Abstract
We investigated genetic variation in CYP2A6 in relation to lung cancer risk among African American smokers, a high-risk population. Previously, we found that CYP2A6, a nicotine/nitrosamine metabolism gene, was associated with lung cancer risk in European Americans, but smoking habits, lung cancer risk and CYP2A6 gene variants differ significantly between European and African ancestry populations. Herein, African American ever-smokers, drawn from two independent lung cancer case–control studies, were genotyped for reduced activity CYP2A6 alleles and grouped by predicted metabolic activity. Lung cancer risk in the Southern Community Cohort Study (n = 494) was lower among CYP2A6 reduced versus normal metabolizers, as estimated by multivariate conditional logistic regression [odds ratio (OR) = 0.44; 95% confidence interval (CI) = 0.26–0.73] and by unconditional logistic regression (OR = 0.62; 95% CI = 0.41–0.94). The association was replicated in an independent study from MD Anderson Cancer Center (n = 407) (OR = 0.64; 95% CI = 0.42–0.98), and pooling the studies yielded an OR of 0.64 (95% CI = 0.48–0.86). Exploratory analyses revealed a significant interaction between CYP2A6 genotype and sex on the risk for lung cancer (Southern Community Cohort Study: P = 0.04; MD Anderson: P = 0.03; Pooled studies: P = 0.002) with a CYP2A6 effect in men only. These findings support a contribution of genetic variation in CYP2A6 to lung cancer risk among African American smokers, particularly men, whereby CYP2A6 genotypes associated with reduced metabolic activity confer a lower risk of developing lung cancer.
Introduction
The smoking-attributable risk for lung cancer is higher among African Americans compared with European Americans, especially among men, despite reporting lower daily cigarette consumption and initiating smoking later in life (1–5). The benefit of lower daily cigarette consumption may be offset by more intensive smoking resulting in greater carcinogen exposure than would be predicted based on cigarettes per day (6–8), and the benefit of a later age of regular smoking may be offset by increased difficulty quitting compared with European Americans (4,9); however, racial differences in the susceptibility to carcinogens within cigarette smoke may also contribute to the disparity in lung cancer risk. Although progress has been made in identifying smoking-related genetic risk factors in other populations, comparatively little is known among populations of African descent (10).
CYP2A6 codes for a nicotine/nitrosamine-metabolizing enzyme (11) and CYP2A6 gene variants have the potential to affect lung cancer risk among smokers by influencing cigarette use (through slower nicotine inactivation) (12), as well as by decreasing the bioactivation of carcinogenic tobacco-specific nitrosamines (13). We and others have found an association between reduced activity CYP2A6 genotypes and lung cancer risk among European and Asian populations (14–16); however, it is unknown what portion of the risk is due to altered smoking amount versus altered carcinogen metabolism.
In contrast to the populations in which CYP2A6 and lung cancer risk have been studied, African Americans report lower cigarette consumption, a greater use of mentholated cigarettes and are at a higher risk of developing lung cancer (1–4). In terms of CYP2A6, African Americans possess different gene variants and more low frequency variants, such as CYP2A6*17, *20, *23-*27 and *35, which are associated with reduced nicotine metabolism in vivo but have not been examined with respect to lung cancer risk (12,17,18). Given the underlying differences in smoking patterns, lung cancer risk and CYP2A6 gene variants, it is challenging to predict how CYP2A6 gene variants may influence lung cancer risk among African American smokers. Thus, we performed the first investigation of CYP2A6 genetic variation and lung cancer risk among African Americans.
Materials and methods
Study participants
Participants were ever-smokers from two independent lung cancer studies. The first was a case–control study nested within the Southern Community Cohort Study (SCCS), a prospective health disparities study across 12 southern states (19). For each incident lung cancer case, 1–2 control subjects were individually matched by race (self-identified), age, sex, and recruitment source and site, which allowed for conditional analyses (20). The original nested lung cancer case control in SCCS from which participants were drawn for the investigation herein included never smokers (20), and exclusion of never smokers disrupted a few matched sets resulting in a smaller sample size in conditional compared with unconditional analyses (n = 445 and 494, respectively) with 3–15 participants missing data for a given smoking variable in either analysis. The second study, drawn from an ongoing case–control investigation of lung cancer risk by the MD Anderson Cancer Center (MDA), comprised control subjects frequency matched to lung cancer patients by smoking behaviours in addition to race (self-identified), age and sex (21). The distribution of sex, age, body mass index (BMI), current/former smoking status, menthol/non-menthol smoking, cigarettes per day and lung cancer histology differed significantly between the SCCS and MDA study populations. In both studies, former smokers are defined as those quit for at least 1 year, whereas current smokers include those quit for less than a year. Institutional Review Boards at Vanderbilt University, MD Anderson Cancer Center and University of Toronto approved the investigation.
CYP2A6 Genotyping
Participants were genotyped for CYP2A6 reduced/null activity alleles predominantly found in African populations, CYP2A6*17, *20, *23-*27, *31, *35, and for those also found in other racial populations, CYP2A6*2, *4, *9 (Supplementary Methods, available at Carcinogenesis Online, and ref. 17). Fisher’s exact test was used to assess departure in genotype frequencies from Hardy–Weinberg equilibrium. Genotype grouping was based on CYP2A6 activity data from the African American smoking population published in Ho et al. (17) and unpublished data for the *1/*31 genotype from that same population, participants. Participants with one or more copies of the CYP2A6*2, *4, *9, *17, *25, *26, *27, *31 and *35 alleles were grouped into a reduced metabolism group and predicted low-risk cancer group, CYP2A6 reduced metabolizers (CYP2A6 RM), CYP2A6 genotypes with ≤75% of the activity of the CYP2A6 *1/*1 genotype with the remaining subjects grouped as CYP2A6 normal metabolizers (CYP2A6 NM), the predicted high-risk group (Supplementary Methods, available at Carcinogenesis Online, and ref.17).
Statistical analysis
Lung cancer odds ratios (ORs) and P values were estimated using logistic regression modeling, and reported ORs were adjusted for predetermined demographic variables (age, sex, BMI) and smoking behaviors variables (cigarettes per day, years of smoking, use of mentholated cigarettes) that have already been established as lung cancer risk factors (20,22,23). Within the SCCS data set, global ancestry was estimated using genotyping data available from the Illumina 1M genotyping array and the software program ADMIXTURE with a two-population model. Supervised analysis was conducted using the CEU and YRI HapMap ancestral populations (24). Lung cancer risk within the SCCS data set was investigated with conditional logistic regression to incorporate the individual matching of cases and controls. Lung cancer risk within SCCS was also modeled with unconditional logistic regression to facilitate comparison with the replication data set, MDA, in which lung cancer risk was investigated using unconditional regression owing to the frequency matching of cases and controls. Individual level data from the two studies were also pooled, as variables were defined in the same manner in both studies (e.g. cigarettes per day, years of smoking, menthol smoking, BMI, current/former smokers) mitigating a need for further data harmonization. A new variable indicating the study, SCCS or MDA, was generated and included as a covariate in the unconditional logistic regression pooled analyses. In all lung cancer risk analyses, the predicted high-risk genotype group, CYP2A6 NM, served as the reference group. The primary analysis consisted of a single comparison, CYP2A6 RM versus NM, based on our biologically founded a priori hypothesis of reduced lung cancer risk among reduced CYP2A6 metabolizers, and as such the significance threshold of P < 0.05 was utilized. Demographic and smoking variables were compared using Wilcoxon or Kruskal–Wallis test for continuous variables and Fisher’s exact test for categorical variables. All statistical analyses were two sided. Statistical analyses were performed with Stata Release 13 (StataCorp LP, College Station, TX).
Results
Characteristics of the study participants included in our investigation are provided (Table I). In SCCS, 1–2 control subjects were individually matched to lung cancer cases by age, sex and recruitment site, but not by smoking behaviours. Among the MDA participants, controls were frequency matched to cases by smoking behaviours in addition to age and sex. Within SCCS, 492 of 494 participants were successfully genotyped for all alleles: 283 (58%) CYP2A6 NMs and 209 (42%) CYP2A6 RMs. Within MDA, 405 of 407 participants were successfully genotyped for all alleles: 246 (61%) CYP2A6 NMs and 159 (39%) CYP2A6 RMs. Genotype frequencies for each CYP2A6 allele did not depart from Hardy–Weinberg equilibrium and are provided in Supplementary Table 1, available at Carcinogenesis Online. Cigarettes per day was significantly lower among CYP2A6 RMs compared with NMs in MDA and the pooled studies and nominally lower in SCCS (Table II).
Table I.
African American study participant characteristics and CYP2A6 genotype activity groups
| Variable | SCCSa, n = 494 | MDAb, n = 407 | Pooled studies, n = 901 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Case patients, n = 204 | Control subjects, n = 290 | P | Case patients, n = 199 | Control subjects, n = 208 | P | Case patients, n = 403 | Control subjects, n = 498 | P | |
| Male | 119 (58%) | 192 (66%) | 0.09 | 103 (52%) | 108 (52%) | 1.0 | 222 (55%) | 200 (60%) | 0.13 |
| Female | 85 (42%) | 98 (34%) | 96 (48%) | 100 (48%) | 181 (45%) | 198 (40%) | |||
| Age entry | 56 (8.7) | 55 (8.5) | 0.24 | 60 (9.4) | 59 (8.9) | 0.46 | 58 (9.3) | 56 (8.9) | 0.07 |
| Age diagnosis | 58 (8.7) | na | 59 (9.4) | na | 59 (9.1) | na | |||
| Body mass index | 26 (5.9) | 29 (6.8) | <0.001 | 27 (6.0) | 30 (6.0) | <0.01 | 27 (6.0) | 29 (6.5) | <0.001 |
| Age start smoking | 17 (5.2) | 18 (5.9) | 0.35 | 18 (5.1) | 19 (4.6) | 0.48 | 18 (5.2) | 18 (5.4) | 0.51 |
| Cigarettes per day | 17 (13) | 14 (12) | 0.006 | 17 (9.3) | 16 (9.8) | 0.26 | 17 (11) | 15 (11) | 0.003 |
| ≤10 cigarettes per day | 95 (47%) | 164 (58%) | 0.05 | 77 (39%) | 88 (42%) | 0.69 | 172 (43%) | 252 (51%) | 0.03 |
| 11–20 cigarettes per day | 77 (38%) | 83 (29%) | 98 (49%) | 94 (45%) | 175 (44%) | 177 (36%) | |||
| >20 cigarettes per day | 31 (15%) | 35 (12%) | 24 (12%) | 26 (12%) | 55 (14%) | 61 (12%) | |||
| Years of smoking | 36 (10) | 30 (12) | <0.001 | 35 (11) | 33 (12) | 0.08 | 35 (11) | 32 (24–39) | <0.001 |
| Pack-years | 30 (25) | 21 (20) | <0.001 | 30 (20) | 27 (21) | 0.07 | 30 (23) | 24 (21) | <0.001 |
| Current smoker | 158 (77%) | 177 (62%) | <0.001 | 110 (55%) | 113 (54%) | 0.92 | 268 (67%) | 290 (59%) | 0.02 |
| Former smoker | 46 (23%) | 110 (38%) | 89 (45%) | 95 (46%) | 135 (33%) | 205 (41%) | |||
| Menthol smoking | 141 (70%) | 223 (78%) | 0.04 | 104 (57%) | 113 (55%) | 0.76 | 245 (63%) | 336 (68%) | 0.13 |
| Non-menthol | 61 (30%) | 62 (22%) | 80 (43%) | 93 (45%) | 141 (37%) | 155 (32%) | |||
| Adenocarcinoma | 50 (25%) | 85 (43%) | 135 (33%) | ||||||
| Squamous cell | 41 (20%) | 54 (27%) | 95 (24%) | ||||||
| Other carcinomasc | 113 (55%) | 60 (30%) | 173 (43%) | ||||||
| CYP2A6 NM | 130 (64%) | 153 (53%) | 0.02 | 130 (66%) | 116 (56%) | 0.05 | 260 (65%) | 269 (54%) | 0.002 |
| CYP2A6 RM | 74 (36%) | 135 (47%) | 68 (34%) | 91 (44%) | 142 (35%) | 226 (46%) | |||
Mean and standard deviation provided for continuous variables. CYP2A6 NM, predicted normal CYP2A6 metabolic activity; CYP2A6 RM, predicted reduced CYP2A6 metabolic activity; squamous: squamous cell carcinoma.
aSCCS: 1–2 control subjects individually matched to each lung cancer patients by age, sex, and recruitment source and site.
bMDA: control subjects frequency matched to lung cancer patients by smoking behaviors in addition to age and sex.
cOther: includes 17 small cell carcinoma cases in SCCS (8.3% of SCCS cases and 4.2% of Pooled cases).
Table II.
Cigarettes per day by CYP2A6 genotype group
| Cigarettes per daya | SCCS, n = 483 | MDA, n = 405 | Pooled studies, n = 888 | |||
|---|---|---|---|---|---|---|
| Mean (95% CI) | P | Mean (95% CI) | P | Mean (95% CI) | P | |
| CYP2A6 NM | 15.6 (14.2–17.1) | 0.40 | 17.1 (15.9–18.3) | 0.04 | 16.3 (15.4–17.3) | 0.04 |
| CYP2A6 RM | 14.6 (12.9–16.2) | 15.4 (13.9–16.9) | 14.9 (13.8–16.1) | |||
CYP2A6 NM, predicted normal CYP2A6 metabolic activity; CYP2A6 RM, predicted reduced CYP2A6 metabolic activity.
aCigarettes per day is not normally distributed and P values were calculated with a non-parametric Wilcoxon rank-sum test.
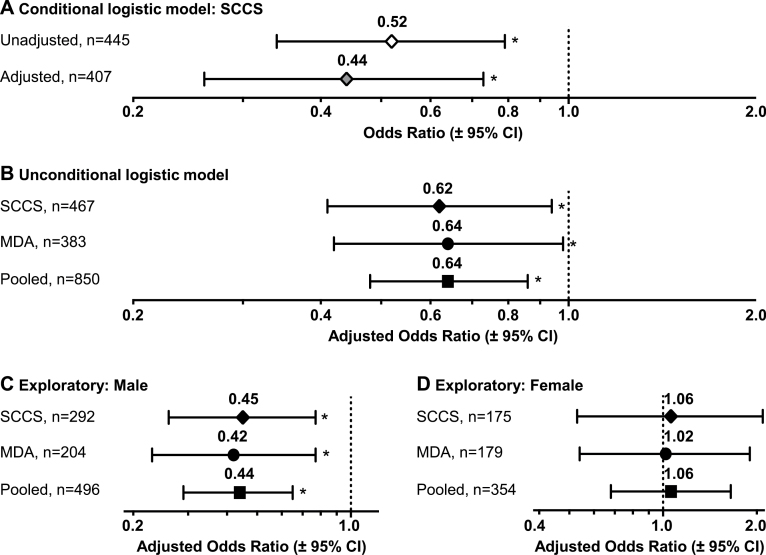
Initially, we utilized the individual matching of control subjects to case patients in SCCS and estimated lung cancer ORs using conditional logistic regression modeling (Figure 1A). CYP2A6 RMs had a significant reduction in lung cancer risk compared with CYP2A6 NMs [OR = 0.52, 95% confidence interval (CI) = 0.34–0.79, P = 0.002], which remained after adjusting for cigarettes per day (cigarettes per day), cigarette type (menthol or non-menthol), years of smoking and BMI (OR = 0.44, 95% CI = 0.26–0.73, P = 0.001, with covariates)—variables independently associated with lung cancer risk (20,22,23).
Fig. 1.
Lung cancer risk by CYP2A6, CYP2A6 reduced metabolizer compared with CYP2A6 normal metabolizer (referent), with odds ratios estimated by (A) conditional logistic regression modeling within SCCS, and by (B) unconditional logistic regression modeling within SCCS, MDA and the Pooled studies, overall and separately within (C) male and (D) female participants. Models (A–D) were all adjusted for log BMI (continuous), log cigarettes per day (continuous), years of smoking (continuous) and type of cigarette (menthol or non-menthol). Models (B–D) were further adjusted for age (continuous) and sex (male or female, B only). Pooled analyses in (B–D) were also adjusted for study site (SCCS or MDA). Lung cancer odds ratios and P values were estimated by logistic regression analysis with P < 0.05 (significance) denoted by an asterisk. All statistical tests were two sided.
Importantly, CYP2A6 risk estimates were not affected by population substructure. Including ancestry estimates, which were available for 388 of 494 participants in SCCS, had a negligible impact on the findings (OR = 0.51, 95% CI = 0.30–0.87, P = 0.01, without covariates; OR = 0.46, 95% CI = 0.24–0.87, P = 0.02, with covariates). The ORs in SCCS were also estimated using unconditional logistic regression with age and sex as additional covariates (Figure 1B): 0.65 (95% CI = 0.45–0.93, P = 0.02) without covariates, 0.62 (95% CI = 0.41–0.94, P = 0.02) with covariates, 0.60 (95% CI = 0.40–0.92, P = 0.02) with ancestry and 0.61 (95% CI = 0.38–0.98, P = 0.04) with ancestry and covariates.
The significant reduction in lung cancer risk among CYP2A6 RMs versus NMs was replicated in MDA (Figure 1B, OR = 0.64, 95% CI = 0.42–0.98, P = 0.04, with covariates). CYP2A6 genotype group and years of smoking were similar across SCCS and MDA participants, whereas other demographic characteristics differed (Table I). However, after pooling SCCS and MDA, there was no evidence of heterogeneity in the CYP2A6 OR by study sample (Mantel–Haenszel test for homogeneity, P = 0.91), and the ORs estimated from unconditional logistic regression were consistent with the results from either study alone (Figure 1B, OR = 0.64, 95% CI = 0.48–0.86, P = 0.003, with covariates).
We found an unexpected interaction between CYP2A6 and sex in lung cancer logistic regression analyses (SCCS: P = 0.04; MDA: P = 0.03; Pooled: P = 0.002, for the interaction term), which was also supported by a Mantel–Haenszel test for homogeneity between CYP2A6 and sex (SCCS: P = 0.01; MDA: P = 0.03; Pooled: P < .001). Subsequent post hoc subgroup analyses revealed a significant association between CYP2A6 and lung cancer risk in men (Figure 1C), and not women (Figure 1D), which was consistent across both studies (Supplementary Table 2A and B, available at Carcinogenesis Online). Exploratory stratified analyses were performed overall and in men and women separately to investigate potential contributing factors to the observed sex-specific effect (Table III). Strata for cigarettes per day, years of smoking and pack-years were generated using a median split based on controls in the pooled data set. Within men, CYP2A6 RMs versus NMs had significantly lower lung cancer risk by cancer histology and by smoking variable (except former smoking), whereas within women none of the ORs by cancer histology or by smoking variable were significant, further supporting a contribution of CYP2A6 to lung cancer risk among men only.
Table III.
Stratified exploration of lung cancer risk by CYP2A6 genotype group and sex
| CYP2A6 RM versus NM (referent) | Pooled studies, n = 897 | Male participants, n = 521 | Female participants, n = 376 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n a | Adj. odds ratiob | P | n a | Adj. odds ratiob | P | n a | Adj. odds ratiob | P | |
| Adenocarcinoma | 598 | 0.69 (0.45–1.06) | 0.09 | 343 | 0.39 (0.20–0.77) | 0.007 | 255 | 1.16 (0.64–2.10) | 0.62 |
| Squamous | 564 | 0.57 (0.35–0.95) | 0.03 | 347 | 0.48 (0.26–0.91) | 0.02 | 217 | 0.66 (0.28–1.58) | 0.35 |
| Current smoker | 529 | 0.60 (0.42–0.87) | 0.007 | 303 | 0.36 (0.21–0.60) | <0.001 | 226 | 1.15 (0.66–2.00) | 0.62 |
| Former smoker | 321 | 0.77 (0.47–1.28) | 0.32 | 193 | 0.59 (0.31–1.13) | 0.11 | 128 | 1.35 (0.58–3.18) | 0.49 |
| Menthol smoker | 559 | 0.68 (0.47–0.98) | 0.04 | 311 | 0.48 (0.29–0.80) | 0.005 | 248 | 1.02 (0.60–1.73) | 0.94 |
| Non-menthol | 291 | 0.52 (0.31–0.88) | 0.01 | 185 | 0.33 (0.17–0.64) | 0.001 | 106 | 0.92 (0.38–2.21) | 0.85 |
| Cigarettes per day: ≤10 | 407 | 0.68 (0.44–1.04) | 0.07 | 203 | 0.43 (0.22–0.83) | 0.01 | 204 | 0.96 (0.54–1.73) | 0.90 |
| Cigarettes per day: >10 | 443 | 0.62 (0.41–0.92) | 0.02 | 293 | 0.42 (0.25–0.71) | 0.001 | 150 | 1.06 (0.52–2.16) | 0.88 |
| Smoke years: ≤32 | 385 | 0.53 (0.34–0.85) | 0.008 | 210 | 0.33 (0.17–0.66) | 0.001 | 175 | 0.86 (0.44–1.65) | 0.64 |
| Smoke years: >32 | 465 | 0.70 (0.47–1.03) | 0.07 | 286 | 0.51 (0.31–0.84) | 0.009 | 179 | 1.16 (0.62–2.20) | 0.64 |
| Pack-years: ≤19 | 378 | 0.55 (0.35–0.87) | 0.01 | 202 | 0.29 (0.15–0.58) | <0.001 | 176 | 1.03 (0.54–1.96) | 0.94 |
| Pack-years: >19 | 472 | 0.72 (0.49–1.06) | 0.10 | 294 | 0.53 (0.32–0.87) | 0.01 | 178 | 1.10 (0.59–2.06) | 0.76 |
CYP2A6 NM, predicted normal CYP2A6 metabolic activity; CYP2A6 RM, predicted reduced CYP2A6 metabolic activity; squamous: squamous cell carcinoma.
a N values indicate the total number of genotyped participants included in stratified analyses and may not add to column total due to missing covariate data; all controls are included in the stratified analyses by cancer histology.
bAll models adjusted for age (continuous), log BMI (continuous), log cigarettes per day (continuous), years of smoking (continuous), type of cigarette (menthol or non-menthol, where applicable), study site (SCCS or MDA) and sex (male or female, where applicable)
Discussion
African Americans suffer disproportionately from smoking-related lung cancer despite reporting lower daily cigarette consumption and initiating smoking later in life (1–5). This is the first report, to our knowledge, of variation in the nicotine/nitrosamine metabolism gene, CYP2A6, with lung cancer risk among African American smokers. Although CYP2A6 gene variants have been associated with lung cancer risk in European and Asian populations (14–16), African Americans possess different gene variants and more low frequency variants (12,17,18), which have not been examined with respect to lung cancer risk. We found that CYP2A6 gene variants with reduced/null activity are associated with a reduction in lung cancer risk among African Americans in two independent populations and that the association is unaffected by correction for percent African ancestry or other smoking-related covariates. Importantly, overall CYP2A6 findings and exploratory findings stratified by sex were consistent across the two studies, which recruited patients from different geographic regions and employed different study designs suggesting that these findings are likely generalizable to the broader African American smoking population.
As in most epidemiological studies, use of self-reported cigarettes per day as a measure of tobacco smoke exposure is a limitation of the current study since cigarettes per day serves as a relatively poor marker of smoking dose, particularly in African American light smokers (6,25). Absence of biochemical data also meant that we were unable to accurately estimate the relative contribution of CYP2A6 to lung cancer due to altered smoking dose (carcinogen exposure) from its potential contribution through altered nitrosamine bioactivation. Sample size was a limitation in the post hoc exploratory analyses, which were undertaken to investigate the novel finding of a CYP2A6–sex interaction on lung cancer risk.
The CYP2A6 sex-specific effect that was observed is in sharp contrast to our prior study in European Americans (14) where we did not observe a significant interaction or heterogeneity in the CYP2A6 ORs by sex. Differences in study design are unlikely to explain the contrasting observations by sex—our prior study also employed an analogous CYP2A6 genotype grouping strategy by predicted metabolic activity, and recruitment of cases and controls was part of the same larger MDA investigation. We can only speculate that genetic or non-genetic factors specific to African American female smokers negate the contribution of CYP2A6 genotype to lung cancer risk. One possibility stemming from exploratory post hoc stratified analyses is a loss of effect of CYP2A6 on the risk of adenocarcinoma among women (Table III)—lower DNA repair capacity and higher DNA adduct loads have been observed in adenocarcinoma in women versus men (26). Biochemical data, such as carcinogen exposure, metabolism and DNA repair capacity, will be instructive as to potential sources for this ethnic–sex-specific finding. Other genetic association studies have furnished evidence of sex-specific genetic effects in respiratory cancers (27–32); however, these studies were all in populations of European descent, and it is unknown whether a sex-specific effect would be observed in additional ethnicities. With respect to CYP2A6, most of the published studies reporting a significant association between CYP2A6 genotype and lung cancer risk included 50–100% male participants (14–16,33–35); hence, there is an overall need to investigate CYP2A6 and lung cancer risk within female smokers of diverse ethnic backgrounds.
In other respects, the CYP2A6 lung cancer risk estimates in the European and African American studies are comparable. In our previous investigation of European Americans, the effect of CYP2A6 appeared strongest among those smoking 20 or fewer cigarettes per day (OR = 0.63; 95% CI = 0.41–0.97, P = 0.04, unadjusted, ref. 14). We observed a similar effect here among African Americans smoking 20 or fewer cigarettes per day (OR = 0.65; 95% CI = 0.49–0.87, P = 0.003, unadjusted). Also consistent with the genotype effect in our European study (14), the association between CYP2A6 and lung cancer risk remained after controlling for cigarettes per day.
In terms of the association between CYP2A6 and cigarettes per day, while cigarettes per day was significantly and substantially lower among CYP2A6 RMs versus NMs in our European American study (14), the difference in cigarettes per day by CYP2A6 genotype group was modest and similar in both the SCCS and the MDA studies (~1–2 cigarettes per day). Cigarettes per day is a particularly poor measure of nicotine intake among African American smokers, who appear to smoke more intensively at lower levels of cigarettes per day and less intensively at higher cigarettes per day to a greater extent than European Americans (6), and differences in cigarettes per day by CYP2A6 among African American light smokers have not been observed in a number of studies (17,25). Among Alaska Native smokers, another population with a high prevalence of light smokers, evidence of titration by CYP2A6 activity was only observed using urinary total nicotine equivalents and not with cigarettes per day (36). Hence, although CYP2A6 may have a limited effect on cigarette consumption among African American smokers, it may still have an effect on nicotine intake (via smoking intensity).
To gauge the consistency of CYP2A6 lung cancer risk estimates across populations, we examined published effect sizes for the CYP2A6 *1/*9 genotype and lung cancer risk since the *9 allele is found at an appreciable frequency among different racial groups (12). The OR for CYP2A6 *1/*9 versus *1/*1 was 0.57 among Europeans (16) and 0.59 among Japanese (15), which is similar to the OR of 0.56 among African Americans in the present investigation. An important caveat to this cross-study comparison is that the smoking behaviors and statistical procedures differed between the studies.
Consistent with our genetic investigation, which implicates altered nicotine/nitrosamine in lung carcinogenesis among smokers, CYP2A6 inhibition studies implicate a role for the enzyme in the development of lung cancer and have generated interest in CYP2A6 as a target for cancer prevention (37,38). Based on our findings and those in other populations, there may be merit in investigating the utility of CYP2A6 genotype in lung cancer screening models to improve cancer risk prediction and to help identify individuals for whom preventative efforts, behavioral or pharmacological, may be particularly beneficial.
Supplementary material
Supplementary Methods and Supplementary Tables 1 and 2 can be found at http://carcin.oxfordjournals.org/
Funding
University Endowed Chair in Addictions for the Department of Psychiatry (R.F.T.); National Institutes of Health (R01 CA092447 supporting the Southern Community Cohort Study, U01DA020830 to R.F.T., P50CA070907 to X.W., R01CA127219 to M.R.S., K07CA172294 to M.C.A.); Canadian Institutes of Health Research (MOP86471 and TMH109787 to R.F.T., Doctoral Award to C.A.W.); Center for Translational and Public Health Genomics of the Duncan Family Institute for Cancer Prevention and Risk Assessment at The University of Texas MD Anderson Cancer Center; Centre for Addiction and Mental Health and the CAMH foundation; Canada Foundation for Innovation (#20289 and #16014); Ontario Ministry of Research and Innovation.
Supplementary Material
Acknowledgements
We thank Christopher I.Amos for assistance initiating the MDA collaboration, Qian Zhou for assistance with CYP2A6 genotyping, Regina Courtney and Jie Wu for help with SCCS sample preparation. Preparation of SCCS samples was conducted at the Survey and Biospecimen Shared Resources, which is supported in part by the Vanderbilt-Ingram Cancer Center (P30CA068485).
Conflict of Interest Statement: R.F.T. has served as a consultant to pharmaceutical companies, primarily on smoking cessation. No other conflicts to declare.
Glossary
Abbreviations:
- BMI
body mass index
- CI
confidence interval
- MDA
MD Anderson Cancer Center
- NM
normal metabolizer
- OR
odds ratio
- RM
reduced metabolizer
- SCCS
Southern Community Cohort Study.
References
- 1. Haiman C.A., et al. (2006). Ethnic and racial differences in the smoking-related risk of lung cancer. N. Engl. J. Med., 354, 333–342. [DOI] [PubMed] [Google Scholar]
- 2. Pinsky P.F. (2006). Racial and ethnic differences in lung cancer incidence: how much is explained by differences in smoking patterns? (United States). Cancer Causes Control, 17, 1017–1024. [DOI] [PubMed] [Google Scholar]
- 3. Trinidad D.R., et al. (2009). Intermittent and light daily smoking across racial/ethnic groups in the United States. Nicotine Tob. Res., 11, 203–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Trinidad D.R., et al. (2011). A nationwide analysis of US racial/ethnic disparities in smoking behaviors, smoking cessation, and cessation-related factors. Am. J. Public Health, 101, 699–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jain R.B. (2014). Trends in serum cotinine concentrations among daily cigarette smokers: data from NHANES 1999-2010. Sci. Total Environ., 472, 72–77. [DOI] [PubMed] [Google Scholar]
- 6. Benowitz N.L., et al. (2011). Racial differences in the relationship between number of cigarettes smoked and nicotine and carcinogen exposure. Nicotine Tob. Res., 13, 772–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. St Helen G., et al. (2013). Racial differences in the relationship between tobacco dependence and nicotine and carcinogen exposure. Addiction, 108, 607–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Melikian A.A., et al. (2007). Gender differences relative to smoking behavior and emissions of toxins from mainstream cigarette smoke. Nicotine Tob. Res., 9, 377–387. [DOI] [PubMed] [Google Scholar]
- 9. Murray R.P., et al. (2001). Experience of Black participants in the Lung Health Study smoking cessation intervention program. Nicotine Tob. Res., 3, 375–382. [DOI] [PubMed] [Google Scholar]
- 10. Schwartz A.G., et al. (2007). The molecular epidemiology of lung cancer. Carcinogenesis, 28, 507–518. [DOI] [PubMed] [Google Scholar]
- 11. Di Y.M., et al. (2009). Structure, function, regulation and polymorphism of human cytochrome P450 2A6. Curr. Drug Metab., 10, 754–780. [DOI] [PubMed] [Google Scholar]
- 12. Mwenifumbo J.C., et al. (2007). Genetic variability in CYP2A6 and the pharmacokinetics of nicotine. Pharmacogenomics, 8, 1385–1402. [DOI] [PubMed] [Google Scholar]
- 13. Rossini A., et al. (2008). CYP2A6 polymorphisms and risk for tobacco-related cancers. Pharmacogenomics, 9, 1737–1752. [DOI] [PubMed] [Google Scholar]
- 14. Wassenaar C.A., et al. (2011). Relationship between CYP2A6 and CHRNA5-CHRNA3-CHRNB4 variation and smoking behaviors and lung cancer risk. J. Natl Cancer Inst., 103, 1342–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fujieda M., et al. (2004). Evaluation of CYP2A6 genetic polymorphisms as determinants of smoking behavior and tobacco-related lung cancer risk in male Japanese smokers. Carcinogenesis, 25, 2451–2458. [DOI] [PubMed] [Google Scholar]
- 16. Gemignani F., et al. (2007). Development of lung cancer before the age of 50: the role of xenobiotic metabolizing genes. Carcinogenesis, 28, 1287–1293. [DOI] [PubMed] [Google Scholar]
- 17. Ho M.K., et al. (2009). Association of nicotine metabolite ratio and CYP2A6 genotype with smoking cessation treatment in African-American light smokers. Clin. Pharmacol. Ther., 85, 635–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mwenifumbo J.C., et al. (2008). Novel and established CYP2A6 alleles impair in vivo nicotine metabolism in a population of Black African descent. Hum. Mutat., 29, 679–688. [DOI] [PubMed] [Google Scholar]
- 19. Signorello L.B., et al. (2005). Southern community cohort study: establishing a cohort to investigate health disparities. J. Natl Med. Assoc., 97, 972–979. [PMC free article] [PubMed] [Google Scholar]
- 20. Blot W.J., et al. (2011). Lung cancer risk among smokers of menthol cigarettes. J. Natl Cancer Inst., 103, 810–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Amos C.I., et al. (2010). Nicotinic acetylcholine receptor region on chromosome 15q25 and lung cancer risk among African Americans: a case-control study. J. Natl Cancer Inst., 102, 1199–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kenfield S.A., et al. (2008). Comparison of aspects of smoking among the four histological types of lung cancer. Tob. Control, 17, 198–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kabat G.C., et al. (2008). Body mass index and waist circumference in relation to lung cancer risk in the Women’s Health Initiative. Am. J. Epidemiol., 168, 158–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Alexander D.H., et al. (2009). Fast model-based estimation of ancestry in unrelated individuals. Genome Res., 19, 1655–1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ho M.K., et al. (2009). Utility and relationships of biomarkers of smoking in African-American light smokers. Cancer Epidemiol. Biomarkers Prev., 18, 3426–3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kiyohara C., et al. (2010). Sex differences in lung cancer susceptibility: a review. Gend. Med., 7, 381–401. [DOI] [PubMed] [Google Scholar]
- 27. Gallagher C.J., et al. (2007). The UDP-glucuronosyltransferase 2B17 gene deletion polymorphism: sex-specific association with urinary 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol glucuronidation phenotype and risk for lung cancer. Cancer Epidemiol. Biomarkers Prev., 16, 823–828. [DOI] [PubMed] [Google Scholar]
- 28. Spitz M.R., et al. (2008). The CHRNA5-A3 region on chromosome 15q24-25.1 is a risk factor both for nicotine dependence and for lung cancer. J. Natl Cancer Inst., 100, 1552–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chen D., et al. (2011). A sex-specific association between a 15q25 variant and upper aerodigestive tract cancers. Cancer Epidemiol. Biomarkers Prev., 20, 658–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tang D.L., et al. (1998). Associations between both genetic and environmental biomarkers and lung cancer: evidence of a greater risk of lung cancer in women smokers. Carcinogenesis, 19, 1949–1953. [DOI] [PubMed] [Google Scholar]
- 31. Larsen J.E., et al. (2005). Risk of non-small cell lung cancer and the cytochrome P4501A1 Ile462Val polymorphism. Cancer Causes Control, 16, 579–585. [DOI] [PubMed] [Google Scholar]
- 32. Timofeeva M.N., et al. (2009). CYP450 polymorphisms as risk factors for early-onset lung cancer: gender-specific differences. Carcinogenesis, 30, 1161–1169. [DOI] [PubMed] [Google Scholar]
- 33. Ariyoshi N., et al. (2002). Genetic polymorphism of CYP2A6 gene and tobacco-induced lung cancer risk in male smokers. Cancer Epidemiol. Biomarkers Prev., 11, 890–894. [PubMed] [Google Scholar]
- 34. Islam M.S., et al. (2013). Lung cancer risk in relation to nicotinic acetylcholine receptor, CYP2A6 and CYP1A1 genotypes in the Bangladeshi population. Clin. Chim. Acta., 416, 11–19. [DOI] [PubMed] [Google Scholar]
- 35. Rotunno M., et al. (2009). Phase I metabolic genes and risk of lung cancer: multiple polymorphisms and mRNA expression. PLoS One, 4, e5652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhu A.Z., et al. (2013). Alaska Native smokers and smokeless tobacco users with slower CYP2A6 activity have lower tobacco consumption, lower tobacco-specific nitrosamine exposure and lower tobacco-specific nitrosamine bioactivation. Carcinogenesis, 34, 93–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Takeuchi H., et al. (2009). 8-Methoxypsoralen, a potent human CYP2A6 inhibitor, inhibits lung adenocarcinoma development induced by 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone in female A/J mice. Mol. Med. Rep., 2, 585–588. [DOI] [PubMed] [Google Scholar]
- 38. Sellers E.M., et al. (2003). The effect of methoxsalen on nicotine and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) metabolism in vivo . Nicotine Tob. Res., 5, 891–899. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.