Abstract
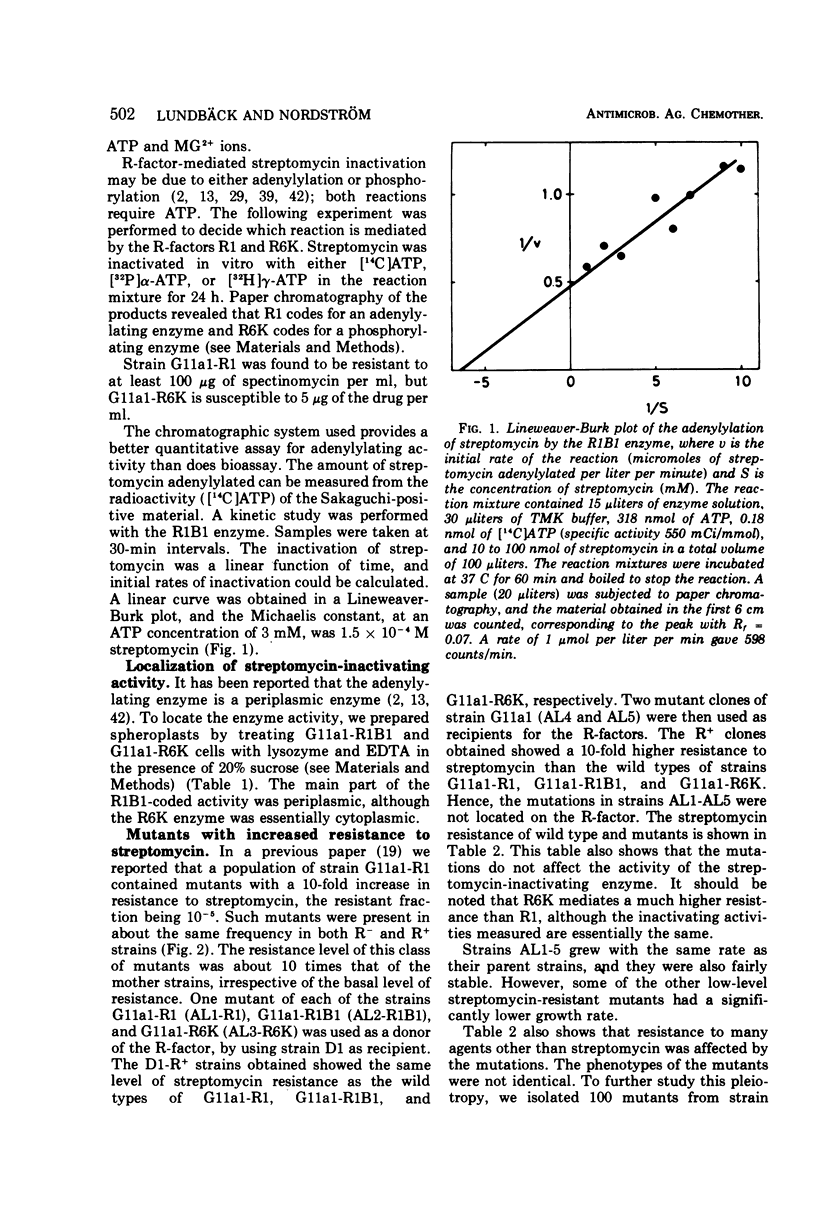
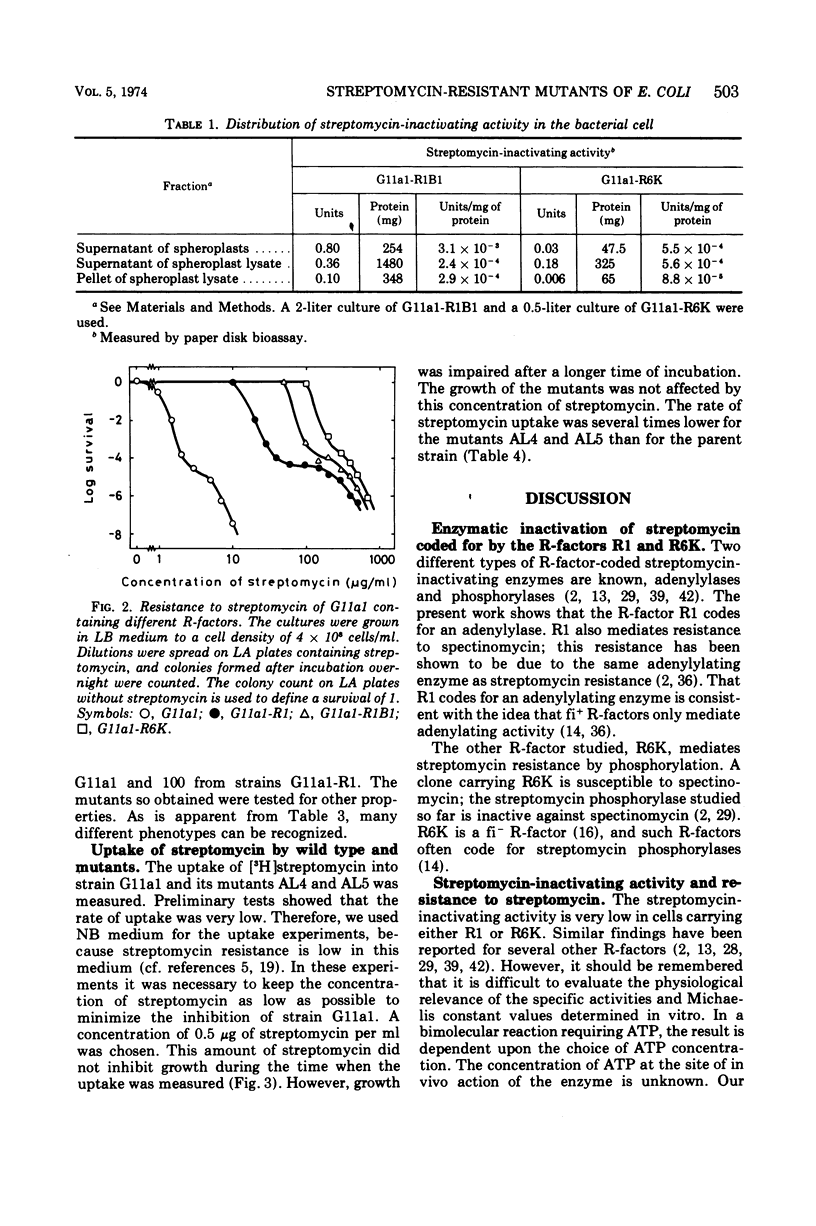
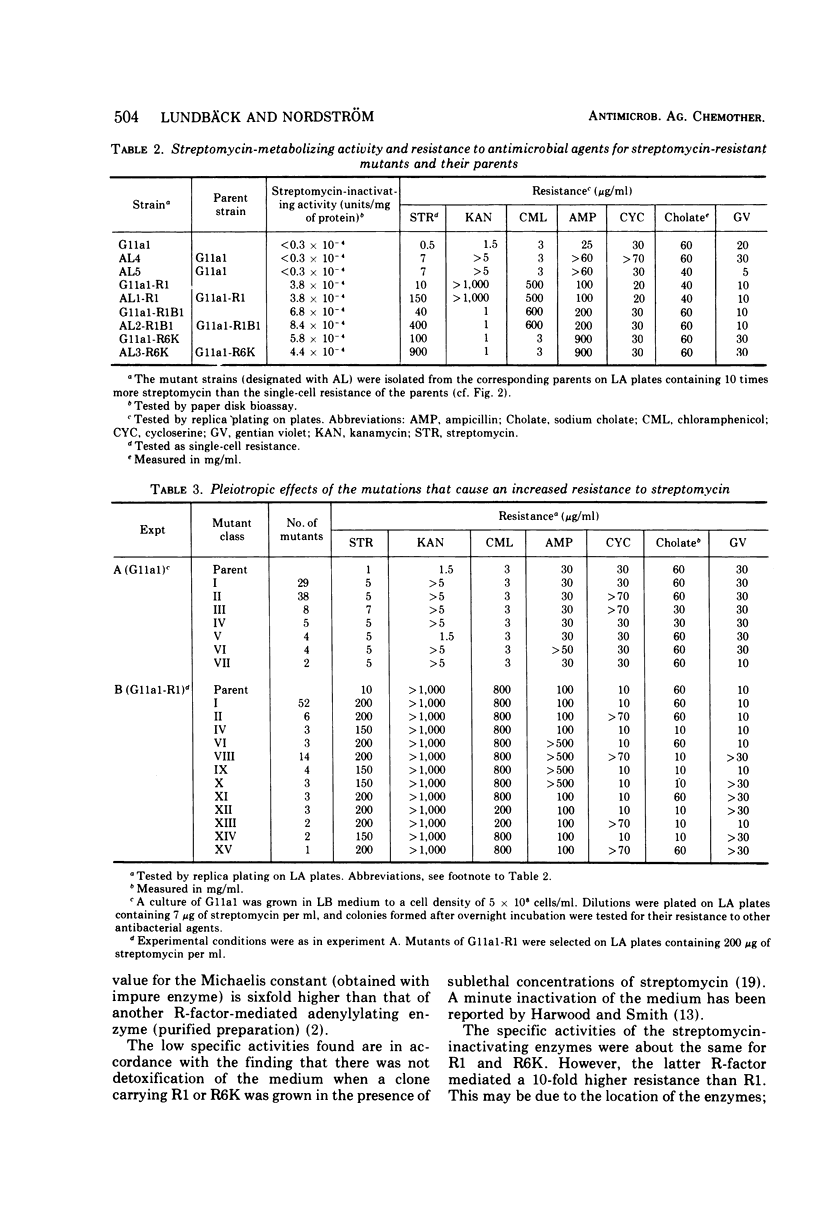
Escherichia coli K-12 carrying the R-factor R1 or R6K is resistant to streptomycin. The resistance is due to R-factor-coded enzymes that metabolize the drug. Streptomycin can be inactivated in two ways, either by adenylylation or by phosphorylation; both reactions require adenosine 5′-triphosphate. In this work we show that the R-factor R1 codes for an enzyme that adenylylates streptomycin and that the enzyme activity is located in the periplasmic volume, whereas the R-factor R6K codes for a streptomycin phosphorylase, which is mainly cytoplasmic. From a strain without any R-factor or carrying different R-factors, mutants were isolated that are 10 times more resistant to streptomycin than the parent strains. This increased resistance is not due to increased amounts of metabolizing enzymes. The mutants have a decreased rate of uptake of streptomycin and an altered response to other antibacterial agents as well. The mutations are located on the chromosome and not on the plasmid. It is likely that the mutations cause changes in the outer layers of the cell envelope and that the increased resistance is due to the synergistic effect of an efficient penetration barrier and a low activity of inactivating enzyme.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERTANI G. Studies on lysogenesis. I. The mode of phage liberation by lysogenic Escherichia coli. J Bacteriol. 1951 Sep;62(3):293–300. doi: 10.1128/jb.62.3.293-300.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste R., Davies J. Mechanisms of antibiotic resistance in bacteria. Annu Rev Biochem. 1973;42:471–506. doi: 10.1146/annurev.bi.42.070173.002351. [DOI] [PubMed] [Google Scholar]
- Benveniste R., Yamada T., Davies J. Enzymatic Adenylylation of Streptomycin and Spectinomycin by R-Factor-Resistant Escherichia coli. Infect Immun. 1970 Jan;1(1):109–119. doi: 10.1128/iai.1.1.109-119.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burman L. G., Nordström K. Colicin tolerance induced by ampicillin or mutation to ampicillin resistance in a strain of Escherichia coli K-12. J Bacteriol. 1971 Apr;106(1):1–13. doi: 10.1128/jb.106.1.1-13.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson K., Bockrath R. C. Physiological Streptomycin Resistance in a Multiauxotroph of Escherichia coli Strain 15 T. J Bacteriol. 1970 Dec;104(3):1294–1298. doi: 10.1128/jb.104.3.1294-1298.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta N., Kontomichalou P. Penicillinase synthesis controlled by infectious R factors in Enterobacteriaceae. Nature. 1965 Oct 16;208(5007):239–241. doi: 10.1038/208239a0. [DOI] [PubMed] [Google Scholar]
- Davies J. E., Rownd R. Transmissible multiple drug resistance in Enterobacteriaceae. Science. 1972 May 19;176(4036):758–768. doi: 10.1126/science.176.4036.758. [DOI] [PubMed] [Google Scholar]
- Demerec M. Origin of Bacterial Resistance to Antibiotics. J Bacteriol. 1948 Jul;56(1):63–74. doi: 10.1128/jb.56.1.63-74.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson-Grennberg K. G., Boman H. G., Jansson J. A., Thorén S. Resistance of Escherichia coli to Penicillins I. Genetic Study of Some Ampicillin-Resistant Mutants. J Bacteriol. 1965 Jul;90(1):54–62. doi: 10.1128/jb.90.1.54-62.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleck A., Begg D. The estimation of ribonucleic acid using ultraviolet absorption measurements. Biochim Biophys Acta. 1965 Nov 8;108(3):333–339. doi: 10.1016/0005-2787(65)90025-0. [DOI] [PubMed] [Google Scholar]
- GILBOE D. D., WILLIAMS J. N., Jr Evaluation of the Sakaguchi reaction for quanitative determination of arginine. Proc Soc Exp Biol Med. 1956 Apr;91(4):535–536. doi: 10.3181/00379727-91-22318. [DOI] [PubMed] [Google Scholar]
- Gundersen W. B. Uptake of 14-C-streptomycin by Escherichia coli carrying the Mu-factor. Acta Pathol Microbiol Scand. 1965;65(4):636–640. doi: 10.1111/apm.1965.65.4.636. [DOI] [PubMed] [Google Scholar]
- Harwood J. H., Smith D. H. Resistance factor-mediated streptomycin resistance. J Bacteriol. 1969 Mar;97(3):1262–1271. doi: 10.1128/jb.97.3.1262-1271.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedges R. W. Resistance to spectinomycin determined by R factors of various compatibility groups. J Gen Microbiol. 1972 Sep;72(2):407–409. doi: 10.1099/00221287-72-2-407. [DOI] [PubMed] [Google Scholar]
- Kontomichalou P., Mitani M., Clowes R. C. Circular R-factor molecules controlling penicillinase synthesis, replicating in Escherichia coli under either relaxed or stringent control. J Bacteriol. 1970 Oct;104(1):34–44. doi: 10.1128/jb.104.1.34-44.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linström E. B., Boman H. G., Steele B. B. Resistance of Escherichia coli to penicillins. VI. Purification and characterization of the chromosomally mediated penicillinase present in ampA-containing strains. J Bacteriol. 1970 Jan;101(1):218–231. doi: 10.1128/jb.101.1.218-231.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundbäck A. K., Lundbäck A., Nordström K. Effect of R-factor-mediated drug-metabolizing enzymes on survival of Escherichia coli K-12 in presence of ampicillin, chloramphenicol, or streptomycin. Antimicrob Agents Chemother. 1974 May;5(5):492–499. doi: 10.1128/aac.5.5.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meynell E., Datta N. The relation of resistance transfer factors to the F-factor (sex-factor) of Escherichia coli K12. Genet Res. 1966 Feb;7(1):134–140. doi: 10.1017/s0016672300009538. [DOI] [PubMed] [Google Scholar]
- Nomura M. Bacterial ribosome. Bacteriol Rev. 1970 Sep;34(3):228–277. doi: 10.1128/br.34.3.228-277.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordström K., Burman L. G., Eriksson-Grennberg K. G. Resistance of Escherichia coli to penicillins. 8. Physiology of a class II ampicillin-resistant mutant. J Bacteriol. 1970 Mar;101(3):659–668. doi: 10.1128/jb.101.3.659-668.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordström K., Eriksson-Grennberg K. G., Boman H. G. Resistance of Escherichia coli to penicillins. 3. AmpB, a locus affecting episomally and chromosomally mediated resistance to ampicillin and chlorampheincol. Genet Res. 1968 Oct;12(2):157–168. doi: 10.1017/s0016672300011770. [DOI] [PubMed] [Google Scholar]
- Nordström K. Increased resistance to several antibiotics by one mutation in an R-factor, R 1a. J Gen Microbiol. 1971 May;66(2):205–214. doi: 10.1099/00221287-66-2-205. [DOI] [PubMed] [Google Scholar]
- Nordström K., Ingram L. C., Lundbäck A. Mutations in R factors of Escherichia coli causing an increased number of R-factor copies per chromosome. J Bacteriol. 1972 May;110(2):562–569. doi: 10.1128/jb.110.2.562-569.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto S., Suzuki Y. Chloramphenicol-, dihydrostreptomycin-, and kanamycin-inactivating enzymes from multiple drug-resistant Escherichia coli carrying episome 'R'. Nature. 1965 Dec 25;208(5017):1301–1303. doi: 10.1038/2081301a0. [DOI] [PubMed] [Google Scholar]
- Ozanne B., Benveniste R., Tipper D., Davies J. Aminoglycoside antibiotics: inactivation by phosphorylation in Escherichia coli carrying R factors. J Bacteriol. 1969 Nov;100(2):1144–1146. doi: 10.1128/jb.100.2.1144-1146.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PERSON S., BOCKRATH R. C., Jr DIFFERENTIAL MUTATION PRODUCTION BY THE DECAY OF INCORPORATED TRITIUM COMPOUNDS IN E. COLI. Biophys J. 1964 Sep;4:355–365. doi: 10.1016/s0006-3495(64)86788-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce L. E., Meynell E. Mutation to high-level streptomycin-resistance in R+ bacteria. J Gen Microbiol. 1968 Jan;50(1):173–176. doi: 10.1099/00221287-50-1-173. [DOI] [PubMed] [Google Scholar]
- REPASKE R. Lysis of gram-negative organisms and the role of versene. Biochim Biophys Acta. 1958 Nov;30(2):225–232. doi: 10.1016/0006-3002(58)90044-1. [DOI] [PubMed] [Google Scholar]
- Roberts L. M., Reeve E. C. Two mutations giving low-level streptomycin resistance in Escherichia coli K 12. Genet Res. 1970 Dec;16(3):359–365. doi: 10.1017/s0016672300002640. [DOI] [PubMed] [Google Scholar]
- STENT G. S., BRENNER S. A genetic locus for the regulation of ribonucleic acid synthesis. Proc Natl Acad Sci U S A. 1961 Dec 15;47:2005–2014. doi: 10.1073/pnas.47.12.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. H., Janjigian J. A., Prescott N., Anderson P. W. Resistance factor-mediated spectinomycin resistance. Infect Immun. 1970 Jan;1(1):120–127. doi: 10.1128/iai.1.1.120-127.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor C. W. STABILIZATION OF PROTOPLASTS AND SPHEROPLASTS BY SPERMINE AND OTHER POLYAMINES. J Bacteriol. 1962 May;83(5):1101–1111. doi: 10.1128/jb.83.5.1101-1111.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takasawa S., Utahara R., Okanishi M., Maeda K., Umezawa H. Studies on adenylylstreptomycin, a product of streptomycin inactivation by E. coli carrying the R-factor. J Antibiot (Tokyo) 1968 Aug;21(8):477–484. doi: 10.7164/antibiotics.21.477. [DOI] [PubMed] [Google Scholar]
- Tseng J. T., Bryan L. E., Van den Elzen H. M. Mechanisms and spectrum of streptomycin resistance in a natural population of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1972 Sep;2(3):136–141. doi: 10.1128/aac.2.3.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Yamada T., Tipper D., Davies J. Enzymatic inactivation of streptomycin by R factor-resistant Escherichia coli. Nature. 1968 Jul 20;219(5151):288–291. doi: 10.1038/219288a0. [DOI] [PubMed] [Google Scholar]
- von Meyenburg Kaspar Transport-limited growth rates in a mutant of Escherichia coli. J Bacteriol. 1971 Sep;107(3):878–888. doi: 10.1128/jb.107.3.878-888.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]


