Abstract
Tetramethylenedisulfotetramine (TETS) is a potent convulsant poison that is thought to trigger seizures by inhibiting the function of the type A gamma-aminobutyric acid receptor (GABAAR). Acute intoxication with TETS can cause vomiting, convulsions, status epilepticus (SE) and even death. Clinical case reports indicate that individuals who survive poisoning may exhibit long-term neuropsychological issues and cognitive deficits. Therefore, the objective of this research was to determine whether a recently described mouse model of acute TETS intoxication exhibits persistent behavioral deficits. Young adult male NIH Swiss mice received a seizure-inducing dose of TETS (0.15 mg/kg, ip) and then were rescued from lethality by administration of diazepam (5 mg/kg, ip) approximately 20 min post-TETS-exposure. TETS-intoxicated mice typically exhibited 2 clonic seizures prior to administration of diazepam with no subsequent seizures post-diazepam injection as assessed using behavioral criteria. Seizures lasted an average of 72 seconds. Locomotor activity, anxiety-like and depression-relevant behaviors and cognition were assessed at 1 week, 1 month and 2 months post-TETS exposure using open field, elevated-plus maze, light↔dark transitions, tail suspension, forced swim and novel object recognition tasks. Interestingly, preliminary validation tests indicated that NIH Swiss mice do not respond to the shock in fear conditioning tasks. Subsequent evaluation of hot plate and tail flick nociception tasks revealed that this strain exhibits significantly decreased pain sensitivity relative to age- and sex-matched C57BL/6J mice, which displayed normal contextual fear conditioning. NIH Swiss mice acutely intoxicated with TETS exhibited no significant anxiety-related, depression-relevant, learning or memory deficits relative to vehicle controls at any of the time points assessed with the exception of significantly increased locomotor activity at 2 months post-TETS intoxication. The general absence of long-term behavioral deficits in TETS-intoxicated mice on these six assays suggests that the neurobehavioral consequences of TETS exposure described in human survivors of acute TETS intoxication are likely due to sustained seizure activity, rather than a direct effect of the chemical itself. Future research efforts are directed towards developing an animal model that better recapitulates the SE and seizure duration reported in humans acutely intoxicated with TETS.
Keywords: behavioral testing, mice, seizures, tetramethylenedisulfotetramine, TETS
1. INTRODUCTION
Tetramethylenedisulfotetramine (TETS) is a GABAAR antagonist that was widely used as a rodenticide until an international ban on its production in 1991 (Whitlow et al., 2005). However, due to its relative ease of synthesis and low production costs, TETS remains available on the international black market. In 2000, the National Poison Control Center of China revealed that 74% of commercial rodenticides in that country contained illegal chemicals, with TETS found in nearly 50% of these products (Banks et al., 2014). TETS is a potent chemical convulsant. The LD50 for TETS in rodents and rabbits is 0.1–0.2 mg/kg ip, and 7–10 mg is considered to be a lethal dose for an adult human (Croddy, 2004, Guan et al., 1993). Severe intoxication produces generalized clonic-tonic convulsions that can progress to status epilepticus (SE), arrhythmias, coma, and death (Barrueto et al., 2003, Li et al., 2012, Lu et al., 2008, Zhang et al., 2011). TETS has been implicated in both accidental and intentional poisoning of as many as 14,000 individuals in China between 1991 and 2010, as well as more than 50 human poisonings in Western countries since 2002 (Li et al., 2012, Zhang et al., 2011). More recently, in March 2014, 30 kindergarten children in Yunnan province were poisoned by a classmate and two of those victims died (Ramzy, 2014).
Clinical case reports indicate that individuals who survive acute intoxication with TETS at levels that cause seizures may experience delayed or long-term effects, including abnormal electroencephalography (EEG), spontaneous recurrent seizures, anxiety and/or depressive disorders and memory impairments that persist for months to years following exposure (Bai et al., 2005, Whitlow et al., 2005, Zhang et al., 2011). Additionally, acute TETS poisoning may cause developmental delays in children and adversely affect cognitive development, as evidenced by lower verbal, performance and overall intelligence scores in children exposed to TETS compared to controls (Bai et al., 2005, Whitlow et al., 2005, Zhang et al., 2011).
We recently characterized a mouse model of acute TETS intoxication (Zolkowska et al., 2012), which confirmed the high potency of TETS as a convulsant in the NIH Swiss mouse. In this model, TETS triggered clonic-tonic seizures, as assessed using both behavioral (Zolkowska et al., 2012) and electroencephalographic (EEG) criteria (Lein and Hammock, data under review). Another group has reported similar findings in C57BL/6J mice and further demonstrated that administration of a high dose of diazepam (5 mg/kg, ip), a GABAAR positive allosteric modulator, protected these mice from further motor seizures and increased survival at 1 h post-TETS exposure (Shakarjian et al., 2012). We recently extended this observation to the NIH Swiss mouse, demonstrating that when administered approximately 20 min post-TETS, diazepam stops further behavioral and EEG seizure outcomes in NIH Swiss mice (Lein and Hammock, data under review).
Histological examination of brains from animals that received sublethal doses of TETS (Zolkowska et al., 2012) revealed neuroinflammation in the hippocampus and cortex evident as increased IBA-1 immunoreactivity on days 1 and 2 post-exposure and increased GFAP immunoreactivity on days 2 and 3 post-exposure. Both GFAP and IBA-1 immunoreactivity returned to control levels by 7 d post-exposure, which was the latest time point examined in that study. Subsequent studies of animals intoxicated with a lethal dose of TETS and then “rescued” from death by diazepam (Lein and Hammock, data under review) found that treatment with diazepam did not mitigate neuroinflammation associated with TETS intoxication: GFAP immunoreactivity was still significantly increased at 2 and 3 d post-exposure but returned to control levels by 7 d post-exposure; however, increased IBA-1 immunoreactivity persisted up to 7 d post-exposure. Neuroinflammation has been associated with various behavioral deficits, including depression, anxiety and impaired memory (Corona et al., 2012, Dantzer et al., 2008, Maes et al., 2009, Smith, 2013). Collectively, these data suggest that TETS-induced neuroinflammation may contribute to the behavioral deficits and memory impairments reported in humans that survive acute TETS intoxication. Therefore, the goal of this research was to characterize the effects of acute TETS intoxication in the NIH Swiss mouse model on cognitive and emotional behaviors. To our knowledge, these are the first experiments addressing TETS-induced behavioral and cognitive deficits in an animal model.
2. METHODS
2.1. Chemicals
Sulfamide, hydrochloric acid, acetone, and hexane were obtained from Thermo Fisher Scientific (Waltham, MA). All chemicals were of the highest purity available. TETS was synthesized as previously described (Zolkowska et al., 2012). A final recrystallization step was performed to ensure no water remained in the crystals and characterization of the final product by gas chromatography-mass spectrometry supported the assigned structure of TETS and its high purity. The product was > 98% pure based on integration of total ion current. USP grade diazepam (in 40% propylene glycol, 10% alcohol, 5% sodium benzoate and 1.5% benzyl alcohol and 43.5% water) manufactured by Hospira was purchased from Western Medical Supply (Arcata, CA).
2.2. Animal Subjects
Animals were maintained in facilities fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care, and all experiments involving animals were approved by the Institutional Care and Use Committee at University of California Davis. Naïve male NIH Swiss mice (6 weeks of age) were purchased from the National Cancer Institute (Bethesda, MD). Upon arrival, mice were housed 4 per cage in clear polycarbonate cages with corncob bedding. A paper fiber nestlet (Ancare, Waupaca, WI) and shredded paper were added to each cage as enrichment. Subjects were housed on a 12 h light/dark cycle, lights on at 7 AM, in a vivarium maintained at 20–22°C and ~45% humidity. Mice received unlimited access to chow and water, and were acclimated to housing conditions for 7 d before beginning any experiments. Separate cohorts of mice were used to assess behavioral and/or cognitive deficits at 1 week, 1 month and 2 months post-vehicle (VEH) or post-TETS administration (Fig. 1). VEH and TETS mice from the 1 week cohort were assessed for post-treatment weight loss before beginning behavior assessments. Both VEH and TETS mice gained weight at a similar rate, approximately 2 grams. Since body weight increases were similar at 1 week post-treatment, weights were not tracked at 1 and 2 months post-treatment. An additional cohort of naïve NIH Swiss mice was used for task validation prior to initiating behavioral studies.
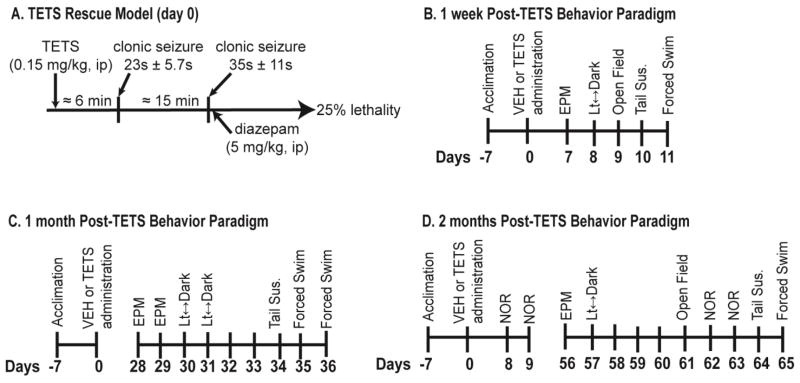
Figure 1. Exposure and testing paradigms.
(A) TETS exposure paradigm. Mice injected with TETS at 0.15 mg/kg exhibit a characteristic pattern of convulsive behavior consisting of 2 brief periods of clonic seizures within 20 min. Lethality is prevented in 75% of the animals by administering diazepam (5 mg/kg, ip) immediately after the second clonic seizure. Onset to clonic seizures (average), seizure length (mean ± S.D.) and percent lethality were determined by combining data from all three experimental cohorts. Separate cohorts of mice were used to assess behavior at varying times post-TETS or VEH exposure: (B) 1 week (n = 11 VEH, 12 TETS), (C) 1 month (n = 18 VEH, 17 TETS) and (D) 2 month (n = 15 VEH, 11 TETS). Abbreviations: EPM, elevated plus-maze; Tail Sus., tail suspension; NOR, novel object recognition.
2.3 Dosing Paradigm
On the day animals were exposed to TETS, stock solutions of TETS were sequentially diluted in warm saline to a final concentration of 0.015 mg/ml in 10% DMSO and administered by the intraperitoneal (ip) route in a volume of 10 ml/kg. Immediately after dosing, animals were observed for up to 60 min for behavioral evidence of seizures. Time to seizure onset and duration of each seizure were recorded for each animal. Diazepam (5 mg/kg in 10% DMSO in saline in a total volume of 5 ml/kg) was administered ip following the second clonic seizure, approximately 20 min post-TETS injection (Figure 1). Vehicle control animals (VEH) were injected ip with the vehicle used for TETS (10% DMSO in saline) at 10 ml/kg followed 20 minutes later with an ip injection of diazepam (5 mg/kg in a total volume of 5 ml/kg).
2.4. Behavioral tasks
To minimize carryover effects, behavioral tasks were performed in the order of least stressful to most stressful in all three cohorts (Fig. 1B, 1C, 1D). All behavioral tasks were performed between 9:00 and 16:00 (7 h) with a minimum of 24 h between tasks. A trained observer blind to experimental treatment groups ran all the behavioral tasks.
Test Validation
Initially, a set of naïve NIH Swiss mice was run through all behavioral tasks to assure that they were capable of performing a given task and that their performance had the capacity to be altered by TETS treatment (Table 1). Interestingly, during this validation testing, we discovered that adult NIH Swiss mice exhibited low freezing scores and minimal reactivity following the tone-shock pairings in the fear conditioning assay (see Supplementary data, Supplementary Figure 1). Therefore, this test was not included in the behavioral battery conducted with the TETS intoxicated animals.
Table 1.
Task validation in naïve NIH Swiss mice.
| TASK VALIDATION | MEAN±SD | N |
|---|---|---|
| Anxiety-Like Behavior | ||
| Elevated Plus Maze | ||
| % Time in Open Arm | 42 ± 6.04 | 9 |
| Open Entries | 16 ±3.6 | 9 |
| Total Entries | 27 ±4.6 | 9 |
| Light ↔Dark Exploration | ||
| Time in Dark (sec) | 310 ±92 | 9 |
| Total Transitions | 51 ±16 | 9 |
| Depression-Like Behavior | ||
| Tail Suspension | ||
| % Time Immobile | 41 ±18 | 8 |
| Forced Swim | ||
| % Time Immobile | 13 ±8.9 | 10 |
Naïve NIH Swiss mice were evaluated on each behavioral task before testing with VEH and TETS treated mice, confirming that NIH Swiss mice responded to each task as predicted from the literature: EPM (Lister, 1987); light↔dark (Holmes et al., 2001, Kulesskaya and Voikar, 2014, Silverman et al., 2010); tail suspension (Cryan et al., 2005); forced swim (Can et al., 2011, Lucki et al., 2001).
Elevated Plus-Maze
The elevated-plus maze (EPM) is a well-established task for assessing anxiety-like conflict behavior in rodents by allowing mice to choose between entering the two open arms of the maze (natural exploratory drive) or staying in the safety of the two closed arms. All four arms are elevated 1 meter from the floor, with the drop-off detectable only in the open arms (Hogg, 1996). The EPM was performed according to previously described procedures (Bailey et al., 2007, Silverman et al., 2011) using a mouse EPM (model ENV-560A) purchased from Med Associates (St. Albans, VT). The EPM contained two open arms (35.5 cm x 6 cm) and two closed arms (35.5 cm x 6 cm) radiating from a central area (6 cm x 6 cm). A 0.5 cm high lip surrounded the edges of the open arms, whereas the closed arms were surrounded by 20 cm high walls. The EPM was cleaned with 70% ethanol before the beginning of the first test session and after each subject mouse was tested, with sufficient time for the ethanol odor to dissipate before the start of the next test session. Room illumination was ~ 30 lux. To begin the test, the mouse was placed in the central area facing the open arm. The mouse was allowed to freely explore for 5 min during which time the activity was recorded by closed circuit television (CCTV). A trained investigator validated the output of the Med Associates software according to previously published methods (File et al., 2004, Walf and Frye, 2007).
Light↔Dark Transitions
The light↔dark transitions test (Crawley and Goodwin, 1980), also termed the light/dark box, assesses anxiety-like conflict behavior in mice by evaluating the tendency of mice to avoid brightly lit areas versus their strong tendency to explore a novel environment. The light↔dark transitions test was performed in accordance with previously described procedures (Brielmaier et al., 2012, Silverman et al., 2011). The test began by placing the mouse in the light side (~320 lux; 28 cm x 27.5 cm x 27 cm) of an automated 2-chambered apparatus, in which the enclosed/dark side (~5 lux; 28 cm x 27.5 cm x 19 cm) was reached by traversing the small opening of the partition between the two chambers. The mouse was allowed to explore freely for 10 minutes. Time in the dark side chamber and total number of transitions between the light and dark side chambers were automatically recorded during the 10 minute session using Labview 8.5.1 software (National Instruments, Austin, TX).
Open Field
General exploratory locomotion was assessed using the novel open field test as previously described (Silverman et al., 2011, Yang et al., 2012). Briefly, a mouse was placed into a novel open field arena composed of plexiglass and measuring 42 cm long by 42 cm wide by 31 cm high. The open field arena was interfaced with VersaMax detection software (AccuScan, Omni-Tech Electronics, Columbus, OH) to detect photobeam breaks. Locomotor activity was monitored for 30 min using photocell detectors. Total distance, center time, horizontal and vertical activities were automatically recorded with VersaMax 400 software (AccuScan).
Tail Suspension
The tail suspension test is a model of “behavioral despair” that was designed to assess antidepressant drug effects. When suspended by the tail, mice actively struggle to escape, then dangle immobile (Steru et al., 1985). Antidepressants decrease time immobile in this test, hence its use to assess depression-like behavior (Cryan and Holmes, 2005, Steru et al., 1985). Here, we measured depression-relevant behavior using an automated tail suspension test for mice (MED-TSS-MS, Med Associates). The mouse was attached by the distal end of its tail to a metal hanger using medical tape. The metal hanger transmits movement of the mouse to a load cell (gain = 16; threshold = 10 – 60), which then transmits this signal to the interfaced computer. Movement data collected for a total of 6 min (resolution = 200 ms) were analyzed using Tail Suspension Software (Med Associates) and used to calculate the percent time immobile.
Forced Swim
The forced swim task measures antidepressant drug response on another measure of “behavioral despair.” When placed in a cylinder of water too deep to escape, mice will first swim, then stop swimming and float. Amount of time the mouse floats, i.e. gives up trying to escape from a tank of water, is calculated as the percent time immobile. (Porsolt et al., 1977). A clear Plexiglas cylinder 20 cm in diameter was filled with water (24 ± 1°C) to a depth of 15 cm. A mouse was placed in the cylinder for a 5 min swim session. Behavior was recorded using a CCTV camera. A trained observer blind to treatment later scored the last 3 minutes (broken into 5 sec intervals) of each swim session for immobility (lack of movement except those necessary to keep the mouse afloat). Data are presented as the percent time immobile.
Novel Object Recognition
The novel object recognition (NOR) task was used to assess recognition memory (Bevins and Besheer, 2006) in VEH and TETS-treated mice. This task was selected after we discovered that NIH Swiss mice appear to be insensitive to fear conditioning (Suppl. Fig. 1). NOR was performed over two days as previously described (Brielmaier et al., 2012, Yang et al., 2012). The same open field arena used to assess locomotor activity was used for NOR. On the first day, the mouse was allowed to habituate to the open field arena by freely exploring for 30 min. After 24 h, the mouse was placed back into the same open field arena for a 10 min habituation session. Subjects were then removed from the area and placed in a holding cage while two identical objects were placed into the open field area (~ 2 min). The subject was placed back into the arena for a 10 min familiarization session during which time spent sniffing each object was recorded. Next, the mouse was removed from the area and placed back in its holding cage during which time the objects were cleaned. After 60 min, the mouse was placed back into the arena with one familiar object and one novel object. During this recognition phase of the test, the animal was allowed to freely explore both objects for 5 min while sniffing behavior was recorded. Objects were small plastic toys of different shapes and colors matched for size and reflective properties. One object was a light brown treasure chest and the second object was a light orange coral. Familiar and novel objects were counterbalanced among test subjects. A trained observer scored videos of the familiarization and recognition sessions for time spent investigating each object. Object investigation was defined as amount of time spent sniffing the object when the nose was deliberately pointed towards the object at less than 2 cm distance. Recognition memory was defined as significantly more time sniffing the novel object than the familiar one during the recognition session. Time spent sniffing the right and the left objects during the familiarization phase confirmed no innate object preference.
2. 5. Statistics
For anxiety-like and depression relevant behaviors, a Student’s t-test was used for comparisons between VEH and TETS groups. When normality failed, a Mann-Whitney U test was utilized to identify significant differences between treatment groups. For locomotor activity, data from each 30 min session time was totaled and VEH versus TETS groups compared using a Student’s t-test. A two-way ANOVA was utilized to determine statistical significance when comparing two factors (treatment, object) during NOR. For all behavioral tasks, mice performing outside two standard deviations from the mean within a specific treatment group were considered statistical outliers and the data point was excluded from analysis for that given behavioral task. For the 1 week EPM measurement, video recording failed for 4 VEH mice and, therefore, these mice could not be scored. Also for this task and time point, 2 TETS treated mice were identified as outliers and were excluded from analysis. Video recording failure during 1 week NOR familiarization phase resulted in 2 fewer VEH animals and 1 less TETS animal out of 15 animals. For the 1 month measurement, 2 TETS treated mice were identified as outliers and excluded from the EPM analysis. Also at this time point, 3 TETS mice were excluded from tail suspension due to the mice escaping from the task and 3 TETS mice were excluded from the light↔dark transitions test due to an unexpected loud noise during testing. At the two month time point, no data was excluded from analysis for any reason. Data are presented as the mean ± standard deviation (SD). For the ANOVA and t-test comparisons the F and t statistics, respectively, are listed with the degrees of freedom in parentheses.
3. RESULTS
3.1. Cohort survival and seizure characteristics in TETS treated mice
Consistent with previous observations (Zolkowska et al., 2012), mice dosed with TETS at 0.15 mg/kg ip displayed a brief period of hyperactivity followed by a period of somnolence, Straub tail, twitches, imbalance followed by a stereotypic pattern of seizure activity consisting of two brief periods of clonic seizures followed by tonic seizures and death (Figure 1A). Administration of diazepam (5 mg/kg, ip) following the second period of clonic seizures prevented the lethal tonic seizures (Figure 1A). Three separate cohorts of TETS-intoxicated mice rescued by diazepam and their vehicle controls were employed to test behavior at three different post-exposure time points (Figure 1B, 1C, 1D). Overall, the seizure duration during the two clonic seizures was similar across the three separate cohorts of mice. In the 1 week cohort, the first clonic seizure lasted for an average of 21 s and the second clonic seizure, an average of 35 s. In the 1 month and 2 month cohorts, the duration of the first clonic seizure averaged 25 and 24 s, respectively, while the duration of the second clonic seizure averaged 37 and 35 s, respectively.
The survival rate was less consistent than the clonic seizure length across cohorts. In the 1 week cohort, 11 of 18 mice (61%) survived acute TETS intoxication at 24 h; in the 1 month cohort, 17 of 18 mice (94%) survived; in the 2 month cohort, 11 of 16 mice (69%) survived. Average 24 h survival after acute TETS exposure was 75% (39/52) across all three cohorts. The 24 h survival rate of mice administered VEH was 100%.
3.2 Acute TETS intoxication alters locomotor activity in mice
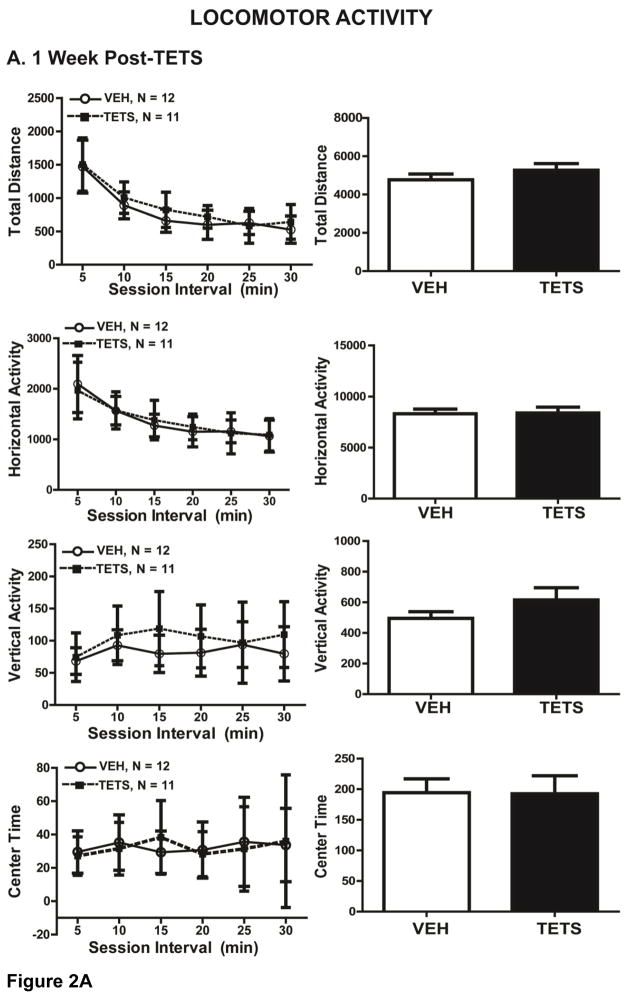
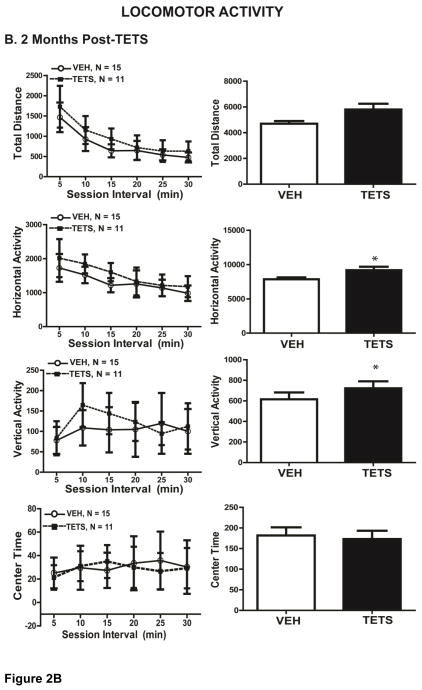
At 1 week post-TETS intoxication (cohort 1), TETS intoxicated mice exhibited novel open field exploratory locomotion that was not significantly different from that of VEH control mice as indicated by horizontal activity (t(21) = 0.924), vertical activity (t(21) = 0.195), total distance (t(21) = 0.286) and center time (t(21) = 0.966) (Figure 2A). At 2 months post-intoxication (cohort 3, Figure 2B), TETS treated mice displayed significantly greater horizontal activity (t(24) = 0.0196) and total distance (t(24) = 0.0235) relative to VEH control mice, suggesting slight hyperactivity. There were no significant differences, however, between VEH and TETS treated mice at 2 months post-exposure for vertical activity (t(24) = 0.286) and center time (t(24) = 0.795) (Figure 2B).
Figure 2. VEH and TETS treated mice exhibited similar open field activity.
Mice injected with TETS or VEH were assessed for open field activity approximately 1 week (A) and 2 months post-exposure (B). Locomotor activity was determined in an open field arena using total distance travelled, horizontal activity, vertical activity and center time and as standard endpoint parameters. The left graph describes each parameter over 5 min time intervals, while the right graph describes total activity over the 30 min testing period. In Figures 2–5, data are presented as the mean ± SD, and the number of mice tested per treatment group appears within the graphs below the respective data. *Indicates statistically significant differences between VEH and TETS treatment groups using Student’s t-test (p < .05).
3.3. Mice intoxicated with TETS do not exhibit anxiety-like and depression-relevant behaviors
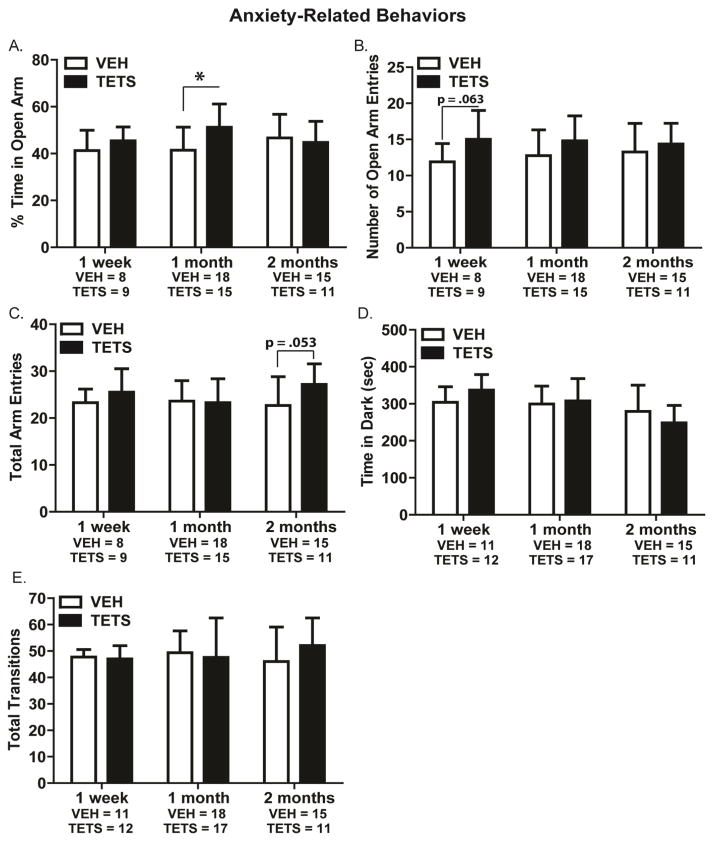
To determine whether acute TETS intoxication altered anxiety-like behavior, we employed the EPM and light↔dark transitions tests in three separate cohorts of mice evaluated at 1 week, 1 month or 2 months post-exposure. At 1 week post-TETS intoxication, there were no significant differences between VEH and TETS treated mice in the EPM with respect to the percent time spent in open arms (t(15) = 0.279) (Figure 3A) and total arm entries (t(15) = 0.281) (Figure 3B). Mice administered TETS showed a trend toward higher open arm entries compared to VEH treated mice (t(15) = 0.0648) (Figure 3C). In the light↔dark transitions test, at 1 week post-exposure, VEH and TETS treated mice exhibited similar time in the dark (t(21) = 0.255) and total transitions (t(21) = 0.909) (Figure 3D and 3E).
Figure 3. TETS treated mice did not exhibit anxiety-like behavior.
Mice intoxicated with TETS and VEH controls were assessed on two standard anxiety-like assays at 1 week, 1 month and 2 months post-exposure. Endpoints assessed in the elevated plus maze (EPM) included: (A) percent time in open arm; (B) open arm entries; and (C) total arm entries. Endpoints assessed in the light↔dark transitions test included (D) time spent in the dark chamber and (E) total number of transitions between the light and dark chambers. [*Indicates a statistically significant difference between VEH and TETS mice as determined by Student’s t-test (p < .05).]
At 1 month post-TETS intoxication, no significant differences were detected between VEH and TETS treated mice in the EPM for open arm entries (t(32) = 0.121) and total arm entries (t(32) = 0.920) (Figure 3B and 3C). In this cohort, however, TETS treated mice spent significantly more time in the open arms as compared to VEH treated mice (t(32) = 0.00624) (Figure 3A). In the light↔dark transitions test at 1 month post-exposure, there were no significant differences between VEH and TETS treated mice with respect to time spent in the dark chamber (t(31) = 0.689) (Figure 3D) or total transitions between the light and dark chambers (t(31) = 0.457) (Figure 3E).
At 2 months post-treatment, the percent time in open arms and open arm entries in the EPM were not significantly different between TETS-treated and VEH control mice (Figure 3A and 3B). In this cohort, the TETS treated mice showed a trend toward more total arm entries than VEH treated mice (t(24) = 0.0530) (Figure 3C). In the light↔dark transition test, TETS intoxicated mice exhibited time in dark (t(24) = 0.220) (Figure 3D) and total transition (t(24) = 0.214) (Figure 3E) values that were not significantly different from VEH control mice.
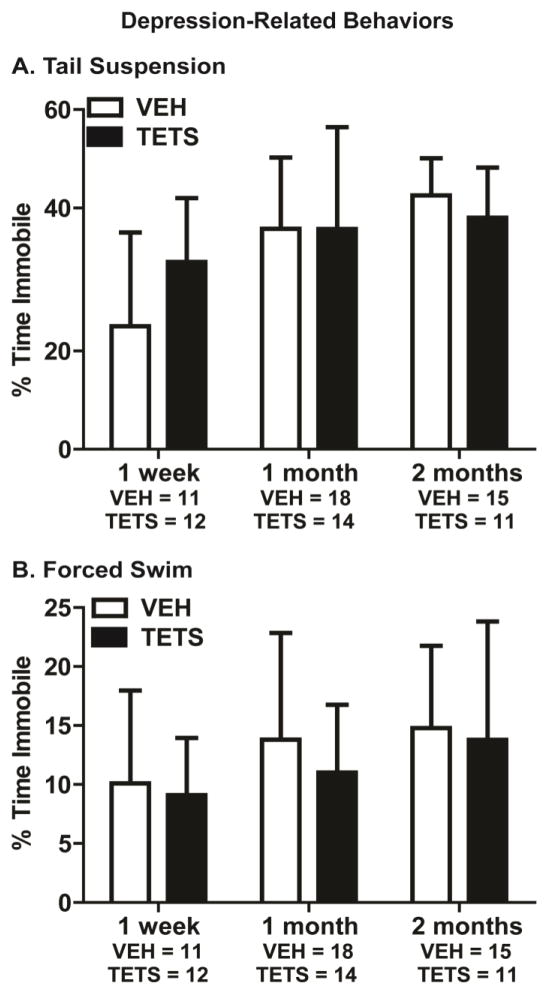
To assess depression-relevant behavior in mice intoxicated with TETS, we employed the tail suspension and Porsolt forced swim tests. During the tail suspension test, immobility was not significantly different between TETS intoxicated mice and VEH control mice at the 1 week (t(21) = 0.468), 1 month (t(31) = 0.988) or 2 month (t(24) = 0.200) time points (Figure 4A). Similarly, the percent time immobile in the forced swim test was not significantly different between TETS intoxicated and VEH control mice at 1 week (t(21) = 0.708), 1 month (t(31) = 0.282) and 2 months (t(24)= 0.504) post-exposure (Figure 4B).
Figure 4. VEH and TETS treated mice exhibited similar depression-related behavior.
TETS-intoxicated mice and VEH control mice were assessed for depression-relevant behavior at 1 week, 1 month and 2 months post-injection. Depression-relevant behavior was determined using (A) tail suspension and (B) forced swim, with percent time immobile as the endpoint measured in both tasks. No statistically significant differences were detected between VEH and TETS treatment groups using Student’s t-test (p < .05).
3.4 Acute TETS intoxication does not impair recognition memory
To assess the effects of acute TETS intoxication on memory, we initially planned to use contextual fear conditioning. However, our initial validation of these tests with the adult male NIH Swiss mouse indicated that this strain did not display the standard immobility or freezing response after fear conditioning. Using the same standard conditioning procedures, age- and sex-matched C57BL/6J mice exhibited normal freezing responses during the contextual and cued phases, demonstrating the expected learning and memory in this task (Supplementary Figure 1). Our finding is similar to that of Clapcote and colleagues who demonstrated that NIH Swiss mice were insensitive to contextual fear conditioning (Clapcote et al., 2005b). Since fear conditioning employs mild foot shocks, a possible explanation is that NIH Swiss mice are less sensitive to the aversive foot shock and therefore did not learn the negative association that subsequently caused freezing. To test this hypothesis, we employed two standard pain sensitivity tasks for mice, hot plate and tail flick nociception. Relative to C57BL/6J mice, the NIH Swiss mice exhibited significantly decreased pain sensitivity (Supplementary Figure 2), which may explain the poor response of the NIH Swiss mice in contextual fear conditioning. Therefore, we selected a non-aversive learning and memory task to determine whether acute TETS intoxication impairs memory in NIH Swiss mice, novel object recognition (NOR).
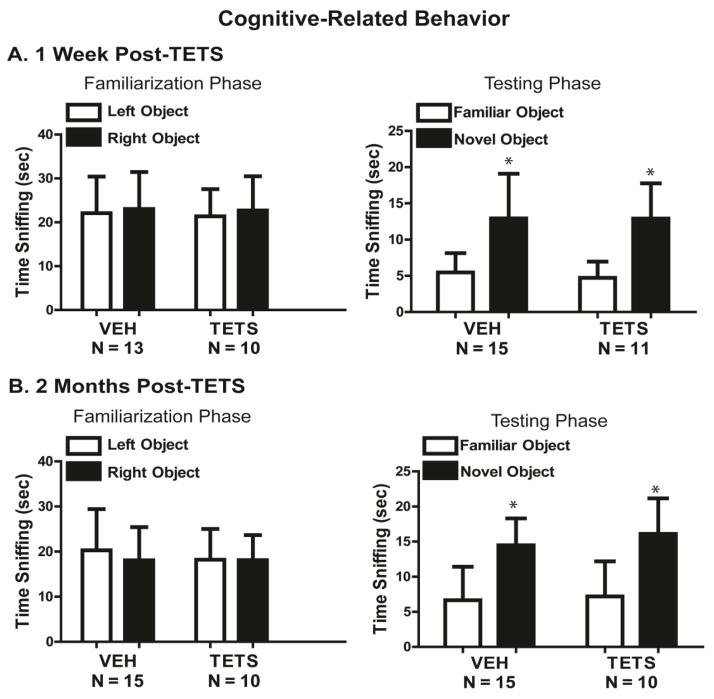
At the 1 week post-treatment time point, both VEH and TETS treated mice recognized the familiar object and spent significantly more time sniffing the novel object (F(1,24) = 38.6, p < .001) (Figure 5A). Normal NOR scores were similarly obtained at 2 months post-treatment (F(1,23) = 48.8, p < .001) (Figure 5B). During the familiarization phase, at 1 week (Figure 5A) and 2 months (Figure 5B) post-exposure, neither TETS intoxicated mice nor VEH control mice displayed a preference for object position during the familiarization phase (1 week: F(1,21) = 0.227, p = .636; 2 months: F(1,23) = 0.322, p = 0.858). On the control measure of location of objects within the open field, there was no significant difference between treatment groups for time sniffing left and right object at 1 week (F(1,21) = 0.0438, p = 0.835) or at 2 months (F(1,23) = 0.111, p = 0.740) (Figure 5A and 5B). On the control measure of total sniff time, there was no significant difference between treatment groups at either time point for total time spent sniffing the familiar versus novel objects (1 week: F(1,24) = 0.102, p = 0.752; 2 months: F(1,23) = 0.552, p = 0.465) (Figure 5).
Figure 5. Acute TETS intoxication did not impair recognition memory.
Recognition memory was assessed in TETS intoxicated mice and vehicle control mice using novel object recognition (NOR). NOR was assessed in the same cohort of animals at (A) 1 week and (B) 2 months post-exposure. During the familiarization phase, object place preference was evaluated using total sniffing time of the right versus left object. During the testing phase, novel object recognition was assessed using total sniffing time of the novel versus familiar objects. During both phases, object locations within the arena were counterbalanced. * indicates a statistically significant difference between familiar and novel object within each treatment using a Student’s t-test (p < .05). No statistically significant treatment-related differences were identified using two-way ANOVA (p < 0.05).
4. DISCUSSION
Clinical case reports indicate that individuals who survive TETS-induced seizures may experience anxiety, depression and/or memory impairments that persist for months to years following exposure (Bai et al., 2005, Whitlow et al., 2005, Zhang et al., 2011). Thus, the major goal of this study was to determine whether TETS-induced seizures cause short- and/or long-term effects on anxiety-like, depression-relevant and/or cognitive behavior in a recently characterized “TETS rescue” mouse model. Previous characterization of this model demonstrated that administration of TETS at 0.15 mg/kg (ip) to adult male NIH Swiss mice causes clonic-tonic seizures that are typically lethal within 25–30 min post-exposure (Zolkowska et al., 2012). However, administration of a high dose of diazepam following the second clonic seizure but before the onset of tonic seizures rescues these TETS intoxicated mice from death and from further seizures, as determined both behaviorally and electroencephalographically (Lein and Hammock, data under review). The results of this study are consistent with the previous studies, as all TETS intoxicated mice used for the behavioral studies described herein were documented to undergo two clonic seizures. The major findings of the behavioral studies were that relative to VEH control mice, TETS intoxicated mice: (1) did not exhibit significant anxiety-like or depression-relevant behaviors or cognitive impairment at 1 week, 1 month or 2 months post-exposure; and (2) displayed increased locomotor activity at 2 months but not at 1 week post-exposure in the novel open field test.
Anxiety-like behavior was assessed using two standard corroborative assays, elevated plus-maze and light↔dark transitions (Crawley, 2007, Crawley, 2008). Our pre-experimentation validation study had indicated that naïve adult male NIH Swiss mice perform as expected in these tests. On almost all parameters, scores were similar in the TETS and vehicle treatment groups. While we did observe that relative to VEH control mice, TETS intoxicated mice demonstrated trends on one parameter, increased open arm entries, at 1 week post-exposure, and on another parameter, more total arm entries, at two months post-exposure in the EPM, these trends were not significant. Further, no treatment trends were seen in the light↔dark transitions test. The lack of robust difference in the time spent in the closed arms and the open arms during EPM might be due to visual deficits caused by the retinal degeneration gene in the background of NIH Swiss mice, since Clapcote and coworkers reported impaired performance by NIH Swiss mice in the Morris water maze, which relies of distal visual cues (Clapcote et al., 2005b). However, it is apparent from watching mice on the EPM that other tactile senses including whiskers are extensively used when the mouse explores the open arms. Consistent with this observation, Milner and Crabbe have shown that visual impairment does not adversely affect the scores on conflict anxiety tasks like the elevated-plus maze (Milner and Crabbe, 2008). Fear conditioning similarly employs visual cues, but these are accompanied by tactile cues from the composition of the floor and shape of the chamber, along with applied olfactory stimuli and the auditory tone. These other sensory modalities represent strong stimuli for mice, leading to our interpretation that differential pain sensitivity is the more likely interpretation of the reduced response of NIH Swiss mice to footshock-induced training on the fear conditioning task. In support of this interpretation, Clapcote and colleagues concluded that the presence of the rd1 mutation, which causes retinal degeneration, had no effect on performance in the fear conditioning procedure (Clapcote et al., 2005a) and a recent paper by Iura and Udo (Iura and Udo, 2014) reported normal fear conditioning in blind mice.
Depression-relevant behavior was assessed using two standard corroborative assays, forced swim and tail suspension. We observed no significant differences between TETS intoxicated mice and VEH controls in the either assay at any post-treatment time point. The small increase in open field exploratory activity was detected at only one time point, 1 month post-treatment, and on only two of the four open field parameters. Cognition was assessed using novel object recognition, a widely used test to assess recognition memory of same versus different small objects in mice (Bevins and Besheer, 2006, Crawley, 2008, Dere et al., 2007). No significant differences between treatment groups were detected on this learning and memory task. Thus, in this specific mouse model, acute intoxication with TETS at levels that cause seizures, as documented by immediate seizure behaviors and EEG recordings in a separate cohort of NIH Swiss mice (Lein and Hammock, data under review), there is no evidence of long-term changes in anxiety-like behavior, depression-relevant behavior, exploratory behavior or impaired cognitive behavior.
Our data suggest that the long-term behavioral and cognitive deficits reported in clinical case studies of patients who survive TETS-induced seizures (Bai et al., 2005, Whitlow et al., 2005, Zhang et al., 2011) either reflect species-dependent differences in response to acute TETS intoxication or, more likely, result from SE-induced brain damage than from a direct effect of TETS itself. Rodent and human models demonstrate that SE induces neuronal damage in areas critical for behavior, learning and memory (Sheppard and Lippe, 2012). Histological analyses of brains from patients who died 11 – 27 d after the first episode of SE demonstrated extensive neuronal damage in the hippocampus, entorhinal cortex, amygdala and dorsomedial thalamic nucleus (Fujikawa et al., 2000). Additionally, baboons showed neuronal damage in the neocortex, thalamus and hippocampus after SE induced by bicuculline (Meldrum et al., 1973). While the definition of SE may vary between studies, many population-based studies define SE as a continuous state of seizing for 5–30 min or longer (Trinka et al., 2012). In humans, the occurrence and severity of SE-induced neurological sequelae, such as developmental delay, hyperactivity and mental retardation, are closely associated with the duration of SE (Eriksson and Koivikko, 1997). Similarly, in rat models of bicuculline-induced SE (Atillo et al., 1983, Soderfeldt et al., 1983a, Soderfeldt et al., 1983b) and SE induced by organophosphate nerve agents (McDonough et al., 1995, Shih et al., 2003), there is a direct correlation between the extent and severity of neuronal damage and the duration of seizures. Thus, the most plausible explanation for why the performance of NIH Swiss mice in our behavioral tests was not impaired by TETS is that the seizures experienced by these mice were not of sufficient duration to cause neuronal damage. In our model, TETS intoxicated mice experienced clonic seizures that lasted 72 s on average, and as we previously reported, these seizures are not associated with overt neuronal injury or cell death as determined by hematoxylin and eosin stain and FluoroJade B labeling (Zolkowska et al., 2012) (Lein and Hammock, data under review).
While we have not observed overt neuronal injury in the brains of mice that have experienced TETS-induced seizures, we have consistently observed delayed neuro-inflammation, evident as reactive astrogliosis and microglial activation (Zolkowska et al., 2012) and Lein and Hammock, data under review]. Behavioral deficits and cognitive decline are associated with neuroinflammation (Corona et al., 2012, Dantzer et al., 2008, Maes et al., 2009, Smith, 2013), and with neuroinflammatory conditions such as Alzheimer’s disease and ageing (Eikelenboom et al., 2002, Gimeno et al., 2009, Kuo et al., 2005, Piazza and Lynch, 2009). Neuroinflammation is characterized by increases in glia in the brain, particularly astrocytes and activated microglia that release inflammatory cytokines such as interleukins and chemokines. Both clinical human data and animal seizure models report neuroinflammation following SE (Drexel et al., 2012, Fujikawa et al., 2000, Liu et al., 2012, Steward, 1994). In these models, neuroinflammation is thought to result from neuronal damage; however, intrahippocampal administration of interleukin-1β (IL-1β) impairs contextual fear conditioning in rats (Hein et al., 2007), and, increases in hippocampal IL-1α, IL-18 and interferon-γ are associated with decreases in long-term potentiation (Griffin et al., 2006). Collectively, these data would suggest that the TETS-induced neuroinflammation observed in our TETS rescue mouse model is not sufficient to impair performance in the anxiety-like, depression-relevant and cognitive tests employed in this study. Further, it raises the question of whether neuroinflammation is an essential component of post-SE brain injury.
To summarize, acute TETS administration did not impair performance of adult male NIH Swiss mice in a battery of tests to assess anxiety, depression and cognition, likely due to short duration of seizure activity and the lack of neuronal lesions and neurodegeneration. These important negative findings could indicate that emotional and cognitive disruptions in humans exposed to TETS are due to non-biological causes, and/or that the present treatments did not sufficiently recapitulate the severity of seizures and brain damage experienced in humans exposed to TETS. Future studies will focus on developing mouse models that more precisely match the degree of TETS-induced SE observed in humans.
Supplementary Material
Highlights.
Survivors of acute TETS poisoning experience persistent psychological problems
Diazepam stops seizures in NIH Swiss mice intoxicated with lethal dose of TETS
TETS mice do not exhibit anxiety or depression-like behavior or cognitive deficits
Behavioral deficits seen in human TETS survivors likely due to sustained seizures
Acknowledgments
FUNDING:
We thank Michael Pride and Jane Hayes (Crawley lab, UC Davis) for their instruction regarding behavioral tasks. We would also like to acknowledge Dr. Michael Rogawski (UC Davis) for the use of his animal procedure room. This research was funded by the CounterACT program, National Institutes of Health Office of the Director and the National Institute of Neurological Disorders and Stroke (U54 NS079202), by the NIEHS Superfund Research Program (P42ES004699) and by the MIND Institute Intellectual and Developmental Disabilities Research Center (U54 HD079125). The funding agencies were not involved in the study design, in the collection, analysis, and interpretation of data, in the writing of the report or in the decision to submit the paper for publication.
Abbreviations
- CCTV
closed circuit television
- EEG
electroencephalography
- EPM
elevated plus maze
- GABAAR
type A gamma-aminobutyric acid receptor
- Ip
intraperitoneal
- NOR
novel object recognition
- PBS
phosphate buffered saline
- SD
standard deviation
- SE
status epilepticus
- TETS
tetramethylenedisulfotetramine
- VEH
vehicle
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Brenna M. Flannery, Email: bflannery@ucdavis.edu.
Jill L. Silverman, Email: jsilverman@ucdavis.edu.
Donald A. Bruun, Email: dabruun@ucdavis.edu.
Kyle R. Puhger, Email: krpuhger@ucdavis.edu.
Mark R. McCoy, Email: mmccoy2@csustan.edu.
Bruce D. Hammock, Email: bdhammock@ucdavis.edu.
Jacqueline N. Crawley, Email: jacqueline.crawley@ucdmc.ucdavis.edu.
Pamela J. Lein, Email: pjlein@ucdavis.edu.
LITERATURE CITED
- Atillo A, Soderfeldt B, Kalimo H, Olsson Y, Siesjo BK. Pathogenesis of brain lesions caused by experimental epilepsy. Light- and electron-microscopic changes in the rat hippocampus following bicuculline-induced status epilepticus. Acta neuropathologica. 1983;59:11–24. doi: 10.1007/BF00690312. [DOI] [PubMed] [Google Scholar]
- Bai H, Zhang SL, Zhang HS, Ji JT, Ma PB, Wang HS, et al. Evaluation of therapeutic project on acute tetramethylene disulphotetramine poisoning and effect on intelligence in children. Zhonghua Yu Fang Yi Xue Za Zhi. 2005;39:95–8. [PubMed] [Google Scholar]
- Bailey KR, Pavlova MN, Rohde AD, Hohmann JG, Crawley JN. Galanin receptor subtype 2 (GalR2) null mutant mice display an anxiogenic-like phenotype specific to the elevated plus-maze. Pharmacology, biochemistry, and behavior. 2007;86:8–20. doi: 10.1016/j.pbb.2006.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks C, Yang D, Lein P, Rowgawski M. Tetramethylenedisulfotetramine. In: Wexler P, editor. Encyclopedia of Toxicology. 3. Elsevier Inc. Academic Press; 2014. pp. 509–11. [Google Scholar]
- Barrueto F, Jr, Furdyna PM, Hoffman RS, Hoffman RJ, Nelson LS. Status epilepticus from an illegally imported Chinese rodenticide: “tetramine”. J Toxicol Clin Toxicol. 2003;41:991–4. doi: 10.1081/clt-120026523. [DOI] [PubMed] [Google Scholar]
- Bevins RA, Besheer J. Object recognition in rats and mice: a one-trial non-matching-to-sample learning task to study ‘recognition memory’. Nature protocols. 2006;1:1306–11. doi: 10.1038/nprot.2006.205. [DOI] [PubMed] [Google Scholar]
- Brielmaier J, Matteson PG, Silverman JL, Senerth JM, Kelly S, Genestine M, et al. Autism-relevant social abnormalities and cognitive deficits in engrailed-2 knockout mice. PloS one. 2012;7:e40914. doi: 10.1371/journal.pone.0040914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Can A, Blackwell RA, Piantadosi SC, Dao DT, O’Donnell KC, Gould TD. Antidepressant-like responses to lithium in genetically diverse mouse strains. Genes, brain, and behavior. 2011;10:434–43. doi: 10.1111/j.1601-183X.2011.00682.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapcote SJ, Lazar NL, Bechard AR, Roder JC. Effects of the rd1 mutation and host strain on hippocampal learning in mice. Behavior genetics. 2005a;35:591–601. doi: 10.1007/s10519-005-5634-5. [DOI] [PubMed] [Google Scholar]
- Clapcote SJ, Lazar NL, Bechard AR, Wood GA, Roder JC. NIH Swiss and Black Swiss mice have retinal degeneration and performance deficits in cognitive tests. Comparative medicine. 2005b;55:310–6. [PubMed] [Google Scholar]
- Corona AW, Fenn AM, Godbout JP. Cognitive and behavioral consequences of impaired immunoregulation in aging. Journal of neuroimmune pharmacology : the official journal of the Society on NeuroImmune Pharmacology. 2012;7:7–23. doi: 10.1007/s11481-011-9313-4. [DOI] [PubMed] [Google Scholar]
- Crawley J, Goodwin FK. Preliminary report of a simple animal behavior model for the anxiolytic effects of benzodiazepines. Pharmacology, biochemistry, and behavior. 1980;13:167–70. doi: 10.1016/0091-3057(80)90067-2. [DOI] [PubMed] [Google Scholar]
- Crawley JN. What’s Wrong With My Mouse? Behavioral Phenotyping of Transgenic and Knockout Mice. 2. New York, NY: John Wiley and Sons; 2007. [Google Scholar]
- Crawley JN. Behavioral phenotyping strategies for mutant mice. Neuron. 2008;57:809–18. doi: 10.1016/j.neuron.2008.03.001. [DOI] [PubMed] [Google Scholar]
- Croddy E. Rat poison and food security in the People’s Republic of China: focus on tetramethylene disulfotetramine (tetramine) Archives of toxicology. 2004;78:1–6. doi: 10.1007/s00204-003-0509-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryan JF, Holmes A. The ascent of mouse: advances in modelling human depression and anxiety. Nature reviews Drug discovery. 2005;4:775–90. doi: 10.1038/nrd1825. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Mombereau C, Vassout A. The tail suspension test as a model for assessing antidepressant activity: review of pharmacological and genetic studies in mice. Neuroscience and biobehavioral reviews. 2005;29:571–625. doi: 10.1016/j.neubiorev.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nature reviews Neuroscience. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dere E, Huston JP, De Souza Silva MA. The pharmacology, neuroanatomy and neurogenetics of one-trial object recognition in rodents. Neuroscience and biobehavioral reviews. 2007;31:673–704. doi: 10.1016/j.neubiorev.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Drexel M, Preidt AP, Sperk G. Sequel of spontaneous seizures after kainic acid-induced status epilepticus and associated neuropathological changes in the subiculum and entorhinal cortex. Neuropharmacology. 2012;63:806–17. doi: 10.1016/j.neuropharm.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eikelenboom P, Bate C, Van Gool WA, Hoozemans JJ, Rozemuller JM, Veerhuis R, et al. Neuroinflammation in Alzheimer’s disease and prion disease. Glia. 2002;40:232–9. doi: 10.1002/glia.10146. [DOI] [PubMed] [Google Scholar]
- Eriksson KJ, Koivikko MJ. Status epilepticus in children: aetiology, treatment, and outcome. Developmental medicine and child neurology. 1997;39:652–8. doi: 10.1111/j.1469-8749.1997.tb07358.x. [DOI] [PubMed] [Google Scholar]
- File SE, Lippa AS, Beer B, Lippa MT. Animal tests of anxiety. In: Crawley Jacqueline N, et al., editors. Current protocols in neuroscience. Unit 8. Chapter 8. 2004. p. 3. [DOI] [PubMed] [Google Scholar]
- Fujikawa DG, Itabashi HH, Wu A, Shinmei SS. Status epilepticus-induced neuronal loss in humans without systemic complications or epilepsy. Epilepsia. 2000;41:981–91. doi: 10.1111/j.1528-1157.2000.tb00283.x. [DOI] [PubMed] [Google Scholar]
- Gimeno D, Kivimaki M, Brunner EJ, Elovainio M, De Vogli R, Steptoe A, et al. Associations of C-reactive protein and interleukin-6 with cognitive symptoms of depression: 12-year follow-up of the Whitehall II study. Psychological medicine. 2009;39:413–23. doi: 10.1017/S0033291708003723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin R, Nally R, Nolan Y, McCartney Y, Linden J, Lynch MA. The age-related attenuation in long-term potentiation is associated with microglial activation. Journal of neurochemistry. 2006;99:1263–72. doi: 10.1111/j.1471-4159.2006.04165.x. [DOI] [PubMed] [Google Scholar]
- Guan FY, Liu YT, Luo Y, Hu XY, Liu F, Li QY, et al. GC/MS identification of tetramine in samples from human alimentary intoxication and evaluation of artificial carbonic kidneys for the treatment of the victims. Journal of analytical toxicology. 1993;17:199–201. doi: 10.1093/jat/17.4.199. [DOI] [PubMed] [Google Scholar]
- Hein AM, Stutzman DL, Bland ST, Barrientos RM, Watkins LR, Rudy JW, et al. Prostaglandins are necessary and sufficient to induce contextual fear learning impairments after interleukin-1 beta injections into the dorsal hippocampus. Neuroscience. 2007;150:754–63. doi: 10.1016/j.neuroscience.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogg S. A review of the validity and variability of the elevated plus-maze as an animal model of anxiety. Pharmacology, biochemistry, and behavior. 1996;54:21–30. doi: 10.1016/0091-3057(95)02126-4. [DOI] [PubMed] [Google Scholar]
- Holmes A, Iles JP, Mayell SJ, Rodgers RJ. Prior test experience compromises the anxiolytic efficacy of chlordiazepoxide in the mouse light/dark exploration test. Behavioural brain research. 2001;122:159–67. doi: 10.1016/s0166-4328(01)00184-x. [DOI] [PubMed] [Google Scholar]
- Iura Y, Udo H. Behavioral analyses of visually impaired Crx knockout mice revealed sensory compensation in exploratory activities on elevated platforms. Behavioural brain research. 2014;258:1–7. doi: 10.1016/j.bbr.2013.10.020. [DOI] [PubMed] [Google Scholar]
- Kulesskaya N, Voikar V. Assessment of mouse anxiety-like behavior in the light-dark box and open-field arena: role of equipment and procedure. Physiology & behavior. 2014;133:30–8. doi: 10.1016/j.physbeh.2014.05.006. [DOI] [PubMed] [Google Scholar]
- Kuo HK, Yen CJ, Chang CH, Kuo CK, Chen JH, Sorond F. Relation of C-reactive protein to stroke, cognitive disorders, and depression in the general population: systematic review and meta-analysis. Lancet neurology. 2005;4:371–80. doi: 10.1016/S1474-4422(05)70099-5. [DOI] [PubMed] [Google Scholar]
- Li JM, Gan J, Zeng TF, Sander JW, Zhou D. Tetramethylenedisulfotetramine intoxication presenting with de novo Status Epilepticus: a case series. Neurotoxicology. 2012;33:207–11. doi: 10.1016/j.neuro.2011.10.008. [DOI] [PubMed] [Google Scholar]
- Lister RG. The use of a plus-maze to measure anxiety in the mouse. Psychopharmacology. 1987;92:180–5. doi: 10.1007/BF00177912. [DOI] [PubMed] [Google Scholar]
- Liu C, Li Y, Lein PJ, Ford BD. Spatiotemporal patterns of GFAP upregulation in rat brain following acute intoxication with diisopropylfluorophosphate (DFP) Current neurobiology. 2012;3:90–7. [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Wang X, Yan Y, Xiao Z, Stephani U. Nongenetic cause of epileptic seizures in 2 otherwise healthy Chinese families: tetramine--case presentation and literature survey. Clinical neuropharmacology. 2008;31:57–61. doi: 10.1097/WNF.0b013e3180d09983. [DOI] [PubMed] [Google Scholar]
- Lucki I, Dalvi A, Mayorga AJ. Sensitivity to the effects of pharmacologically selective antidepressants in different strains of mice. Psychopharmacology. 2001;155:315–22. doi: 10.1007/s002130100694. [DOI] [PubMed] [Google Scholar]
- Maes M, Yirmyia R, Noraberg J, Brene S, Hibbeln J, Perini G, et al. The inflammatory & neurodegenerative (I&ND) hypothesis of depression: leads for future research and new drug developments in depression. Metabolic brain disease. 2009;24:27–53. doi: 10.1007/s11011-008-9118-1. [DOI] [PubMed] [Google Scholar]
- McDonough JH, Jr, Dochterman LW, Smith CD, Shih TM. Protection against nerve agent-induced neuropathology, but not cardiac pathology, is associated with the anticonvulsant action of drug treatment. Neurotoxicology. 1995;16:123–32. [PubMed] [Google Scholar]
- Meldrum BS, Vigouroux RA, Brierley JB. Systemic factors and epileptic brain damage. Prolonged seizures in paralyzed, artificially ventilated baboons. Archives of neurology. 1973;29:82–7. doi: 10.1001/archneur.1973.00490260026003. [DOI] [PubMed] [Google Scholar]
- Milner LC, Crabbe JC. Three murine anxiety models: results from multiple inbred strain comparisons. Genes, brain, and behavior. 2008;7:496–505. doi: 10.1111/j.1601-183X.2007.00385.x. [DOI] [PubMed] [Google Scholar]
- Piazza A, Lynch MA. Neuroinflammatory changes increase the impact of stressors on neuronal function. Biochemical Society transactions. 2009;37:303–7. doi: 10.1042/BST0370303. [DOI] [PubMed] [Google Scholar]
- Porsolt RD, Bertin A, Jalfre M. Behavioral despair in mice: a primary screening test for antidepressants. Archives internationales de pharmacodynamie et de therapie. 1977;229:327–36. [PubMed] [Google Scholar]
- Ramzy A. The New York Times. 2014. Two Children Die in Mass Poisoning at Chinese Kindergarten. [Google Scholar]
- Shakarjian MP, Veliskova J, Stanton PK, Velisek L. Differential antagonism of tetramethylenedisulfotetramine-induced seizures by agents acting at NMDA and GABA(A) receptors. Toxicology and applied pharmacology. 2012;265:113–21. doi: 10.1016/j.taap.2012.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard E, Lippe S. Cognitive outcome of status epilepticus in children. Epilepsy research and treatment. 2012;2012:984124. doi: 10.1155/2012/984124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih TM, Duniho SM, McDonough JH. Control of nerve agent-induced seizures is critical for neuroprotection and survival. Toxicology and applied pharmacology. 2003;188:69–80. doi: 10.1016/s0041-008x(03)00019-x. [DOI] [PubMed] [Google Scholar]
- Silverman JL, Turner SM, Barkan CL, Tolu SS, Saxena R, Hung AY, et al. Sociability and motor functions in Shank1 mutant mice. Brain research. 2011;1380:120–37. doi: 10.1016/j.brainres.2010.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman JL, Yang M, Turner SM, Katz AM, Bell DB, Koenig JI, et al. Low stress reactivity and neuroendocrine factors in the BTBR T+tf/J mouse model of autism. Neuroscience. 2010;171:1197–208. doi: 10.1016/j.neuroscience.2010.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. Review: the long-term consequences of microglial activation following acute traumatic brain injury. Neuropathology and applied neurobiology. 2013;39:35–44. doi: 10.1111/nan.12006. [DOI] [PubMed] [Google Scholar]
- Soderfeldt B, Kalimo H, Olsson Y, Siesjo B. Histopathological changes in the rat brain during bicuculline-induced status epilepticus. Advances in neurology. 1983a;34:169–75. [PubMed] [Google Scholar]
- Soderfeldt B, Kalimo H, Olsson Y, Siesjo BK. Bicuculline-induced epileptic brain injury. Transient and persistent cell changes in rat cerebral cortex in the early recovery period. Acta neuropathologica. 1983b;62:87–95. doi: 10.1007/BF00684924. [DOI] [PubMed] [Google Scholar]
- Steru L, Chermat R, Thierry B, Simon P. The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology. 1985;85:367–70. doi: 10.1007/BF00428203. [DOI] [PubMed] [Google Scholar]
- Steward O. Electroconvulsive seizures upregulate astroglial gene expression selectively in the dentate gyrus. Brain research Molecular brain research. 1994;25:217–24. doi: 10.1016/0169-328x(94)90156-2. [DOI] [PubMed] [Google Scholar]
- Trinka E, Hofler J, Zerbs A. Causes of status epilepticus. Epilepsia. 2012;53 (Suppl 4):127–38. doi: 10.1111/j.1528-1167.2012.03622.x. [DOI] [PubMed] [Google Scholar]
- Walf AA, Frye CA. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nature protocols. 2007;2:322–8. doi: 10.1038/nprot.2007.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitlow KS, Belson M, Barrueto F, Nelson L, Henderson AK. Tetramethylenedisulfotetramine: old agent and new terror. Ann Emerg Med. 2005;45:609–13. doi: 10.1016/j.annemergmed.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Yang M, Bozdagi O, Scattoni ML, Wohr M, Roullet FI, Katz AM, et al. Reduced excitatory neurotransmission and mild autism-relevant phenotypes in adolescent Shank3 null mutant mice. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:6525–41. doi: 10.1523/JNEUROSCI.6107-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Su M, Tian DP. Tetramine poisoning: A case report and review of the literature. Forensic Sci Int. 2011;204:e24–7. doi: 10.1016/j.forsciint.2010.06.025. [DOI] [PubMed] [Google Scholar]
- Zolkowska D, Banks CN, Dhir A, Inceoglu B, Sanborn JR, McCoy MR, et al. Characterization of seizures induced by acute and repeated exposure to tetramethylenedisulfotetramine. J Pharmacol Exp Ther. 2012;341:435–46. doi: 10.1124/jpet.111.190579. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.