Abstract
Chronic heavy alcohol consumption is a risk factor for cortical bone fractures in males. The increase in fracture risk may be due, in part, to reduced bone quality. Intracortical (osteonal) bone remodeling is the principle mechanism for maintaining cortical bone quality. However, it is not clear how alcohol abuse impacts intracortical bone remodeling. This study investigated the effects of long-duration heavy alcohol consumption on intracortical bone remodeling in a non-human primate model. Following a 4-month induction period, male rhesus macaques (Macaca mulatta, n = 21) were allowed to voluntarily self-administer water or alcohol (4% ethanol w/v) for 22 h/d, 7 d/wk for 12 months. Control monkeys (n = 13) received water and an isocaloric maltose-dextrin solution. Tetracycline hydrochloride was administered orally 17 and 3 days prior to sacrifice for determination of active mineralization sites. Animals in the alcohol group consumed 2.7 ± 0.2 g alcohol/kg/d (mean ± SE) during the 12 months of self-administration, resulting in a mean daily blood alcohol concentration of 77 ± 9 mg/dl from samples taken at 7 h after the start of a daily session. However, blood alcohol concentration varied widely from day to day, with peak levels exceeding 250 mg/dl, modeling a binge-drinking pattern of alcohol consumption. The skeletal response to alcohol was determined by densitometry, microcomputed tomography and histomorphometry. Significant differences in tibial bone mineral content, bone mineral density, and cortical bone architecture (cross-sectional volume, cortical volume, marrow volume, cortical thickness, and polar moment of inertia) in the tibial diaphysis were not detected with treatment. However, cortical porosity was lower (1.8 ± 0.5 % versus 0.6 ± 0.1 %, p = 0.021) and labeled osteon density was lower (0.41 ± 0.2/mm2 versus 0.04 ± 0.01/mm2, p < 0.003) in alcohol-consuming monkeys compared to controls, indicating a reduced rate of intracortical bone remodeling. In concordance, plasma CTx was lower (2.5 ± 0.3 ng/ml versus 1.7 ± 0.1 ng/ml, p = 0.028) in the alcohol group. These results suggest that chronic heavy alcohol consumption may negatively impact bone health, in part, by suppressing intracortical bone remodeling.
Keywords: Haversian remodeling, histomorphometry, microcomputed tomography, ethanol, non-human primate
1. Introduction
Chronic heavy alcohol consumption is associated with an increase in all cause fracture risk [1–5]. A low bone mineral density (BMD) is often observed in chronic heavy alcohol consumers and when detected is generally associated with decreased bone formation [6–14]. In contrast, the precise role of bone resorption in the etiology of this condition is less clear [6, 7, 11, 12, 15–18]. Because alcohol consumption often results in an overall reduction in the rate of bone remodeling, bone loss may occur when the reduction in bone formation exceeds the reduction in bone resorption [19–21].
Although alcoholics often exhibit reduced BMD, the specific effects of alcohol on the human skeleton are poorly defined. This is due, in part, to complications associated with co-morbidities, such as smoking, poor diet and alcohol-induced disease [22]. In addition, alcohol intervention studies are exceedingly difficult to perform in humans. As a result, most intervention studies have been conducted using experimental animal models, with rodents being the mainstay for assessing the specific effects of alcohol on bone metabolism [23–25]. To date, rodent studies have focused on the effects of alcohol on cortical bone accrual and cancellous bone turnover. However, because intracortical (osteonal) bone remodeling is absent in small rodents, these animals are not ideal for evaluation of the effects of alcohol on cortical bone turnover [26].
In humans, cortical bone comprises the great majority (~80%) of bone mass and plays a fundamental role in the mechanical function of the skeleton. Cortical bone fractures are common in young adult and middle aged males and are more prevalent in chronic heavy alcohol consumers [26, 27]. Interestingly, heavy alcohol consumers are more likely to sustain low trauma fractures than non-heavy consumers [27]. Because the ability of a bone to resist fracture depends not only on bone mass, but also on factors such as architecture and intrinsic properties of the bone material [28], a deficit in cortical bone quality caused by detrimental changes in architecture and material properties can increase fracture risk independent of reduced BMD [29–31]. In this regard, intracortical remodeling is essential for maintenance of cortical bone quality [32].
Intracortical bone remodeling is initiated by osteoclast-mediated resorption on a quiescent bone surface to form a cutting cone, which is followed spatially and temporally by a closing cone lined by osteoblasts. In a completed osteon, bone fills most of the cavity that was created by the action of osteoclasts, leaving only a Haversian canal [33]. An increase in the overall rate of bone remodeling, or a remodeling imbalance where bone formation fails to keep pace with bone resorption, can result in increased cortical bone porosity and decreased BMD [34]. The effect of heavy alcohol consumption on intracortical bone remodeling rate and balance is unknown. The purpose of this study was to evaluate the specific impact of alcohol on intracortical bone remodeling and cortical porosity in a non-human primate model for chronic heavy alcohol consumption. The study focused on late adolescent to young adult male monkeys to model the human population that is most prone to chronic heavy alcohol consumption [35].
2. Materials & Methods
2.1 Animals
The study population was comprised of a total of 34 late adolescent/young adult (5.4 ± 0.1 years old at initiation of alcohol protocol) male rhesus macaques (Macaca mulatta). Epiphyseal closure in rhesus macaque males is complete by 6.5 years of age [36]. Animals were pooled from 3 cohorts of monkeys selected from a colony born and reared in captivity at the Oregon National Primate Research Center at Oregon Health and Sciences University. The first cohort was sent to necropsy in May 2010 (n = 5 control, 8 alcohol), the second in March 2012 (n = 4 control, 8 alcohol), and the third in July 2012 (n = 4 control, 5 alcohol). All of the animals from each cohort were subjected to the same experimental design (described below). Throughout the study, monkeys were housed individually under constant temperature (20–22 °C) and humidity (65%) and an 11-h light cycle (light 0700–1800 hrs) in a room allowing visual, auditory, and olfactory contact with other monkeys. Body weights were recorded weekly throughout the study. Alcohol intake and blood alcohol concentration data for some of these subjects have been published elsewhere [37, 38].
2.2 Experimental Design
Induction phase
The experimental design is described in detail elsewhere [39]. Briefly, monkeys were trained to self-administer food and beverage (using an operant panel integrated into the side of their cage) and to submit their leg for blood sampling. Monkeys in the treatment group were then induced to drink increasing volumes of an alcohol solution (4% w/v ethanol mixed in deionized water) in a step-wise fashion over 4 consecutive 30-day periods for a total of 120 days. To induce drinking, a 1 g flavored food pellet was delivered every 5 min and water was the only fluid available. After water consumption became associated with the delivery of the pellet, the monkeys underwent a 30-day session where water was the only drinking fluid provided. During the second 30-day interval, animals drank a predetermined volume of alcohol solution corresponding to 0.5 g/kg/d alcohol, followed by volumes of alcohol corresponding to 1.0 and 1.5 g/kg/d during the third and fourth 30-day intervals, respectively. Monkeys were allowed to drink only 4% w/v alcohol until the required dose of alcohol was reached (e.g., 0.5 g/kg/d), at which point animals were allowed to drink only water. This step-wise increase in alcohol induction was done to circumvent alcohol taste aversion and increase the opportunity to associate drinking alcohol with its intoxicating effects.
Voluntary drinking phase
Following the 120-day induction, monkeys in the alcohol group (n = 21) were given simultaneous access to both water and alcohol (4% w/v) and allowed to voluntarily self-administer alcohol and/or water for 22 h/d (1100–0900 hrs each day), 7 d/wk for 12 months. Control animals (n = 13) were allowed to self-administer a volume of maltose-dextrin solution isocaloric to the mean volume of alcohol consumed by the alcohol group. Consumption was recorded daily by using weighing scales (Ohaus Corp., Parsippay, NJ) to measure the change in the mass of containers dispensing the solutions.
Blood samples were collected every 5 days from the saphenous vein of monkeys in the alcohol group just before the lights were turned off (between 1800 and 1900 hrs), which corresponded to 7 h into the 22 h sessions. Blood samples were sealed in airtight vials containing 0.5 ml of distilled water and 0.02 ml of 10% isopropanol (internal standard), and stored at −4°C until analysis.
Food consisted of 1g banana-flavored pellets (carbohydrate, 63%; fat, 4%; protein, 22%; PJ Noyes, Lancaster, NH). Over the 12-month duration of the experiment, the monkeys were required to eat their daily allotment of food in no fewer than 3 “meals,” with at least 2 hours between each meal. A meal was defined by the proportion of daily food allotted to each monkey and the pace of the animal to obtain the food. The meal ended if one-third of the daily food allotment was obtained at a time, or if the monkey took longer than 2 minutes to obtain a pellet.
Fluorochrome tetracycline hydrochloride (20 mg/kg) was administered orally (17 and 3 days) prior to sacrifice for determination of active mineralization sites and rates of bone formation. At necropsy, the right tibia with attached fibula was harvested from each animal, placed into 70% alcohol, and stored at 4°C until analysis.
2.3 Blood Alcohol Concentrations
Blood alcohol concentrations were assayed in plasma using a gas chromatograph (Hewlett-Packard 5890 Series II, Avondale, PA) equipped with a headspace autosampler, flame ionization detector, and a Hewlett Packard 3392A integrator.
2.4 Blood Marker of Bone Resorption
Carboxyterminal cross-linking telopeptide of type 1 collagen (CTx), a marker of bone resorption, was measured in samples collected at necropsy using Serum CrossLaps ELISA (Immunodiagnostic Systems, Fountain Hills, AZ).
2.5 Blood Vitamin D
25-Hydroxyvitamin D was measured in plasma samples collected at necropsy using an electrochemiluminescence binding assay (Roche Diagnostics, Indianapolis, IN).
2.6 Dual-Energy X-Ray Absorptiometry
Bone mineral content (BMC, g), bone area (cm2), and areal BMD (g/cm2) in tibia/fibula (the tibia and fibula of each animal were analyzed together) were determined post mortem using a dual-energy X-ray absorptiometry (DXA) scanner (Hologic Discovery A, Waltham, MA) and Hologic APEX System Software, Version 3.1.1. In addition to analysis of total tibia/fibula, a subregion in the distal tibia diaphysis (~2.5 mm in length, enclosing the region evaluated by μCT and histomorphometry; please see below) was evaluated. Quality control check was performed against the Anthropomorphic Spine Phantom and Small Animal Step Phantom provided by the manufacturer. The coefficient of variation evaluating test-retest reliability for DXA scans in our laboratory is 1.0% for BMC, area, and areal BMD. The least significant difference is 0.003–0.006 g/cm2, depending on skeletal site, at the 95% confidence level.
2.7 Microcomputed Tomography
Microcomputed tomography (μCT) was used for nondestructive 3-dimensional evaluation of cortical bone architecture and porosity in tibial diaphysis. Tibial length was measured as the distance between the proximal tip of the intercondylar eminence and the distal tip of the medial malleolus. The distal third of the tibia was then excised using an IsoMet® Low Speed Saw (Buehler, Lake Bluff, IL) and scanned in 70% ethanol at a voxel size of 30 × 30 × 30 μm (55 kVp, 145 μA, and 200 ms, 500 projections/rotation) on a Scanco μCT40 scanner (Scanco Medical AG, Basserdorf, Switzerland). Evaluations were conducted with filtering parameters sigma and support set to 0.8 and 1, respectively. Thirty-three consecutive slices (1.0 mm) of cortical bone (at proximal end of the distal third of the tibia) were analyzed at a threshold of 245 (gray scale of 0–1000) determined empirically. This threshold corresponds to 374 mg hydroxyapatite/cm3. Cortical measurements included (1) cross-sectional tissue volume (cortical and marrow volume, mm3), (2) cortical volume (mm3), (3) marrow volume (mm3), (4) cortical thickness (mm), and (5) polar moment of inertia (an estimate of bone strength in torsion, mm4).
Subsequently, a 1-mm thick cross-section of bone (for further μCT evaluation) and a 50-μm thick cross-section of bone (for histomorphometric evaluation) were removed from the proximal end of the scanned portion of the tibia using the IsoMet® Low Speed Saw. The 1-mm thick section was cut into 4 quadrants (sectioned at 45° angles to the cranial-caudal and medial-lateral axes to generate cranial, lateral, caudal, and medial quadrants) and each quadrant was scanned at a voxel size of 6 × 6 × 6 μm to provide adequate resolution for assessment of intracortical porosity. Cutting the cross-section into quadrants was essential due to constraints on specimen size for scanning at 6 μm voxel size. Sixty-eight consecutive slices (408 μm) were evaluated in each quadrant. Inverse thresholding was used to create an image of the canal size and distribution throughout the bone specimen. Direct measurements included cortical porosity (canal volume/bone volume, %), canal thickness (μm), canal number (mm−1), and canal spacing (μm). Results are reported for each quadrant and as a mean for the 4 quadrants.
2.8 Quantitative Bone Histomorphometry
The 50-μm thick cross-sections were ground on a roughened glass surface (using 220-grit aluminum oxide powder) to an approximate thickness of 25 μm for histomorphometric evaluation. Fluorochrome-based measurements of intracortical bone remodeling included (1) labeled osteon density (number of single- and double-labeled osteons/bone area, number/mm2), (2) mineral apposition rate (the distance between two fluorochrome markers that comprise a double label in an osteon divided by the 14-d interval between consecutive labels, μm/d), (3) bone formation rate (osteonal double + ½ single labeled perimeter multiplied by mineral apposition rate and expressed per bone area, %/year), and (4) formation period (osteonal wall thickness/mineral apposition rate, d). Activation frequency is not reported because it approached 0 in the alcohol group. In addition, the number and size (area bounded by a cement line) of incomplete osteons (i.e., actively remodeling osteons where the formation phase of the remodeling cycle has not been completed) was determined. The osteon count was normalized to bone area (number/mm2).
Fluorochrome-based measurements of endocortical and periosteal bone formation included (1) mineralizing perimeter (mineralizing perimeter/bone perimeter: bone perimeter covered with double plus half single label normalized to bone perimeter, %), (2) mineral apposition rate (the distance between two fluorochrome markers that comprise a double label divided by the 14-d interlabel interval, μm/day), and (3) bone formation rate (bone formation rate/bone perimeter: calculated by multiplying mineralizing perimeter by mineral apposition rate normalized to bone perimeter, μm2/μm/y).
All histomorphometric data were collected using an Olympus BH2 Microscope (Olympus, Shinjuku, Tokyo, Japan) equipped with an Olympus DP71 microscope digital camera (Olympus, Shinjuku, Tokyo, Japan) and attached to a computer system with OsteoMeasure software (OsteoMetrics, Atlanta, GA).
2.9 Statistical Analysis
A two-tailed t-test was used to evaluate the effects of alcohol on bone mass, cortical architecture, and parameters of intracortical bone remodeling. A Wilcoxon rank sum test was used when t-test assumptions of normality or homogeneity of variance were violated. Data analysis was conducted using R version 2.12 (R Development Core Team, 2010). A p-value of ≤ 0.05 was considered significant. All data are expressed as mean ± SE.
3. Results
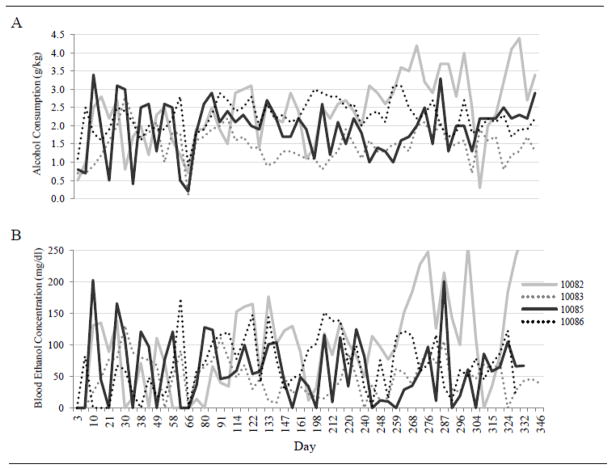
Characteristics (age, body weight, alcohol consumption, and blood alcohol concentration) of the study population are shown in Table 1. There were no significant differences in age or body weight between control and alcohol-consuming monkeys. By design, monkeys in the control group did not consume alcohol. Monkeys in the alcohol group consumed an average of 2.7 g/kg/d over the 12-month study, resulting in an average blood alcohol concentration (measured prior to lights out) of 77 mg/dl. Blood alcohol concentration varied considerably when measured every fifth day (Figure 1), modeling a binge-drinking pattern of alcohol consumption [40–42].
Table 1.
Study Population
| Control | Alcohol | t test P | |
|---|---|---|---|
|
|
|
||
| Age (years) | 6.7 ± 0.3 | 6.7 ± 0.2 | NS |
| Body weight (kg) | 8.5 ± 0.5 | 8.7 ± 0.2 | NS |
| Alcohol consumption (g/kg/d) | - | 2.7 ± 0.2 | |
| Blood alcohol concentration (mg/dL) | - | 77 ± 9 | |
Data are mean ± SE
Figure 1.
Variation in daily alcohol consumption (A) and corresponding blood alcohol concentrations taken every fifth day at seven hours following onset of session (B) over the 12-month duration of study in four representative male rhesus macaques.
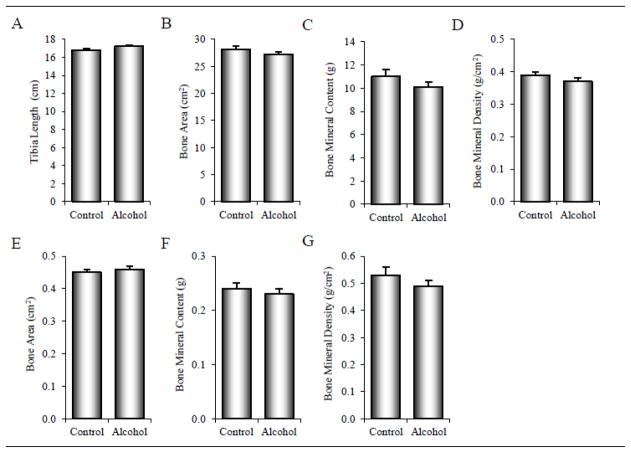
The effects of alcohol consumption on tibial length, total tibia/fibula bone mass and density, and bone mass and density in a subregion of the distal tibia enclosing the region evaluated by μCT and histomorphometry are shown in Figure 2. Significant differences between control and alcohol-consuming monkeys were not detected for any measured or derived parameters.
Figure 2.
Effects of 12 months of chronic alcohol consumption on tibial length (A), total tibia/fibula bone area (B), bone mineral content (C), and bone mineral density (D) and on bone area (E), bone mineral content (F), and bone mineral density (G) in a subregion of the distal tibia enclosing the region evaluated by μCT and histomorphometry in male rhesus macaques. Data are mean ± SE.
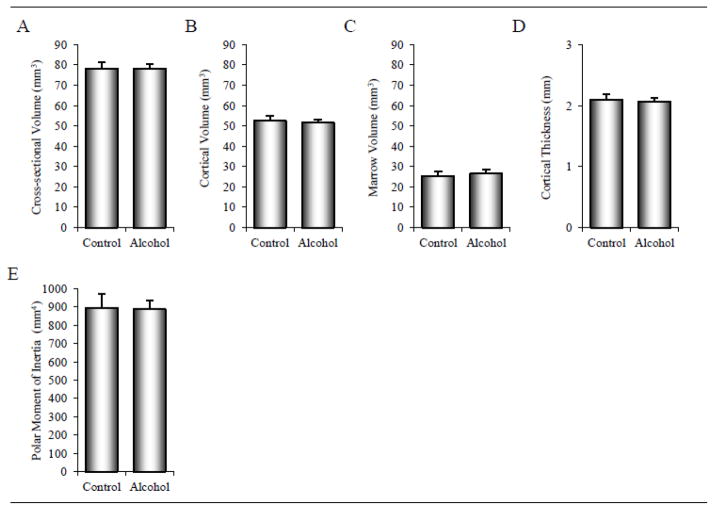
The effects of alcohol consumption on cortical bone architecture in the tibial diaphysis are shown in Figure 3. Significant differences between control and alcohol groups were not detected for cross-sectional volume (A), cortical volume (B), marrow volume (C), cortical thickness (D), or polar moment of inertia (E).
Figure 3.
Effects of 12 months of chronic alcohol consumption on tibial diaphysis cross-sectional volume (A), cortical volume (B), marrow volume (C), cortical thickness (D), and polar moment of inertia (E) in male rhesus macaques. Data are mean ± SE.
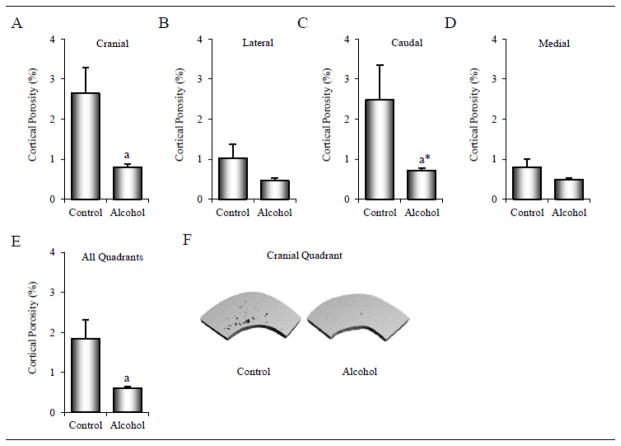
The effects of alcohol consumption on cortical porosity in the tibial diaphysis are shown in Figure 4 and Table 2. Cortical porosity was 68% lower (2.6 ± 0.6% versus 0.8 ± 0.1%, p = 0.015) in the cranial quadrant (A) and tended to be lower (71%, 2.5 ± 0.9% versus 0.7 ± 0.1%, p = 0.062) in the caudal quadrant (C) in the alcohol group compared to the control group. Significant differences in porosity between control and alcohol-consuming monkeys were not detected in either the lateral (B) or medial (D) quadrants. Cortical porosity for the entire cross-section (4 quadrants combined, E) was 67% lower (1.8 ± 0.5% versus 0.6 ± 0.1%, p = 0.021) in the alcohol-consuming monkeys compared to the control monkeys. The lower cortical porosity in the cranial quadrant was associated with lower canal thickness and number and higher canal spacing (Table 2). Canal thickness was also lower in the alcohol-consuming monkeys compared to control monkeys in the lateral and caudal quadrants and in total cross-section (the four quadrants combined). μCT images illustrating differences in cortical porosity in a representative cranial quadrant from a control and an alcohol-consuming monkey are shown in Figure 4F.
Figure 4.
Effects of 12 months of chronic alcohol consumption on cortical porosity in the tibial diaphysis in male rhesus macaques. Tibial cross-sections were divided into four quadrants and porosity assessed in the cranial (A), lateral (B), caudal (C), and medial quadrants (D) and as a mean for the 4 quadrants (E). Representative μCT images (6 × 6 × 6 μm voxel size) of cranial quadrants depicting cortical porosity are shown for a control monkey and an alcohol-consuming monkey (F). Data are mean ± SE. aDifferent from Control, p < 0.05; a*p < 0.1.
Table 2.
Effects of 12 months of chronic alcohol consumption on canal thickness, number, and spacing in the cranial, lateral, caudal, and medial quadrants and in the total cross-section (four quadrants) of the tibial diaphysis.
| Control | Alcohol | t test P | |
|---|---|---|---|
|
|
|
||
| Cranial quadrant | |||
| Canal thickness (μm) | 65 ± 8 | 32 ± 2 | 0.002 |
| Canal number (mm−1) | 3.8 ± 0.08 | 3.6 ± 0.04 | 0.016 |
| Canal spacing (μm) | 271 ± 4 | 284 ± 3 | 0.027 |
| Lateral quadrant | |||
| Canal thickness (μm) | 45 ± 9 | 22 ± 1 | 0.023 |
| Canal number (mm−1) | 3.5 ± 0.02 | 3.5 ± 0.02 | NS |
| Canal spacing (μm) | 290 ± 2 | 291 ± 2 | NS |
| Caudal quadrant | |||
| Canal thickness (μm) | 56 ± 11 | 25 ± 1 | 0.018 |
| Canal number (mm−1) | 3.7 ± 0.06 | 3.6 ± 0.03 | NS |
| Canal spacing (μm) | 279 ± 4 | 283 ± 3 | NS |
| Medial quadrant | |||
| Canal thickness (μm) | 34 ± 7 | 23 ± 1 | NS |
| Canal number (mm−1) | 3.5 ± 0.03 | 3.5 ± 0.05 | NS |
| Canal spacing (μm) | 295 ± 3 | 297 ± 6 | NS |
| All quadrants | |||
| Canal thickness (μm) | 52 ± 7 | 26 ± 1 | 0.003 |
| Canal number (mm−1) | 3.7 ± 0.04 | 3.6 ± 0.03 | 0.06 |
| Canal spacing (μm) | 283 ± 2 | 288 ± 2 | NS |
Data are mean ± SE
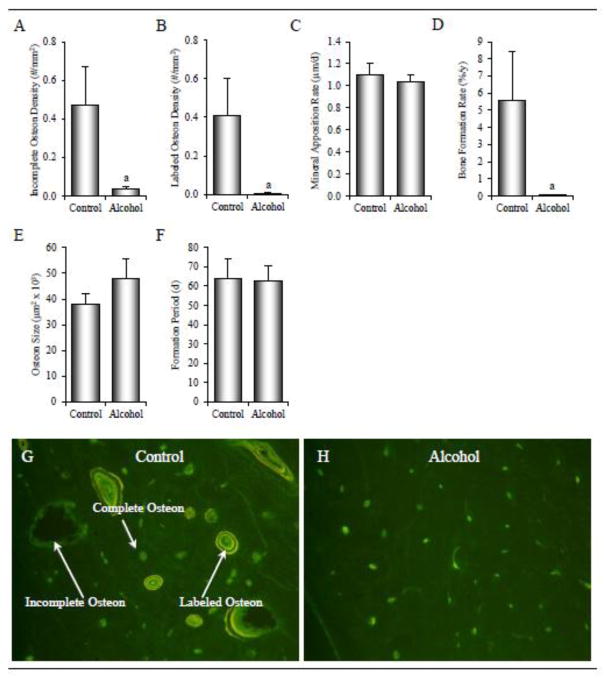
The effects of alcohol consumption on intracortical bone remodeling in the tibial diaphysis are shown in Figure 5. The density of incomplete osteons was 91% lower (0.47 ± 0.2/mm2 versus 0.04 ± 0.01/mm2, p < 0.001) in the alcohol group than in the control group (A). Additionally, labeled osteon density was 98% lower (0.41 ± 0.2/mm2 versus 0.01 ± 0.01/mm2, p < 0.003) in alcohol-consuming monkeys relative to controls (B). Differences in mineral apposition rate were not detected with treatment (C). Bone formation rate was 98% lower (5.6 ± 2.8 %/year versus 0.10 ± 0.00 %/year, p < 0.019) in the alcohol group than the control group (D). Significant differences between control and alcohol-consuming monkeys were not detected for osteon size (E) or formation period (F). Photomicrographs of histological sections illustrating differences in intracortical remodeling in a control and alcohol monkey are shown in Figure 5 (G–H).
Figure 5.
Effects of 12 months of chronic alcohol consumption on tibial diaphysis incomplete osteon density (A), labeled osteon density (B), osteonal mineral apposition rate (C), osteonal bone formation rate (D), osteonal size (E), and osteonal formation period (F) in male rhesus macaques. Photomicrographs depicting intracortical remodeling are shown in a control monkey (E) and an alcohol-consuming monkey (F). Data are mean ± SE. aDifferent from Control, p < 0.05.
The effects of alcohol on bone formation in periosteal and endocortical envelopes in the tibial diaphysis are shown in Figure 6. Periosteal mineralizing perimeter tended to be lower (p < 0.074) in alcohol-consuming in comparison to control monkeys (A). Differences in periosteal mineral apposition rate (B) and bone formation rate (C) were not detected with treatment. Alcohol resulted in 91% lower endocortical mineralizing perimeter (19.6 ± 7.0% versus 1.7 ± 1.4, p < 0.001, D), 47% lower mineral apposition rate (0.86 ± 0.07 versus 0.46 ± 0.06, p = 0.004; E), and 95% lower bone formation rate (6.3 ± 2.4 μm2/μm/y versus 0.3 ± 0.3; p = 0.001, F) in the alcohol group compared to the control group.
Figure 6.
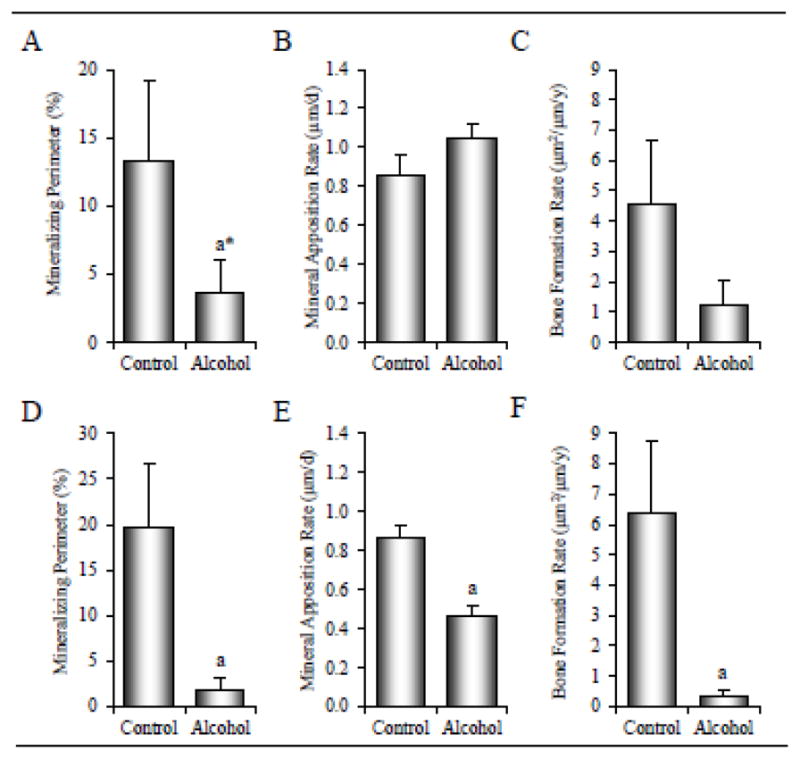
Effects of 12 months of chronic alcohol consumption on tibial diaphysis periosteal mineralizing perimeter (A), mineral apposition rate (B), and bone formation rate (C), and on endocortical mineralizing perimeter (D), mineral apposition rate (E), and bone formation rate (F) in male rhesus macaques. Data are mean ± SE. aDifferent from Control, p < 0.05; a*p < 0.1.
The effects of alcohol consumption on plasma CTx and 25-hydroxyvitamon D are shown in Figure 7. Alcohol-consuming monkeys had 31% lower plasma CTx levels compared to control monkeys (2.5 ± 0.3 ng/ml versus 1.7 ± 0.1 ng/ml, p = 0.028) (A). Significant differences in 25-hydroxyvitamin D were not detected with treatment (B).
Figure 7.
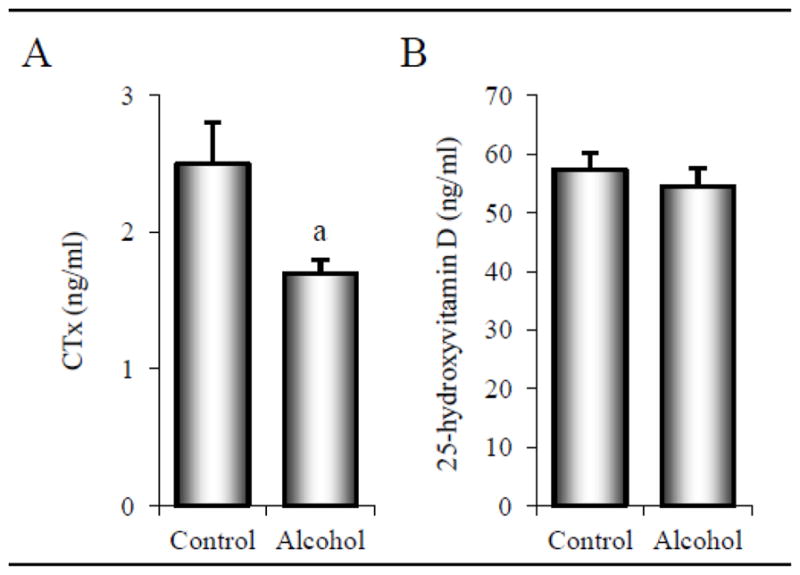
Effects of 12 months of chronic alcohol consumption on plasma levels of CTx (A) and 25-hydroxyvitamin D (B) in male rhesus macaques. Data are mean ± SE. aDifferent from Control, p < 0.05.
4. Discussion
We investigated the effects of 12 months of daily voluntary alcohol consumption on bone density, cortical bone architecture, and intracortical bone remodeling in tibiae of young adult male rhesus macaques. To our knowledge, this is the first study to examine the effects of chronic alcohol consumption on indices of intracortical bone remodeling using an animal model. An average daily alcohol intake of 2.7 ± 0.2 g/kg, resulting in average daily blood alcohol concentration of 77 ± 9 mg/dl, had no effect on tibial BMC and areal BMD nor on cortical bone mass or architecture in tibial diaphysis. In contrast, alcohol consumption resulted in reduced density of fluorochrome-labeled osteons and incomplete osteons, reduced intracortical porosity, and reduced endocortical bone formation. Finally, alcohol consumption resulted in reduced plasma level of the bone resorption marker CTx.
Macaques have greater than 95% gene homology with humans and share many similarities in physiology [35], including bone physiology. Furthermore, attributes of alcohol consumption in macaques closely resembles attributes of alcohol consumption in humans [35]. This includes similar absorption and metabolism of alcohol and the propensity to voluntarily consume large quantities of alcohol [35]. Based on the aforementioned features, macaques should be an excellent model to evaluate the effects of alcohol on the skeleton.
Consistent with prior studies in cynomolgus macaques [39], rhesus macaques in the current study frequently engaged in episodes of binge drinking. Although average daily blood alcohol concentrations were only moderately high, the levels were highly variable, with day-to-day levels ranging from 0 to over 250 mg/dl (a blood alcohol concentration which can result in severe motor impairment and unconsciousness). The patterns of alcohol consumption and the daily blood alcohol concentrations exhibited by the monkeys in this study are similar to drinking patterns and blood alcohol concentrations of alcoholic men given 24 h/d free access to alcohol for 10–60 consecutive days in a hospital ward [40–42].
Bone fracture risk is influenced by a number of parameters including bone mass, architecture, and quality. The findings of the present cross sectional analysis demonstrate that cortical bone mass and architecture in young adult male rhesus macaques were not significantly affected by 15 months (3 month induction phase plus 12 month voluntary drinking phase) of chronic heavy alcohol consumption. Many studies in human alcoholics have associated chronic heavy alcohol consumption with low BMD [3, 4, 43–50]. In the majority, subjects had alcoholism-related comorbidities, such as liver or kidney disease, pancreatitis, smoking, impaired ability to carry out activities of daily living, or poor nutrition, which could have alcohol-independent detrimental skeletal effects [3, 47, 51–53]. Additionally, not all studies, particularly ones that excluded individuals with alcohol-related secondary diseases, have demonstrated an association between chronic heavy alcohol intake and low BMD. Laitinen et al. (1993), for example, reported that an average consumption of 186 ± 85 g/d (~13 standard drinks/d) in women without cirrhosis had no effect on BMD [43]. This level of alcohol consumption would greatly exceed moderate drinking (< 2 standard drinks/d in women). Similar findings have also been reported in men [4]. Although low BMD is a predictor of fracture risk in the general population, the increase in low trauma, as well as all cause fractures in heavy alcohol consumers exceeds values predicted by the extent of bone loss [46, 54]. These findings suggest that chronic heavy alcohol consumption may reduce bone strength by a mechanism that does not necessarily lead to a reduction in BMD.
Intracortical bone formation is normally coupled to bone resorption, suggesting that the reduced intracortical bone formation in alcohol-consuming monkeys may be caused by near-complete suppression of initiation of new secondary osteons. The observed reductions in intracortical porosity, incomplete osteon density, and blood level of CTx, with normal osteonal mineral apposition rate in the alcohol-consuming monkeys support this interpretation as does μCT analysis indicating that the reduction in intracortical porosity was due to reduced canal size; alcohol consumption decreased (by 52%) overall canal size with only a tendency to lower (by 3%) canal number. Alternatively, resorption may have been initiated and terminated prematurely. However, incomplete osteons in the alcohol-consuming monkeys were not smaller nor was the formation interval reduced when compared to controls. Thus, the tissue level reduction in intracortical bone formation observed in alcohol-consuming monkeys was largely due to decreased activation of osteonal bone remodeling.
As evidenced by the decrease in cortical bone porosity, osteons that were in the process of formation prior to dietary intervention were completed by the end of study. This is not surprising because the formation interval (~2 months) of the bone remodeling cycle was much shorter than the treatment interval. Several classes of pharmaceuticals (e.g., bisphosphonates, estrogens, and selective estrogen receptor modulators) preserve bone mass and quality during high turnover states, such as following menopause, by reducing initiation of bone remodeling [55]. Consumption of moderate quantities of alcohol may confer a similar benefit in postmenopausal women [56]. However, highly suppressed intracortical bone remodeling, of the magnitude observed in the present study, is associated with failure to repair microdamage resulting in reduced bone quality [57, 58].
In the present study, the dramatic decrease in cortical bone turnover in the femur was associated with site-specific reductions in porosity. The decrease in porosity was not associated with a change in areal BMD. Thus, prolonged suppression of intracortical bone remodeling is a plausible mechanism by which chronic heavy alcohol consumption could lead to increased fracture risk independent of BMD. If our presumption is correct, conventional screening with DXA may not detect an alcohol-induced loss of bone strength.
Chronic heavy alcohol consumption in humans is consistently associated with reduced biochemical markers of global bone formation [9, 17, 43, 45–47, 59, 60]. However, these markers provide little insight as to region- or compartment-specific skeletal effects. Histomorphometric analysis of biopsies allows for accurate assessment of local bone formation. Analyses of human transiliac biopsies in chronic heavy alcohol consumers show reduced cancellous bone formation [6, 16, 48, 61, 62]. Unfortunately, biopsy studies are uncommon and we are aware of only one study that has reported the effects of alcohol on indices of intracortical bone remodeling [61]. Although the alcoholics in the aforementioned study had reduced cortical thickness associated with increased endocortical bone resorption, all of the chronic alcohol abusers had pancreatitis and many had additional comorbidities that could influence bone metabolism, including poorly controlled type 2 diabetes, iron overload, liver disease and low serum magnesium and vitamin D levels [63]. Thus, the specific effect of chronic heavy alcohol consumption on cortical bone turnover in humans remains to be established.
Rodents have been extremely useful for modeling the effects of alcohol consumption on bone. High levels of alcohol, whether administered continuously in a liquid diet [64, 65], intermittently as a model for binge drinking [66], or by total enteral nutrition [67], have been shown to suppress bone growth in rats. Although small rodents do not normally exhibit intracortical bone remodeling, they do remodel cortical bone at the endocortical surface and several important bone regulating hormones (e.g., estrogen and parathyroid hormone) have been shown to modify marrow cavity volume and cortical bone thickness by regulating endocortical bone formation and resorption [68, 69]. Under certain circumstances, including chronically elevated parathyroid hormone levels, intracortical bone turnover can be induced in rodents, resulting in greatly increased intracortical porosity [70]. Few studies have investigated the effects of alcohol on cortical bone architecture in skeletally mature rodents, but feeding high levels of alcohol (35% caloric intake) to 6-month-old male rats for 16 weeks was shown to have no effect on intracortical porosity [71] or on marrow volume [72]. These findings suggest that as in male rhesus macaques, chronic heavy alcohol consumption does not result in loss of cortical bone in adult male rats.
It is likely that alcohol acts on bone through a combination of mechanisms, both direct and indirect [23–25], although the cellular and molecular mechanisms underlying the skeletal effects of alcohol are not fully understood. High concentrations of alcohol directly affect osteoblasts, osteoclasts, and osteocytes in culture [23]. Furthermore, alcohol may act as an endocrine disruptor by altering levels of and/or skeletal response to vitamin D, sex hormones (e.g., estrogen and testosterone), parathyroid hormone, pituitary-derived hormones (e.g., growth hormone), and adipocyte-derived hormones (e.g., leptin) [24, 25]. Alcohol self-administration lowered dehydroepiandrosterone-sulfate and selectively altered its adrenocortical regulation in rhesus macaques but did not lower testosterone levels [73]. Low 25-hydroxyvitamin D levels, due to poor nutritional status, are often found in chronic alcohol consumers [46, 47]. However, the present study suggests that low 25-hydroxyvitamin D levels are not required for alcohol to suppress intracortical remodeling. Given the effects of alcohol on intracortical bone remodeling observed in the present study, further investigation into the cellular and molecular mechanisms is warranted.
In summary, our results indicate that 12 months of voluntary heavy alcohol consumption in male rhesus macaques had no effect on tibial mass and density or cortical bone architecture. However, this pattern and duration of alcohol consumption resulted in reduced intracortical bone remodeling which resulted in decreased cortical bone porosity. These changes, were they to continue indefinitely, could impact bone quality and lead to reduced bone mechanical properties independent of BMD.
Highlights.
Chronic alcohol abuse is associated with an increase in all cause fracture risk
Effects of 12 months of voluntary alcohol consumption on intracortical bone remodeling were evaluated in young adult male rhesus macaques.
Significant differences in tibial BMC, BMD, and cortical bone architecture in the diaphysis were not detected with treatment.
Labeled osteon density was significantly lower in alcohol-consuming monkeys compared to controls, indicating a lower rate of intracortical bone remodeling.
The results suggest that chronic heavy alcohol consumption may negatively impact bone health, in part, by suppressing intracortical bone remodeling.
Acknowledgments
We thank Teresa Johnson for editorial assistance. This work was supported by NIH grants AA022454 (to UTI) and AA109431, AA13510, and OD11092 (to KAG).
Footnotes
Disclosure summary: The authors have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Berg K. Association between alcohol consumption and both osteoporotic fracture and bone density. Am J Med. 2008;121:406–18. doi: 10.1016/j.amjmed.2007.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chon KS, Sartoris DJ, Brown SA, Clopton P. Alcoholism-associated spinal and femoral bone loss in abstinent male alcoholics, as measured by dual X-ray absorptiometry. Skeletal Radiol. 1992;21:431–6. doi: 10.1007/BF00190985. [DOI] [PubMed] [Google Scholar]
- 3.Gonzalez-Reimers E, Alvisa-Negrin J, Santolaria-Fernandez F, Ros-Vilamajo R, Martin-Gonzalez MC, Hernandez-Betancor I, Garcia-Valdecasas-Campelo E, Gonzalez-Diaz A. Prognosis of osteopenia in chronic alcoholics. Alcohol. 2011;45:227–38. doi: 10.1016/j.alcohol.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 4.Santori C, Ceccanti M, Diacinti D, Attilia ML, Toppo L, D’Erasmo E, Romagnoli E, Mascia ML, Cipriani C, Prastaro A, Carnevale V, Minisola S. Skeletal turnover, bone mineral density, and fractures in male chronic abusers of alcohol. J Endocrinol Invest. 2008;31:321–6. doi: 10.1007/BF03346365. [DOI] [PubMed] [Google Scholar]
- 5.Yuan Z, Dawson N, Cooper GS, Einstadter D, Cebul R, Rimm AA. Effects of alcohol-related disease on hip fracture and mortality: a retrospective cohort study of hospitalized Medicare beneficiaries. Am J Public Health. 2001;91:1089–93. doi: 10.2105/ajph.91.7.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bikle DD, Stesin A, Halloran B, Steinbach L, Recker R. Alcohol-induced bone disease: relationship to age and parathyroid hormone levels. Alcohol Clin Exp Res. 1993;17:690–5. doi: 10.1111/j.1530-0277.1993.tb00821.x. [DOI] [PubMed] [Google Scholar]
- 7.Schnitzler CM, Solomon L. Bone changes after alcohol abuse. S Afr Med J. 1984;66:730–4. [PubMed] [Google Scholar]
- 8.Gonzalez-Calvin JL, Garcia-Sanchez A, Bellot V, Munoz-Torres M, Raya-Alvarez E, Salvatierra-Rios D. Mineral metabolism, osteoblastic function and bone mass in chronic alcoholism. Alcohol Alcohol. 1993;28:571–9. [PubMed] [Google Scholar]
- 9.Labib M, Abdel-Kader M, Ranganath L, Teale D, Marks V. Bone disease in chronic alcoholism: the value of plasma osteocalcin measurement. Alcohol Alcohol. 1989;24:141–4. doi: 10.1093/oxfordjournals.alcalc.a044877. [DOI] [PubMed] [Google Scholar]
- 10.Laitinen K, Lamberg-Allardt C, Tunninen R, Harkonen M, Valimaki M. Bone mineral density and abstention-induced changes in bone and mineral metabolism in noncirrhotic male alcoholics. Am J Med. 1992;93:642–50. doi: 10.1016/0002-9343(92)90197-j. [DOI] [PubMed] [Google Scholar]
- 11.Laitinen K, Lamberg-Allardt C, Tunninen R, Karonen SL, Ylikahri R, Valimaki M. Effects of 3 weeks’ moderate alcohol intake on bone and mineral metabolism in normal men. Bone Miner. 1991;13:139–51. doi: 10.1016/0169-6009(91)90081-a. [DOI] [PubMed] [Google Scholar]
- 12.Laitinen K, Tahtela R, Luomanmaki K, Valimaki MJ. Mechanisms of hypocalcemia and markers of bone turnover in alcohol-intoxicated drinkers. Bone Miner. 1994;24:171–9. doi: 10.1016/s0169-6009(08)80134-1. [DOI] [PubMed] [Google Scholar]
- 13.Nielsen HK, Lundby L, Rasmussen K, Charles P, Hansen C. Alcohol decreases serum osteocalcin in a dose-dependent way in normal subjects. Calcif Tissue Int. 1990;46:173–8. doi: 10.1007/BF02555040. [DOI] [PubMed] [Google Scholar]
- 14.Rico H, Cabranes JA, Cabello J, Gomez-Castresana F, Hernandez ER. Low serum osteocalcin in acute alcohol intoxication: a direct toxic effect of alcohol on osteoblasts. Bone Miner. 1987;2:221–5. [PubMed] [Google Scholar]
- 15.Bikle DD, Genant HK, Cann C, Recker RR, Halloran BP, Strewler GJ. Bone disease in alcohol abuse. Ann Intern Med. 1985;103:42–8. doi: 10.7326/0003-4819-103-1-42. [DOI] [PubMed] [Google Scholar]
- 16.Crilly RG, Anderson C, Hogan D, Delaquerriere-Richardson L. Bone histomorphometry, bone mass, and related parameters in alcoholic males. Calcif Tissue Int. 1988;43:269–76. doi: 10.1007/BF02556634. [DOI] [PubMed] [Google Scholar]
- 17.Diez A, Puig J, Serrano S, Marinoso ML, Bosch J, Marrugat J, Mellibovsky L, Nogues X, Knobel H, Aubia J. Alcohol-induced bone disease in the absence of severe chronic liver damage. J Bone Miner Res. 1994;9:825–31. doi: 10.1002/jbmr.5650090608. [DOI] [PubMed] [Google Scholar]
- 18.Lalor BC, France MW, Powell D, Adams PH, Counihan TB. Bone and mineral metabolism and chronic alcohol abuse. Q J Med. 1986;59:497–511. [PubMed] [Google Scholar]
- 19.Harding A, Dunlap J, Cook S, Mattalino A, Azar F, O’Brien M, Kester M. Osteoporotic correlates of alcoholism in young males. Orthopedics. 1988;11:279–82. doi: 10.3928/0147-7447-19880201-07. [DOI] [PubMed] [Google Scholar]
- 20.Odvina CV, Safi I, Wojtowicz CH, Barengolts EI, Lathon P, Skapars A, Desai PN, Kukreja SC. Effect of heavy alcohol intake in the absence of liver disease on bone mass in black and white men. J Clin Endocrinol Metab. 1995;80:2499–503. doi: 10.1210/jcem.80.8.7629250. [DOI] [PubMed] [Google Scholar]
- 21.Pumarino H, Gonzalez P, Oviedo S, Lillo R, Bustamante E. Assessment of bone status in intermittent and continuous alcoholics, without evidence of liver damage. Rev Med Chil. 1996;124:423–30. [PubMed] [Google Scholar]
- 22.Kanis J, Johnell O, Gullberg B, Allander E, Elffors L, Ranstam J, Dequeker J, Dilsen G, Gennari C, Vaz AL, Lyritis G, Mazzuoli G, Miravet L, Passeri M, Perez Cano R, Rapado A, Ribot C. Risk factors for hip fracture in men from southern Europe: the MEDOS study. Mediterranean Osteoporosis Study. Osteoporos Int. 1999;9:45–54. doi: 10.1007/s001980050115. [DOI] [PubMed] [Google Scholar]
- 23.Turner RT. Skeletal response to alcohol. Alcohol Clin Exp Res. 2000;24:1693–701. [PubMed] [Google Scholar]
- 24.Turner RT, Doran E, Iwaniec UT. Detrimental effects of alcohol on bone growth. In: Lin Y, editor. Osteogenesis. Rijeka, Croatia: InTech; 2012. pp. 57–92. [Google Scholar]
- 25.Maurel DB, Boisseau N, Benhamou CL, Jaffre C. Alcohol and bone: review of dose effects and mechanisms. Osteoporos Int. 2012;23:1–16. doi: 10.1007/s00198-011-1787-7. [DOI] [PubMed] [Google Scholar]
- 26.Court-Brown CM, Bugler KE, Clement ND, Duckworth AD, McQueen MM. The epidemiology of open fractures in adults. A 15-year review Injury. 2012;43:891–7. doi: 10.1016/j.injury.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 27.Nyquist F, Berglund M, Nilsson BE, Obrant KJ. Nature and healing of tibial shaft fractures in alcohol abusers. Alcohol Alcohol. 1997;32:91–5. doi: 10.1093/oxfordjournals.alcalc.a008240. [DOI] [PubMed] [Google Scholar]
- 28.Bouxsein ML, Seeman E. Quantifying the material and structural determinants of bone strength. Best Pract Res Clin Rheumatol. 2009;23:741–53. doi: 10.1016/j.berh.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 29.Nyman JS, Roy A, Tyler JH, Acuna RL, Gayle HJ, Wang X. Age-related factors affecting the postyield energy dissipation of human cortical bone. J Orthop Res. 2007;25:646–55. doi: 10.1002/jor.20337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oei L, Zillikens MC, Dehghan A, Buitendijk GH, Castano-Betancourt MC, Estrada K, Stolk L, Oei EH, van Meurs JB, Janssen JA, Hofman A, van Leeuwen JP, Witteman JC, Pols HA, Uitterlinden AG, Klaver CC, Franco OH, Rivadeneira F. High bone mineral density and fracture risk in type 2 diabetes as skeletal complications of inadequate glucose control: the Rotterdam Study. Diabetes Care. 2013;36:1619–28. doi: 10.2337/dc12-1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tjhia CK, Stover SM, Rao DS, Odvina CV, Fyhrie DP. Relating micromechanical properties and mineral densities in severely suppressed bone turnover patients, osteoporotic patients, and normal subjects. Bone. 2012;51:114–22. doi: 10.1016/j.bone.2012.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gabet Y, Bab I. Microarchitectural changes in the aging skeleton. Curr Osteoporos Rep. 2011;9:177–83. doi: 10.1007/s11914-011-0072-1. [DOI] [PubMed] [Google Scholar]
- 33.Parfitt AM. Osteonal and hemi-osteonal remodeling: the spatial and temporal framework for signal traffic in adult human bone. J Cell Biochem. 1994;55:273–86. doi: 10.1002/jcb.240550303. [DOI] [PubMed] [Google Scholar]
- 34.Bell KL, Loveridge N, Lindsay PC, Lunt M, Garrahan N, Compston JE, Reeve J. Cortical remodeling following suppression of endogenous estrogen with analogs of gonadotrophin releasing hormone. J Bone Miner Res. 1997;12:1231–40. doi: 10.1359/jbmr.1997.12.8.1231. [DOI] [PubMed] [Google Scholar]
- 35.Vivian JA, Green HL, Young JE, Majerksy LS, Thomas BW, Shively CA, Tobin JR, Nader MA, Grant KA. Induction and maintenance of ethanol self-administration in cynomolgus monkeys (Macaca fascicularis): long-term characterization of sex and individual differences. Alcohol Clin Exp Res. 2001;25:1087–97. [PubMed] [Google Scholar]
- 36.Cheverud JM. Epiphyseal union and dental eruption Macaca mulatta. Am J Phys Anthropol. 1981;56:157–67. doi: 10.1002/ajpa.1330560207. [DOI] [PubMed] [Google Scholar]
- 37.Helms CM, Rau A, Shaw J, Stull C, Gonzales SW, Grant KA. The effects of age at the onset of drinking to intoxication and chronic ethanol self-administration in male rhesus macaques. Psychopharmacology (Berl) 2014;231:1853–61. doi: 10.1007/s00213-013-3417-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kroenke CD, Rohlfing T, Park B, Sullivan EV, Pfefferbaum A, Grant KA. Monkeys that voluntarily and chronically drink alcohol damage their brains: a longitudinal MRI study. Neuropsychopharmacology. 2014;39:823–30. doi: 10.1038/npp.2013.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grant KA, Leng X, Green HL, Szeliga KT, Rogers LS, Gonzales SW. Drinking typography established by scheduled induction predicts chronic heavy drinking in a monkey model of ethanol self-administration. Alcohol Clin Exp Res. 2008;32:1824–38. doi: 10.1111/j.1530-0277.2008.00765.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mello NK, Mendelson JH. A quantitative analysis of drinking patterns in alcoholics. Arch Gen Psychiatry. 1971;25:527–39. doi: 10.1001/archpsyc.1971.01750180047009. [DOI] [PubMed] [Google Scholar]
- 41.Mello NK, Mendelson JH. Experimentally induced intoxication in alcoholics: a comparison between programed and spontaneous drinking. J Pharmacol Exp Ther. 1970;173:101–16. [PubMed] [Google Scholar]
- 42.Majchrowicz E, Mendelson JH. Blood concentrations of acetaldehyde and ethanol in chronic alcoholics. Science. 1970;168:1100–2. doi: 10.1126/science.168.3935.1100. [DOI] [PubMed] [Google Scholar]
- 43.Laitinen K, Karkkainen M, Lalla M, Lamberg-Allardt C, Tunninen R, Tahtela R, Valimaki M. Is alcohol an osteoporosis-inducing agent for young and middle-aged women? Metabolism. 1993;42:875–81. doi: 10.1016/0026-0495(93)90063-t. [DOI] [PubMed] [Google Scholar]
- 44.Malik P, Gasser RW, Kemmler G, Moncayo R, Finkenstedt G, Kurz M, Fleischhacker WW. Low bone mineral density and impaired bone metabolism in young alcoholic patients without liver cirrhosis: a cross-sectional study. Alcohol Clin Exp Res. 2009;33:375–81. doi: 10.1111/j.1530-0277.2008.00847.x. [DOI] [PubMed] [Google Scholar]
- 45.Alvisa-Negrin J, Gonzalez-Reimers E, Santolaria-Fernandez F, Garcia-Valdecasas-Campelo E, Valls MR, Pelazas-Gonzalez R, Duran-Castellon MC, de Los Angeles Gomez-Rodriguez M. Osteopenia in alcoholics: effect of alcohol abstinence. Alcohol Alcohol. 2009;44:468–75. doi: 10.1093/alcalc/agp038. [DOI] [PubMed] [Google Scholar]
- 46.Gonzalez-Reimers E, Alvisa-Negrin J, Santolaria-Fernandez F, Candelaria Martin-Gonzalez M, Hernandez-Betancor I, Fernandez-Rodriguez CM, Vina-Rodriguez J, Gonzalez-Diaz A. Vitamin D and nutritional status are related to bone fractures in alcoholics. Alcohol Alcohol. 2011;46:148–55. doi: 10.1093/alcalc/agq098. [DOI] [PubMed] [Google Scholar]
- 47.Santolaria F, Gonzalez-Reimers E, Perez-Manzano JL, Milena A, Gomez-Rodriguez MA, Gonzalez-Diaz A, de la Vega MJ, Martinez-Riera A. Osteopenia assessed by body composition analysis is related to malnutrition in alcoholic patients. Alcohol. 2000;22:147–57. doi: 10.1016/s0741-8329(00)00115-4. [DOI] [PubMed] [Google Scholar]
- 48.Diamond T, Stiel D, Lunzer M, Wilkinson M, Posen S. Ethanol reduces bone formation and may cause osteoporosis. Am J Med. 1989;86:282–8. doi: 10.1016/0002-9343(89)90297-0. [DOI] [PubMed] [Google Scholar]
- 49.Kim MJ, Shim MS, Kim MK, Lee Y, Shin YG, Chung CH, Kwon SO. Effect of chronic alcohol ingestion on bone mineral density in males without liver cirrhosis. Korean J Intern Med. 2003;18:174–80. doi: 10.3904/kjim.2003.18.3.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kouda K, Iki M, Fujita Y, Tamaki J, Yura A, Kadowaki E, Sato Y, Moon JS, Morikawa M, Tomioka K, Okamoto N, Kurumatani N. Alcohol intake and bone status in elderly Japanese men: baseline data from the Fujiwara-kyo osteoporosis risk in men (FORMEN) study. Bone. 2011;49:275–80. doi: 10.1016/j.bone.2011.04.010. [DOI] [PubMed] [Google Scholar]
- 51.Duggan SN, O’Sullivan M, Hamilton S, Feehan SM, Ridgway PF, Conlon KC. Patients with chronic pancreatitis are at increased risk for osteoporosis. Pancreas. 2012;41:1119–24. doi: 10.1097/MPA.0b013e31824abb4d. [DOI] [PubMed] [Google Scholar]
- 52.Matsui T, Yokoyama A, Matsushita S, Ogawa R, Mori S, Hayashi E, Roh S, Higuchi S, Arai H, Maruyama K. Effect of a comprehensive lifestyle modification program on the bone density of male heavy drinkers. Alcohol Clin Exp Res. 2010;34:869–75. doi: 10.1111/j.1530-0277.2010.01159.x. [DOI] [PubMed] [Google Scholar]
- 53.George J, Ganesh HK, Acharya S, Bandgar TR, Shivane V, Karvat A, Bhatia SJ, Shah S, Menon PS, Shah N. Bone mineral density and disorders of mineral metabolism in chronic liver disease. World J Gastroenterol. 2009;15:3516–22. doi: 10.3748/wjg.15.3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kanis JA, Johansson H, Johnell O, Oden A, De Laet C, Eisman JA, Pols H, Tenenhouse A. Alcohol intake as a risk factor for fracture. Osteoporos Int. 2005;16:737–42. doi: 10.1007/s00198-004-1734-y. [DOI] [PubMed] [Google Scholar]
- 55.Marrone JA, Maddalozzo GF, Branscum AJ, Hardin K, Cialdella-Kam L, Philbrick KA, Breggia AC, Rosen CJ, Turner RT, Iwaniec UT. Moderate alcohol intake lowers biochemical markers of bone turnover in postmenopausal women. Menopause. 2012;19:974–9. doi: 10.1097/gme.0b013e31824ac071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Das S, Crockett JC. Osteoporosis - a current view of pharmacological prevention and treatment. Drug Des Devel Ther. 2013;7:435–48. doi: 10.2147/DDDT.S31504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li J, Sato M, Jerome C, Turner CH, Fan Z, Burr DB. Microdamage accumulation in the monkey vertebra does not occur when bone turnover is suppressed by 50% or less with estrogen or raloxifene. J Bone Miner Metab. 2005;23(Suppl):48–54. doi: 10.1007/BF03026323. [DOI] [PubMed] [Google Scholar]
- 58.Mashiba T, Hirano T, Turner CH, Forwood MR, Johnston CC, Burr DB. Suppressed bone turnover by bisphosphonates increases microdamage accumulation and reduces some biomechanical properties in dog rib. J Bone Miner Res. 2000;15:613–20. doi: 10.1359/jbmr.2000.15.4.613. [DOI] [PubMed] [Google Scholar]
- 59.Malik P, Gasser RW, Moncayo R, Kemmler G, Wolfgang Fleischhacker W. Markers of bone resorption and formation during abstinence in male alcoholic patients. Alcohol Clin Exp Res. 2012;36:2059–64. doi: 10.1111/j.1530-0277.2012.01834.x. [DOI] [PubMed] [Google Scholar]
- 60.Nyquist F, Ljunghall S, Berglund M, Obrant K. Biochemical markers of bone metabolism after short and long time ethanol withdrawal in alcoholics. Bone. 1996;19:51–4. doi: 10.1016/8756-3282(96)00110-x. [DOI] [PubMed] [Google Scholar]
- 61.Schnitzler CM, Mesquita JM, Shires R. Cortical and trabecular bone microarchitecture and turnover in alcohol-induced chronic pancreatitis: a histomorphometric study. J Bone Miner Metab. 2010;28:456–67. doi: 10.1007/s00774-009-0151-x. [DOI] [PubMed] [Google Scholar]
- 62.Chappard D, Plantard B, Petitjean M, Alexandre C, Riffat G. Alcoholic cirrhosis and osteoporosis in men: a light and scanning electron microscopy study. J Stud Alcohol. 1991;52:269–74. doi: 10.15288/jsa.1991.52.269. [DOI] [PubMed] [Google Scholar]
- 63.Haaber AB, Rosenfalck AM, Hansen B, Hilsted J, Larsen S. Bone mineral metabolism, bone mineral density, and body composition in patients with chronic pancreatitis and pancreatic exocrine insufficiency. Int J Pancreatol. 2000;27:21–7. doi: 10.1385/IJGC:27:1:21. [DOI] [PubMed] [Google Scholar]
- 64.Turner RT, Aloia RC, Segel LD, Hannon KS, Bell NH. Chronic alcohol treatment results in disturbed vitamin D metabolism and skeletal abnormalities in rats. Alcohol Clin Exp Res. 1988;12:159–62. doi: 10.1111/j.1530-0277.1988.tb00152.x. [DOI] [PubMed] [Google Scholar]
- 65.Sampson HW, Chaffin C, Lange J, DeFee B., 2nd Alcohol consumption by young actively growing rats: a histomorphometric study of cancellous bone. Alcohol Clin Exp Res. 1997;21:352–9. [PubMed] [Google Scholar]
- 66.Lauing K, Himes R, Rachwalski M, Strotman P, Callaci JJ. Binge alcohol treatment of adolescent rats followed by alcohol abstinence is associated with site-specific differences in bone loss and incomplete recovery of bone mass and strength. Alcohol. 2008;42:649–56. doi: 10.1016/j.alcohol.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Brown EC, Perrien DS, Fletcher TW, Irby DJ, Aronson J, Gao GG, Hogue WJ, Skinner RA, Suva LJ, Ronis MJ, Hakkak R, Badger TM, Lumpkin CK., Jr Skeletal toxicity associated with chronic ethanol exposure in a rat model using total enteral nutrition. J Pharmacol Exp Ther. 2002;301:1132–8. doi: 10.1124/jpet.301.3.1132. [DOI] [PubMed] [Google Scholar]
- 68.Turner RT, Evans GL, Sluka JP, Adrian MD, Bryant HU, Turner CH, Sato M. Differential responses of estrogen target tissues in rats including bone to clomiphene, enclomiphene, and zuclomiphene. Endocrinology. 1998;139:3712–20. doi: 10.1210/endo.139.9.6177. [DOI] [PubMed] [Google Scholar]
- 69.Sibonga JD, Iwaniec UT, Shogren KL, Rosen CJ, Turner RT. Effects of parathyroid hormone (1–34) on tibia in an adult rat model for chronic alcohol abuse. Bone. 2007;40:1013–20. doi: 10.1016/j.bone.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 70.Lotinun S, Evans GL, Bronk JT, Bolander ME, Wronski TJ, Ritman EL, Turner RT. Continuous parathyroid hormone induces cortical porosity in the rat: effects on bone turnover and mechanical properties. J Bone Miner Res. 2004;19:1165–71. doi: 10.1359/JBMR.040404. [DOI] [PubMed] [Google Scholar]
- 71.Johnson TL, Gaddini GW, Branscum AJ, Olson DA, Caroline-Westerlind K, Turner RT, Iwaniec UT. Effects of chronic heavy alcohol consumption and endurance exercise on cancellous and cortical bone microarchitecture in adult male rats. Alcohol Clin Exp Res. doi: 10.1111/acer.12366. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Reed AH, McCarty HL, Evans GL, Turner RT, Westerlind KC. The effects of chronic alcohol consumption and exercise on the skeleton of adult male rats. Alcohol Clin Exp Res. 2002;26:1269–74. doi: 10.1097/01.ALC.0000023984.47311.6E. [DOI] [PubMed] [Google Scholar]
- 73.Helms CM, Park B, Grant KA. Adrenal steroid hormones and ethanol self-administration in male rhesus macaques. Psychopharmacology (Berl) 2014;231:3425–36. doi: 10.1007/s00213-014-3590-6. [DOI] [PMC free article] [PubMed] [Google Scholar]