Abstract
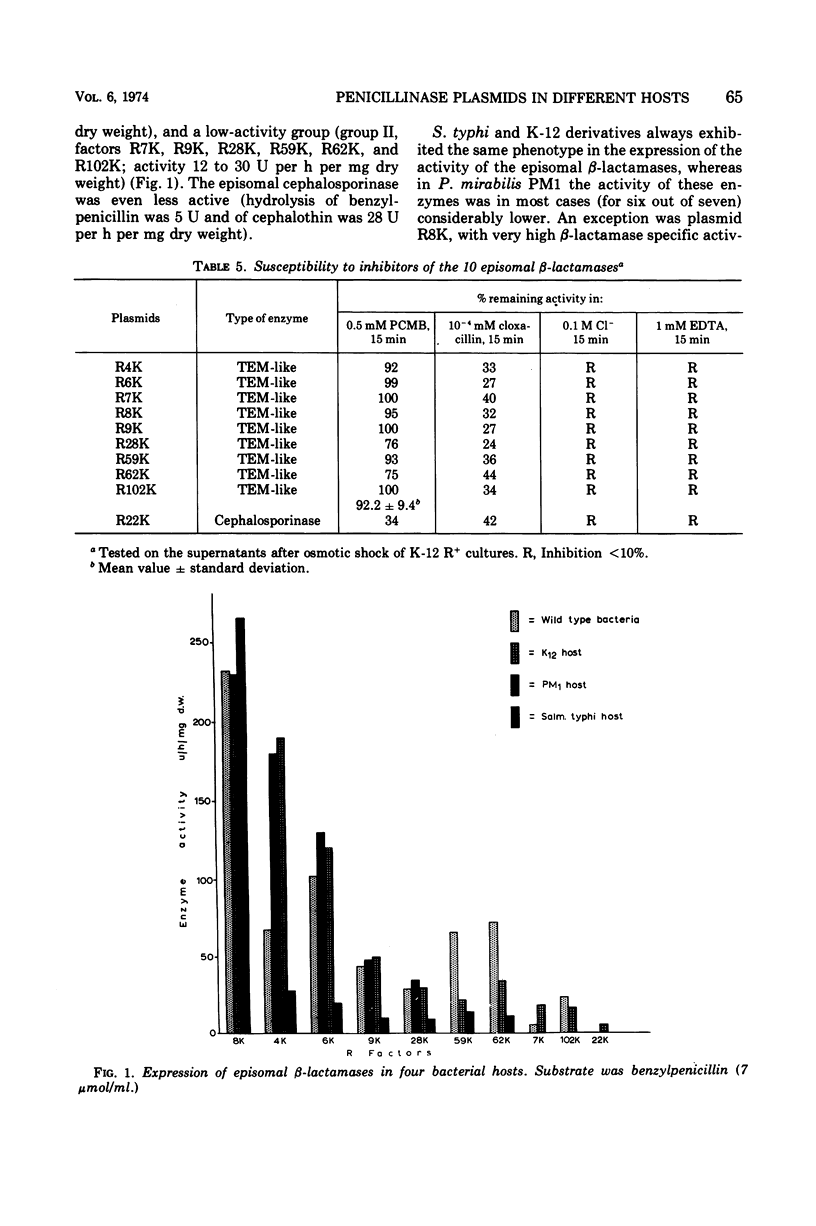
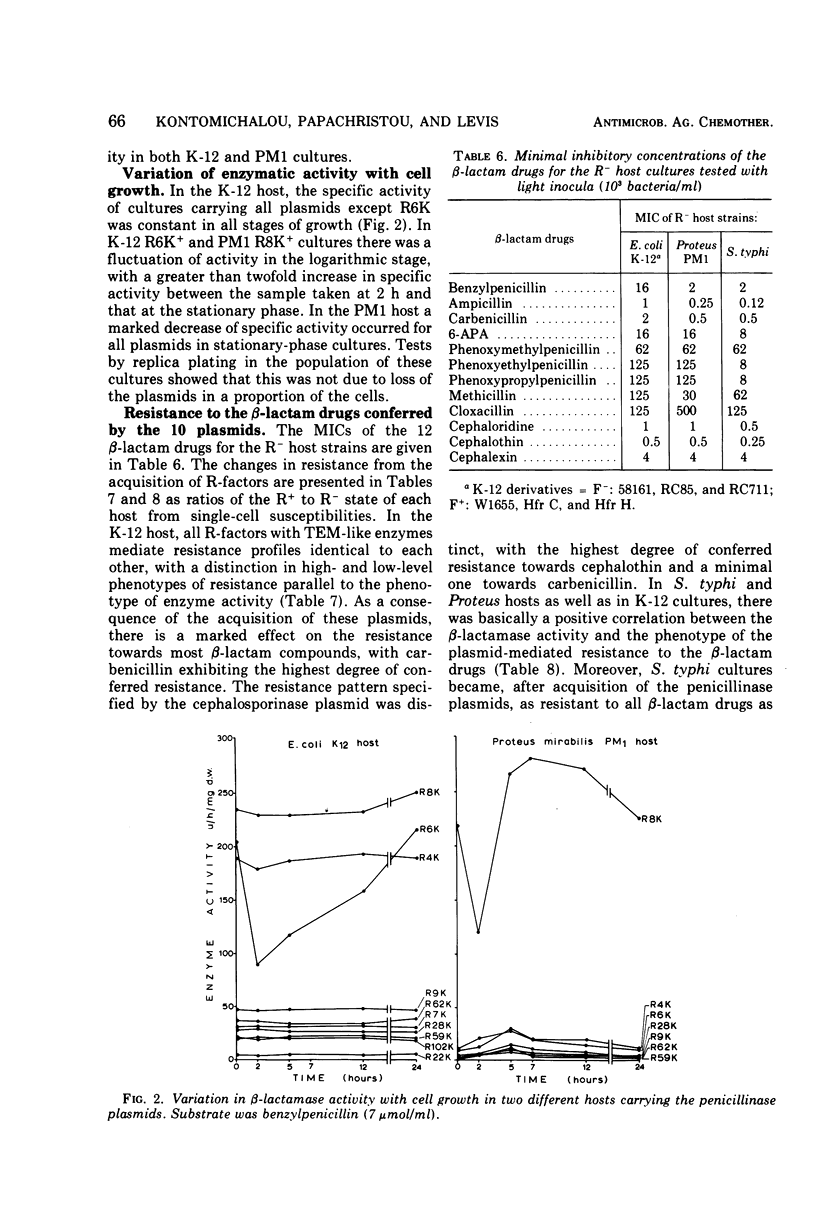
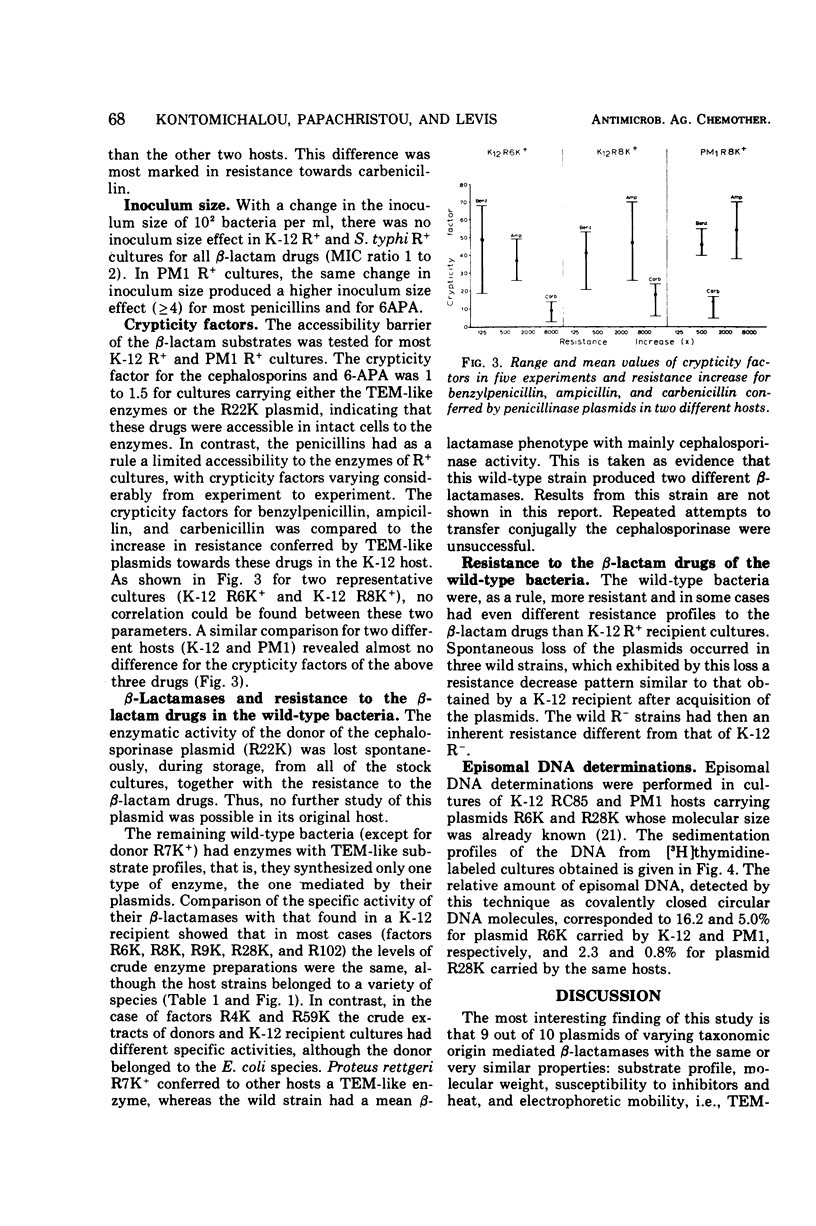
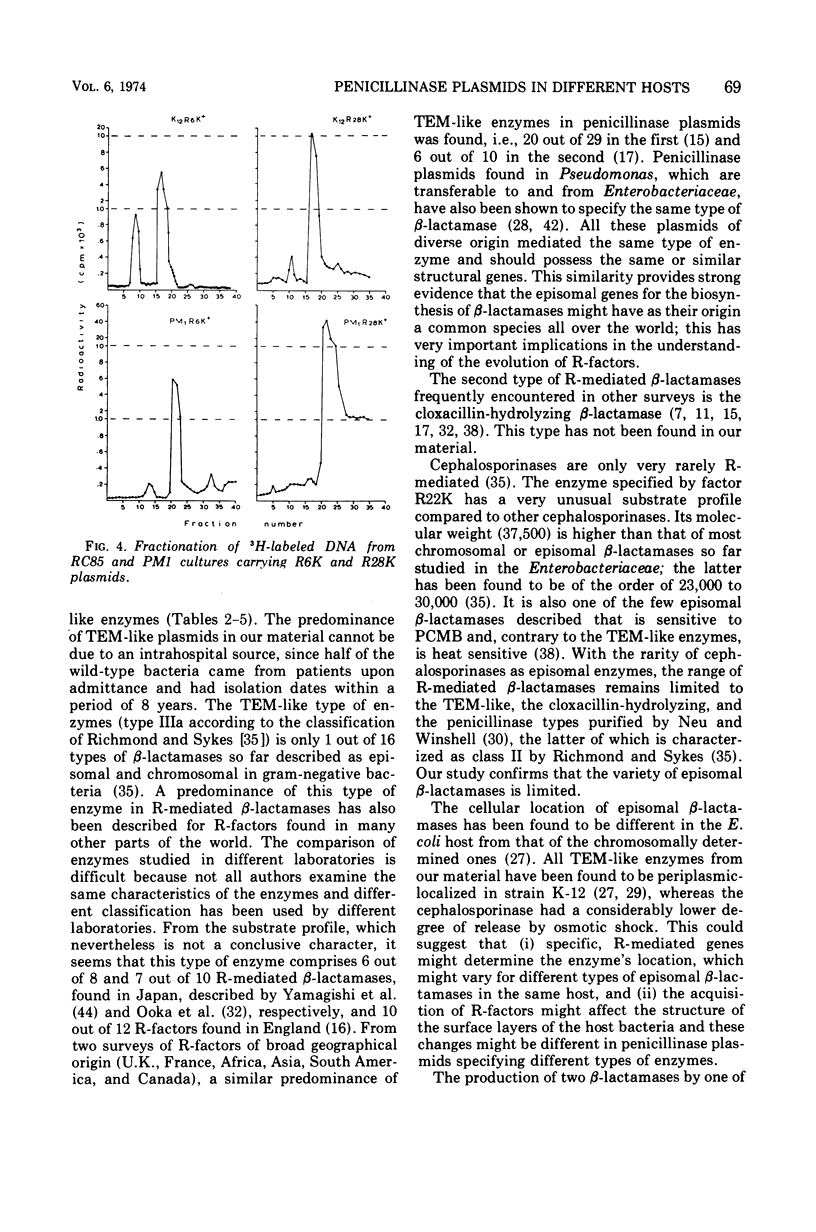
Ten penicillinase plasmids of varying taxonomic origin were studied after transfer to a variety of bacterial hosts. Nine of the ten plasmids specified enzymes with the following identical, or very similar, properties: substrate profile, molecular weight, susceptibility to heat and inhibitors, and electrophoretic mobility, i.e., TEM-like enzymes. The tenth R-mediated β-lactamase was a cephalosporinase. Plasmids with TEM-like enzymes mediated resistance patterns identical towards the β-lactam drugs, whereas the resistance pattern of the cephalosporinase plasmid was distinctly different. Expression of enzyme and resistance had a dual R-factor and host specificity. Escherichia coli K-12 and Salmonella typhi constituted one group of the same R-factor phenotype expressions. Most, but not all, penicillinase plasmids exhibited in Proteus PM1 a considerably lower order of β-lactamase activity and an even lower order of resistance to the β-lactam drugs than the previous two hosts. This difference was most pronounced for the resistance to carbenicillin, which was mediated by the plasmids specifying the synthesis of TEM-like enzymes. Release by osmotic shock was complete in the host E. coli K-12 for the TEM-like enzymes, but was lower for the cephalosporinase and minimal or negative in the PM1 host. Crypticity factor for benzylpenicillin, ampicillin, and carbenicillin was not related to the increase in resistance mediated by the penicillinase plasmids in both K-12 and PM1 hosts. Inoculum size effects for the penicillins and 6-aminopenicillanic acid were higher in PM1 than in K-12 R+ cultures. The expression of penicillinase plasmids in wild-type bacteria was strain specific and not species specific. For two plasmids of different phenotypes for β-lactamase activity (and resistance) in K-12 and PM1 hosts, a positive correlation was found between their phenotype and the relative amount of episomal deoxyribonucleic acid, as detected by ethidium bromide density gradient centrifugation. This is interpreted as indicating differences in the mode of replication of the plasmids in the two hosts.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. The gel-filtration behaviour of proteins related to their molecular weights over a wide range. Biochem J. 1965 Sep;96(3):595–606. doi: 10.1042/bj0960595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clowes R. C. Molecular structure of bacterial plasmids. Bacteriol Rev. 1972 Sep;36(3):361–405. doi: 10.1128/br.36.3.361-405.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coetzee J. N. Genetics of the Proteus group. Annu Rev Microbiol. 1972;26:23–54. doi: 10.1146/annurev.mi.26.100172.000323. [DOI] [PubMed] [Google Scholar]
- Dale J. W., Smith J. T. The purification and properties of the -lactamase specified by the resistance factor R-1818 in Escherichia coli and Proteus mirabilis. Biochem J. 1971 Jul;123(4):493–500. doi: 10.1042/bj1230493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta N., Kontomichalou P. Penicillinase synthesis controlled by infectious R factors in Enterobacteriaceae. Nature. 1965 Oct 16;208(5007):239–241. doi: 10.1038/208239a0. [DOI] [PubMed] [Google Scholar]
- Datta N., Richmond M. H. The purification and properties of a penicillinase whose synthesis is mediated by an R-factor in Escherichia coli. Biochem J. 1966 Jan;98(1):204–209. doi: 10.1042/bj0980204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies J. E., Rownd R. Transmissible multiple drug resistance in Enterobacteriaceae. Science. 1972 May 19;176(4036):758–768. doi: 10.1126/science.176.4036.758. [DOI] [PubMed] [Google Scholar]
- Evans J., Galindo E., Olarte J., Falkow S. Beta-lactamase of R factors. J Bacteriol. 1968 Oct;96(4):1441–1442. doi: 10.1128/jb.96.4.1441-1442.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FALKOW S., WOHLHIETER J. A., CITARELLA R. V., BARON L. S. TRANSFER OF EPISOMIC ELEMENTS TO PROTEUS. I. TRANSFER OF F-LINKED CHROMOSOMAL DETERMINANTS. J Bacteriol. 1964 Jan;87:209–219. doi: 10.1128/jb.87.1.209-219.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkow S., Tompkins L. S., Silver R. P., Guerry P., Le Blanc D. J. The problems of drug-resistant pathogenic bacteria. The replication of R-factor DNA in Escherichia coli K-12 following conjugation. Ann N Y Acad Sci. 1971 Jun 11;182:153–171. doi: 10.1111/j.1749-6632.1971.tb30654.x. [DOI] [PubMed] [Google Scholar]
- HAMILTON-MILLER J. M. Penicillinase from Klebsiella aerogenes. A comparison with penicillinases from gram-positive species. Biochem J. 1963 Apr;87:209–214. doi: 10.1042/bj0870209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedges R. W., Datta N., Kontomichalou P., Smith J. T. Molecular specificities of R factor-determined beta-lactamases: correlation with plasmid compatibility. J Bacteriol. 1974 Jan;117(1):56–62. doi: 10.1128/jb.117.1.56-62.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack G. W., Richmond M. H. A comparative study of eight distinct beta-lactamases synthesized by gram-negative bacteria. J Gen Microbiol. 1970 Apr;61(1):43–61. doi: 10.1099/00221287-61-1-43. [DOI] [PubMed] [Google Scholar]
- Jenkins P. H., Drabble W. T. -lactamases of R factors derived from Shigella and Salmonella strains. J Bacteriol. 1971 Oct;108(1):159–165. doi: 10.1128/jb.108.1.159-165.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kontomichalou P., Mitani M., Clowes R. C. Circular R-factor molecules controlling penicillinase synthesis, replicating in Escherichia coli under either relaxed or stringent control. J Bacteriol. 1970 Oct;104(1):34–44. doi: 10.1128/jb.104.1.34-44.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kontomichalou P. Studies on resistance transfer factors. II. Transmissible resistance to eight antibacterial drugs in a strain of Escherichia coli. Pathol Microbiol (Basel) 1967;30(2):185–200. doi: 10.1159/000161658. [DOI] [PubMed] [Google Scholar]
- Kontomichalou P. Studies on resistance transfer factors. Pathol Microbiol (Basel) 1967;30(1):71–93. doi: 10.1159/000161646. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lindqvist R. C., Nordström K. Resistance of Escherichia coli to penicillins. VII. Purification and characterization of a penicillinase mediated by the R factor R1. J Bacteriol. 1970 Jan;101(1):232–239. doi: 10.1128/jb.101.1.232-239.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melling J., Scott G. K. Preparation of gram quantities of a purified R-factor-mediated penicillinase from Escherichia coli strain W3310. Biochem J. 1972 Nov;130(1):55–62. doi: 10.1042/bj1300055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meynell E., Datta N. The relation of resistance transfer factors to the F-factor (sex-factor) of Escherichia coli K12. Genet Res. 1966 Feb;7(1):134–140. doi: 10.1017/s0016672300009538. [DOI] [PubMed] [Google Scholar]
- Neu H. C. -lactamase production by Pseudomonas aeruginosa. Antimicrob Agents Chemother (Bethesda) 1970;10:534–538. [PubMed] [Google Scholar]
- Neu H. C., Chou J. Release of surface enzymes in Enterobacteriaceae by osmotic shock. J Bacteriol. 1967 Dec;94(6):1934–1945. doi: 10.1128/jb.94.6.1934-1945.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu H. C. The surface localization of penicillinases in Escherichia coli and Salmonella typhimurium. Biochem Biophys Res Commun. 1968 Jul 26;32(2):258–263. doi: 10.1016/0006-291x(68)90378-1. [DOI] [PubMed] [Google Scholar]
- Neu H. C., Winshell E. B. Purification and characterization of penicillinases from Salmonella typhimurium and Escherichia coli. Arch Biochem Biophys. 1970 Aug;139(2):278–290. doi: 10.1016/0003-9861(70)90479-0. [DOI] [PubMed] [Google Scholar]
- Nisioka T., Mitani M., Clowes R. C. Molecular recombination between R-factor deoxyribonucleic acid molecules in Escherichia coli host cells. J Bacteriol. 1970 Jul;103(1):166–177. doi: 10.1128/jb.103.1.166-177.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooka T., Hashimoto H., Mitsuhashi S. Comparison of penicillinases produced by R factors isolated from ampicillin-resistant gram-negative bacteria. Jpn J Microbiol. 1970 Mar;14(2):123–128. doi: 10.1111/j.1348-0421.1970.tb00499.x. [DOI] [PubMed] [Google Scholar]
- POLLOCK M. R., TORRIANI A. M. Purification et caractéristiques physicochimiques de la pénicillinase de Bacillus cereus. C R Hebd Seances Acad Sci. 1953 Jul 20;237(3):276–278. [PubMed] [Google Scholar]
- Rownd R., Kasamatsu H., Mickel S. The molecular nature and replication of drug resistance factors of the Enterobacteriaceae. Ann N Y Acad Sci. 1971 Jun 11;182:188–206. doi: 10.1111/j.1749-6632.1971.tb30656.x. [DOI] [PubMed] [Google Scholar]
- Rownd R., Nakaya R., Nakamura A. Molecular nature of the drug-resistance factors of the Enterobacteriaceae. J Mol Biol. 1966 Jun;17(2):376–393. doi: 10.1016/s0022-2836(66)80149-3. [DOI] [PubMed] [Google Scholar]
- Rubin F. A., Smith D. H. Characterization of R factor beta-lactamases by the acidimetric method. Antimicrob Agents Chemother. 1973 Jan;3(1):68–73. doi: 10.1128/aac.3.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawai T., Takahashi K., Yamagishi S., Mitsuhashi S. Variant of penicillinase mediated by an R factor in Escherichia coli. J Bacteriol. 1970 Nov;104(2):620–629. doi: 10.1128/jb.104.2.620-629.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver R. P., Falkow S. Specific labeling and physical characterization of R-factor deoxyribonucleic acid in Escherichia coli. J Bacteriol. 1970 Oct;104(1):331–339. doi: 10.1128/jb.104.1.331-339.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. T. R-factor gene expression gram-negative bacteria. J Gen Microbiol. 1969 Jan;55(1):109–120. doi: 10.1099/00221287-55-1-109. [DOI] [PubMed] [Google Scholar]
- Sykes R. B., Richmond M. H. Intergeneric transfer of a beta-lactamase gene between Ps. aeruginosa and E. coli. Nature. 1970 Jun 6;226(5249):952–954. doi: 10.1038/226952a0. [DOI] [PubMed] [Google Scholar]
- Yamagishi S., O'Hara K., Sawai T., Mitsuhashi S. The purification and properties of penicillin beta-lactamases mediated by transmissible R factors in Escherichia coli. J Biochem. 1969 Jul;66(1):11–20. doi: 10.1093/oxfordjournals.jbchem.a129111. [DOI] [PubMed] [Google Scholar]
- Zinder N. D. RNA phages. Annu Rev Microbiol. 1965;19:455–472. doi: 10.1146/annurev.mi.19.100165.002323. [DOI] [PubMed] [Google Scholar]


