Abstract
While colistin is considered a last resort for the treatment of multidrug-resistant Gram-negative bacterial infections, there has been an increase in its use due to the increasing prevalence of drug-resistant infections worldwide. The pharmacology of colistin is complex, and pharmacokinetic data are limited, especially in patients requiring renal replacement therapy. As a result, dosing for patients who require renal replacement remains a challenge. Here, we present pharmacokinetic data for colistin from two burn patients (37 and 68 years old) infected with colistin-susceptible isoclonal Acinetobacter baumannii and receiving continuous venovenous hemofiltration (CVVH). To our knowledge, we are the first to examine data from before and during CVVH (for one patient), allowing analysis of the effect of CVVH on colistin pharmacokinetics. Pharmacokinetic/pharmacodynamic analysis indicated that a dose increase from 1.5 to 2.2 mg/kg of body weight colistin base activity on CVVH was insufficient to satisfy the target parameter of an AUC24/MIC (area under the concentration-time curve over 24 h in the steady state divided by the MIC) of ≥60 at an MIC of ≥1 μg/ml in one patient with residual endogenous renal function. Plasma concentrations of colistin ranged from 0 to 15 μg/ml, with free colistin levels ranging from 0.4 to 2.2 μg/ml. While both patients resolved their clinical infections and survived to discharge, colistin-resistant colonizing isolates resulted from therapy in one patient. The variabilities observed in colistin concentrations and pharmacokinetic characteristics highlight the importance of pharmacokinetic monitoring of antibiotics in patients undergoing renal replacement therapy.
INTRODUCTION
An increase in the prevalence of multidrug-resistant Gram-negative infections worldwide (1, 2) and the lack of new antibiotics for the treatment of multidrug-resistant Gram-negative infections (3) have led to the increased use of polymyxin antibiotics (4), the most common of which are polymyxin B and polymyxin E (also known as colistin). Colistin is a multicomponent lipopeptide containing predominantly colistin A and colistin B, which differ only in the fatty acid chain attached to a cyclic decapeptide moiety (5). To reduce toxicity (3), colistin is administered primarily as the prodrug colistin methanesulfonate sodium (CMS), which is produced by sulfomethylation of five amino acid residues in colistin (6). Following parenteral administration, CMS undergoes hydrolysis in vivo to form a complex mixture of colistin and partially sulfomethylated derivatives (3). The rate of CMS conversion to formed colistin has not been well characterized in vivo but is thought to be lower than the rate of renal CMS elimination by unimpaired kidney function (6). In contrast, formed colistin undergoes predominantly nonrenal clearance through mechanisms yet to be elucidated (6).
Due to the complicated nature of CMS conversion to colistin in vivo, a lack of consensus on dosing guidelines (7, 8), and concerns about potential nephrotoxicity (6, 9), colistin has long been considered an agent of last resort for the treatment of Gram-negative infections. As the use of colistin predates modern preclinical testing requirements, pharmacokinetic data for colistin are limited. Given the increasing prevalence of multidrug-resistant Gram-negative infections and global use of colistin (2), more data are needed on the pharmacokinetics of colistin, particularly during continuous renal replacement therapies (CRRT), such as continuous venovenous hemofiltration (CVVH), which is being used more often to treat critically ill patients for its hemodynamic compatibility (10–13). Advances in high-performance liquid chromatography (HPLC) have allowed for the separation and identification of colistin in biological samples (5, 14–16). Here, we report plasma pharmacokinetic data for formed colistin in two burn patients treated for multidrug-resistant Acinetobacter baumannii infections undergoing CVVH, one of whom also underwent pharmacokinetic characterization prior to CVVH.
(A portion of this material was presented at ID Week 2013, Infectious Diseases Society of America, San Francisco, CA.)
CASE REPORT
Patient 1.
A 68-year-old male with diabetes mellitus and hypertension was transferred to the burn intensive care unit (ICU) at the U.S. Army Institute of Surgical Research after suffering 2nd- and 3rd-degree burns to the chest, back, and upper extremities, totaling 11% of the total body surface area (TBSA), as well as moderate inhalational injury, from a house fire. He arrived mechanically ventilated and experienced two episodes of ventilator-associated pneumonia (VAP) during his hospital stay, diagnosed on hospital days (HD) 14 and 30, with serial recovery of A. baumannii from the respiratory tract. The initial isolate, recovered on hospital day 12, was tested for colistin susceptibility and demonstrated an MIC of 0.38 μg/ml by epsilometer test (Etest) and resistance to all other antimicrobials tested. Additional isolates of A. baumannii were recovered from the respiratory tract up to hospital day 70 (see Fig. S1 in the supplemental material). Both episodes of VAP were treated with 14 days of intravenous (i.v.) CMS (Coly-Mycin M; Parkedale Pharmaceuticals, Rochester, MI) (4.4 mg colistin base activity [CBA]/kg of body weight/day in 2 divided doses) and nebulized CMS therapy (75 mg every 8 h). During the period of VAP treatment with colistin, he also received vancomycin titrated to renal function for skin graft donor site cellulitis and levofloxacin 750 mg i.v. for 3 days for empirical treatment of sepsis prior to the second episode of VAP (HD 26). During the second episode, empirical imipenem-cilastatin was added for Gram-negative rods recovered in bronchoalveolar lavage cultures, found to be multidrug/carbapenem-resistant A. baumannii and pan-susceptible Klebsiella pneumoniae. In addition to colistin, the patient received imipenem-cilastatin for 10 days.
On the day of pharmacokinetic sampling (HD 23, day 10 of the initial course of systemic and day 7 of nebulized CMS therapy), the patient received both i.v. CMS (2.2 mg CBA/kg every 12 h, infused over 30 min) and nebulized CMS (75 mg every 8 h). During sampling, the patient received CVVH (which was indicated for renal support) at a delivered dose of 38.1 ml/kg/h using a Prismaflex device (Gambro, Lakewood, CO) with an HF1400 polyarylethersulfone filter. Replacement fluid (RFP401; NxStage) was infused prefilter at 2 liters/h and postfilter at 2 liters/h, with a blood flow rate (Qb) of 300 ml/min and an ultrafiltration rate set to zero. The 24-h urine output on the day of sampling was 915 ml (0.2 ml/kg/h). The patient's VAP resolved clinically, with improvement in chest X-ray infiltrates and minimal ventilator settings, and he was discharged to an acute respiratory care facility 77 days after admission.
Patient 2.
A 37-year-old previously healthy male member of the U.S. military sustained 3rd-degree burns totaling 51% TBSA in a helicopter crash while overseas. After preliminary stabilization care at a combat support hospital, he underwent aeromedical evacuation to the United States and was admitted to the burn ICU at the U.S. Army Institute of Surgical Research 4 days after injury. Acute Kidney Injury Network stage 2 (17) and rhabdomyolysis were present upon admission. Orthopedic injuries included fractures of the humerus and patella. Blood cultures drawn upon admission, and subsequently bronchoalveolar lavage fluid, contained A. baumannii, susceptible only to colistin with an MIC of 0.75 μg/ml, as determined by Etest. Additional isolates were recovered from superficial cultures of intact and debrided skin from various body sites (see Fig. S1 in the supplemental material).
CMS was infused over 30 min at 2.9 mg CBA/kg/day (in 2 divided doses) initially and increased to 4.4 mg CBA/kg/day (2 divided doses) after CVVH was prescribed at 35 ml/kg/h for therapy of acute kidney injury (AKI) with metabolic acidosis. CMS was also given by nebulizer three times daily (75 mg every 8 h). During colistin therapy, the patient also received empirical treatment with vancomycin titrated to renal function and imipenem-cilastatin 250 to 500 mg every 6 h, although the recovered A. baumannii isolates were resistant to imipenem. In addition, doxycycline 100 mg daily was given for terminal malaria prophylaxis. During pharmacokinetic sampling, the patient received CVVH at a delivered dose of 28.4 ± 3.7 ml/kg/h using an NxStage device (NxStage Medical, Lawrence, MA) with a CAR500 polyethersulfone filter. Replacement fluid (RFP401; NxStage) was infused prefilter only at 3 liters/h, with a Qb of 250 ml/min and an ultrafiltration rate of 300 ml/h. The 24-h urine output on the day of sampling was 2,263 ml (0.9 ml/kg/h). Patient 2 survived to hospital discharge 154 days after admission with no clinical evidence of recurrent VAP. However, additional colonizing isolates of A. baumannii were recovered, including a colistin-resistant isolate (MIC, 24 μg/ml) from the respiratory tract (HD 47) which appeared to revert to being colistin susceptible (MIC, 1 μg/ml, on HD 52). An isolate with high-level colistin resistance (MIC, >256 g/ml) was recovered on a rectal surveillance swab on HD 101. Both colistin-resistant isolates remained resistant to all other first-line antimicrobials, and all isolates shared the identical pulsed-field gel electrophoresis (PFGE) pattern (see Fig. S1 in the supplemental material).
MATERIALS AND METHODS
Materials.
All drugs and chemicals were reagent grade and were obtained from Sigma (St. Louis, MO) unless otherwise noted.
Pharmacokinetic sampling and analysis.
Pharmacokinetic sampling of single dose curves was performed following the surrogate's informed consent as part of an ongoing prospective observational pharmacokinetic (PK) and pharmacodynamic (PD) study in trauma and burn patients approved by the U.S. Army Medical Research and Materiel Command Institutional Review Board. Three milliliters of whole blood was sampled at 0 (predose), 1, 2, 4, 8, and 12 h from the start of infusion. The samples obtained during CVVH therapy included prefilter blood and ultrafiltrate specimens. Plasma was separated from whole blood by centrifugation at 2,000 × g for 10 min at 4°C, and the plasma or ultrafiltrate sample was immediately frozen at −80°C until analysis. Pharmacokinetic parameters were estimated by noncompartmental analysis using WinNonLin software, version 6.3 (Pharsight, Cary, NC). The total (bound plus free) colistin AUC24/MIC (area under the concentration-time curve over 24 h in the steady state divided by the MIC) of ≥60, derived from a large population pharmacokinetic analysis (18), was selected as the target value to determine adequacy of therapy. When calculating the sieving coefficient (Sc), prefilter blood concentrations were multiplied by the following factor to correct for the dilution effects of prefilter replacement fluids: Qb/(Qb + Qrep), representing prefilter flow rates of blood (Qb) and replacement fluid (Qrep). The Sc was calculated as the ratio of the area under the curve (AUC) for the time-concentration curve for the ultrafiltrate to the dilution-corrected AUC of plasma (19, 20).
Sample preparation.
Colistin derivatization and isolation from plasma were performed as previously described (15). Briefly, 200 μl plasma was spiked with netilmicin (100 ng, internal standard) and loaded under vacuum onto a solid-phase extraction column (Sep-Pak C18; Waters, Milford, MA) previously conditioned with methanol (1 ml) and 1% sodium bicarbonate (1 ml, pH 10). After washing with sodium bicarbonate (500 μl), 30 μl of freshly prepared 9-fluorenylmethyl chloroformate (FMOC-Cl) was added and drawn into the extraction column. The derivatization reaction was allowed to proceed for 10 min before drying the column under vacuum. Reaction products were eluted with acetone (900 μl) and mixed with sodium borate (0.2 M, 600 μl, pH 8.5). A total of 20 μl of each sample was assayed by HPLC. Calibration curves were prepared by creating solutions of blank human plasma containing 0.1 to 10 μg/ml CBA, which were processed in parallel to the samples. Free (unbound) colistin was determined by filter centrifugation (Centrifree ultrafiltrate [UF], 30 kDa; Millipore, Billerica, MA) of plasma at 1,500 × g for 30 min. Despite reports of extensive nonspecific binding to membranes of similar chemistry but smaller (10-kDa) pore size (21), our pilot studies recovered 100% of a 1-μg/ml solution of colistin in phosphate-buffered saline.
HPLC conditions.
The concentration of colistin in plasma was determined by HPLC as previously reported (14, 15, 22, 23). Briefly, the HPLC (Dionex Ultimate 3000; Sunnyvale, CA) consisted of a binary pump, sample injection loop, thermal-controlled column compartment (25°C), and UV-diode array with fluorescence detectors. The stationary phase was a reversed-phase Luna C18 column (150 by 4.6 mm, 100 Å, 5-μm particle size; Phenomenex, Torrance, CA), and the mobile phase consisted of an acetonitrile-methanol-tetrahydrofuran-water mixture (78:10:8:4) run in the isocratic mode at 1 ml/min. FMOC-colistin was detected by fluorescence using excitation and emission wavelengths of 260 and 315 nm, respectively. The limit of detection for the assay was 100 ng/ml. Colistin peaks in the patient samples were identified by their retention times compared to blank human plasma samples spiked with colistin, and colistin A and B peaks were interpreted according to their elution order as previously reported (16). The total colistin concentration was determined by adding the peak areas of colistin A and B and dividing by the peak area of the internal standard.
Bacterial characterization.
Isolates of A. baumannii were identified using the Vitek 2 (bioMérieux, Durham, NC) and Phoenix (BD, Franklin Lakes, NJ) automated microbiology systems. Colistin MICs were determined by Etest (bioMérieux) following the testing and interpretive methods of the Clinical and Laboratory Standards Institute (CLSI; susceptible, ≤2 μg/ml). Clonal relationships were assessed by pulsed-field gel electrophoresis (PFGE) following the Pulsenet protocol (CDC) with modifications for Acinetobacter as previously described (24). Briefly, genomic DNA was digested with ApaI (New England BioLabs, Ipswich, MA) for 4 h at 25°C and separated on a 1% agarose gel in Tris-borate-EDTA buffer. PFGE was performed with a CHEF-DR III system (Bio-Rad, Hercules, CA) and a gradient of 6 V/cm at a 120° angle, with the pulse time increasing from 7 to 20 s. Electrophoresis was run at 14°C for 18.5 h. DNA from Salmonella enterica (ATCC BAA-664) samples was used as a molecular size standard. The gels were stained with 1 mg/ml ethidium bromide, destained with water, imaged with the U:Genius system (Syngene, Frederick, MD), and analyzed with BioNumerics software (Applied Maths, Austin, TX). The PFGE patterns were interpreted and grouped into pulsed-field types using established criteria (25).
RESULTS
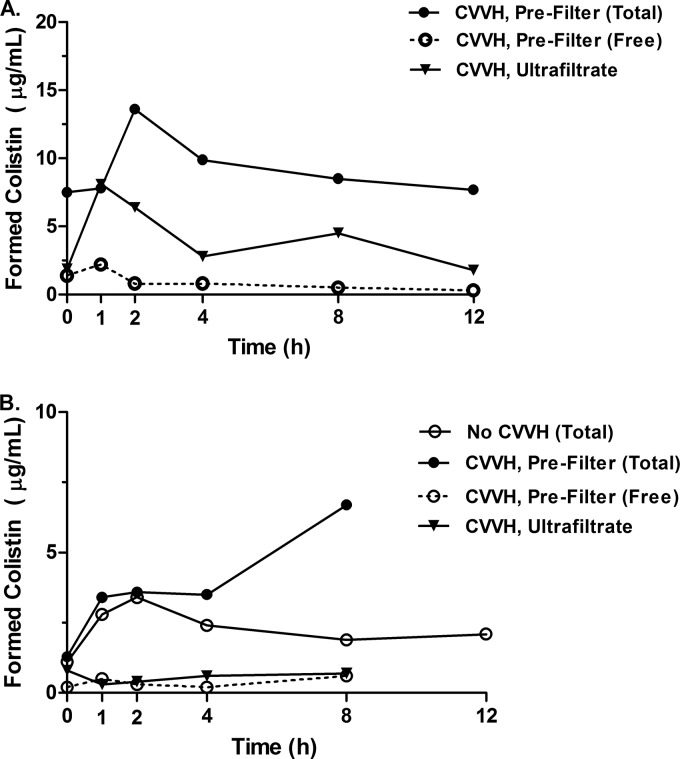
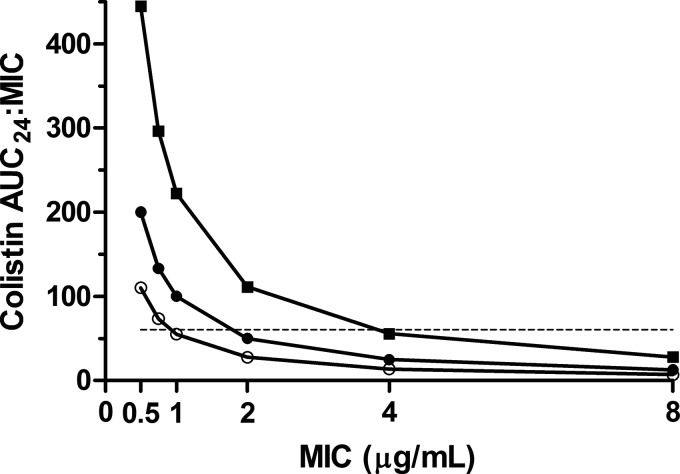
Pharmacokinetic parameters of colistin (Table 1) were estimated using noncompartmental analysis from the time-concentration curves for colistin (Fig. 1). In the plasma samples, the proportions of colistin A and B were approximately 65 to 68% and 10 to 14%, respectively, before and during CVVH. Interestingly, colistin B was cleared across the filter membrane slightly more readily than colistin A (difference from plasma, −14.2% for A versus +16.5% for B). AUC24/MIC curves were calculated for hypothetical MICs, indicating that the increased clearance associated with CVVH has the potential to compromise PK/PD targets, particularly with higher MICs and preserved renal function (Fig. 2). A PFGE analysis of A. baumannii isolates indicated that the same strain was present in all sample locations throughout the hospital stay for each patient (Dice coefficient, ≥95%; see Fig. S1 in the supplemental material). Colistin MICs ranged from 0.75 to 1.0 μg/ml for patient 1. MICs for susceptible isolates from patient 2 ranged from 0.5 to 1.5 μg/ml, with two colistin-resistant colonizing isolates having MICs from 24 to >256 μg/ml.
TABLE 1.
Patient characteristics and pharmacokinetic data for colistin in plasma samples from patients undergoing CVVH
| Characteristica | Patient 1 | Patient 2 |
|
|---|---|---|---|
| Before CVVH | During CVVH | ||
| Age (yr)/gender | 68/male | 37/male | |
| % TBSA burn | 11 | 51 | |
| CVVH delivered dose (mean ± SD) (ml/kg/h) | 38.1 ± 11.0 | 28.0 ± 3.7 | |
| Weight (kg) | 136.4 | 86.6 | 102.3 |
| Urine output (ml/kg/h) | 0.2 | 1.6 | 0.9 |
| Machine and filter | Prismaflex, HF1400 | NxStage, CAR500 | |
| Prefilter fluid replacement (liters/h) | 2 | 3 | |
| CMS dose (mg/kg) | 2.2 | 1.5 | 2.2 |
| Colistin Cmax (μg/ml) | 13.6 (2.2 free) | 3.4 (0.4 free) | 6.7 (0.6 free) |
| Css.avg (mg/ml) | 9.3 | 4.2 | 2.3 |
| AUC24 (μg · h/ml) | 222.2 (18.2 free) | 55.0 (6.0 free) | 100.0 (8.7 free)b |
| V (liters) | 86.1 | 99.7 | |
| t1/2 (h) | 22 | 11.9 | |
| Total clearance (liters/h) | 2.7 | 4.7 | |
| CVVH clearance (liters/h) | 1.4 | 0.04 | |
| % CVVH clearance | 51.6 | ||
| Sieving coefficient | 0.49 | 0.17b | |
| % Free colistin (mean ± SD) | 11.9 ± 9.2 | 13.7 ± 6.4 | 10.9 ± 4.6 |
| % Colistin A (mean ± SD) | 64.8 ± 1.4 | 67.7 ± 0.4 | 68.6 ± 2.1 |
| % Colistin B (mean ± SD) | 14.1 ± 1.7 | 10.9 ± 4.6 | 10.0 ± 2.4 |
| MIC at which AUC24/MIC was ≥60 (μg/ml) | 2 | 0.75 | 1 |
Cmax, maximum concentration; t1/2, half-life.
Only 0- to 8-h data available due to filter clotting.
FIG 1.
Time-concentration curves for CMS and colistin in patients undergoing CVVH. Concentrations over time in prefilter plasma samples for total and free colistin and ultrafiltrate fluid for patient 1 (A) and patient 2 (B). Additionally, colistin concentrations over time before CVVH are shown for patient 2. For patient 2, 12-h sampling was not performed during CVVH due to filter clotting.
FIG 2.
Achievement of the pharmacodynamic target of total colistin AUC24/MIC of ≥60 (dashed line) versus hypothetical MIC using plasma AUC values in two burn patients, one of whom was sampled before and during CVVH therapy. Filled squares, patient 1 treated with 2.2 mg CBA/kg with a delivered CVVH dose of 38.1 ± 11.0 ml/kg/h over 12 h; filled circles, patient 2 treated with 1.5 mg CBA/kg without CVVH; open circles, patient 2 treated with 2.2 mg CBA/kg with a delivered CVVH dose of 28.0 ± 3.7 ml/kg/h over 8 h. Both patients also received nebulized CMS (75 mg every 8 h) during sampling.
DISCUSSION
Colistin, approved for parenteral use in the United States as the prodrug colistin methanesulfonate, has seen a resurgence of use in the last decade as a drug of last resort for multidrug-resistant Gram-negative organisms. As it was developed prior to modern regulatory requirements, which include rigorous pharmacokinetic study, significant uncertainties persist regarding its disposition in humans. Methods for the analysis of colistin by HPLC have been described (14–16, 22, 23), resulting in an increase in pharmacokinetic studies in patients. The most comprehensive pharmacokinetic study to date included 851 sample observations from 105 subjects (18), of which 12 were treated with hemodialysis and 4 were treated with CRRT; of these, 3 were treated with continuous venovenous hemodiafiltration (CVVHDF) and 1 received CVVH. Since CVVHDF uses passive diffusion across a concentration gradient in addition to hemofiltration (the sole method for clearance in CVVH), the equivalences of these modes with respect to CMS/colistin clearance are unknown. Additionally, recent reports have described CMS and colistin pharmacokinetics in patients undergoing CVVHDF (26) and dialysis (27), but to our knowledge, this is the first study to report on the pharmacokinetics of colistin in the same individual prior to and during CVVH therapy. Evaluation of the pharmacokinetic data for colistin before and during CVVH affords the opportunity to directly study the impact of CVVH on colistin pharmacokinetics.
After administration, colistin is a complex mixture of at least 30 hydrolysis products of the prodrug CMS, with colistin A and B serving as the major products (6, 14). The pharmacokinetic dispositions of CMS and colistin in humans are equally complex, especially under conditions of CVVH therapy, with CMS being cleared renally but colistin being cleared by poorly defined nonrenal mechanisms. Additionally, the in vivo rate and extent of conversion from CMS to colistin are unknown but are thought to be lower than the renal clearance of CMS by unimpaired kidneys (6), and there is uncertainty as to whether CMS hydrolysis is spontaneous or enzymatically catalyzed (3, 6). Despite the fact that the CVVH circuit and apparatus can functionally increase the volume of distribution (V), the V of patient 1 (during CVVH) was slightly lower than that of patient 2 (without CVVH). This may reflect a reduced circulating plasma volume due to fluid losses from ultrafiltration. CRRT further complicates the situation, as data describing the rate of CRRT clearance of CMS and colistin or to what extent CRRT clearance of CMS may be offset by a reduced glomerular filtration rate are sparse. Other than monitoring of urine output, there are no methods currently available to determine the degree of intrinsic renal function while receiving CRRT. Additionally, there are various modes of CRRT utilized with various doses that are prescribed. The use of intermittent modes of renal replacement in many units with hybrid techniques, such as sustained low-efficiency dialysis, also complicates the picture (28–30). As a result, CMS dosing for patients who require renal replacement remains a challenge.
While this study provides valuable in vivo pharmacokinetic data for colistin in patients undergoing CVVH and, for the first time, pharmacokinetic data for colistin before and during CVVH, some limitations of this study require caution in drawing broad conclusions. The small sample size (two patients) and lack of data for CMS limit the statistical comparisons and reduce the power of analyses. Unfortunately, filter clotting in patient 2 during CVVH, which may have caused a rise in the plasma colistin concentration prior to clotting, prevented calculation of the full suite of PK parameters, because a terminal clearance slope was not determined. Further, although samples were kept cold during the analytical procedures, some spontaneous conversion of CMS to colistin in ultrafiltrate and plasma samples during or after collection is possible (14), particularly in the ultrafiltrate that is sampled from fluid collected at ambient temperature over a long sampling interval. However, concentrations of formed colistin in the ultrafiltrate were similar at the beginning and end of sampling. Additionally, the patients were treated with nebulized CMS during CVVH and pharmacokinetic sampling, which may complicate the interpretation of colistin plasma concentrations, but inhaled colistin has been shown to have minimal systemic absorption at doses higher than those observed here (31, 32). Due to the inherent limitations in the system of care at the U.S. Army Institute of Surgical Research, we were not able to ascertain the age of the CVVH filters at the time of sampling. This is relevant because the sieving efficiency of individual filters is known to degrade over time (33), likely as a result of proteinaceous fouling (34). Thus, newly placed filters in the CVVH circuit may have enhanced solute clearance relative to older filters. While the delivered CVVH doses during colistin PK sampling reflect those typically used for renal support (10–13), higher doses have been used for sepsis and other states of shock (13, 35, 36). The impact of higher CVVH doses on colistin and other antimicrobials remains to be determined.
A previous analysis, based upon population pharmacokinetics derived from three nonburned CVVHDF patients and one CVVH patient, has suggested that a daily dose of 192 mg CBA (irrespective of body weight) is necessary for each 1.0-μg/ml steady-state average colistin concentration (Css.avg) during continuous renal replacement (18). In our study, patient 1 received 600 mg daily during CVVH, but this resulted in a plasma Css.avg above the predicted value (predicted Css.avg, 3.1 μg/ml; measured Css.avg, 9.3 μg/ml). In contrast, the Css.avg for patient 2, who received 450 mg daily during CVVH, was accurately predicted (predicted Css.avg, 2.3 μg/ml; measured Css.avg, 2.3 μg/ml). This difference may be attributable to the greater preservation of endogenous renal function in patient 2, as reflected by urinary output. In addition, potential drug interactions impairing the clearance of colistin are impossible to assess, given that the mechanism for colistin clearance (which is nonrenal) remains unknown.
Colistin has been reported to have 59 to 74% protein binding (26 to 41% unbound fraction) in a study of nonburned critically ill patients (37), whereas we observed slightly higher protein binding of 80 to 90%. The increased protein binding we observed relative to previous reports may be due to prolonged elevation in alpha-1-acid glycoprotein, as occurs in burn patients (38, 39) and to which colistin has moderate binding affinity (40). This highlights the need for further detailed studies examining free colistin pharmacokinetics in burn patients, as a previous population pharmacokinetic model derived from burn patients did not incorporate protein binding data (41) and the unbound fraction has been identified as a relevant PK/PD parameter (42). The values before and during CVVH were similar for patient 2 (Table 1), suggesting that CVVH did not affect the protein binding status of colistin. This highlights the uncertain and variable results of CMS dosing and suggests a need for therapeutic drug monitoring to achieve optimal levels during CVVH, particularly for oliguric or anuric patients.
Colistin MIC values before and during therapy were below the CLSI “susceptible” breakpoint of 2 μg/ml. It is notable, therefore, that for patient 2, an increase in the CMS dose from 2.9 to 4.4 mg CBA/kg/day (2 divided doses) during CVVH was insufficient to achieve an AUC24/MIC of ≥60 when the MIC was ≥1 μg/ml (Fig. 2). Thus, isolates determined to be susceptible according to current CLSI interpretive criteria may receive inadequate therapy despite achieving the predicted plasma concentration. Indeed, Garonzik et al. (18) were prudent to acknowledge that their recommended maintenance doses may not be reliably effective against isolates with colistin MICs of >0.5 μg/ml. Published reports of treatment-associated colistin resistance are accumulating in the literature (43–45), perhaps corroborating in vitro data suggesting that no regimen of colistin monotherapy (including continuous infusion) can prevent colistin resistance in A. baumannii (46). Additionally, colistin resistance arose in patient 2 despite concurrent therapy with vancomycin and imipenem, which in vitro data suggest may be able to prevent the emergence of resistance when given concurrently with colistin (46).
Despite its limitations, this study provides an opportunity to examine the total clearance and fractional CVVH-related clearance rates for colistin and adds to the existing pharmacokinetic data for an increasingly common antibiotic for increasingly common multidrug-resistant bacterial infections. We observed significant variability in colistin concentrations resulting from recommended dosing strategies, with high and low concentration excursions in the setting of CVVH, suggesting risks for toxicity and compromised PK/PD target attainment, respectively. In our opinion, this study highlights the importance of antibiotic PK/PD monitoring in intensive care unit (ICU) patients, particularly those undergoing continuous renal replacement therapy.
Supplementary Material
ACKNOWLEDGMENTS
This study was conducted under a protocol reviewed and approved by the U.S. Army Medical Research and Materiel Command Institutional Review Board in accordance with approved protocol H-09-059.
This work was supported by Defense Medical Research & Development Program (DMRDP) Military Infectious Disease Clinical Trial Award (MID-CTA) D_MIDCTA_I_12_J2_299 and, in part, by an appointment to the Postgraduate Research Participation Program at the U.S. Army Institute of Surgical Research administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the U.S. Department of Energy and USAMRMC.
We gratefully acknowledge the invaluable contributions of Doug Johnson, Kristie Harnisch, Crystal Rosemann, Lance Ferguson, and the participating research subjects, without whom this research would not be possible.
The opinions or assertions contained herein are the private views of the authors and are not to be construed as official or as reflecting the views of the Department of the Army or the Department of Defense.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.03783-14.
REFERENCES
- 1.Walsh TR, Toleman MA. 2012. The emergence of pan-resistant Gram-negative pathogens merits a rapid global political response. J Antimicrob Chemother 67:1–3. doi: 10.1093/jac/dkr378. [DOI] [PubMed] [Google Scholar]
- 2.Wertheim H, Van Nguyen K, Hara GL, Gelband H, Laxminarayan R, Mouton J, Cars O. 2013. Global survey of polymyxin use: a call for international guidelines. J Glob Antimicrob Resist 1:131–134. doi: 10.1016/j.jgar.2013.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bergen PJ, Li J, Rayner CR, Nation RL. 2006. Colistin methanesulfonate is an inactive prodrug of colistin against Pseudomonas aeruginosa. Antimicrob Agents Chemother 50:1953–1958. doi: 10.1128/AAC.00035-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zavascki AP. 2014. Polymyxins for the treatment of extensively-drug-resistant Gram-negative bacteria: from pharmacokinetics to bedside. Expert Rev Anti Infect Ther 12:531–533. doi: 10.1586/14787210.2014.902307. [DOI] [PubMed] [Google Scholar]
- 5.Orwa JA, Govaerts C, Busson R, Roets E, Van Schepdael A, Hoogmartens J. 2001. Isolation and structural characterization of colistin components. J Antibiot (Tokyo) 54:595–599. doi: 10.7164/antibiotics.54.595. [DOI] [PubMed] [Google Scholar]
- 6.Nation RL, Velkov T, Li J. 2014. Colistin and polymyxin B: peas in a pod, or chalk and cheese? Clin Infect Dis 59:88–94. doi: 10.1093/cid/ciu213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li J, Nation RL, Turnidge JD. 2006. Defining the dosage units for colistin methanesulfonate: urgent need for international harmonization. Antimicrob Agents Chemother 50:4231 (Reply, 50:4231–4232.) doi: 10.1128/AAC.00756-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nation RL, Li J, Cars O, Couet W, Dudley MN, Kaye KS, Mouton JW, Paterson DL, Tam VH, Theuretzbacher U, Tsuji BT, Turnidge JD. 2014. Consistent global approach on reporting of colistin doses to promote safe and effective use. Clin Infect Dis 58:139–141. doi: 10.1093/cid/cit680. [DOI] [PubMed] [Google Scholar]
- 9.Koch-Weser J, Sidel VW, Federman EB, Kanarek P, Finer DC, Eaton AE. 1970. Adverse effects of sodium colistimethate: manifestations and specific reaction rates during 317 courses of therapy. Ann Intern Med 72:857–868. doi: 10.7326/0003-4819-72-6-857. [DOI] [PubMed] [Google Scholar]
- 10.Jamal JA, Mat-Nor MB, Mohamad-Nor FS, Udy AA, Lipman J, Roberts JA. 2014. A national survey of renal replacement therapy prescribing practice for acute kidney injury in Malaysian intensive care units. Nephrology (Carlton) 19:507–512. doi: 10.1111/nep.12276. [DOI] [PubMed] [Google Scholar]
- 11.Legrand M, Darmon M, Joannidis M, Payen D. 2013. Management of renal replacement therapy in ICU patients: an international survey. Intensive Care Med 39:101–108. doi: 10.1007/s00134-012-2706-x. [DOI] [PubMed] [Google Scholar]
- 12.Friedrich JO, Wald R, Bagshaw SM, Burns KE, Adhikari NK. 2012. Hemofiltration compared to hemodialysis for acute kidney injury: systematic review and meta-analysis. Crit Care 16:R146. doi: 10.1186/cc11458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chung KK, Lundy JB, Matson JR, Renz EM, White CE, King BT, Barillo DJ, Jones JA, Cancio LC, Blackbourne LH, Wolf SE. 2009. Continuous venovenous hemofiltration in severely burned patients with acute kidney injury: a cohort study. Crit Care 13:R62. doi: 10.1186/cc7801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li J, Milne RW, Nation RL, Turnidge JD, Coulthard K. 2003. Stability of colistin and colistin methanesulfonate in aqueous media and plasma as determined by high-performance liquid chromatography. Antimicrob Agents Chemother 47:1364–1370. doi: 10.1128/AAC.47.4.1364-1370.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li J, Milne RW, Nation RL, Turnidge JD, Coulthard K, Johnson DW. 2001. A simple method for the assay of colistin in human plasma, using pre-column derivatization with 9-fluorenylmethyl chloroformate in solid-phase extraction cartridges and reversed-phase high-performance liquid chromatography. J Chromatogr B Biomed Sci Appl 761:167–175. doi: 10.1016/S0378-4347(01)00326-7. [DOI] [PubMed] [Google Scholar]
- 16.Li J, Milne RW, Nation RL, Turnidge JD, Coulthard K, Valentine J. 2002. Simple method for assaying colistin methanesulfonate in plasma and urine using high-performance liquid chromatography. Antimicrob Agents Chemother 46:3304–3307. doi: 10.1128/AAC.46.10.3304-3307.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, Levin A, Acute Kidney Injury Network . 2007. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care 11:R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garonzik SM, Li J, Thamlikitkul V, Paterson DL, Shoham S, Jacob J, Silveira FP, Forrest A, Nation RL. 2011. Population pharmacokinetics of colistin methanesulfonate and formed colistin in critically ill patients from a multicenter study provide dosing suggestions for various categories of patients. Antimicrob Agents Chemother 55:3284–3294. doi: 10.1128/AAC.01733-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Colton CK, Henderson LW, Ford CA, Lysaght MJ. 1975. Kinetics of hemodiafiltration. I. In vitro transport characteristics of a hollow-fiber blood ultrafilter. J Lab Clin Med 85:355–371. [PubMed] [Google Scholar]
- 20.Henderson LW, Colton CK, Ford CA. 1975. Kinetics of hemodiafiltration. II. Clinical characterization of a new blood cleansing modality. J Lab Clin Med 85:372–391. [PubMed] [Google Scholar]
- 21.Li J, Milne RW, Nation RL, Turnidge JD, Smeaton TC, Coulthard K. 2003. Use of high-performance liquid chromatography to study the pharmacokinetics of colistin sulfate in rats following intravenous administration. Antimicrob Agents Chemother 47:1766–1770. doi: 10.1128/AAC.47.5.1766-1770.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li J, Rayner CR, Nation RL, Deans R, Boots R, Widdecombe N, Douglas A, Lipman J. 2005. Pharmacokinetics of colistin methanesulfonate and colistin in a critically ill patient receiving continuous venovenous hemodiafiltration. Antimicrob Agents Chemother 49:4814–4815. doi: 10.1128/AAC.49.11.4814-4815.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Markantonis SL, Markou N, Fousteri M, Sakellaridis N, Karatzas S, Alamanos I, Dimopoulou E, Baltopoulos G. 2009. Penetration of colistin into cerebrospinal fluid. Antimicrob Agents Chemother 53:4907–4910. doi: 10.1128/AAC.00345-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Durmaz R, Otlu B, Koksal F, Hosoglu S, Ozturk R, Ersoy Y, Aktas E, Gursoy NC, Caliskan A. 2009. The optimization of a rapid pulsed-field gel electrophoresis protocol for the typing of Acinetobacter baumannii, Escherichia coli and Klebsiella spp. Jpn J Infect Dis 62:372–377. [PubMed] [Google Scholar]
- 25.Tenover FC, Arbeit RD, Goering RV, Mickelsen PA, Murray BE, Persing DH, Swaminathan B. 1995. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J Clin Microbiol 33:2233–2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karvanen M, Plachouras D, Friberg LE, Paramythiotou E, Papadomichelakis E, Karaiskos I, Tsangaris I, Armaganidis A, Cars O, Giamarellou H. 2013. Colistin methanesulfonate and colistin pharmacokinetics in critically ill patients receiving continuous venovenous hemodiafiltration. Antimicrob Agents Chemother 57:668–671. doi: 10.1128/AAC.00985-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Strunk AK, Schmidt JJ, Baroke E, Bode-Boger SM, Martens-Lobenhoffer J, Welte T, Kielstein JT. 2014. Single- and multiple-dose pharmacokinetics and total removal of colistin in a patient with acute kidney injury undergoing extended daily dialysis. J Antimicrob Chemother 69:2008–2010. doi: 10.1093/jac/dku075. [DOI] [PubMed] [Google Scholar]
- 28.Bohler J, Donauer J, Keller F. 1999. Pharmacokinetic principles during continuous renal replacement therapy: drugs and dosage. Kidney Int (Suppl):S24–S28. [PubMed] [Google Scholar]
- 29.Choi G, Gomersall CD, Tian Q, Joynt GM, Freebairn R, Lipman J. 2009. Principles of antibacterial dosing in continuous renal replacement therapy. Crit Care Med 37:2268–2282. doi: 10.1097/CCM.0b013e3181aab3d0. [DOI] [PubMed] [Google Scholar]
- 30.Roberts JA, Lipman J. 2009. Pharmacokinetic issues for antibiotics in the critically ill patient. Crit Care Med 37:840–851; quiz, 859. doi: 10.1097/CCM.0b013e3181961bff. [DOI] [PubMed] [Google Scholar]
- 31.Lu Q, Girardi C, Zhang M, Bouhemad B, Louchahi K, Petitjean O, Wallet F, Becquemin MH, Le Naour G, Marquette CH, Rouby JJ. 2010. Nebulized and intravenous colistin in experimental pneumonia caused by Pseudomonas aeruginosa. Intensive Care Med 36:1147–1155. doi: 10.1007/s00134-010-1879-4. [DOI] [PubMed] [Google Scholar]
- 32.Lu Q, Luo R, Bodin L, Yang J, Zahr N, Aubry A, Golmard JL, Rouby JJ, Nebulized Antibiotics Study Group . 2012. Efficacy of high-dose nebulized colistin in ventilator-associated pneumonia caused by multidrug-resistant Pseudomonas aeruginosa and Acinetobacter baumannii. Anesthesiology 117:1335–1347. doi: 10.1097/ALN.0b013e31827515de. [DOI] [PubMed] [Google Scholar]
- 33.Claure-Del Granado R, Macedo E, Chertow GM, Soroko S, Himmelfarb J, Ikizler TA, Paganini EP, Mehta RL. 2011. Effluent volume in continuous renal replacement therapy overestimates the delivered dose of dialysis. Clin J Am Soc Nephrol 6:467–475. doi: 10.2215/CJN.02500310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dana AP, Ahmar CA, Clapp WL, Ross EA. 2011. A new form of myeloma “kidney”: shortened hemofilter survival and implications for membrane filtration plasmapheresis. Clin Nephrol 75:120–124. doi: 10.5414/CNP75120. [DOI] [PubMed] [Google Scholar]
- 35.Laurent I, Adrie C, Vinsonneau C, Cariou A, Chiche JD, Ohanessian A, Spaulding C, Carli P, Dhainaut JF, Monchi M. 2005. High-volume hemofiltration after out-of-hospital cardiac arrest: a randomized study. J Am Coll Cardiol 46:432–437. doi: 10.1016/j.jacc.2005.04.039. [DOI] [PubMed] [Google Scholar]
- 36.Rimmele T, Kellum JA. 2012. High-volume hemofiltration in the intensive care unit: a blood purification therapy. Anesthesiology 116:1377–1387. doi: 10.1097/ALN.0b013e318256f0c0. [DOI] [PubMed] [Google Scholar]
- 37.Mohamed AF, Karaiskos I, Plachouras D, Karvanen M, Pontikis K, Jansson B, Papadomichelakis E, Antoniadou A, Giamarellou H, Armaganidis A, Cars O, Friberg LE. 2012. Application of a loading dose of colistin methanesulfonate in critically ill patients: population pharmacokinetics, protein binding, and prediction of bacterial kill. Antimicrob Agents Chemother 56:4241–4249. doi: 10.1128/AAC.06426-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martyn JA, Abernethy DR, Greenblatt DJ. 1984. Plasma protein binding of drugs after severe burn injury. Clin Pharmacol Ther 35:535–539. doi: 10.1038/clpt.1984.73. [DOI] [PubMed] [Google Scholar]
- 39.Pos O, van der Stelt ME, Wolbink GJ, Nijsten MW, van der Tempel GL, van Dijk W. 1990. Changes in the serum concentration and the glycosylation of human alpha 1-acid glycoprotein and alpha 1-protease inhibitor in severely burned persons: relation to interleukin-6 levels. Clin Exp Immunol 82:579–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Azad MA, Huang JX, Cooper MA, Roberts KD, Thompson PE, Nation RL, Li J, Velkov T. 2012. Structure-activity relationships for the binding of polymyxins with human alpha-1-acid glycoprotein. Biochem Pharmacol 84:278–291. doi: 10.1016/j.bcp.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee J, Han S, Jeon S, Hong T, Song W, Woo H, Yim DS. 2013. Population pharmacokinetic analysis of colistin in burn patients. Antimicrob Agents Chemother 57:2141–2146. doi: 10.1128/AAC.00271-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dudhani RV, Turnidge JD, Coulthard K, Milne RW, Rayner CR, Li J, Nation RL. 2010. Elucidation of the pharmacokinetic/pharmacodynamic determinant of colistin activity against Pseudomonas aeruginosa in murine thigh and lung infection models. Antimicrob Agents Chemother 54:1117–1124. doi: 10.1128/AAC.01114-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim Y, Bae IK, Lee H, Jeong SH, Yong D, Lee K. 2014. In vivo emergence of colistin resistance in Acinetobacter baumannii clinical isolates of sequence type 357 during colistin treatment. Diagn Microbiol Infect Dis 79:362–366. doi: 10.1016/j.diagmicrobio.2014.03.027. [DOI] [PubMed] [Google Scholar]
- 44.Lesho E, Yoon EJ, McGann P, Snesrud E, Kwak Y, Milillo M, Onmus-Leone F, Preston L, St Clair K, Nikolich M, Viscount H, Wortmann G, Zapor M, Grillot-Courvalin C, Courvalin P, Clifford R, Waterman PE. 2013. Emergence of colistin-resistance in extremely drug-resistant Acinetobacter baumannii containing a novel pmrCAB operon during colistin therapy of wound infections. J Infect Dis 208:1142–1151. doi: 10.1093/infdis/jit293. [DOI] [PubMed] [Google Scholar]
- 45.Snitkin ES, Zelazny AM, Gupta J, Program NCS, Palmore TN, Murray PR, Segre JA. 2013. Genomic insights into the fate of colistin resistance and Acinetobacter baumannii during patient treatment. Genome Res 23:1155–1162. doi: 10.1101/gr.154328.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cai Y, Chai D, Wang R, Liang B, Bai N. 2012. Colistin resistance of Acinetobacter baumannii: clinical reports, mechanisms and antimicrobial strategies. J Antimicrob Chemother 67:1607–1615. doi: 10.1093/jac/dks084. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.