Abstract
Cytokine signals are central to the differentiation of thymocytes and their stepwise progression through defined developmental stages. The intensity and duration of cytokine signals are regulated by the suppressor of cytokine signalling (SOCS) proteins. A clear role for SOCS1 during the later stages of thymopoiesis has been established, but little is known about its role during early thymopoiesis, nor the function of its closest relative, SOCS3. Here, we find that both SOCS1 and SOCS3 are expressed during early thymopoiesis, with expression coincident during the double negative (DN)2 and DN3 stages. We examined thymocyte differentiation in vitro by co-culture of SOCS-deficient bone marrow cells with OP9 cells expressing the Notch ligand Delta-like1 (OP9-DL1). Cells lacking SOCS1 were retarded at the DN3:DN4 transition and appeared unable to differentiate into double positive (DP) thymocytes. Cells lacking both SOCS1 and SOCS3 were more severely affected, and displayed an earlier block in T cell differentiation at DN2, the stage at which expression of SOCS1 and SOCS3 coincides. This indicates that, in addition to their specific roles, SOCS1 and SOCS3 share overlapping roles during thymopoiesis. This is the first demonstration of functional redundancy within the SOCS family, and has uncovered a vital role for SOCS1 and SOCS3 during two important checkpoints in early T cell development.
Keywords: Signal transduction, thymus, T cells, transgenic/knockout mice, thymopoiesis, SOCS proteins
INTRODUCTION
The function of the thymus is to generate T lymphocytes expressing T cell receptors of sufficient diversity to combat challenge by every possible microorganism, while eradicating potentially autoreactive T cells. Thymocyte progenitor cells progressively mature in the thymus through a series of well-defined stages that can be distinguished by differential expression of cell surface markers (Godfrey et al., 1993). Immature double negative (CD4−CD8−, DN) thymocytes can be classified into 4 stages according to their expression of CD25 and CD44; DN1 (CD25− CD44+), DN2 (CD25+ CD44+), DN3 (CD25+ CD44−) and DN4 (CD25− CD44−) (Godfrey et al., 1993). Expression of both CD4 and CD8 is then upregulated (double positive, DP), and those thymocytes surviving selection proceed to the mature single positive (SP) stage and enter peripheral lymphoid tissues. Recently, a culture system has been developed for in vitro differentiation of T cells. Co-culture of progenitor cells with OP9 cells expressing the Notch ligand Delta-like1 (OP9-DL1) has been shown to induce differentiation through all stages of thymopoiesis to SP cells, and is therefore a useful model for studying T lymphopoiesis in vitro (Schmitt and Zuniga-Pflucker, 2002).
Cytokines play an essential role in T cell development, particularly during the early stages of thymopoiesis (Yarilin and Belyakov, 2004). IL-7, in particular, is critical, as T cell development is severely impaired in mice lacking either IL-7 or the IL-7 receptor (Peschon et al., 1994; von Freeden-Jeffry et al., 1995). Cytokines induce highly potent signals that can disrupt immune homeostasis in the absence of appropriate regulation (Fehniger et al., 2001; Kopf et al., 1994; Sadlack et al., 1993; von Freeden-Jeffry et al., 1995). The suppressor of cytokine signalling (SOCS) proteins are key regulators of cytokine signalling (Alexander and Hilton, 2004). Of the eight members of the SOCS family, SOCS1 and SOCS3 appear to be the most important for the regulation of T cell development and function (Fletcher and Starr, 2005). In addition, SOCS1 and SOCS3 are more closely related to each other than to other family members, and share similar functions in vitro, indicating that they may also have overlapping roles in vivo (Starr et al., 1997).
SOCS1 is highly expressed in the thymus (Chong et al., 2003). Overexpression of SOCS1 in T cells in vivo leads to a profound reduction in thymic cellularity, due to a block in development at the TN2:TN3 transition (Fujimoto et al., 2000). This phenotype is reminiscent of mice lacking the common γ chain receptor subunit shared by IL-7, IL-15 and other cytokines (Cao et al., 1995), suggesting that SOCS1, when overexpressed, regulates thymopoiesis by limiting responses to these cytokines. Similarly, studies of SOCS1-deficient mice have suggested that SOCS1 is critical for regulating both IL-7 and IL-15 signalling in the thymus (Chong et al., 2003; Cornish et al., 2003b; Ilangumaran et al., 2003).
Comparatively little is known about the role of SOCS3 during T cell development. SOCS3 is expressed in peripheral T cells and regulates responses to the gp130 cytokines IL-6 and IL-27 (Brender et al., 2007). Overexpression of SOCS3 in vivo has little impact on thymopoiesis, with thymic subsets represented in the appropriate ratio, but overall thymic cellularity is slightly reduced in these mice (Matsumoto et al., 2003).
Here, we assess the roles of SOCS1 and SOCS3 during thymopoiesis, both by analysing early thymic differentiation in SOCS-deficient mice in vivo, and using the OP9-DL1 co-culture system in vitro. We find that despite apparently normal thymopoiesis in vivo in the absence of SOCS proteins, progression through the DN3:DN4 and DN:DP stages of thymopoiesis was retarded in SOCS1-deficient cells in vitro. These defects were compounded by the additional removal of SOCS3, leading to an earlier block in differentiation at the DN2:DN3 transition, a stage at which SOCS1 and SOCS3 are co-expressed. This is the first demonstration of shared functions between SOCS family members, and suggests that both SOCS1 and SOCS3 regulate critical checkpoints during early thymopoiesis.
MATERIALS AND METHODS
Mice
Socs1ΔLck/ΔLck, Socs3ΔLck/ΔLck, Socs1+/−, Socs3+/−, Socs1−/−Ifnγ−/− and Ifnγ−/− (obtained from The Jackson Laboratory, Bar Harbor, ME) mice on a C57BL/6 background have previously been described (Alexander et al., 1999; Brender et al., 2007; Chong et al., 2003; Dalton et al., 1993; Roberts et al., 2001; Starr et al., 1998). Mice floxed at both Socs1 and Socs3 loci were generated by breeding Socs1fl/fl mice with Socs3fl/fl mice to produce progeny containing two floxed alleles at each locus (Socs1fl/flSocs3fl/fl). Mice were routinely housed in clean conventional facilities, and were used for experiments when 3–12 weeks of age. All mouse studies were approved by the St Vincent’s Health Animal Ethics Committee.
PCR to monitor excision of Socs alleles
The conditional and excised Socs1 alleles were identified by PCR of genomic DNA using the following primers and cycling conditions: Socs1, 5′-TTC TGG AAA GCT AGC ACC ACG-3′ (reverse), 5′-GCA TCC CTC TTA ACC CGG TAC-3′ (forward, SOCS1 coding) and 5′-GGT TTA AGA GCC TGA TGC AGG-3′ (forward), 35 cycles at 94°C for 30 s, 59.5°C for 30 s and 72°C for 60 s. PCR of genomic DNA to distinguish conditional and excised Socs3 alleles was performed as described (Croker et al., 2003).
Socs expression
As part of the targeting strategy for both the Socs1−/− and Socs3−/− mice, each Socs gene was replaced by the lacZ reporter gene under control of Socs regulatory sequences (Roberts et al., 2001; Starr et al., 1998). β-galactosidase (β-gal) activity was used, therefore, as a surrogate marker of Socs gene expression, and was analysed by the FACSgal assay. Briefly, single cell suspensions were stained with rat mAbs specific for the cell surface markers of interest (BD Biosciences, San Diego, CA) and then incubated with an equal volume of the β-gal substrate, 2 mM fluorescein di-β-D-galactopyranoside (FDG; Sigma-Aldrich, St. Louis, MO) under hypotonic conditions for 2 min at 37°C, immediately placed on ice, and incubated on ice for 2 hr prior to the addition of propidium iodide (1 μg/ml) and analysis by flow cytometry (Brender et al., 2007). β-gal converts FDG to fluorescein, which accumulates in the cytoplasm and can be detected in the FL1 channel. Single positive (SP) thymic subsets were gated on CD3hi to ensure that expression was assayed in mature thymocytes. The FACSgal assay was performed in parallel with cells from age- and sex-matched C57BL/6 wildtype mice as a control.
Cell sorting and flow cytometry
For analysis of DN thymocyte populations, single cell suspensions of thymi from young (3–6 week old) mice (1 × 107 cells/sample) were incubated on ice for 1 hr with biotinylated rat antibodies specific for CD4 (GK1.5), CD8 (53-6.7), Ly-76 (TER119), B220 (RA3-6B2), Mac1 (M1/70) and Gr1 (RB6-8C5) (BD Biosciences). Cells were then washed and incubated with Dynabeads coated with anti-rat IgG and mature cells depleted using a magnet, according to the manufacturer’s instructions (Dynal Biotech). The enriched DN thymocyte preparation was then stained with fluorochrome-conjugated antibodies specific for CD25 (PC61) and CD44 (IM7), and streptavidin-PerCP (BD Biosciences), washed, and analysed by flow cytometry or DN1-4 subsets sorted using a FACS Aria (BD Biosciences). Mature thymocytes and non-T cells were excluded from the analysis and sorting by gating out PerCP-positive cells. Mature thymocyte subsets were analysed by staining whole thymocyte suspensions with antibodies specific for CD4 and CD8.
Retroviral infection of bone marrow stem cells
Mice were injected i.p. with 150 mg/kg of 5-fluorouracil (Mayne Pharma) and bone marrow (BM) was harvested from femurs 4 days later. Erythrocytes were lysed as described (Cornish et al., 2003b). BM cells were cultured in Stem Cell Medium (SCM; RPMI containing 10% (v/v) fetal bovine serum (FBS), 50 ng/ml Flt3L, 50 ng/ml SCF, 10 ng/ml IL-6, 5 ng/ml IL-3, 1 U/ml penicillin/streptomycin and 1 mM L-glutamine). Cells were cultured at 5×105 cells/ml overnight in a humidified 37°C incubator with 5% CO2.
An MSCV-Cre recombinase-IRES-eGFP plasmid was constructed by ligation of a cDNA fragment encoding Cre recombinase into the MSCV-IRES-eGFP plasmid backbone (Van Parijs et al., 1999). The construct (MCG) was introduced into GP+E86 packaging cells by standard techniques, and high titer producer cell clones (MCG4) were isolated by limiting dilution (Markowitz et al., 1988).
MCG4 producer cells were plated at 4×105 cells/25cm2 flask in RPMI containing 10% (v/v) FBS the day prior to use. BM cells (1.75×106) rested overnight were then co-cultured for 2 days with MCG4 cells expressing Cre retrovirus in 7 ml SCM. BM cells were harvested from the MCG4 monolayer by gentle pipetting, resuspended in 5ml fresh SCM and cultured overnight, prior to co-culture with OP9-DL1 cells.
OP9-DL1 co-cultures
The murine Delta-like 1-transduced OP9 (OP9-DL1) stromal cell line has been described previously (Schmitt and Zuniga-Pflucker, 2002). OP9-DL1 cells were plated at 1×105 cells/well in 6 well plates the day prior to use, or 2×105 cells/well on the day of use, in modified α-MEM medium supplemented with 20% (v/v) FBS, 2mM L-glutamine and 10 μM β-mercaptoethanol. BM cells (1×103–1×104 sorted GFP+ retrovirus-infected cells, or 1×105 cells/well for established cultures or unsorted BM) were co-cultured with OP9-DL1 cells in 3 ml of modified α-MEM medium supplemented with 0.2 ng/ml IL-7 and 5 ng/ml Flt3L.
Haemopoietic cells were harvested periodically from the OP9-DL1 stromal layer by gentle pipetting and subcultured onto freshly prepared OP9-DL1 cell layers. GFP+ cells expressing the retroviral Cre construct were sorted within the first 6 days of co-culture. Lin− cells were sorted within 20 days of co-culture by staining with antibodies specific for mature cells, as described above. For periodic monitoring of differentiation, haemopoietic cells were distinguished from stromal cells by staining with anti-CD45, and mature thymocytes and residual non-T cells were excluded by staining with antibodies specific for CD3, CD4, CD8, TER119, B220, Mac1 and Gr1. Double negative and mature thymocyte populations were analysed as described above. Dead cells were excluded by staining with Sytox blue (Invitrogen).
RESULTS
Expression of SOCS1 and SOCS3 is coincident during early thymopoiesis
The expression of SOCS1 in the thymus has been well characterised. SOCS1 is expressed throughout thymocyte development, with expression highest in the DP subset (Chong et al., 2003; Trop et al., 2001). Although SOCS3 expression has been studied in peripheral T lymphocytes and during T cell activation (Matsumoto et al., 2003; Yu et al., 2003), little is known of SOCS3 expression during thymopoiesis. We used SOCS1+/− and SOCS3+/− mice to examine the expression of SOCS1 and SOCS3 during early thymic development. In these mice, which are healthy and have no apparent abnormalities (Roberts et al., 2001; Starr et al., 1998), the lacZ reporter gene is under the control of the Socs1 or Socs3 promoter, respectively, and thus provides a surrogate marker of Socs gene expression. Immature DN (CD4−CD8−) thymocyte populations were divided into DN1-4 subsets based on CD44 and CD25 expression (Godfrey et al., 1993) (Figure 1A), and β-galactosidase activity of each gated subset was analysed. Unlike SOCS1, which was expressed uniformly during DN2-4 (Figure 1B) (Chong et al., 2003; Trop et al., 2001), SOCS3 was expressed only in DN2 and DN3, with expression declining to baseline in DN4 and SOCS3 expression was only upregulated again in mature T cells in the periphery (Figure 1C and data not shown). Expression of SOCS1 and SOCS3, therefore, was coincident in the thymus only during DN2 and DN3 stages of early thymopoiesis.
Figure 1. Expression of SOCS1 and SOCS3 in early thymocytes.

(A) Flow cytometric analysis of DN subsets from wt thymus, showing the gates used for each subset. The expression of Socs1 (B) and Socs3 (C) in DN thymocytes from Socs1+/− or Socs3+/− mice, respectively, was monitored by measuring β-gal activity (filled histograms). The background β-gal activity in wt thymocytes is indicated by the black line in each analysis (B,C). Data are representative of 3 mice in each group.
Early thymopoiesis in vivo is unaffected by the absence of SOCS1 or SOCS3
To assess whether the expression of SOCS1 and SOCS3 during early thymopoiesis is critical for normal T cell development in vivo, we analysed the proportion of different thymocyte subsets in Socs1ΔLck/ΔLck and Socs3ΔLck/ΔLck mice, in which there is Cre-mediated deletion of Socs1 or Socs3, respectively, during early thymopoiesis. Both Socs1ΔLck/ΔLck and Socs3ΔLck/ΔLck mice are healthy and have normal lifespans (Brender et al., 2007; Chong et al., 2003). Socs1ΔLck/ΔLck, Socs3ΔLck/ΔLck and wt mice showed similar frequencies of DN1-4 subsets, suggesting that neither SOCS1 nor SOCS3 are critical regulators of the TN stages of thymocyte development at homeostasis in vivo (Figure 2A). The proportion of mature thymocytes was similar in Socs1ΔLck/ΔLck, Socs3ΔLck/ΔLck mice and wt mice, with the exception that Socs1ΔLck/ΔLck mice showed enhanced differentiation of CD8+ T cells (Figure 2B), most likely due to unregulated IL-7 signalling (Chong et al., 2003).
Figure 2. Early thymopoiesis is normal in Socs1ΔLck/ΔLck and Socs3ΔLck/ΔLck mice.
(A) The DN profile of thymocytes from wt, Socs1ΔLck/ΔLck and Socs3ΔLck/ΔLck mice was generated by exclusion of mature thymocytes, and analysis of CD44 and CD25 expression on the resulting gated progenitor T cell population. (B) The mature thymocyte subsets of the mice used in (A) are shown. Profiles shown are representative of 3 mice of each genotype. Numbers indicate the percentage of cells in each quadrant or gated population. Sorted DN2-4 subsets from resting Socs1ΔLck/ΔLck (C) or Socs3ΔLck/ΔLck (D) mice were genotyped by PCR to determine the efficiency of Cre-mediated excision of the conditional Socs loci. The conditional (fl) and excised (Δ) alleles are indicated. C, control DNA
In these mice, Cre expression is driven by the Lck proximal promoter. Although Lck mRNA is found in all DN thymocyte subsets, Lck protein is undetectable in DN1 and DN2 subsets, but is upregulated at least 20-fold between DN2 and DN3 (Buckland et al., 2000). Similarly, in transgenic mice where expression of green fluorescent protein (GFP) is driven by the Lck promoter, GFP protein is undetectable until the DN2:DN3 transition (Buckland et al., 2000). It is likely, therefore, that excision of the Socs alleles would be minimal at earlier DN stages in these mice. To assess this, we sorted cells at DN stages 2–4 by flow cytometry and, using PCR, determined the degree to which excision of the loci had occurred. As shown in Figure 2C, excision of the Socs1 locus was initiated during the DN3 stage and was essentially complete by DN4, whereas excision of the Socs3 locus was not observed at DN3 but was mostly complete by DN4 (Figure 2D). Thus, study of early thymopoiesis using the Lck promoter to drive expression of the Cre transgene in vivo is problematic.
Perturbed thymopoiesis in vitro in the absence of SOCS1 and SOCS3
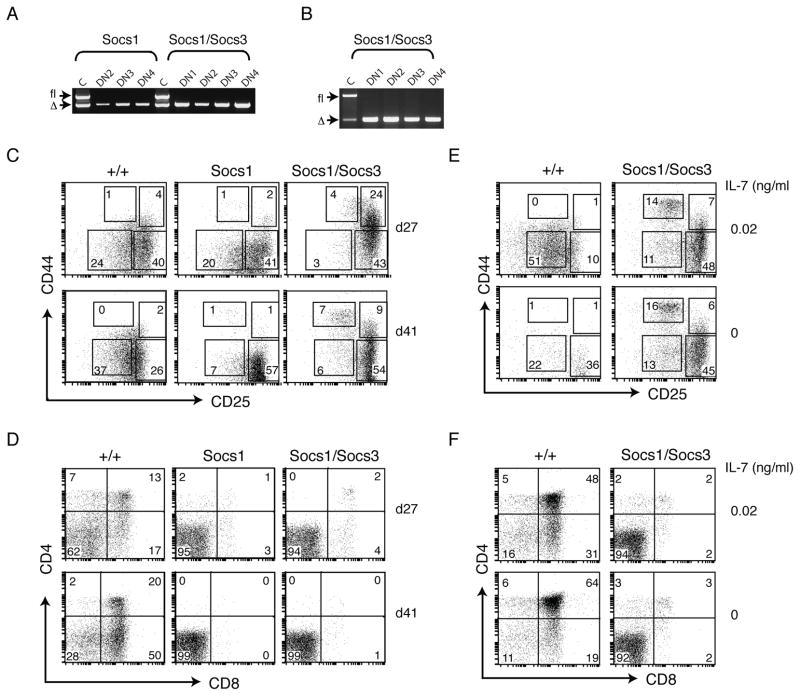
In order to overcome the difficulties analyzing early T cell development with Lck-mediated deletion of Socs alleles, and to determine whether SOCS1 and SOCS3 have overlapping roles during early thymopoiesis, we designed a strategy of retroviral-mediated deletion of Socs alleles in bone marrow progenitor cells, followed by in vitro differentiation by co-culture with OP9-DL1 cells. Wt, Socs1fl/fl, Socs3fl/fl and Socs1fl/flSocs3fl/fl mice were injected with the cytotoxic drug 5-fluorouracil (5-FU) to mobilize stem cells in the bone marrow. Bone marrow cells were co-cultured with packaging cells expressing a retroviral Cre construct. Lin− cells expressing Cre were then co-cultured with OP9-DL1 cells and differentiation was monitored periodically by flow cytometry. Using this strategy, excision of the Socs alleles was highly efficient, even at the earliest DN stages (Figure 3A,B).
Figure 3. Both SOCS1 and SOCS3 regulate early thymocyte differentiation in OP9-DL1 co-cultures.
(A,B) Retroviral Cre-mediated excision of Socs alleles is highly efficient. Cre+ Lin− DN cells of the indicated genotypes were sorted and genotyped by PCR to assess the efficiency of Socs allele excision. Bands corresponding to the conditional (fl) and excised (Δ) alleles of Socs1 (A) and Socs3 (B) are indicated. C, control DNA. (C) Cre+ Lin− progenitor cells were cultured on OP9-DL1 stroma in 0.2 ng/ml IL-7 for the indicated times, stained with Ab to CD25 and CD44 and analysed by flow cytometry. Numbers indicate the percentage of Lin− cells in each DN subset. (D) Flow cytometry analysis of cultures shown in (C), stained with Ab to CD4 and CD8, showing the mature thymocyte subsets. Data are representative of two independent experiments showing similar results. (E,F) Cre+ Lin− progenitor wt and SOCS1ΔSOCS3Δ cultures shown in (C,D) were co-cultured for 37 days on OP9-DL1 stroma in the presence of 0.2 ng/ml IL-7, then washed free of IL-7 and co-cultured for a further 4 days on OP9-DL1 either in the presence of 0.02 ng/ml IL-7, or without added IL-7. Cells were stained with Abs to either CD25 and CD44 (E) or CD4 and CD8 (F), showing DN or mature thymic subsets, respectively. Numbers indicate the percentage of cells in each subset.
Although progression of SOCS3-deficient (SOCS3Δ) cells through the DN stages was comparable with that of wt cells (data not shown), there were marked differences in the differentiation of SOCS1-deficient (SOCS1Δ) cells. At day 27 of culture, the proportion of each DN subset was similar between wt and SOCS1Δ samples (Figure 3C). Unlike wt cells, however, very few SOCS1Δ cells had differentiated into CD4+CD8+ DP thymocytes, suggesting that development at this stage of thymopoiesis is perturbed in the absence of SOCS1 (Figure 3D). After 41 days of culture, the development of SOCS1Δ DN3 cells appeared to be delayed or blocked, with a substantially reduced proportion of cells progressing to DN4 compared to wt cells (Figure 3C). Alternatively, this result could reflect reduced viability of the DN4 cells as they fail to differentiate into DP cells.
To investigate whether SOCS1 and SOCS3 have shared roles during early T cell development that may be masked by deletion of either regulator on its own, we assessed T cell differentiation in cells lacking both SOCS1 and SOCS3 (SOCS1ΔSOCS3Δ). Deletion of both Socs genes resulted in a block in differentiation at an even earlier stage than cells lacking SOCS1 alone, with progression from DN2 to DN3 retarded in SOCS1ΔSOCS3Δ cells (Figure 3C). This defect was apparent earlier than SOCS1Δ cells, from day 27 of culture. Similar to SOCS1Δ cells, very few SOCS1ΔSOCS3Δ cells had progressed to DN4 and DP stages (Figure 3C,D). In view of the coincident expression of SOCS1 and SOCS3 during DN2 and DN3, this result suggests that in addition to a non-redundant role for SOCS1 during the DN3:DN4 transition, regulation of the DN2:DN3 transition is crucially shared by SOCS1 and SOCS3.
IL-7 has been shown to be important for increasing thymocyte progenitor survival and growth (von Freeden-Jeffry et al., 1995). Although the inclusion of IL-7 in cultures promotes proliferation of thymocyte progenitors early in the culture period, high IL-7 levels inhibit differentiation at both the DN2:DN3 and DP stages of development (Balciunaite et al., 2005; DeLuca and Clark, 2002; El Kassar et al., 2004; Huang et al., 2005). Since SOCS1 negatively regulates IL-7 signalling (Chong et al., 2003; Cornish et al., 2003a; Ramanathan et al., 2006; Yu et al., 2006), we wished to investigate whether the block in early thymocyte differentiation in the absence of SOCS1 was due to hypersensitivity to IL-7.
OP9-DL1 co-cultures of wt and SOCS1ΔSOCS3Δ cells maintained for 37 days in 0.2 ng/ml IL-7 were washed free of IL-7, divided into three separate cultures and co-cultured for a further 4 days with OP9-DL1 in either 0.2 or 0.02 ng/ml IL-7, or in the absence of IL-7 (compare Figure 3C,D, day 41 cultured in 0.2 ng/ml IL-7 with Figure 3E,F, day 41 cultured in 0.02 and 0 ng/ml IL-7). Withdrawal of IL-7 led to an increase in the differentiation of cells from DN3 to DN4. This occurred to a greater extent in the wt cultures (0.2 compared with 0.02 ng/ml IL-7), and did not substantially alleviate the DN3:DN4 block in SOCS1ΔSOCS3Δ cultures (Figure 3E). In the absence of IL-7 there were very few cells remaining at the DN stages, particularly in wt cultures, which may result from reduced viability of immature thymocytes in the absence of IL-7, enhanced differentiation into DP cells, or a combination of the two (Figure 3E). Indeed, the proportion of DP cells in wt cultures was substantially increased by IL-7 withdrawal (Figure 3F). There was only a minor increase in the proportion of DP cells in SOCS1ΔSOCS3Δ cultures deprived of IL-7, indicating that IL-7 withdrawal was unable to rescue the block in differentiation at this stage in these cells (Figure 3F). Withdrawal of IL-7 also affected cell proliferation or survival, with total numbers of live thymocytes in the cultures decreasing with reduced IL-7 concentrations (data not shown). Thus, perturbed thymocyte differentiation in the absence of SOCS proteins in OP9-DL1 co-cultures is unlikely to result solely from hypersensitivity to IL-7.
Lymphoid progenitors from SOCS1−/− mice show perturbed thymopoiesis in vitro
Mice lacking SOCS1 die as neonates from a severe inflammatory disorder that can be rescued by deriving the mice on an IFNγ-deficient background (Alexander et al., 1999; Starr et al., 1998). Early thymopoiesis has been shown to occur normally in Socs1−/−Ifng−/− mice in vivo (Cornish et al., 2003b; Ilangumaran et al., 2003). To verify that SOCS1-deficient lymphoid progenitors have altered thymopoiesis in vitro, we co-cultured BM cells from Socs1−/−Ifng−/− mice with OP9-DL1 stromal cells. With these samples, differentiation on OP9-DL1 stroma was more rapid than for the retrovirus-infected BM cells, which required sorting Cre+ cells prior to co-culture, since larger numbers of unfractionated BM could be plated initially. Similar to cells with Cre-mediated Socs1 deletion, differentiation of Socs1−/−Ifng−/− lymphoid progenitors appeared normal at earlier culture times (day 6, 13, Figure 4A) but after this time progression from DN3 to DN4 was retarded compared to Ifng−/− controls (day 16, Figure 4A). The ability of SOCS1-deficient DN cells to progress to the DP stage was similarly blocked in Socs1−/−Ifng−/− cultures (Figure 4B). These results confirm that the OP9-DL1 co-culture system of thymic differentiation is highly sensitive to defects in thymopoiesis that may not be evident in vivo.
Figure 4. Perturbed thymocyte differentiation in culture of progenitor cells from Socs1−/− mice.
Bone marrow cells from Ifnγ−/− and Socs1−/−Ifnγ−/− mice were co-cultured with OP9-DL1 stroma for the indicated times, and analysed by flow cytometry. Cells were stained with Abs to either CD25 and CD44 (A) or CD4 and CD8 (B), showing DN or mature thymic subsets, respectively. Numbers indicate the percentage of cells in each subset.
DISCUSSION
Cytokines play crucial regulatory roles throughout the life of a T lymphocyte, from controlling early events during thymopoiesis to regulating T cell activation and homeostasis in the periphery (Hunter, 2005; Malek et al., 1999; Marsden et al., 2006). These are finely tuned signals that are modulated by a variety of mechanisms, including inhibition of cytokine signalling pathways by the SOCS proteins (Alexander and Hilton, 2004).
Both SOCS1 and SOCS3 appear to play key roles in regulating cytokine signalling in T cells (Brender et al., 2007; Chong et al., 2003; Cornish et al., 2003a; Davey et al., 2005; Fujimoto et al., 2000; Gagnon et al., 2007; Ilangumaran et al., 2003; Ramanathan et al., 2006; Yu et al., 2006). Although the role of SOCS1 in the thymus has been well studied, little is known about the function of SOCS3 during thymopoiesis. Mice with global SOCS3 deficiency die before birth due to placental insufficiency (Marine et al., 1999; Roberts et al., 2001). The generation of mice with conditional deletion of SOCS3 in lymphocytes has enabled the assessment of thymopoiesis in the absence of SOCS3, which appears to be normal both in vitro and in vivo (Figure 2)(Brender et al., 2007; Chen et al., 2006). Our data demonstrate, however, that analysis of early thymopoiesis is difficult in mice with conditional gene deletion mediated by the Lck promoter, and indicate that the viral infection model established in this study is a feasible way to delete SOCS genes in early thymocytes.
Despite apparently normal thymopoiesis in the absence of SOCS1 in vivo, early thymic differentiation of SOCS1-deficient progenitor T cells in vitro was perturbed at both the DN3:DN4 and DN:DP transitions in vitro. This perturbation could result from a block in differentiation and/or reduced viability of DN4 cells. The appearance of normal proportions of DN3 and DN4 cells in SOCS1-deficient cultures at earlier, but not later, timepoints suggests that DN4 cells may be dying, perhaps as they fail to receive the correct signals to induce development into DP cells.
These defects were observed not only in cultures using retroviral Cre-mediated SOCS1 deletion, but also using bone marrow from Socs1−/−Ifng−/− mice. The detection of similar perturbations in thymopoiesis in vitro using tissue from Socs1−/−Ifng−/− mice confirms that the OP9-DL1 co-culture system is highly sensitive to defects in thymopoiesis, despite the appearance of normal thymopoiesis in vivo in Socs1−/−Ifng−/− mice (Cornish et al., 2003b; Ilangumaran et al., 2003). The disparity between thymopoiesis in vitro and in vivo may reflect the complexity of thymus organization and contribution from stromal cells, which may compensate for the absence of SOCS1 in vivo. Alternatively, the OP9-DL1 system, which focuses on development from haemopoietic stem cells to CD4+CD8+ thymocytes, may be able to detect small differences in precursor T cell potential not observed with in vivo studies that were performed once T cell homeostasis had already been established.
In the absence of both SOCS1 and SOCS3, an earlier block in thymocyte differentiation was seen at the DN2:DN3 transition, the stage at which expression of these proteins is coincident. This suggests that the functions of SOCS1 and SOCS3 overlap during this stage of thymopoiesis, and that each molecule can compensate functionally for the absence of each other at this time. Studies of mice lacking SOCS family members have shown each SOCS protein has specific and non-redundant roles (Alexander and Hilton, 2004), and this is the first study to provide evidence for additional overlapping roles between SOCS1 and SOCS3.
What role could SOCS1 have at the DN3:DN4 and DN:DP transitions to regulate early thymopoiesis? At the DN3 stage, TCRβ-chain rearrangement is initiated, and cells are only able to progress to DN4 once a functional pre-TCR complex is expressed on the cell surface (β-selection checkpoint) (Borowski et al., 2002). Similarly, signalling through the pre-TCR is critical for promoting survival, proliferation and differentiation to the DP stage (Borowski et al., 2002). It is possible that the absence of SOCS1 may affect signalling through or appropriate expression of the pre-TCR, resulting in cells failing the β-selection test. Indeed, a role for SOCS1 during this stage of development has been seen in overexpression studies. Thymocyte development is blocked at the DN3:DN4 transition in transgenic mice expressing SOCS1 under the control of the Lck promoter (Fujimoto et al., 2000). Similarly, overexpression of SOCS1 in foetal thymic organ cultures interfered with progression through DN3, but in contrast to results shown here, differentiation of DN3 thymocytes occurred normally, but cell proliferation was impeded (Trop et al., 2001). The authors suggested SOCS1 is critical for maintaining DN3 thymocytes in a cytokine-unresponsive state until signals through the pre-TCR complex downregulate SOCS1 expression and relieve this inhibition (Trop et al., 2001).
The transition from DN to the DP stage is accompanied by a burst of proliferation, which is dependent on signalling through the pre-TCR complex (Hoffman et al., 1996). IL-7 signalling promotes early thymocyte proliferation, and also survival by inducing expression of the anti-apoptotic molecule Bcl-2 (von Freeden-Jeffry et al., 1997). Several studies have established SOCS1, but not SOCS3, to be a key regulator of cytokines that signal through the γc chain receptor, including IL-7 (Brender et al., 2007; Chong et al., 2003; Cornish et al., 2003a; Fujimoto et al., 2000; Ramanathan et al., 2006; Yu et al., 2006). Indeed, SOCS1 expression is highest in DP thymocytes, in which it is thought to suppress cytokine-induced survival signals received by DP cells prior to successful completion of positive selection (Chong et al., 2003; Yu et al., 2006).
The requirement for IL-7 during early thymic differentiation is well established, as mice unable to respond to IL-7 exhibit a profound reduction in thymic cellularity (Peschon et al., 1994; Puel et al., 1998; von Freeden-Jeffry et al., 1995). Mice transgenic for the Il-7 gene, however, have a severe block at the DP stage of thymic development, illustrating that responses to IL-7 need to be tightly controlled, and that excessive IL-7 or expression at an inappropriate stage can be detrimental to development (El Kassar et al., 2004). This has also been illustrated in OP9-DL1 co-cultures, where early thymic differentiation was blocked at the DN2:DN3 and DN:DP transitions (similar to that of SOCS1ΔSOCS3Δ cells) in the presence of high, but not low, IL-7 concentrations (Balciunaite et al., 2005; Huang et al., 2005). In the absence of SOCS1, thymocytes would be hyper-responsive to IL-7, resulting in cells detecting a higher or prolonged IL-7 signal, and conceivably this could be contributing to the blocks in development seen in the OP9-DL1 co-cultures described here. SOCS3, however, does not appear to regulate IL-7 signals, suggesting that other factors may be inhibiting differentiation in SOCS1ΔSOCS3Δ cells (Brender et al., 2007). Our results also suggest that hypersensitivity to IL-7 is unlikely to be the sole cause of perturbed differentiation in these cultures, since IL-7 withdrawal failed to substantially relieve the DN2:DN3 and DN:DP development blocks.
In summary, our data reveal a requirement for SOCS1 in the regulation of early thymic differentiation that is distinct from suppression of IL-7 signals. Further, although thymopoiesis in the absence of SOCS3 appears normal, we show that defects in early thymic differentiation are more pronounced in the absence of both SOCS1 and SOCS3, suggesting that they have shared functions during this process. The full spectrum of SOCS functions, therefore, may only be realised fully once mice have been generated that lack two or more SOCS family members.
Acknowledgments
The authors thank Melanie Rowe and Rebecca Branch for animal husbandry, Catherine Li for cell sorting assistance, Wei Wei for construction of the MSCV-Cre-IRES-eGFP retrovirus and Drs Sebastian Carotta and Marnie Blewitt for advice regarding retroviral infection. This work was supported by the Australian National Health and Medical Research Council (Program Grant 461219) and NIH RO1 CA22556; R.S. was supported by a Sylvia and Charles Viertel Senior Medical Research Fellowship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alexander WS, Hilton DJ. The role of suppressors of cytokine signaling (SOCS) proteins in regulation of the immune response. Annu Rev Immunol. 2004;22:503–29. doi: 10.1146/annurev.immunol.22.091003.090312. [DOI] [PubMed] [Google Scholar]
- Alexander WS, Starr R, Fenner JE, Scott CL, Handman E, Sprigg NS, Corbin JE, Cornish AL, Darwiche R, Owczarek CM, Kay TW, Nicola NA, Hertzog PJ, Metcalf D, Hilton DJ. SOCS1 is a critical inhibitor of interferon gamma signaling and prevents the potentially fatal neonatal actions of this cytokine. Cell. 1999;98:597–608. doi: 10.1016/s0092-8674(00)80047-1. [DOI] [PubMed] [Google Scholar]
- Balciunaite G, Ceredig R, Fehling HJ, Zuniga-Pflucker JC, Rolink AG. The role of Notch and IL-7 signaling in early thymocyte proliferation and differentiation. Eur J Immunol. 2005;35:1292–1300. doi: 10.1002/eji.200425822. [DOI] [PubMed] [Google Scholar]
- Borowski C, Martin C, Dounari F, Haughn L, Aifantis I, Grassi F, von Boehmer H. On the brink of becoming a T cell. Curr Opin Immunol. 2002;14:200–206. doi: 10.1016/s0952-7915(02)00322-9. [DOI] [PubMed] [Google Scholar]
- Brender C, Tannahill GM, Jenkins BJ, Fletcher J, Columbus R, Saris CJM, Ernst M, Nicola NA, Hilton DJ, Alexander WS, Starr R. SOCS3 regulates CD8 T cell proliferation by inhibition of IL-6 and IL-27. Blood. 2007;110:2528–2536. doi: 10.1182/blood-2006-08-041541. [DOI] [PubMed] [Google Scholar]
- Buckland J, Pennington DJ, Bruno L, Owen MJ. Co-ordination of the expression of the protein tyrosine kinase p56(lck) with the pre-T cell receptor during thymocyte development. Eur J Immunol. 2000;30:8–18. doi: 10.1002/1521-4141(200001)30:1<8::AID-IMMU8>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Cao X, Shores EW, Hu-Li J, Anver MR, Kelsall BL, Russell SM, Drago J, Noguchi M, Grinberg A, Bloom ET. Defective lymphoid development in mice lacking expression of the common cytokine receptor gamma chain. Immunity. 1995;2:223–238. doi: 10.1016/1074-7613(95)90047-0. [DOI] [PubMed] [Google Scholar]
- Chen Z, Laurence A, Kanno Y, Pacher-Zavisin M, Zhu BM, Tato C, Yoshimura A, Hennighausen L, O’Shea JJ. Selective regulatory function of Socs3 in the formation of IL-17-secreting T cells. Proc Natl Acad Sci U S A. 2006;103:8137–8142. doi: 10.1073/pnas.0600666103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong MM, Cornish AL, Darwiche R, Stanley EG, Purton JF, Godfrey DI, Hilton DJ, Starr R, Alexander WS, Kay TW. Suppressor of cytokine signaling-1 is a critical regulator of interleukin-7-dependent CD8+ T cell differentiation. Immunity. 2003;18:475–87. doi: 10.1016/s1074-7613(03)00078-5. [DOI] [PubMed] [Google Scholar]
- Cornish AL, Chong MM, Davey GM, Darwiche R, Nicola NA, Hilton DJ, Kay TW, Starr R, Alexander WS. Suppressor of cytokine signaling-1 regulates signaling in response to interleukin-2 and other gamma c-dependent cytokines in peripheral T cells. J Biol Chem. 2003a;278:22755–61. doi: 10.1074/jbc.M303021200. [DOI] [PubMed] [Google Scholar]
- Cornish AL, Davey GM, Metcalf D, Purton JF, Corbin JE, Greenhalgh CJ, Darwiche R, Wu L, Nicola NA, Godfrey DI, Heath WR, Hilton DJ, Alexander WS, Starr R. Suppressor of cytokine signaling-1 has IFN-gamma-independent actions in T cell homeostasis. J Immunol. 2003b;170:878–86. doi: 10.4049/jimmunol.170.2.878. [DOI] [PubMed] [Google Scholar]
- Croker BA, Krebs DL, Zhang JG, Wormald S, Willson TA, Stanley EG, Robb L, Greenhalgh CJ, Forster I, Clausen BE, Nicola NA, Metcalf D, Hilton DJ, Roberts AW, Alexander WS. SOCS3 negatively regulates IL-6 signaling in vivo. Nat Immunol. 2003;4:540–5. doi: 10.1038/ni931. [DOI] [PubMed] [Google Scholar]
- Dalton DK, Pitts-Meek S, Keshav S, Figari IS, Bradley A, Stewart TA. Multiple defects of immune cell function in mice with disrupted interferon-gamma genes. Science. 1993;259:1739–42. doi: 10.1126/science.8456300. [DOI] [PubMed] [Google Scholar]
- Davey GM, Starr R, Cornish AL, Burghardt JT, Alexander WS, Carbone FR, Surh CD, Heath WR. SOCS-1 regulates IL-15-driven homeostatic proliferation of antigen-naive CD8 T cells, limiting their autoimmune potential. J Exp Med. 2005;202:1099–108. doi: 10.1084/jem.20050003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLuca D, Clark DR. Interleukin-7 negatively regulates the development of mature T cells in fetal thymus organ cultures. Developmental and Comparative Immunology. 2002;26:365–384. doi: 10.1016/s0145-305x(01)00085-4. [DOI] [PubMed] [Google Scholar]
- El Kassar N, Lucas PJ, Klug DB, Zamisch M, Merchant M, Bare CV, Choudhury B, Sharrow SO, Richie E, Mackall CL, Gress RE. A dose effect of IL-7 on thymocyte development. Blood. 2004;104:1419–1427. doi: 10.1182/blood-2004-01-0201. [DOI] [PubMed] [Google Scholar]
- Fehniger TA, Suzuki K, Ponnappan A, VanDeusen JB, Cooper MA, Florea SM, Freud AG, Robinson ML, Durbin J, Caligiuri MA. Fatal leukemia in interleukin 15 transgenic mice follows early expansions in natural killer and memory phenotype CD8+ T cells. J Exp Med. 2001;193:219–231. doi: 10.1084/jem.193.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher J, Starr R. The role of suppressors of cytokine signalling in thymopoiesis and T cell activation. Int J Biochem Cell Biol. 2005;37:1774–86. doi: 10.1016/j.biocel.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Fujimoto M, Naka T, Nakagawa R, Kawazoe Y, Morita Y, Tateishi A, Okumura K, Narazaki M, Kishimoto T. Defective thymocyte development and perturbed homeostasis of T cells in STAT-induced STAT inhibitor-1/suppressors of cytokine signaling-1 transgenic mice. J Immunol. 2000;165:1799–806. doi: 10.4049/jimmunol.165.4.1799. [DOI] [PubMed] [Google Scholar]
- Gagnon J, Ramanathan S, Leblanc C, Ilangumaran S. Regulation of IL-21 signaling by suppressor of cytokine signaling-1 (SOCS1) in CD8+ T lymphocytes. Cellular Signalling. 2007;19:806–816. doi: 10.1016/j.cellsig.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Godfrey DI, Kennedy J, Suda T, Zlotnik A. A developmental pathway involving four phenotypically and functionally distinct subsets of CD3-CD4-CD8- triple-negative adult mouse thymocytes defined by CD44 and CD25 expression. Journal of Immunology. 1993;150:4244–4252. [PubMed] [Google Scholar]
- Hoffman ES, Passoni L, Crompton T, Leu TMJ, Schatz DG, Koff A, Owen MJ, Hayday AC. Productive T-cell receptor b-chain gene rearrangement: coincident regulation of cell cycle and clonality during development in vivo. Genes Dev. 1996;10:948–962. doi: 10.1101/gad.10.8.948. [DOI] [PubMed] [Google Scholar]
- Huang J, Garrett KP, Pelayo R, Zuniga-Pflucker JC, Petrie HT, Kincade PW. Propensity of adult lymphoid progeniors to progress to DN2/3 stage thymocytes with Notch receptor ligation. Journal of Immunology. 2005;175:4858–4865. doi: 10.4049/jimmunol.175.8.4858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter CA. New IL-12-family members: IL-23 and IL-27, cytokines with divergent functions. Nat Rev Immunol. 2005;5:521–531. doi: 10.1038/nri1648. [DOI] [PubMed] [Google Scholar]
- Ilangumaran S, Ramanathan S, Ning T, La Rose J, Reinhart B, Poussier P, Rottapel R. Suppressor of cytokine signaling 1 attenuates IL-15 receptor signaling in CD8+ thymocytes. Blood. 2003;102:4115–22. doi: 10.1182/blood-2003-01-0175. [DOI] [PubMed] [Google Scholar]
- Kopf M, Baumann H, Freer G, Freudenberg M, Lamers M, Kishimoto T, Zinkernagel R, Bluethmann H, Kohler G. Impaired immune and acute-phase responses in interleukin-6-deficient mice. Nature. 1994;368:339–42. doi: 10.1038/368339a0. [DOI] [PubMed] [Google Scholar]
- Malek TR, Porter BO, He YW. Multiple gamma c-dependent cytokines regulated T-cell development. Immunol Today. 1999;20:71–76. doi: 10.1016/s0167-5699(98)01391-7. [DOI] [PubMed] [Google Scholar]
- Marine JC, McKay C, Wang D, Topham DJ, Parganas E, Nakajima H, Pendeville H, Yasukawa H, Sasaki A, Yoshimura A, Ihle JN. SOCS3 is essential in the regulation of fetal liver erythropoiesis. Cell. 1999;98:617–27. doi: 10.1016/s0092-8674(00)80049-5. [DOI] [PubMed] [Google Scholar]
- Markowitz D, Goff S, Bank A. A safe packaging cell line for gene transfer: separating viral genes on two different plasmids. J Virol. 1988;62:1120–1124. doi: 10.1128/jvi.62.4.1120-1124.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsden VS, Kappler JW, Marrack P. Homeostasis of the memory T cell pool. Int Arch Allergy Immunol. 2006;139:63–74. doi: 10.1159/000090000. [DOI] [PubMed] [Google Scholar]
- Matsumoto A, Seki Y, Watanabe R, Hayashi K, Johnston JA, Harada Y, Abe R, Yoshimura A, Kubo M. A role of suppressor of cytokine signaling 3 (SOCS3/CIS3/SSI3) in CD28-mediated interleukin 2 production. J Exp Med. 2003;197:425–36. doi: 10.1084/jem.20020939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peschon JJ, Morrissey PJ, Grabstein KH, Ramsdell FJ, Maraskovsky E, Gliniak BC, Park LS, Ziegler SF, Williams DE, Ware CB, Meyer JD, Davison DL. Early lymphocyte expansion is severly impaired in interleukin 7 receptor-deficient mice. J Exp Med. 1994;180:1955–1960. doi: 10.1084/jem.180.5.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puel A, Ziegler SF, Buckley RH, Leonard WJ. Defective IL7R expression in T(−)B(+)NK(+) severe combined immunodeficiency. Nat Genet. 1998;20:394–397. doi: 10.1038/3877. [DOI] [PubMed] [Google Scholar]
- Ramanathan S, Gagnon J, Leblanc C, Rottapel R, Ilangumaran S. Suppressor of cytokine signaling 1 stringently regulates distinct functions of IL-7 and IL-15 in vivo during T lymphocyte development and homeostasis. J Immunol. 2006;176:4029–4041. doi: 10.4049/jimmunol.176.7.4029. [DOI] [PubMed] [Google Scholar]
- Roberts AW, Robb L, Rakar S, Hartley L, Cluse L, Nicola NA, Metcalf D, Hilton DJ, Alexander WS. Placental defects and embryonic lethality in mice lacking suppressor of cytokine signaling 3. Proc Natl Acad Sci U S A. 2001;98:9324–9. doi: 10.1073/pnas.161271798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadlack B, Merz H, Schorle H, Schimpl A, Feller AC, Horak I. Ulcerative colitis-like disease in mice with a disrupted interleukin-2 gene. Cell. 1993;75:253–261. doi: 10.1016/0092-8674(93)80067-o. [DOI] [PubMed] [Google Scholar]
- Schmitt TM, Zuniga-Pflucker JC. Induction of T cell development from hematopoietic progenitor cells by delta-like-1 in vitro. Immunity. 2002;17:749–756. doi: 10.1016/s1074-7613(02)00474-0. [DOI] [PubMed] [Google Scholar]
- Starr R, Metcalf D, Elefanty AG, Brysha M, Willson TA, Nicola NA, Hilton DJ, Alexander WS. Liver degeneration and lymphoid deficiencies in mice lacking suppressor of cytokine signaling-1. Proc Natl Acad Sci USA. 1998;95:14395–9. doi: 10.1073/pnas.95.24.14395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr R, Willson TA, Viney EM, Murray LJ, Rayner JR, Jenkins BJ, Gonda TJ, Alexander WS, Metcalf D, Nicola NA, Hilton DJ. A family of cytokine-inducible inhibitors of signalling. Nature. 1997;387:917–21. doi: 10.1038/43206. [DOI] [PubMed] [Google Scholar]
- Trop S, De Sepulveda P, Zuniga-Pflucker JC, Rottapel R. Overexpression of suppressor of cytokine signaling-1 impairs pre-T-cell receptor-induced proliferation but not differentiation of immature thymocytes. Blood. 2001;97:2269–2277. doi: 10.1182/blood.v97.8.2269. [DOI] [PubMed] [Google Scholar]
- Van Parijs L, Refaeli Y, Lord JD, Nelson BH, Abbas AK, Baltimore D. Uoupling IL-2 signals that regulate T cell proliferation, survival, and Fas-mediated activation-induced cell death. Immunity. 1999;11:281–288. doi: 10.1016/s1074-7613(00)80103-x. [DOI] [PubMed] [Google Scholar]
- von Freeden-Jeffry U, Solvason N, Howard M, Murray R. The earliest T lineage-committed cells depend on IL-7 for Bcl-2 expression and normal cell cycle progression. Immunity. 1997;7:147–154. doi: 10.1016/s1074-7613(00)80517-8. [DOI] [PubMed] [Google Scholar]
- von Freeden-Jeffry U, Vieira P, Lucian LA, McNeil T, Burdach SE, Murray R. Lymphopenia in interleukin (IL)-7 gene-deleted mice identifies IL-7 as a nonredundant cytokine. J Exp Med. 1995;181:1519–1526. doi: 10.1084/jem.181.4.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarilin AA, Belyakov IM. Cytokines in the thymus: production and biological effects. Curr Med Chem. 2004;11:447–464. doi: 10.2174/0929867043455972. [DOI] [PubMed] [Google Scholar]
- Yu CR, Mahdi RM, Ebong S, Vistica BP, Gery I, Egwuagu CE. Suppressor of cytokine signaling 3 regulates proliferation and activation of T-helper cells. J Biol Chem. 2003;278:29752–9. doi: 10.1074/jbc.M300489200. [DOI] [PubMed] [Google Scholar]
- Yu Q, Park J-H, Doan Ll, Erman B, Feigenbaum L, Singer A. Cytokine signal transduction is suppressed in preselection double-positive thymocytes and restored by positive selection. Journal of Experimental Medicine. 2006;203:165–175. doi: 10.1084/jem.20051836. [DOI] [PMC free article] [PubMed] [Google Scholar]