Abstract
One hundred twenty-four strains of Bacteroides fragilis were examined for susceptibility to tetracycline disks and by minimal inhibitory concentration (MIC) determinations. MIC values and zone sizes around 30-μg tetracycline disks were determined by using selected test conditions which included Mueller-Hinton agar supplemented with sheep blood, vitamin K, and hemin and an incubation temperature of 35 C in an atmosphere of 80% N2, 10% H2, and 10% CO2. Strains were separated into two distinct populations by geometric mean MIC and dis tests. Of the 124 strains, 78 were resistant and 46 were susceptible. The resistant strains had geometric mean MIC's of 8 μg/ml or greater, whereas the geometric mean MIC's of sensitive strains were 5 μg/ml or less. The disk test proved to be more reproducible than the MIC test and completely separated the resistant and susceptible populations. An interpretive scheme for B. fragilis to tetracycline was statistically derived on the basis of the distribution of zone sizes of susceptible and resistant strains: resistant, 18 mm or less; indeterminant, 19 to 20 mm; and susceptible, 21 mm or greater. These zone sizes compared closely with the Kirby-Bauer criteria for aerobic bacteria.
Full text
PDF

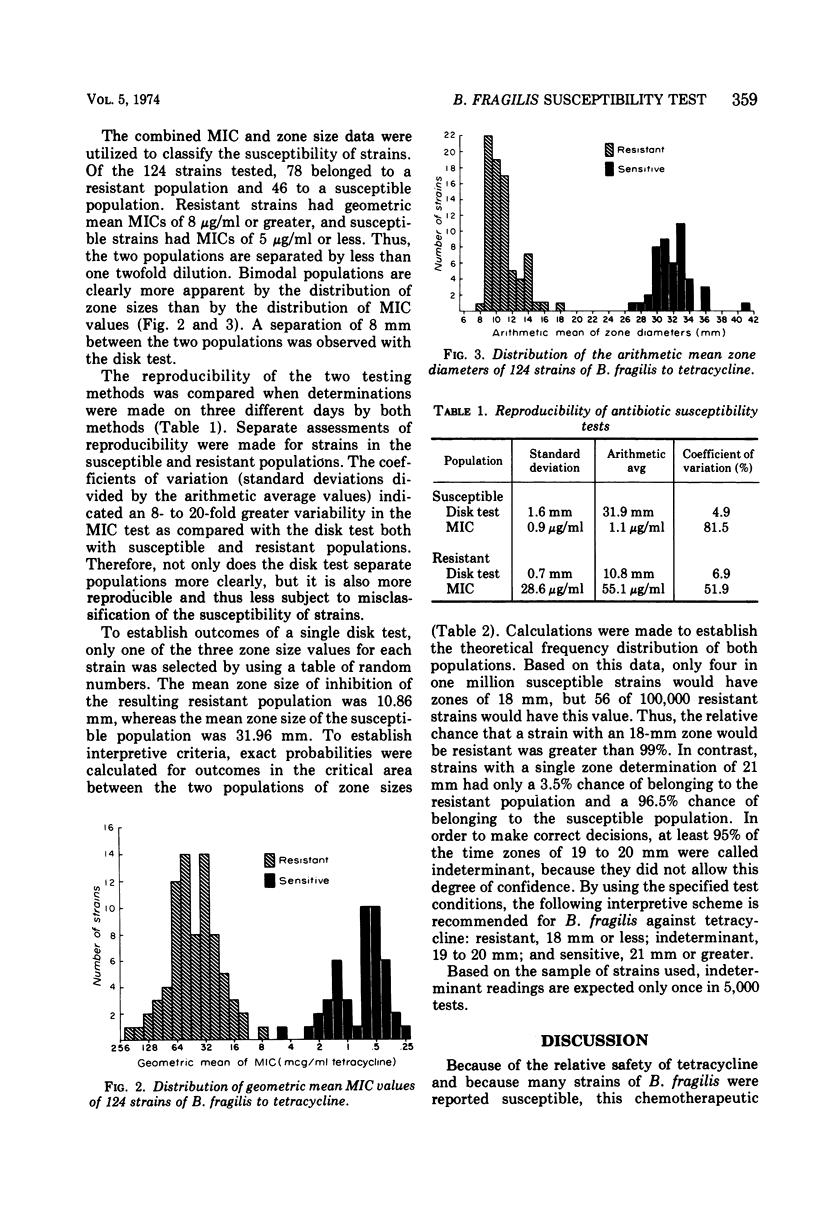
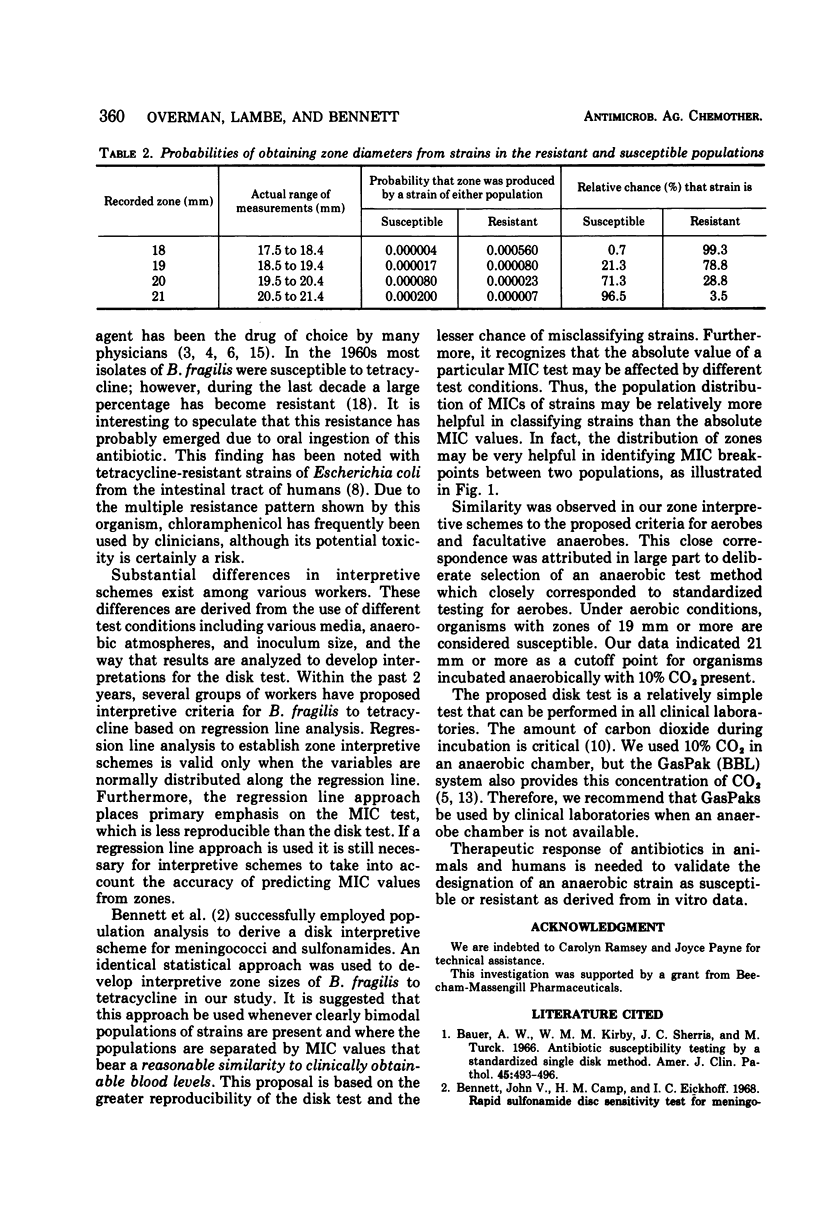

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BORNSTEIN D. L., WEINBERG N., SWARTZ M. N., KUNZ L. J. ANAEROBIC INFECTIONS--REVIEW OF CURRENT EXPERIENCE. Medicine (Baltimore) 1964 May;43:207–232. doi: 10.1097/00005792-196405000-00003. [DOI] [PubMed] [Google Scholar]
- Bauer A. W., Kirby W. M., Sherris J. C., Turck M. Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol. 1966 Apr;45(4):493–496. [PubMed] [Google Scholar]
- Bodner S. J., Koenig M. G., Goodman J. S. Bacteremic Bacteroides infections. Ann Intern Med. 1970 Oct;73(4):537–544. doi: 10.7326/0003-4819-73-4-537. [DOI] [PubMed] [Google Scholar]
- GILLESPIE W. A., GUY J. Bacteroides in intra-abdominal sepsis: their sensitivity to antibiotics. Lancet. 1956 Jun 30;270(6931):1039–1041. doi: 10.1016/s0140-6736(56)90802-9. [DOI] [PubMed] [Google Scholar]
- Gardner M., Martin W. J. Simplified method of anaerobic incubation. Appl Microbiol. 1971 Jun;21(6):1092–1092. doi: 10.1128/am.21.6.1092-1092.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsh D. C., Burton G. C., Blenden D. C. Effect of oral tetracycline on the occurrence of tetracycline-resistant strains of Escherichia coli in the intestinal tract of humans. Antimicrob Agents Chemother. 1973 Jul;4(1):69–71. doi: 10.1128/aac.4.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingham H. R., Selkon J. B., Codd A. A., Hale J. H. The effect of carbon dioxide on the sensitivity of Bacteroides fragilis to certain antibiotics in vitro. J Clin Pathol. 1970 Apr;23(3):254–258. doi: 10.1136/jcp.23.3.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kislak J. W. The susceptibility of Bacteroides fragilis to 24 antibiotics. J Infect Dis. 1972 Mar;125(3):295–299. doi: 10.1093/infdis/125.3.295. [DOI] [PubMed] [Google Scholar]
- Marcoux J. A., Zabransky R. J., Washington J. A., 2nd, Wellman W. E., Martin W. J. Bacteroides bacteremia. Minn Med. 1970 Nov;53(11):1169–1176. [PubMed] [Google Scholar]
- Martin W. J., Gardner M., Washington J. A., 2nd In vitro antimicrobial susceptibility of anaerobic bacteria isolated from clinical specimens. Antimicrob Agents Chemother. 1972 Feb;1(2):148–158. doi: 10.1128/aac.1.2.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobles E. R., Jr Bacteroides infections. Ann Surg. 1973 May;177(5):601–606. [PMC free article] [PubMed] [Google Scholar]
- Rotheram E. B., Jr, Schick S. F. Nonclostridial anaerobic bacteria in septic abortion. Am J Med. 1969 Jan;46(1):80–89. doi: 10.1016/0002-9343(69)90060-6. [DOI] [PubMed] [Google Scholar]
- Sutter V. L., Kwok Y. Y., Finegold S. M. Standardized antimicrobial disc susceptibility testing of anaerobic bacteria. I. Susceptibility of Bacteroides fragilis to tetracycline. Appl Microbiol. 1972 Feb;23(2):268–275. doi: 10.1128/am.23.2.268-275.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TYNES B. S., FROMMEYER W. B., Jr Bacteroides septicemia. Cultural, clinical, and therapeutic features in a series of twenty-five patients. Ann Intern Med. 1962 Jan;56:12–26. doi: 10.7326/0003-4819-56-1-12. [DOI] [PubMed] [Google Scholar]
- Thornton G. F., Cramer J. A. Antibiotic susceptibility of bacteroides species. Antimicrob Agents Chemother (Bethesda) 1970;10:509–513. [PubMed] [Google Scholar]
- Wikins T. D., Holdeman L. V., Abramson I. J., Moore W. E. Standardized single-disc method for antibiotic susceptibility testing of anaerobic bacteria. Antimicrob Agents Chemother. 1972 Jun;1(6):451–459. doi: 10.1128/aac.1.6.451. [DOI] [PMC free article] [PubMed] [Google Scholar]


