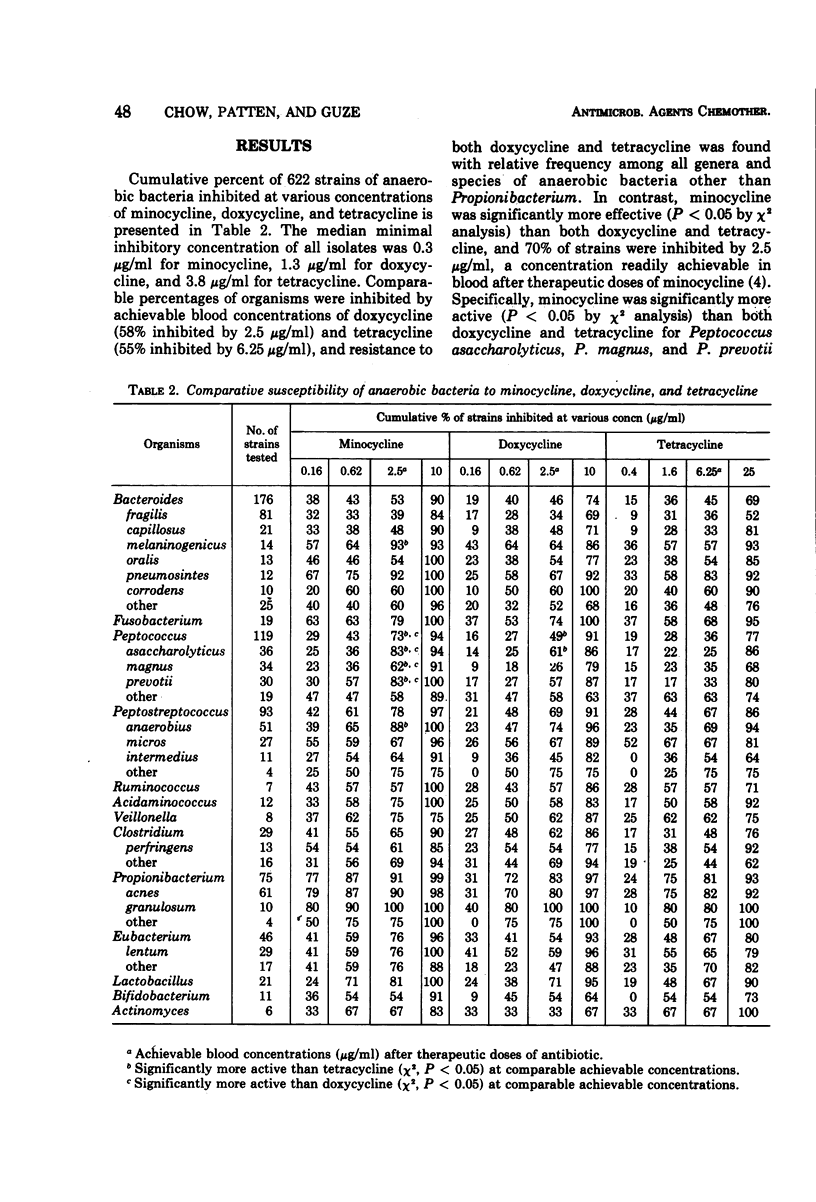
Abstract
The comparative susceptibility of 622 recent clinical isolates of anaerobic bacteria to minocycline, doxycycline, and tetracycline was determined by an agar-dilution technique. In addition to Bacteroides fragilis, a variety of other anaerobic bacteria was resistant to achievable blood concentrations of tetracycline (55% inhibited by 6.25 μg/ml) and doxycycline (58% inhibited by 2.5 μg/ml). In contrast, minocycline was significantly more active (P < 0.05) than both doxycycline and tetracycline, and 70% of strains were inhibited by achievable blood concentrations of this antibiotic (2.5 μg/ml). The enhanced activity of minocycline was particularly striking for Peptococcus asaccharolyticus, P. magnus, P. prevotii, Peptostreptococcus anaerobius, and Bacteroides melaninogenicus. Further evaluation of the clinical efficacy of minocycline against anaerobic infections is indicated.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- English A. R. Alpha-6-deoxyoxytetracycline. I. Some biological properties. Proc Soc Exp Biol Med. 1966 Aug-Sep;122(4):1107–1112. doi: 10.3181/00379727-122-31338. [DOI] [PubMed] [Google Scholar]
- Kawamori Y., Nishizawa N. [Clinical and laboratory research on minocycline]. Jpn J Antibiot. 1969 Dec;22(6):426–429. [PubMed] [Google Scholar]
- Kelly R. G., Kanegis L. A. Metabolism and tissue distribution of radioisotopically labelled minocycline. Toxicol Appl Pharmacol. 1967 Jul;11(1):171–183. doi: 10.1016/0041-008x(67)90036-1. [DOI] [PubMed] [Google Scholar]
- Keusch G. T., O'Connell C. J. The susceptibility of bacteroides to the penicillins and cephalothin. Am J Med Sci. 1966 Apr;251(4):428–432. doi: 10.1097/00000441-196604000-00007. [DOI] [PubMed] [Google Scholar]
- Kuck N. A., Redin G. S., Forbes M. Activity of minocycline and other tetracyclines against tetracycline-sensitive and -resistant staphylococci. Proc Soc Exp Biol Med. 1971 Feb;136(2):479–481. doi: 10.3181/00379727-136-35292. [DOI] [PubMed] [Google Scholar]
- Leibowitz B. J., Hakes J. L., Cahn M. M., Levy E. J. Doxycycline blood levels in normal subjects after intravenous and oral administration. Curr Ther Res Clin Exp. 1972 Dec;14(12):820–832. [PubMed] [Google Scholar]
- Martin W. J., Gardner M., Washington J. A., 2nd In vitro antimicrobial susceptibility of anaerobic bacteria isolated from clinical specimens. Antimicrob Agents Chemother. 1972 Feb;1(2):148–158. doi: 10.1128/aac.1.2.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapico F. L., Kwok Y. Y., Sutter V. L., Finegold S. M. Standardized antimicrobial disc susceptibility testing of anaerobic bacteria: in vitro susceptibility of Clostridium perfringens to nine antibiotics. Antimicrob Agents Chemother. 1972 Oct;2(4):320–325. doi: 10.1128/aac.2.4.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steigbigel N. H., Reed C. W., Finland M. Susceptibility of common pathogenic bacteria to seven tetracycline antibiotics in vitro. Am J Med Sci. 1968 Mar;255:179–195. doi: 10.1097/00000441-196803000-00005. [DOI] [PubMed] [Google Scholar]
- Sutter V. L., Kwok Y. Y., Finegold S. M. Standardized antimicrobial disc susceptibility testing of anaerobic bacteria. I. Susceptibility of Bacteroides fragilis to tetracycline. Appl Microbiol. 1972 Feb;23(2):268–275. doi: 10.1128/am.23.2.268-275.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tally F. P., Armfield A. Y., Dowell V. R., Jr, Kwok Y. Y., Sutter V. L., Finegold S. M. Susceptibility of Clostridium ramosum to antimicrobial agents. Antimicrob Agents Chemother. 1974 Jun;5(6):589–593. doi: 10.1128/aac.5.6.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikins T. D., Holdeman L. V., Abramson I. J., Moore W. E. Standardized single-disc method for antibiotic susceptibility testing of anaerobic bacteria. Antimicrob Agents Chemother. 1972 Jun;1(6):451–459. doi: 10.1128/aac.1.6.451. [DOI] [PMC free article] [PubMed] [Google Scholar]


