Abstract
Objective
To demonstrate outer retinal tubulation (ORT) in various degenerative retinal disorders.
Methods
This was a retrospective review of the multimodal imaging of 29 eyes of 15 patients with various retinal dystrophies and inflammatory maculopathies manifesting ORT. The morphologic features of ORT and its evolution over time were analyzed using spectral-domain optical coherence tomography (SD-OCT) data.
Results
Outer retinal tubulation was identified as round or ovoid structures with hyper-reflective borders in pattern dystrophy (6 eyes), acute zonal occult outer retinopathy (5 eyes), retinitis pigmentosa (4 eyes), Stargardt disease (4 eyes), gyrate atrophy (2 eyes), choroideremia (2 eyes), and various other degenerative conditions. These structures appeared to develop from the invagination of photoreceptors at the junction of intact and atrophic outer retina. During follow-up, the number and distribution of ORT largely remained stable. As zones of atrophy enlarged, the frequency of ORT appeared to increase. The ORT structures were found in fewer than 10% of patients with retinitis pigmentosa, Stargardt, or pattern dystrophy.
Conclusion
Outer retinal tubulation is found in various degenerative retinal disorders that share in common damage to the outer retina and/or retinal pigment epithelium. The presence of ORT may be in an indicator of underlying disease stage and severity.
Keywords: ORT, Outer retinal tubulation, Retinal Degeneration, Retinal Dystrophy, Spectral OCT, Tubulation
Introduction
Spectral-domain optical coherence tomography (SD-OCT) has revolutionized our ability to image the retina and analyze structural changes within the retina and choroid that develop with disease progression. With SD-OCT’s enhanced resolution and ability to follow specific retinal features over time using eye-tracking, new findings not appreciated with time-domain OCT can now be more carefully characterized.
The term ‘outer retinal tubulation’ (ORT) was first used to describe a SD-OCT finding of branching tubular structures within the outer nuclear layer, seen primarily in eyes with neovascular age-related macular degeneration (AMD). Zweifel et al1 originally described the SD-OCT appearance of ORT as circular or ovoid structures with hyperreflective borders, resembling the findings of cystoid macular edema (CME) and subretinal fluid. These lesions often contain hyper-reflective material believed to represent malformed photoreceptor outer segments. Dr. Christine Curcio and colleagues were the first to identify this entity in a histopathologic study of eyes with advanced AMD. They noted that surviving photoreceptors appeared to reorganize into interconnecting tubes over disciform scars.2 In the original OCT series, ORT was found predominantly in eyes with choroidal neovascularization (CNV) or subretinal fibrosis; a few additional cases of ORT were seen in eyes without these features. Here, we demonstrate ORT in a variety of degenerative retinal disorders that share the common feature of significant outer retinal atrophy.
Methods
We conducted a retrospective review of consecutive patients seen by two physicians (KBF and ST) in vitreoretinal referral practices located in New York City, New York. Eyes of patients with retinal dystrophies, degenerations, or inflammatory disorders other than AMD, in whom ORT was identified with SD-OCT, were included. Diagnoses were established based on clinical phenotypes, family histories, electrophysiologic testing, and genetic testing. Patients were excluded from the study if their macular disorder was associated with CNV or subretinal fibrosis. The study period, which started at the time each eye was imaged for the first time with SD-OCT, extended from April 2008 to March 2012. The study had Western Institutional Review Board approval and was compliant with the Health Insurance Portability and Accountability Act.
All patients had undergone complete ocular examinations including best-corrected Snellen visual acuity (VA), slit-lamp biomicroscopy, and fundus photography. Fundus autofluorescence (FAF) imaging was obtained in most patients. The SD-OCT images were obtained from high-density horizontal raster scans with the Heidelberg Spectralis (Spectralis; Heidelberg Engineering, Heidelberg, Germany) which simultaneously acquires SD-OCT and near-infrared reflectance and/or FAF images, thereby enabling precise identification of corresponding sites of pathology between the different imaging modalities.
The presence of ORT was identified in each case and, when serial SD-OCT studies were available, the evolution of ORT over time was evaluated. Patient characteristics at the time of the first SD-OCT, including age, sex, ethnicity, and visual acuity, were recorded. The prevalence of ORT for certain disorders was obtained by dividing the number of diagnosed patients with ORT by the total number of diagnosed patients evaluated within the same time period with SD-OCT.
Results
The ORT structures were identified in 29 eyes of 15 patients with macular disorders without associated CNV or subretinal fibrosis. Diagnoses included pattern dystrophy (6 eyes), retinitis pigmentosa (RP) (4 eyes), Stargardt disease (4 eyes), gyrate atrophy (2 eyes), choroideremia (2 eyes), Bietti crystalline dystrophy (2 eyes), maternally inherited diabetes and deafness (MIDD) (2 eyes), thioridazine toxicity (2 eyes) and acute zonal occult outer retinopathy (AZOOR) (5 eyes). ORT was seen bilaterally in all cases except in 1 patient with AZOOR who had unilateral disease. The mean age of the patients was 51 years (range, 13–84), 60% of patients studied were male (9) and 40% were female (6). One patient was Asian, 3 patients were Hispanic, and the remaining 11 were Caucasian. The median visual acuity was 20/40 (range 20/20 to counting fingers).
Outer retinal tubulation was identified in all cases as round or ovoid structures with hyper-reflective borders surrounding a central lumen with mixed reflectivity. Outer retinal tubulation was seen both at the peripheral edge of, or centrally within, zones of outer retinal atrophy (Figure 1). The number of ORT structures in study eyes ranged from 1 to10 on any particular SD-OCT line scan. The vertical height of ORT ranged from 35 to 115 microns, and there was often elevation and/or distortion of the overlying retina related to the presence of underlying ORT. The ORT structures appeared to arise from the gradual reorganization and invagination of the photoreceptor layer with the inner segment-outer segment (IS/OS) junction, and occasionally, the external limiting membrane, appearing to form their hyperreflective border (Figure 2). During follow-up, the number and distribution of ORT largely remained stable; yet, both gain and loss of ORT were observed in some cases using the eye-tracking feature of SD-OCT and close analysis of serial sections in the OCT image sets. In some cases, as zones of atrophy enlarged, the number of ORT structures appeared to increase (Figure 3). Longer ovoid ORT structures were occasionally observed to evolve into small round rosettes during follow-up (Figure 2). In several eyes, ORT was detected in near proximity to sites of CME (Figure 4). In one patient with extensive atrophy due to Stargardt disease, there were subfoveal ORT structures in both eyes, and the visual acuity was 20/40 in the right eye and 20/30 in the left (not shown).
Figure 1.
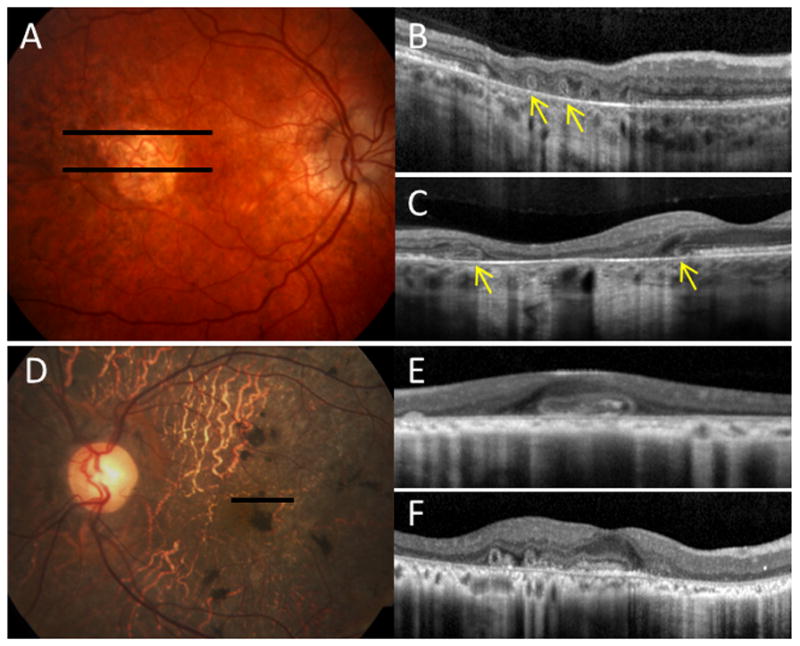
Various features of outer retinal tubulation (ORT) in retinal degenerative disorders. A, Color photograph of the right eye of a patient with pattern dystrophy. Spectral-domain optical coherence tomography (SD-OCT) scans corresponding to the solid black lines, showing circular ORT structures with hyper-reflective borders (yellow arrows) within B, a zone of geographic atrophy, and C, at the junction of preserved and disrupted outer retina. D, Color photograph of the left eye of a patient with thioridazine toxicity. E, corresponding SD-OCT scan, and F, scan of the fellow eye, showing both oblong and circular ORT structures. The retina overlying the ORT structures in B and F appears slightly elevated and distorted.
Figure 2.
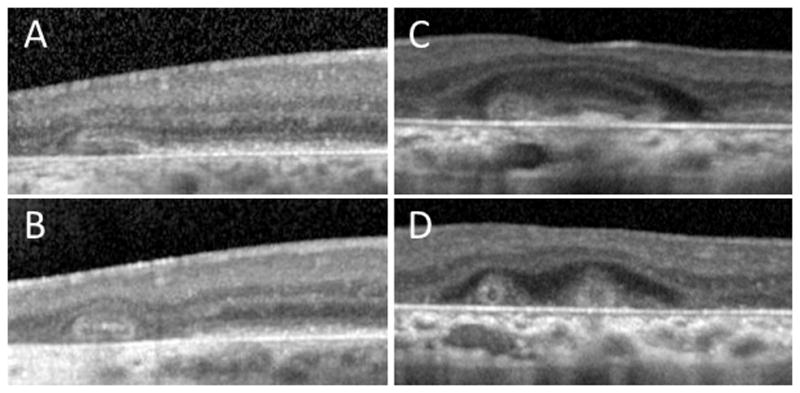
Evolution of outer retinal tubulation (ORT) over time. Eye-tracked spectral-domain optical coherence tomography (SD-OCT) scans for varying conditions at baseline (top row) and follow-up (bottom row). A, B, SD-OCT scans of an eye with choroideremia at baseline and 35 months later, showing the development of ORT from the gradual invagination of outer retinal structures, at the junction of intact and atrophic retina. C,D, SD-OCT scans of an eye with Stargardt disease, showing long and ovoid ORT structures evolving into smaller round structures, over a period of 5 months.
Figure 3.
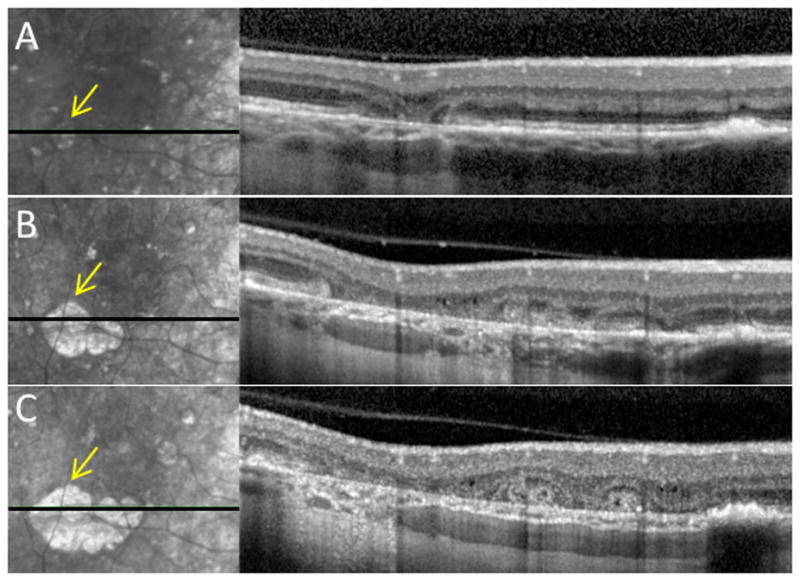
Development of outer retinal tubulation (ORT) with progressive atrophy in an eye with pattern dystrophy. Progressive infrared photographs (left) and corresponding spectral-domain optical coherence tomography (SD-OCT) scans (right) at A, baseline, B, 29 months, and C, 42 months. As the area of outer retinal atrophy enlarges (yellow arrows), there is progressive appearance of the ORT structures.
Figure 4.
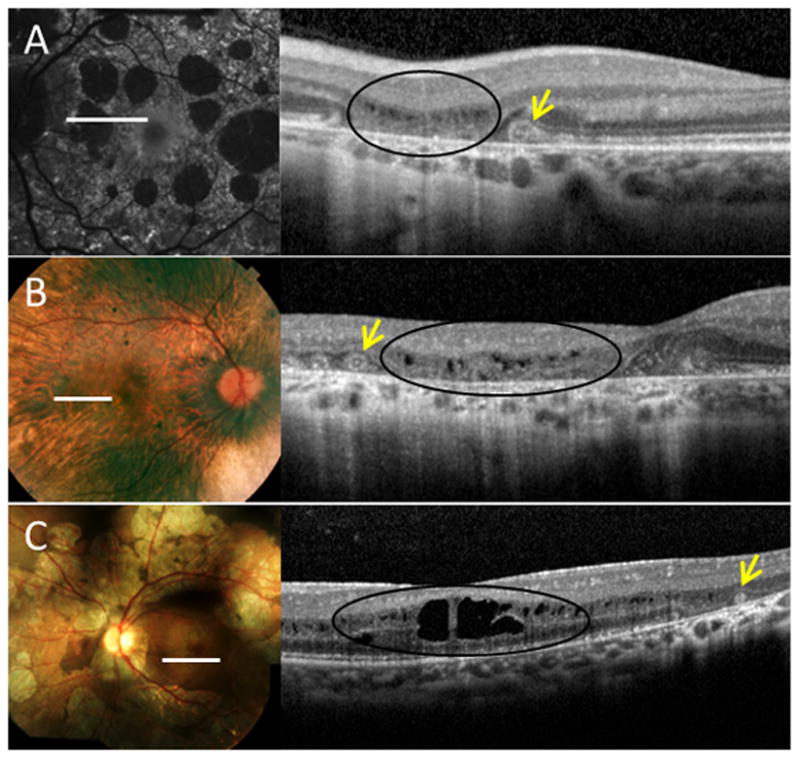
Outer retinal tubulation (ORT) in near proximity to sites of cystoid macular edema (CME) in various degenerative disorders. A, Fundus autofluorescence (left) and corresponding spectral-domain optical coherence tomography (SD-OCT) (right) of an eye with pattern dystrophy, which highlights the distinguishing features of ORT (arrows), such as hyperreflective borders and positioning within the outer nuclear layer, from the findings of CME (black ovals). B-C, Fundus photographs (left) and SD-OCTs (right) of eyes with B, choroideremia, and C, gyrate atrophy.
The ORT structures seen by SD-OCT were not detectable clinically. In some cases, the presence of ORT seen with SD-OCT corresponded to sites of increased autofluorescence with FAF imaging, relative to the hypoautofluorescent surround from geographic atrophy (Figure 5). Among 100 patients with RP evaluated with SD-OCT, ORT was identified in only 2 patients with extensive geographic atrophy without hyper-autofluorescent macular rings. Among 50 patients followed with Stargardt disease and 30 patients with pattern dystrophy, ORT structures were seen in 2 and 3 patients, respectively.
Figure 5.
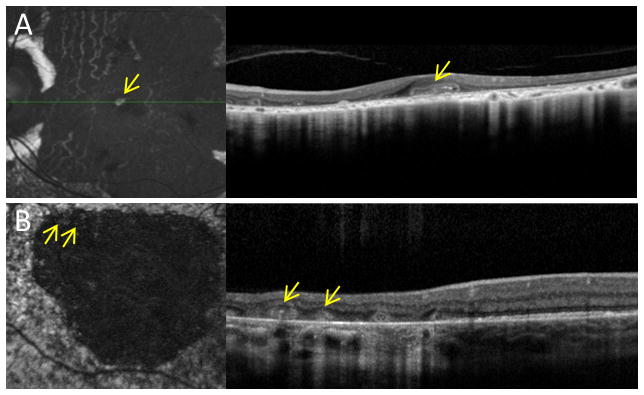
Fundus autofluorescence imaging showing ORT-containing hyperautofluorescent material within larger atrophic areas. Autofluorescence (left) with corresponding spectral-domain optical coherence tomography scans (right), of A, left eye of a 51 year-old man with thioridazine toxicity, and B, right eye of a 41 year-old man with pattern dystrophy.
Discussion
Outer retinal tubulation has previously been described in disorders primarily associated with CNV or subretinal fibrosis such as neovascular AMD and angioid streaks due to pseudoxanthoma elasticum1,3,4; it has also been reported in select conditions associated with geographic atrophy without CNV such as non-neovascular AMD and gyrate atrophy.5 In this series, we demonstrate ORT in an expanded spectrum of degenerative retinal disorders in which outer retinal damage to the photoreceptors (as in RP and AZOOR) or the retinal pigment epithelium (RPE) (as in gyrate atrophy and choroideremia) appears to be the unifying feature. Although the pathogenic mechanism leading to the formation of the tubules remains uncertain, ORT seems to represent a common final pathway in various retinal disorders whether they are inflammatory, degenerative, or neovascular in etiology.
It has been proposed, based on former histopathologic studies,2 that ORT assemble into rosette-like structures from degenerating photoreceptor cells due to a misguided reparative event, in which outward folding of the photoreceptor layer enables new lateral connections to form with reconstitution of the IS/OS junction.1 Histopathologic and immunofluorescence studies of autopsy eyes from a patient with autosomal dominant retinitis pigmentosa have shown patches of remaining photoreceptors arranged in rosettes, composed mostly of rods, with lumens of containing malformed photoreceptor elements.6 Rosettes seen histopathologically in mouse models with degenerated retinas, corresponded to tubular structures seen with OCT.7 In adult rat retinas, induced injury to photoreceptors with sparing of the RPE resulted in IS/OS degeneration and migration of photoreceptor nuclei in a circular pattern with resultant rosette-like structures.8 Yet, in other studies, in the absence of viable RPE in murine eyes, rosette formation was also observed and shown to promote photoreceptor survival.9 Although it is not entirely clear from these studies whether the triggering event for rosette formation is primary RPE or photoreceptor damage, it is conceivable that critical injury to either could result in loss of adhesion between the photoreceptor outer segments and the RPE, or secondary damage to the neighboring tissue, which may then allow the subsequent process of invagination to occur.
The process of ORT formation likely begins with outward folding of photoreceptors at the junction of intact and disrupted IS/OS, and eventual formation of a long ovoid tubular complex which, over time, may reassemble into smaller round rosettes. In a former series, ORT structures were identified in areas of normal retinal thickness and were not observed in eyes with complete photoreceptor loss, as in end-stage RP.1 In our series, however, we observed ORT not only near junctions of intact and absent IS/OS, where they appear to originate, but also as islands of residual photoreceptors within more expansive zones of atrophy, as the outer retina is progressively damaged. With the earlier development of atrophy, more ORT were observed to arise, but the number and distribution of ORT also appeared largely stable over time, as shown in cases of neovascular AMD (Jung JJ, Freund KB, In Press, Archives of Ophthalmology, 2012), suggesting that by the time ORT is detected, the underlying process, to some extent, may be less progressive. In a practice that imaged numerous patients with RP, Stargardt disease, and pattern dystrophy, less than 10% of patients with any of these conditions were found to have ORT. The relatively small incidence of ORT in disorders primarily affecting the choroid and RPE (such as pattern dystrophy) or photoreceptors (such as RP), suggests that ORT is a late stage finding in patients with advanced disease of the photoreceptors, RPE, or both. The absence of hyperautofluorescent rings in the RP patients with ORT supports this notion10, and thus, the presence of ORT by SD-OCT, may be another prognostic feature of disease severity.
Recognition of ORT as an OCT feature of various maculopathies is also important as it could possibly be mistaken for CME with lower resolution time-domain OCT. Cystoid changes, found mostly within the inner nuclear layer, are frequently seen in RP and less commonly in other entities such as gyrate atrophy, and are fairly responsive to therapy with carbonic anhydrase inhibitors.11,12 The presence of hyperreflective borders surrounding ORT and the localization within the outer retina can help distinguish ORT structures from CME and enable sparing of treatment in certain cases.
Sites of ORT seen with SD-OCT occasionally correlated with sites of increased autofluorescence with FAF, relative to the adjacent areas of atrophy and hypoautofluorescence. The autofluorescence patterns seen, together with the finding of preserved visual function in eyes with subfoveal ORT shown here and elsewhere,5 may suggest some residual degree of function over the sites of ORT structures. Despite the disruption in photoreceptor continuity that accompanies ORT formation, the photoreceptors within these structures may have some limited and transient viability.
In the original OCT series, ORT was observed predominantly in eyes with CNV or subretinal fibrosis, and the presence of intraretinal and subretinal exudation from CNV was believed to damage the photoreceptor layer which would then trigger the process of tubulation. In some cases, it was hypothesized that fluid leakage from CNV filled the tubular network. Besides AMD, inflammatory diseases with CNV such as multifocal choroiditis, among others, were found to have ORT.1 In selecting cases for this series, we also observed ORT in other macular dystrophies with extensive subretinal fibrosis including Best’s disease (1 eye) and enhanced-Scone syndrome (2 eyes). Although CNV and fibrosis are common features of eyes with ORT, the myriad of diseases reported here without antecedent exudation suggests that the presence of CNV is not required for ORT development. Significant disturbance of the photoreceptors and/or pigment epithelium, with or without exudative changes and scarring, appears sufficient for the process of tubulation to take place.
Summary Statement.
Here, we demonstrate outer retinal tubulation in a variety of degenerative retinal disorders without choroidal neovascularization.
Acknowledgments
Supported in part by the Macula Foundation, Inc. The funding organization had no role in the design or conduct of this research.
Footnotes
Financial Disclosure(s): K. Bailey Freund Genentech: Research Support, Consultant (H), Regeneron, Consultant (H).
References
- 1.Zweifel SA, Engelbert M, Laud K, et al. Outer retinal tubulation: a novel optical coherence tomography finding. Arch Ophthalmol. 2009;127(12):1596–602. doi: 10.1001/archophthalmol.2009.326. [DOI] [PubMed] [Google Scholar]
- 2.Curcio CA, Medeiros NE, Millican CL. Photoreceptor loss in age-related macular degeneration. Invest Ophthalmol Vis Sci. 1996;37(7):1236–49. [PubMed] [Google Scholar]
- 3.Wolff B, Matet A, Vasseur V, et al. En face OCT imaging for the diagnosis of outer retinal tubulations in age-related macular degeneration. J Ophthalmol. 2012;2012:542417. doi: 10.1155/2012/542417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ellabban AA, Hangai M, Yamashiro K, et al. Tomographic fundus features in pseudoxanthoma elasticum: comparison with neovascular age-related macular degeneration in Japanese patients. Eye. 2012;26(8):1086–94. doi: 10.1038/eye.2012.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sergouniotis PI, Davidson AE, Lenassi E, et al. Retinal structure, function, and molecular pathologic features in gyrate atrophy. Ophthalmology. 2012;119(3):596–605. doi: 10.1016/j.ophtha.2011.09.017. [DOI] [PubMed] [Google Scholar]
- 6.Tulvatana W, Adamian M, Berson EL, Dryja TP. Photoreceptor rosettes in autosomal dominant retinitis pigmentosa with reduced penetrance. Arch Ophthalmol. 1999;117(3):399–402. doi: 10.1001/archopht.117.3.399. [DOI] [PubMed] [Google Scholar]
- 7.Fischer MD, Huber G, Beck SC, et al. Noninvasive, in vivo assessment of mouse retinal structure using optical coherence tomography. PLoS One. 2009;4(10):e7507. doi: 10.1371/journal.pone.0007507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pittler SJ, Fliesler SJ, Fisher PL, et al. In vivo requirement of protein prenylation for maintenance of retinal cytoarchitecture and photoreceptor structure. J Cell Biol. 1995;130(2):431–9. doi: 10.1083/jcb.130.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ma N, Streilein JW. Contribution of microglia as passenger leukocytes to the fate of intraocular neuronal retinal grafts. Invest Ophthalmol Vis Sci. 1998;39(12):2384–93. [PubMed] [Google Scholar]
- 10.Lima LH, Burke T, Greenstein VC, et al. Progressive constriction of the hyperautofluorescent ring in retinitis pigmentosa. Am J Ophthalmol. 2012;153(4):718–27. doi: 10.1016/j.ajo.2011.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ganesh A, Stroh E, Manayath GJ, et al. Macular cysts in retina dystrophy. Curr Opin Ophthalomol. 2011;22(5):332. doi: 10.1097/ICU.0b013e328349229e. [DOI] [PubMed] [Google Scholar]
- 12.Oliveira TL, Andrade RE, Muccioli C, et al. Cystoid macular edema in gyrate atrophy of the choroid and retina: a fluorescein angiography and optical coherence tomography evaluation. Am J Ophthalmol. 2005;140(1):147–9. doi: 10.1016/j.ajo.2004.12.083. [DOI] [PubMed] [Google Scholar]


