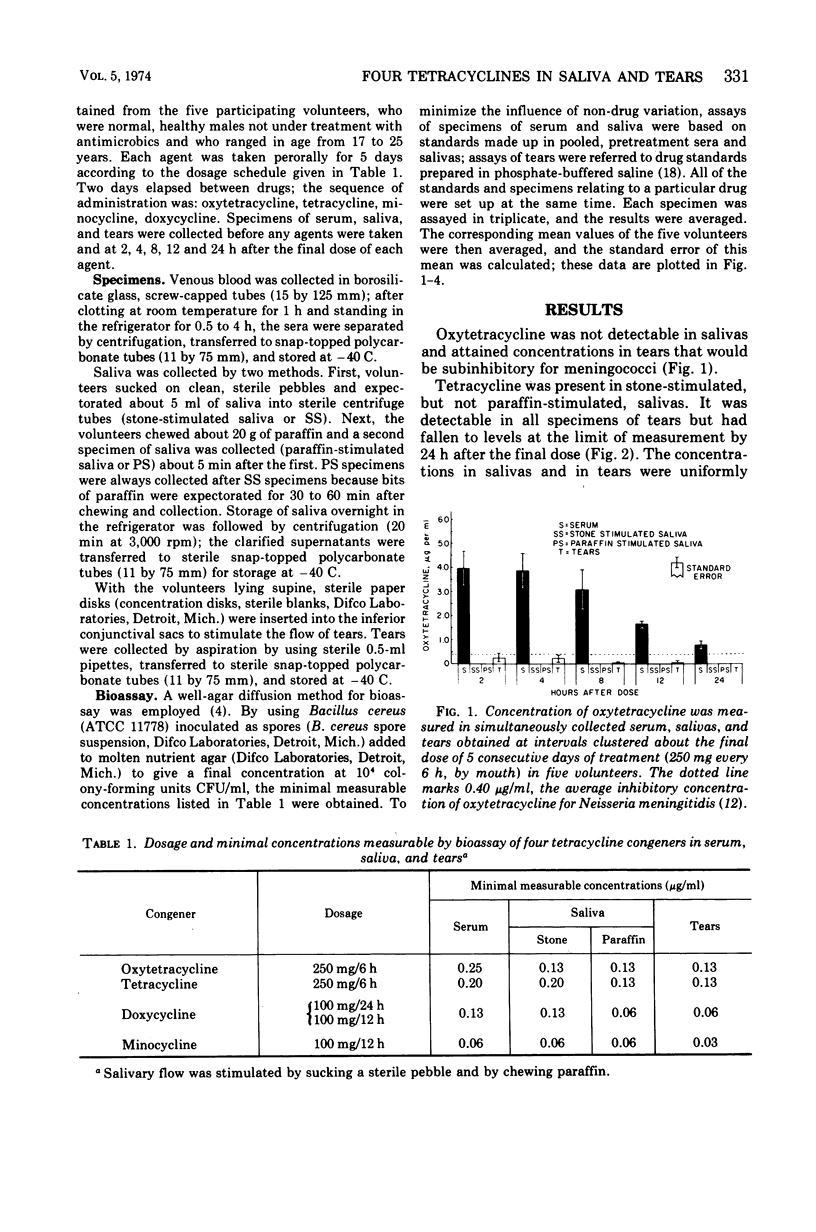
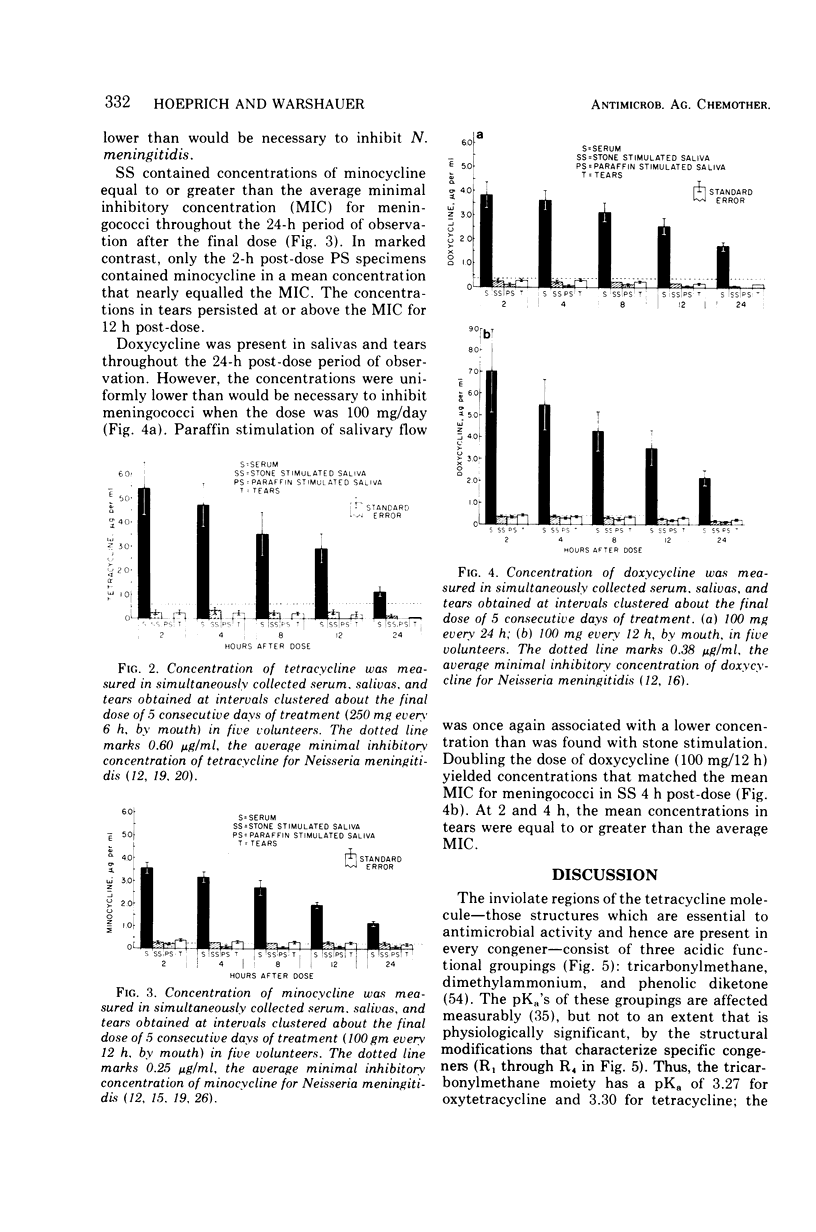
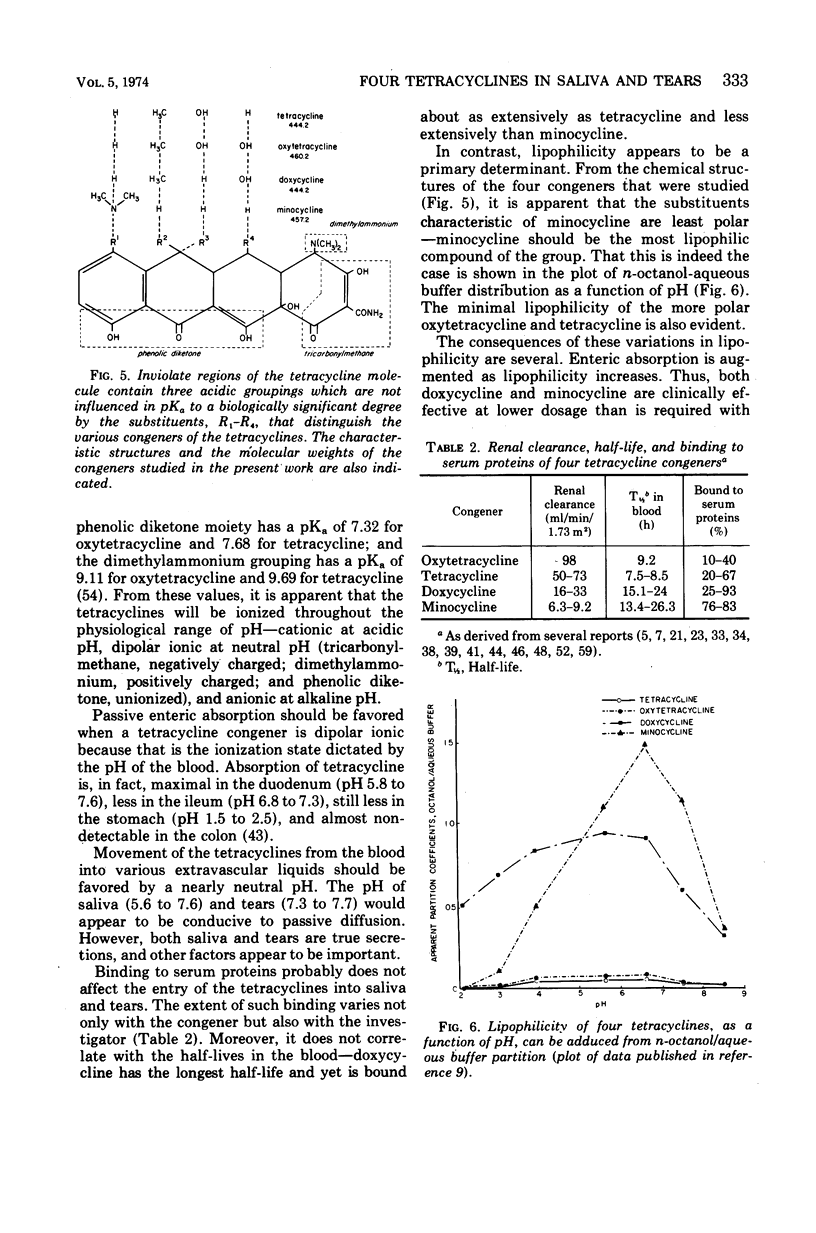
Although meningococci are susceptible to the tetracyclines by testing in vitro, oxytetracycline (OC) and doxycycline (DC) have failed to eliminate carriage, whereas minocycline (MC) has been effective. Because these congeners differ in lipophilicity, they and tetracycline (TC) were studied in volunteers by assay of serum, saliva, and tears obtained after 5 days of treatment. OC and TC were undetectable or attained concentrations subinhibitory for meningococci in saliva and tears. The concentrations of MC in saliva and tears were equal to or greater than the average minimal inhibitory concentration as long as 12 h post-dose. Near inhibitory concentrations resulted with DC at 100 mg/day; yet, doubling the dose to 100 mg/12 h did not yield concentrations that exceeded the average minimal inhibitory concentration for meningococci. The previous reports of failure or meager entry of DC and MC into saliva probably reflected extraction of these drugs in the paraffin chewed by subjects to stimulate salivary flow. The efficiency of entry of the tetracyclines into the secretions of the noninflamed upper respiratory tract correlates with lipophilicity at physiological pH, enabling prediction of meningococcal chemoprophylactic efficacy.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander C. E., Sanborn W. R., Cherriere G., Crocker W. H., Jr, Ewald P. E., Kay C. R. Sulfadiazine-resistant group A Neisseria meningitidis. Science. 1968 Sep 6;161(3845):1019–1019. doi: 10.1126/science.161.3845.1019. [DOI] [PubMed] [Google Scholar]
- Artenstein M. S., Lamson T. H., Evans J. R. Attempted prophylaxis against meningococcal infection using intramuscular penicillin. Mil Med. 1967 Dec;132(12):1009–1011. [PubMed] [Google Scholar]
- BROWN J. W., CONDIT P. K. MENINGOCOCCAL INFECTIONS. FORT ORD AND CALIFORNIA. Calif Med. 1965 Mar;102:171–180. [PMC free article] [PubMed] [Google Scholar]
- Beam W. E., Jr, Newberg N. R., Devine L. F., Pierce W. E., Davies J. A. The effect of rifampin on the nasopharyngeal carriage of Neisseria meningitidis in a military population. J Infect Dis. 1971 Jul;124(1):39–46. doi: 10.1093/infdis/124.1.39. [DOI] [PubMed] [Google Scholar]
- Bennet J. V., Young L. S. Trends in meningococcal disease. J Infect Dis. 1969 Nov;120(5):634–635. doi: 10.1093/infdis/120.5.634. [DOI] [PubMed] [Google Scholar]
- Bennett J. V., Brodie J. L., Benner E. J., Kirby W. M. Simplified, accurate method for antibiotic assay of clinical specimens. Appl Microbiol. 1966 Mar;14(2):170–177. doi: 10.1128/am.14.2.170-177.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett J. V., Mickelwait J. S., Barrett J. E., Brodie J. L., Kirby W. M. Comparative serum binding of four tetracyclines under simulated in vivo conditions. Antimicrob Agents Chemother (Bethesda) 1965;5:180–182. [PubMed] [Google Scholar]
- Bernard B., Yin E. J., Simon H. J. Clinical pharmacologic studies with minocycline. J Clin Pharmacol New Drugs. 1971 Sep-Oct;11(5):332–348. [PubMed] [Google Scholar]
- Colaizzi J. L., Klink P. R. pH-Partition behavior of tetracyclines. J Pharm Sci. 1969 Oct;58(10):1184–1189. doi: 10.1002/jps.2600581003. [DOI] [PubMed] [Google Scholar]
- DULBECCO R., VOGT M. Plaque formation and isolation of pure lines with poliomyelitis viruses. J Exp Med. 1954 Feb;99(2):167–182. doi: 10.1084/jem.99.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deal W. B., Sanders E. Efficacy of rifampin in treatment of meningococcal carriers. N Engl J Med. 1969 Sep 18;281(12):641–645. doi: 10.1056/NEJM196909182811203. [DOI] [PubMed] [Google Scholar]
- Deal W. B., Sanders E. Therapeutic trial of cephalexin in meningococcal carriers. Antimicrob Agents Chemother (Bethesda) 1969;9:441–444. [PubMed] [Google Scholar]
- Devine L. F., Hagerman C. R. Spectra of susceptibility of Neisseria meningitidis to antimicrobial agents in vitro. Appl Microbiol. 1970 Feb;19(2):329–334. doi: 10.1128/am.19.2.329-334.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devine L. F., Johnson D. P., Hagerman C. R., Pierce W. E., Rhode S. L., 3rd, Peckinpaugh R. O. Rifampin. Levels in serum and saliva and effect on the meningococcal carrier state. JAMA. 1970 Nov 9;214(6):1055–1059. doi: 10.1001/jama.214.6.1055. [DOI] [PubMed] [Google Scholar]
- Devine L. F., Johnson D. P., Hagerman C. R., Pierce W. E., Rhode S. L., 3rd, Peckinpaugh R. O. The effect of coumermycin A on the meningococcal carrier state. Am J Med Sci. 1970 Sep;260(3):165–170. doi: 10.1097/00000441-197009000-00004. [DOI] [PubMed] [Google Scholar]
- Devine L. F., Johnson D. P., Hagerman C. R., Pierce W. E., Rhode S. L., 3rd, Peckinpaugh R. O. The effect of minocycline on meningococcal nasopharyngeal carrier state in naval personnel. Am J Epidemiol. 1971 May;93(5):337–345. doi: 10.1093/oxfordjournals.aje.a121266. [DOI] [PubMed] [Google Scholar]
- Devine L. F., Knowles R. C., Pierce W. E., Peckinpaugh R. O., Hagerman C. R., Lytle R. I. Proposed model for screening antimicrobial agents for potential use in eliminating meningococci from the nasopharynx of healthy carriers. Antimicrob Agents Chemother (Bethesda) 1968;8:307–314. [PubMed] [Google Scholar]
- Dowd J. M., Blink D., Miller C. H., Frank P. F., Pierce W. E. Antibiotic prophylaxis of carriers of sulfadiazine-resistant meningococci. J Infect Dis. 1966 Oct;116(4):473–480. doi: 10.1093/infdis/116.4.473. [DOI] [PubMed] [Google Scholar]
- EICKHOFF T. C., FINLAND M. CHANGING SUSCEPTIBILITY OF MENINGOCOCCI TO ANTIMICROBIAL AGENTS. N Engl J Med. 1965 Feb 25;272:395–398. doi: 10.1056/NEJM196502252720804. [DOI] [PubMed] [Google Scholar]
- Eickhoff T. C. In-vitro and in-vivo studies of resistance to rifampin in meningococci. J Infect Dis. 1971 Apr;123(4):414–420. doi: 10.1093/infdis/123.4.414. [DOI] [PubMed] [Google Scholar]
- Fabre J., Kunz J. P., Virieux C., Laurencet J. L., Pitton J. S. Le comportement de la doxycycline CHEZ L'homme. Chemotherapy. 1968;13(Suppl):23–40. [PubMed] [Google Scholar]
- Fabre J., Milek E., Kalfopoulos P., Mérier G. La cinétique des tétracyclines chez l'homme. I. Absorption digestive et concentrations sériques. Schweiz Med Wochenschr. 1971 May 1;101(17):593–598. [PubMed] [Google Scholar]
- Fabre J., Pitton J. S., Kunz J. P. Distribution and excretion of doxycycline in man. Chemotherapy. 1966;11(2):73–85. doi: 10.1159/000220439. [DOI] [PubMed] [Google Scholar]
- Fabre J., Pitton J. S., Virieux C., Laurencet F. L., Bernhardt J. P., Godel J. C. La doxycycline. Absorption, distribution et excrétion d'un nouvel antibiotique à large spectre chez l'homme. Schweiz Med Wochenschr. 1967 Jul 15;97(28):915–924. [PubMed] [Google Scholar]
- Giromini M., Wasem R., Mérier G., Fabre J. Influence de l'anurie et des hémodialyses sur le comportement des antibiotiques. Praxis. 1969 Sep 23;58(38):1181–1187. [PubMed] [Google Scholar]
- Guttler R. B., Counts G. W., Avent C. K., Beaty H. N. Effect of rifampin and minocycline on meningococcal carrier rates. J Infect Dis. 1971 Aug;124(2):199–205. doi: 10.1093/infdis/124.2.199. [DOI] [PubMed] [Google Scholar]
- Hinton N. A. The effect of oral tetracycline HCl and doxycycline on the intestinal flora. Curr Ther Res Clin Exp. 1970 Jun;12(6):341–352. [PubMed] [Google Scholar]
- Hoeprich P. D. Prediction of antimeningococcic chemoprophylactic efficacy. J Infect Dis. 1971 Feb;123(2):125–133. doi: 10.1093/infdis/123.2.125. [DOI] [PubMed] [Google Scholar]
- Hoshiwara I., Ostler H. B., Hanna L., Cignetti F., Coleman V. R., Jawetz E. Doxycycline treatment of chronic trachoma. JAMA. 1973 Apr 9;224(2):220–223. [PubMed] [Google Scholar]
- IVLER D., THRUPP L. D., LEEDOM J. M., WEHRLE P. F., PORTNOY B. AMPICILLIN IN THE TREATMENT OF ACUTE BACTERIAL MENINGITIS. Antimicrob Agents Chemother (Bethesda) 1963;161:335–345. [PubMed] [Google Scholar]
- KUNIN C. M. Comparative serum binding, distribution and excretion of tetracycline and a new analogue, methacycline. Proc Soc Exp Biol Med. 1962 Jun;110:311–315. doi: 10.3181/00379727-110-27501. [DOI] [PubMed] [Google Scholar]
- KUNIN C. M., DORNBUSH A. C., FINLAND M. Distribution and excretion of four tetracycline analogues in normal young men. J Clin Invest. 1959 Nov;38:1950–1963. doi: 10.1172/JCI103974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly R. G., Kanegis L. A. Metabolism and tissue distribution of radioisotopically labelled minocycline. Toxicol Appl Pharmacol. 1967 Jul;11(1):171–183. doi: 10.1016/0041-008x(67)90036-1. [DOI] [PubMed] [Google Scholar]
- Lefèvre M., Sirol J., Vandekerkove M., Faucon R. Données biologiques et cliniques concernant le comportement de Neisseria meningitidis A vis-à-vis de quelques sulfamides et antibiotiques: observations faites à Fort-Lamy (Tchad) lors d'une poussée saisonnière de meningite cérébro-spinale. Bull World Health Organ. 1969;40(3):331–342. [PMC free article] [PubMed] [Google Scholar]
- Little P. J., Bailey R. R. Tetracyclines and renal failure. N Z Med J. 1970 Sep;72(460):183–184. [PubMed] [Google Scholar]
- MILLAR J. W., SIESS E. E., FELDMAN H. A., SILVERMAN C., FRANK P. IN VIVO AND IN VITRO RESISTANCE TO SULFADIAZINE IN STRAINS OF NEISSERIA MENINGITIDIS. JAMA. 1963 Oct 12;186:139–141. doi: 10.1001/jama.1963.63710020008016. [DOI] [PubMed] [Google Scholar]
- Macdonald H., Kelly R. G., Allen E. S., Noble J. F., Kanegis L. A. Pharmacokinetic studies on minocycline in man. Clin Pharmacol Ther. 1973 Sep-Oct;14(5):852–861. doi: 10.1002/cpt1973145852. [DOI] [PubMed] [Google Scholar]
- Mahon W. A., Wittenberg J. V., Tuffnel P. G. Studies on the absorption and distribution of doxycycline in normal patients and in patients with severely impaired renal function. Can Med Assoc J. 1970 Nov 7;103(10):1031–1034. [PMC free article] [PubMed] [Google Scholar]
- Mannhart M., Dettli L., Spring P. Die Elimination des Doxyzyklins und ihre Beeinflussung durch die Hämodialyse bei anurischen Patienten. Schweiz Med Wochenschr. 1971 Jan 30;101(4):123–127. [PubMed] [Google Scholar]
- Mérier G., Laurencet F. L., Rudhardt M., Chuit A., Fabre J. Behaviour of doxycycline in renal insuffciency. Helv Med Acta. 1969 Nov;35(2):124–134. [PubMed] [Google Scholar]
- PINDELL M. H., CULL K. M., DORAN K. M., DICKISON H. L. Absorption and excretions studies on tetracycline. J Pharmacol Exp Ther. 1959 Apr;125(4):287–294. [PubMed] [Google Scholar]
- Rosenblatt J. E., Barrett J. E., Brodie J. L., Kirby W. M. Comparison of in vitro activity and clinical pharmacology of doxycycline with other tetracyclines. Antimicrob Agents Chemother (Bethesda) 1966;6:134–141. [PubMed] [Google Scholar]
- SUKHOVA A. G., PRAKHOV N. V. PSEUDOCHOLINESTERASE ACTIVITY IN STORED BLOOD AND PLASMA. Fed Proc Transl Suppl. 1965 Jan-Feb;24:180–182. [PubMed] [Google Scholar]
- Sanders E. Use of sulfonamide carbonic anhydrase inhibitors in treatment of meningococcal carriers: rationale and report of a clinical trial of ethoxzolamide. Am J Med Sci. 1967 Nov;254(5):709–716. doi: 10.1097/00000441-196711000-00017. [DOI] [PubMed] [Google Scholar]
- Schach von Wittenau M. Some pharmacokinetic aspects of doxycycline metabolism in man. Chemotherapy. 1968;13(Suppl):41–50. doi: 10.1159/000220581. [DOI] [PubMed] [Google Scholar]
- Schach von Wittenau M., Twomey T. M. The disposition of doxycyline by man and dog. Chemotherapy. 1971;16(4):217–228. doi: 10.1159/000220730. [DOI] [PubMed] [Google Scholar]
- Shibata K., Hanai T., Kato T., Ito T., Fujii M. [Laboratory and clinical studies on minocycline in the surgical field]. Jpn J Antibiot. 1969 Dec;22(6):458–462. [PubMed] [Google Scholar]
- Singer R. C. Sulfonamide-resistant meningococcal disease. Med Clin North Am. 1967 May;51(3):719–727. doi: 10.1016/s0025-7125(16)33040-1. [DOI] [PubMed] [Google Scholar]
- Steigbigel N. H., Reed C. W., Finland M. Absorption and excretion of five tetracycline analogues in normal young men. Am J Med Sci. 1968 May;255:296–312. doi: 10.1097/00000441-196805000-00005. [DOI] [PubMed] [Google Scholar]
- Stein W., Schoog M., Franz H. E. Doxycyclin-Serumspiegel bei niereninsuffizienten Patienten. Arzneimittelforschung. 1969 May;19(5):827–828. [PubMed] [Google Scholar]
- Vassiliadis P., Kanellakis A., Papadakis J. Sulphadiazine-resistant group A meningococci isolated during the 1968 meningitis epidemic in Greece. J Hyg (Lond) 1969 Jun;67(2):279–288. doi: 10.1017/s0022172400041681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidmer C. E., Dunkel T. B., Pettyjohn F. S., Smith C. D., Leibovitz A. Effectiveness of rifampin in eradicating the meningococcal carrier state in a relatively closed population: emergence of resistant strains. J Infect Dis. 1971 Aug;124(2):172–178. doi: 10.1093/infdis/124.2.172. [DOI] [PubMed] [Google Scholar]
- Wiggins G. L., McLaughlin J. V., Bickham S. T., Jones W. L., Balows A. Susceptibility of Neisseria meningitidis strains from the civilian population to sulfadiazine, penicillin, and rifampin. Appl Microbiol. 1970 Dec;20(6):893–898. doi: 10.1128/am.20.6.893-898.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von WITTENAU M., YEARY R. The excretion and distribution in body fluids of tetracyclines after intravenous administration to dogs. J Pharmacol Exp Ther. 1963 May;140:258–266. [PubMed] [Google Scholar]


