Abstract
Background
Overall mortality rates from coronary heart disease (CHD) in the U.S. have declined in recent decades, yet the rate has plateaued among younger women. The potential for further improvement in mortality rates among young women through changes in lifestyle is unknown.
Objectives
To estimate the proportion of CHD cases and clinical cardiovascular disease (CVD) risk factors among young women that might be attributable to poor adherence to a healthy lifestyle.
Methods
We conducted a prospective analysis among 88,940 women, aged 27–44 years at baseline, in the Nurses’ Health Study II and followed from 1991 – 2011. Lifestyle factors were updated repeatedly by questionnaire. A healthy lifestyle was defined as not smoking, normal body mass index (BMI), physical activity ≥ 2.5 hours/week, television ≤ 7 hours/week, diet in top 40% of the Alternative Healthy Eating Index-2010, and 0.1 – 14.9 g/day of alcohol. To estimate the proportion of CHD and clinical CVD risk factors (diabetes, hypertension, hypercholesterolemia) that could be attributed to poor adherence to a healthy lifestyle, we calculated the population attributable risk percent.
Results
During 20 years of follow-up, we documented 456 incident CHD cases. In multivariable-adjusted models, non-smoking, healthy BMI, exercise, and healthy diet were independently and significantly associated with lower CHD risk. Compared to women with no healthy lifestyle factors, the hazard ratio (HR) for CHD for women with 6 lifestyle factors was 0.08 (95% CI: 0.03 to 0.22). Approximately 73% (95% CI: 39%to 89%) of CHD cases were attributable to poor adherence to a healthy lifestyle. Similarly, 46% (95% CI: 43%to 49%) of clinical CVD risk factor cases were attributable to poor lifestyle.
Conclusions
Primordial prevention through maintenance of a healthy lifestyle among young women may substantially lower the burden of CVD.
Keywords: epidemiology, coronary disease, risk factors, hypertension, diabetes, hypercholesterolemia
INTRODUCTION
Although overall mortality rates from coronary heart disease (CHD) in the U.S. have declined steadily in the last four decades, the decline in CHD mortality rates among adults aged 35–54 years has slowed (1,2). Of particular concern is that the mortality rate among women aged 35–44 years increased on average by 1.3% (95% CI 0.2 to 2.5) per year between 1997 and 2002 and predicted CHD risk among young adults and women has declined only modestly since then (2,3). The lack of more significant improvements in CHD incidence and mortality among younger adults may be explained, in part, by adverse risk factor profiles. While hypertension rates, management of hypercholesterolemia, and tobacco use have improved, these positive changes may be offset by increases in the prevalence of obesity and type 2 diabetes (2,4).
Young women with a favorable cardiovascular disease (CVD) risk profile have a very low risk of CHD mortality compared with others (5) and recent evidence suggests that maintaining a healthy lifestyle throughout young adulthood is strongly associated with a low risk profile in middle age (6). Primordial prevention, defined as prevention of the development of clinical risk factors (7), through maintenance or adoption of a healthy lifestyle will sustain women in a low CVD risk profile and, consequently, reduce their incidence of CHD (1). Furthermore, it could reduce the substantial economic burden of the medical management of intermediate CVD-related conditions as hypertension, diabetes mellitus, and hyperlipidemia are among the top ten leading diagnoses for direct health expenditures in the U.S. (1) Finally, targeting younger women for primordial prevention of CHD may be more feasible, before the development of adverse risk factor profiles common in older adults.
The purpose of this analysis was to estimate the proportion of cases of CHD and clinical CVD risk factors – diabetes, hypertension, or high cholesterol – among younger women, who are attributable to poor adherence to a healthy lifestyle.
METHODS
Study Population
The Nurses’ Health Study II (NHSII) was established in 1989 when 116,430 registered nurses, aged 25–42 years, completed a baseline questionnaire that included lifestyle assessment and medical history. Follow-up biennial questionnaires were sent to participants to collect updated information on potential risk factors and newly diagnosed diseases. This study was approved by the Institutional Review Board at Brigham and Women’s Hospital. Informed consent was implied by completion of the questionnaire.
Participants first completed a validated semi-quantitative food frequency questionnaire (FFQ) (8) in 1991, which served as the baseline for this analysis. Women were excluded if they did not complete the FFQ (n = 18,075), left more than 70 food items blank (n = 236), or reported energy intake <600 kcal/d or >3500 kcal/d (n = 2101). Women with CVD, cancer, or diabetes prior to 1991 (n = 4,001) or missing physical activity (n = 511) or body mass index (BMI) (n = 2,566) at baseline were excluded, leaving 88,940 women for the CHD analysis. For the analysis of clinical CVD risk factors, we additionally excluded 19,693 women with a diagnosis of hypertension or hypercholesterolemia prior to 1991, leaving 69,247 women in the CVD analysis.
Assessment of Lifestyle Factors
Information on weight, height (baseline only), smoking status, and physician diagnosis of disease was obtained biennially. Leisure-time physical activity (including television watching) was assessed in 1991, 1997, 2001, 2005, and 2009 with a previously validated questionnaire (9) on time per week spent on various activities over the previous year. The total hours per week spent engaged in moderate- to vigorous-intensity (≥3 METs) physical activity (10) was calculated.
The FFQ was completed every four years beginning in 1991 (8). For each food item, participants were asked how often a specified portion was consumed during the past year. Nutrient intake was calculated by multiplying the nutrient content of each food by the frequency of intake and summed across all food items (11). All nutrients were adjusted for total energy intake by regressing nutrient intake on total energy (12).
Definition of Optimal Lifestyle
We considered 6 factors to define optimal lifestyle: smoking, diet, physical activity, television watching, BMI, and alcohol consumption. These factors were selected based on the evidence for their association with CHD and current recommendations for CVD prevention. For each lifestyle factor, a participant received 1 point if she met the criteria for optimal and 0 points if she did not.
For smoking, the optimal group was defined as those who were not currently smoking. Based on current guidelines, we defined optimal physical activity as engaging in at least 2.5 hours per week of moderate- to vigorous-intensity exercise (13). As there is not yet a recommendation for T.V. watching, we decided a priori that optimal would be defined as 7 hours/week or less (14). We defined optimal BMI as 18.5–24.9 kg/m2 (15).
For alcohol, women were classified as being optimal if they consumed an average of 0.1 to 14.9 g of alcohol per day (approximately l drink), which is consistent with current guidelines (16) among non-pregnant women who choose to drink (as pregnant women should not consume alcohol). It should be noted that ‘optimal’ in this case is with regards to cardiovascular risk, as alcohol may increase risk of other outcomes.
For diet, we defined optimal as an Alternate Healthy Eating Index-2010 (AHEI-2010) score (17) in the top 40% of the cohort distribution (corresponding to a score ≥47) as there are no numeric targets established for the AHEI-2010 (see footnote in Table 1). The AHEI is an evidence-based measure of diet quality that is associated with low risk of chronic disease (17,18). As we decided a priori to include alcohol as a separate factor, it is not included in the AHEI-2010 score in this analysis.
Table 1.
Hazard ratio (95% confidence interval) of coronary heart disease by healthy lifestyle factors
| Healthy Lifestyle Factor | Person-years, % | No. of Cases | Age-adjusted HR | P-value for Linear Trend | MV-adjusted HR* | P-value for Linear Trend |
|---|---|---|---|---|---|---|
| Smoking, cigs/day | ||||||
| ≥ 25 | 1.2 | 22 | 1.00 | <.001 | 1.00 | <.001 |
| 15 – 24 | 3.3 | 53 | 0.88 (0.54, 1.46) | 1.02 (0.61, 1.68) | ||
| 1 – 14 | 4.5 | 47 | 0.57 (0.34, 0.96) | 0.75 (0.45, 1.27) | ||
| Former | 25.2 | 116 | 0.21 (0.13, 0.34) | 0.29 (0.18, 0.47) | ||
| Never | 65.8 | 218 | 0.18 (0.11, 0.28) | 0.23 (0.15, 0.36) | ||
| Physical activity, h/wk | ||||||
| < 0.2 | 20.2 | 149 | 1.00 | <.001 | 1.00 | 0.004 |
| 0.2 – 0.9 | 15.7 | 86 | 0.86 (0.66, 1.12) | 1.01 (0.77, 1.32) | ||
| 1 – 2.4 | 20.5 | 85 | 0.64 (0.49, 0.83) | 0.80 (0.61, 1.06) | ||
| 2.5 – 4.9 | 19.7 | 67 | 0.52 (0.39, 0.69) | 0.74 (0.55, 1.00) | ||
| ≥ 5 | 23.9 | 69 | 0.43 (0.32, 0.57) | 0.67 (0.50, 0.91) | ||
| Alternative Healthy Eating Index-2010 score† | ||||||
| < 36 | 16.9 | 79 | 1.00 | <.001 | 1.00 | 0.003 |
| 36 – 41.9 | 21.1 | 117 | 1.01 (0.76, 1.35) | 1.15 (0.86, 1.54) | ||
| 42 – 46.9 | 20.2 | 109 | 0.91 (0.68, 1.22) | 1.12 (0.83, 1.51) | ||
| 47 – 52.9 | 20.4 | 85 | 0.67 (0.49, 0.92) | 0.89 (0.65, 1.22) | ||
| ≥ 53 | 21.4 | 66 | 0.45 (0.32, 0.63) | 0.68 (0.48, 0.96) | ||
| Body mass index, kg/m2 | ||||||
| ≥ 35.0 | 9.9 | 97 | 1.00 | <.001 | 1.00 | <.001 |
| 30.0 – 34.9 | 12.9 | 86 | 0.69 (0.51, 0.92) | 0.85 (0.63, 1.14) | ||
| 25.0 – 29.9 | 26.4 | 133 | 0.53 (0.41, 0.69) | 0.75 (0.57, 0.99) | ||
| 18.5 – 24.9 | 49.2 | 135 | 0.35 (0.27, 0.45) | 0.58 (0.43, 0.78) | ||
| < 18.5 | 1.5 | 5 | 0.48 (0.20, 1.18) | 0.72 (0.29, 1.80) | ||
| Alcohol, g/day | ||||||
| 0 | 39.7 | 225 | 1.00 | <.001 | 1.00 | 0.006‡ |
| 0.1 – 4.9 | 35.5 | 136 | 0.69 (0.56, 0.86) | 0.75 (0.60, 0.93) | ||
| 5.0 –14.9 | 17.9 | 65 | 0.60 (0.46, 0.79) | 0.73 (0.55, 0.97) | ||
| 15.0 – 29.9 | 4.9 | 18 | 0.54 (0.33, 0.87) | 0.67 (0.41, 1.10) | ||
| ≥ 30 | 2.0 | 12 | 0.81 (0.45, 1.45) | 0.74 (0.41, 1.34) | ||
| TV watching, h/wk | ||||||
| ≥ 20.0 | 7.2 | 59 | 1.00 | 0.001 | 1.00 | 0.60 |
| 10.0 – 19.9 | 19.4 | 86 | 0.58 (0.41, 0.80) | 0.71 (0.51, 0.99) | ||
| 5.0 – 9.9 | 27.4 | 124 | 0.62 (0.46, 0.85) | 0.83 (0.60, 1.14) | ||
| 1.1 – 4.9 | 33.6 | 135 | 0.57 (0.42, 0.77) | 0.84 (0.61, 1.15) | ||
| ≤1.0 | 12.3 | 52 | 0.58 (0.40, 0.84) | 0.94 (0.64, 1.38) | ||
The models were stratified by age (in months) and time period and included parental history of MI before 60 years of age, aspirin use, menopausal status, postmenopausal hormone use, parity, oral contraceptive use, and history of hypertension or hypercholesterolemia at baseline. All healthy lifestyle factors were included simultaneously in the same model.
The AHEI-2010 includes 11 components: high intake of vegetables, fruits, whole grains, nuts and legumes, long-chain (n-3) fats (EPA + DHA), and polyunsaturated fatty acids; moderate intake of alcohol; and low intake of sugar-sweetened beverages and fruit juice, red and processed meat, trans fat, and sodium. The AHEI-2010 score ranges from 0 to 100 (after excluding alcohol) with higher scores representing better adherence.
P for non-linearity = 0.03.
Outcome Ascertainment
Incident CHD
The primary endpoint was incident CHD, which included nonfatal myocardial infarction (MI) and fatal CHD diagnosed after the return of the 1991 questionnaire and before June 2011. Self-reported MIs were confirmed by medical records according to World Health Organization criteria that included symptoms plus either diagnostic ECG changes or elevated cardiac enzymes (19). Fatal CHD was confirmed by hospital or autopsy records or if CHD was listed as the cause of death on the death certificate and evidence of previous CHD was available.
Clinical CVD Risk Factors
Additionally, we examined the outcome of diagnosis with at least one of three physician-diagnosed clinical CVD risk factors – type 2 diabetes, hypertension, or hypercholesterolemia – reported after the return of the 1991 questionnaire and through 2011. Type 2 diabetes was defined as a self-report of incident diabetes confirmed by a validated supplemental questionnaire using the 1997 American Diabetes Association criteria (20). Incident hypertension and hypercholesterolemia were self-reported from biennial questionnaires. The calendar year of diagnosis was recorded and used to estimate a time to event month assignment for the purposes of survival analysis, based on the month of questionnaire return (21).
Statistical Analysis
All analyses were performed using SAS statistical software, version 9.3 (SAS Institute Inc., Cary, NC). Each eligible participant contributed person-time from the return of the 1991 questionnaire until the date of diagnosis of the first event (CHD or clinical CVD risk factor), death, or June 2011.
To obtain the best estimate of long-term dietary intake and to reduce measurement error, we used the cumulative average of diet scores from repeated dietary assessments as described previously (12). For all other healthy lifestyle factors and covariates, we used simple updated levels of each variable in which outcomes were predicted from the most recent questionnaire. For example, events that occurred between 1991 and 1993 were examined in relation to exposures reported on the 1991 questionnaire; events occurring between 1993 and 1995 were examined in relation to exposures reported on the 1993 questionnaire; and so forth. We skipped any questionnaire cycle during which a participant was pregnant.
To examine the association between healthy lifestyle factors and CHD or clinical CVD risk factors, separate Cox proportional hazards models were used to estimate hazard ratios (HR) of each outcome (CHD or clinical CVD risk factor). The models were stratified by age (in months) and time period and adjusted for parental history of MI before 60 years of age, aspirin use, menopausal status, postmenopausal hormone use, parity, and oral contraceptive use. Additionally, the models for CHD included history of hypertension or hypercholesterolemia at baseline. Tests for linear trend were computed using category medians, except for smoking for which an ordinal variable was used. Statistical significance was defined as p < 0.05. We also examined possible non-linear relations between healthy lifestyle factors and each outcome non-parametrically with restricted cubic splines (22).
To estimate the proportion of CHD and clinical CVD risk factors attributable to poor adherence to a healthy lifestyle, we calculated the population attributable risk percent (PAR%), an estimate of the percentage of cases in this population that would not have occurred if all women had been in the optimal group, assuming a causal relationship (23). To allow valid calculation of the PAR%, pooled logistic regression models were used with age and time period included explicitly in the model.
RESULTS
During 20 years of follow-up, 456 women had documented incident CHD and 31,691 reported a physician diagnosis of one or more clinical CVD risk factors (n = 2,749 with diabetes, n = 16,978 with hypertension, and n = 23,971 with hypercholesterolemia). The mean (SD) age of the population at baseline was 37.1 (4.5) years, while the mean (SD) age at diagnosis of CHD was 50.3 (5.9) years and the mean (SD) age at diagnosis with a clinical CVD risk factor was 46.8 (6.2) years.
Association between Healthy Lifestyle Factors and CHD, Clinical CVD Risk Factors
In multivariable-adjusted analyses (Table 1), not smoking, higher physical activity, higher AHEI-2010 diet score, and lower BMI were associated with lower risk of CHD (P for linear trend <0.01 for each). The relationship with alcohol was J-shaped, with the lowest risk being among women with alcohol intake of 15.0 – 29.9 g/day. Television watching was not significantly associated with CHD after adjusting for the other healthy lifestyle factors. Similar results were seen for clinical CVD risk factors: not smoking, higher physical activity, higher AHEI-2010 score, lower BMI, and not watching television were each associated with lower risk of a diagnosed clinical CVD risk factor (diabetes, hypertension, or hypercholesterolemia) (P for linear trend <0.001 for each, Table 2). Again, the association with alcohol intake appeared J-shaped, with the lowest risk among women who reported 5.0 – 14.9 g/day (P for non-linearity <0.0001).
Table 2.
Hazard ratio (95% CI) of diagnosis with a clinical CVD risk factor (diabetes, hypertension, or hypercholesterolemia*) by healthy lifestyle factors
| Healthy Lifestyle Factor | Person-years, % | No. of Cases | Age-adjusted HR | P-value for Linear Trend | MV-adjusted HR† | P-value for Linear Trend |
|---|---|---|---|---|---|---|
| Smoking, cigs/day | ||||||
| ≥ 25 | 1.1 | 411 | 1.00 | <.001 | 1.00 | <.001 |
| 15 – 24 | 3.3 | 1109 | 0.94 (0.84, 1.05) | 1.01 (0.90, 1.13) | ||
| 1 – 14 | 4.8 | 1445 | 0.81 (0.73, 0.91) | 0.92 (0.83, 1.03) | ||
| Former | 24.2 | 8365 | 0.83 (0.75, 0.92) | 0.95 (0.86, 1.05) | ||
| Never | 66.5 | 20,361 | 0.80 (0.72, 0.88) | 0.91 (0.82, 1.00) | ||
| Physical activity, h/wk | ||||||
| < 0.2 | 18.0 | 7015 | 1.00 | <.001 | 1.00 | <.001 |
| 0.2 – 0.9 | 15.6 | 5125 | 0.91 (0.88, 0.95) | 0.98 (0.94, 1.01) | ||
| 1 – 2.4 | 21.0 | 6662 | 0.87 (0.84, 0.90) | 0.98 (0.94, 1.01) | ||
| 2.5 – 4.9 | 20.7 | 6046 | 0.77 (0.75, 0.80) | 0.93 (0.90, 0.96) | ||
| ≥ 5 | 24.7 | 6843 | 0.68 (0.66, 0.70) | 0.88 (0.85, 0.91) | ||
| Alternative Healthy Eating Index-2010 score | ||||||
| < 36 | 17.7 | 5446 | 1.00 | <.001 | 1.00 | <.001 |
| 36 – 41.9 | 21.6 | 6950 | 0.92 (0.89, 0.96) | 0.95 (0.91, 0.98) | ||
| 42 – 46.9 | 20.0 | 6576 | 0.89 (0.86, 0.92) | 0.93 (0.90, 0.97) | ||
| 47 – 52.9 | 19.8 | 6514 | 0.84 (0.81, 0.88) | 0.91 (0.88, 0.95) | ||
| ≥ 53 | 20.8 | 6205 | 0.72 (0.69, 0.74) | 0.83 (0.80, 0.86) | ||
| Body mass index, kg/m2 | ||||||
| ≥ 35.0 | 5.9 | 3840 | 1.00 | <.001 | 1.00 | <.001 |
| 30.0 – 34.9 | 9.5 | 5231 | 0.82 (0.79, 0.86) | 0.85 (0.82, 0.89) | ||
| 25.0 – 29.9 | 24.3 | 9729 | 0.61 (0.59, 0.63) | 0.65 (0.62, 0.67) | ||
| 18.5 – 24.9 | 58.3 | 12,637 | 0.36 (0.35, 0.37) | 0.39 (0.38, 0.41) | ||
| < 18.5 | 2.0 | 254 | 0.23 (0.21, 0.27) | 0.26 (0.23, 0.29) | ||
| Alcohol, g/day | ||||||
| 0 | 38.5 | 12,788 | 1.00 | <.001 | 1.00 | 0.07‡ |
| 0.1 – 4.9 | 36.7 | 10,945 | 0.89 (0.87, 0.91) | 0.94 (0.91, 0.96) | ||
| 5.0 – 14.9 | 18.5 | 5469 | 0.79 (0.76, 0.81) | 0.90 (0.87, 0.93) | ||
| 15.0 – 29.9 | 4.6 | 1659 | 0.83 (0.79, 0.87) | 0.96 (0.91, 1.01) | ||
| ≥ 30 | 1.8 | 830 | 1.06 (0.99, 1.14) | 1.17 (1.09, 1.26) | ||
| TV watching, h/wk | ||||||
| ≥ 20.0 | 5.8 | 2399 | 1.00 | <.001 | 1.00 | <.001 |
| 10.0 – 19.9 | 18.2 | 6486 | 0.89 (0.85, 0.93) | 0.99 (0.94, 1.03) | ||
| 5.0 – 9.9 | 27.5 | 8787 | 0.80 (0.77, 0.84) | 0.94 (0.90, 0.99) | ||
| 1.1 – 4.9 | 35.0 | 10,422 | 0.74 (0.71, 0.78) | 0.92 (0.88, 0.97) | ||
| ≤1.0 | 13.6 | 3597 | 0.64 (0.60, 0.67) | 0.85 (0.80, 0.89) | ||
Total number of cases = 31,691; diabetes n = 2749, hypertension n =16,978, hypercholesterolemia n = 23,971.
The models were stratified by age (in months) and time period and included parental history of MI before 60 years of age, aspirin use, menopausal status, postmenopausal hormone use, parity, and oral contraceptive use. All healthy lifestyle factors were included simultaneously in the same model.
P for non-linearity <.001.
When the healthy lifestyle factors were collapsed into binary categories of optimal vs. not optimal, each lifestyle factor remained significantly associated with lower risk of incident CHD (except television watching) and diagnosis with a clinical CVD risk factor in multivariable-adjusted models including all lifestyle factors simultaneously (Table 3). Among women in the optimal category for all 6 lifestyle factors, comprising approximately 5% of the study population, the hazard ratio (HR) for CHD was 0.08 (95% CI 0.03 to 0.22) and for presence of ≥1 clinical CVD risk factor was 0.34 (95% CI 0.30 to 0.38), compared to women not in the optimal category for any lifestyle factor.
Table 3.
Hazard ratio (95% CI) of diagnosis with a clinical CVD risk factor (diabetes, hypertension, or hypercholesterolemia) or coronary heart disease (CHD) by optimal lifestyle factor status
|
|
|||||||
|---|---|---|---|---|---|---|---|
| CHD (n = 456) | Clinical CVD risk factor (n = 31,691) | ||||||
|
| |||||||
| Lifestyle Factor | Definition of optimal | Person-years at optimal level, % | MV-adjusted HR* | PAR, % | Person-years at optimal level, % | MV-adjusted HR* | PAR, % |
| Smoking | Not currently smoking | 91.0% | 0.29 (0.23, 0.35) | 19.2 (13.6, 24.6) | 90.7% | 0.97 (0.93, 1.00) | 0.4 (0.1, 0.8) |
| Physical activity | ≥ 2.5 hrs/wk | 43.7% | 0.72 (0.58, 0.88) | 20.0 (7.4, 32.0) | 45.4% | 0.88 (0.85, 0.90) | 8.2 (6.8, 9.6) |
| AHEI-2010 score | AHEI score ≥ 47 | 41.8% | 0.69 (0.57, 0.85) | 19.5 (8.1, 30.4) | 40.6% | 0.90 (0.88, 0.92) | 5.4 (4.0, 6.8) |
| BMI | 18.5 – 24.9 kg/m2 | 49.2% | 0.68 (0.55, 0.84) | 22.9 (10.2, 34.9) | 58.3% | 0.54 (0.53, 0.55) | 29.8 (28.6, 31.0) |
| Alcohol | 0.1 – 14.9 g/day | 53.4% | 0.77 (0.64, 0.93) | 12.6 (3.1, 21.8) | 55.2% | 0.91 (0.89, 0.93) | 4.6 (3.5, 5.7) |
| T.V. watching | ≤ 7 hrs/wk | 45.9% | 1.04 (0.86, 1.26) | -------- | 48.6% | 0.91 (0.89, 0.93) | 5.9 (4.6, 7.2) |
| All 6 factors | ----- | 4.6% | 0.08 (0.03, 0.22) | 72.7 (39.1, 89.2) | 5.4% | 0.34 (0.30, 0.38) | 46.1 (42.7, 49.3) |
The models were stratified by age (in months) and time period and included parental history of MI before 60 years of age, aspirin use, menopausal status, postmenopausal hormone use, parity, and oral contraceptive use. For CHD, the model also includes history of hypertension or hypercholesterolemia at baseline. All healthy lifestyle factors were included simultaneously in the same model.
Population Attributable Risk Percent for CHD, Clinical CVD Risk Factors
Table 3 additionally provides the PAR% for each healthy lifestyle factor separately as well as all six factors combined. For women in the optimal category for all six lifestyle factors, the PAR% for CHD was 72.7% (95% CI: 39.1, to 89.2), suggesting that almost three quarters of all confirmed CHD events in this cohort of younger women could have been prevented if all women were in the optimal group. For diagnosis of at least one clinical CVD risk factor, the PAR% was 46.1% (95% CI: 42.7 to 49.3). As a sensitivity analysis, we restricted cases of incident hypertension and hypercholesterolemia only to participants also reporting medication use and the PAR% was 52.7% (95% CI: 48.7 to 56.5).
Lifestyle Factors and CHD Risk in Women with and without Clinical CVD Risk Factors
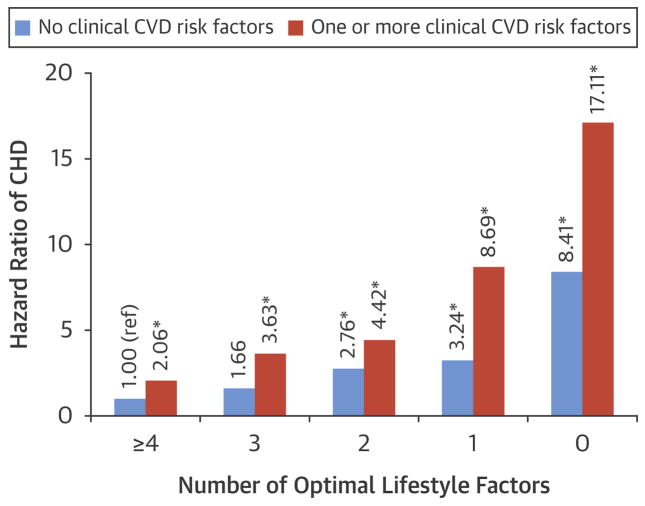
The Figure shows the association between the number of optimal lifestyle factors and CHD among women with no clinical CVD risk factors and women with one or more risk factors. Overall, for a given number of optimal lifestyle factors, women with one or more clinical CVD risk factors (diabetes, hypertension, or hypercholesterolemia) were at higher risk of CHD compared to women with no risk factors. However, even among women diagnosed with a CVD risk factor, adherence to 4 or more healthy lifestyle factors was associated with a substantially lower risk of CHD (HR = 2.06, 95% CI 1.20 to 3.52) compared to adherence to zero healthy lifestyle factors (HR = 17.11, 95% CI 9.29 to 31.52) (P for difference based on Wald test < .001). Additionally, the PAR% for all lifestyle factors combined was slightly higher in women with at least one clinical CVD risk factor (71.1%, 95% CI: 36.3 to 88.5) compared to women with no CVD risk factors (64.9%, 95% CI: 24.6 to 86.1) (Online Supplemental Table 1).
Figure. Hazard ratios for coronary heart disease according to number of optimal lifestyle factors among women who have no CVD risk factors and one or more risk factors.
The models were stratified by age and period and included parental history of MI, aspirin use, menopausal status, postmenopausal hormone use, parity, and oral contraceptive use.
A ‘*’ indicates values significant at p = 0.05. P for interaction = 0.48.
When we stratified by age, the PAR% for CHD was 66.5% (95% CI: 12.0 to 90.2) for all six factors among women less than 50 years of age and 78.5% (95% CI: 26.8 to 95.1) among women 50 years and older. Finally, we examined the association between the lifestyle factors and each of the clinical CVD risk factors separately (Online Supplemental Table 2). The PAR% for all 6 lifestyle factors combined was 92.9% (95% CI: 87.6 to 96.0) for diabetes, 57.0% (95% CI: 52.8 to 60.9) for hypertension, and 40.0% (95% CI: 35.5 to 44.3) for hypercholesterolemia.
DISCUSSION
In this large, prospective study of young U.S. women, adhering to a healthy lifestyle was associated with decreased risk of incident CHD as well as decreased risk of diagnosis with one or more clinical CVD risk factors, including type 2 diabetes, hypertension, and hypercholesterolemia (Central Illustration). Women who engaged in all 6 healthy lifestyle choices had a 92% lower risk of CHD and 66% lower risk of a clinical CVD risk factor. If these associations are causal, over 70% of CHD and nearly half of CVD risk factor diagnoses in this cohort may have been prevented if all women had been in the optimal category for all 6 lifestyle factors. Thus, even among young women, healthy lifestyle plays an important role in the primordial prevention of CHD.
Central Illustration. Hazard Ratios (95% CI) for Coronary Heart Disease or Diagnosis With a Clinical CVD Risk Factor (Diabetes, Hypertension, or Hypercholesterolemia) for Optimal Levels of Lifestyle Factors.
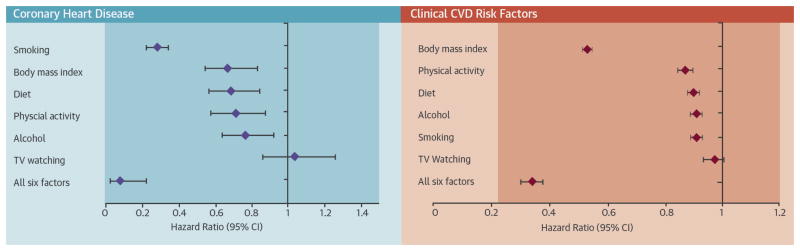
The models were stratified by age (in months) and time period and included parental history of MI before 60 years of age, aspirin use, menopausal status, postmenopausal hormone use, parity, and oral contraceptive use. For CHD, the model also included history of hypertension or hypercholesterolemia at baseline. All healthy lifestyle factors were included simultaneously in the same model. CHD = coronary heart disease; MI = myocardial infarction.
This study is timely given recent indication that the CHD mortality rate in women aged 35–44 years may not be declining as it is in other groups (2). Furthermore, there is limited evidence regarding associations between modifiable lifestyle factors and CHD in young women. Existing evidence for CHD risk factors in young women comes primarily from small case-control studies and is limited to oral contraceptive use (24), smoking (25), diabetes (25), hypertension (25), alcohol (26), and coffee (27).
To our knowledge, this is the first study to investigate the association between lifestyle factors and risk of both clinical CVD risk factors and incident CHD. Focusing on primordial prevention through healthy lifestyle could importantly address the economic burden of the medical management of clinical CVD risk factors (1,7) as well the substantial health care costs of subsequent CHD, stroke, eye disease, and premature death (1,4,7,28,29). In the American Heart Association’s 2020 Impact Goals, ideal cardiovascular health is characterized by 7 health metrics (1) including 4 health (lifestyle) behaviors (not smoking, physical activity, diet, normal body weight) and 3 health (risk) factors (optimal total cholesterol, blood pressure, and fasting blood glucose in absence of drug treatment). Based on data from NHANES 2009–2010, among adults 20–39 years of age, 32.2% had total cholesterol ≥200 mg/dL, 35.7% had a blood pressure of ≥120/80 mmHg, and 26.5% had fasting blood glucose ≥100 mg/dL. The percentage of individuals that do not have ideal levels of risk factors rises to 63.2%, 59.3%, and 46.0%, respectively, among adults 40–59 years old (1). Previous evidence suggests that approximately 70% of incident CVD can be explained by elevated levels of risk factors (30). Our data suggest that adhering to four healthy lifestyle behaviors, with the addition of light drinking and possibly limited television, would substantially decrease the burden of both CVD risk factors and CHD among young women in the United States.
We found that healthy lifestyle was equally important in preventing CHD among women who had already developed at least 1 clinical CVD risk factor. This is an important public health message and consistent with previous work among men where a healthy lifestyle prevented the majority of CHD events, even among those already taking medications for hypertension and hypercholesterolemia (31).
Strengths of our study include the prospective design, minimal loss to follow-up, detailed information on a large number of lifestyle factors collected multiple times during follow-up, and the large number of self-reported physician-diagnosed clinical CVD-related conditions and confirmed CHD cases despite the relatively young age of study participants.
Our study also has several limitations that should be considered. As in any observational study, the possibility of residual confounding cannot be eliminated; however we were able to adjust for many known risk factors. Our study population, consisting of predominantly white nurses, is not representative of the general population. Thus, we cannot necessarily generalize our results to other populations with different educational levels, incomes, or distributions of race and ethnicity. Nonetheless, the prevalence of CVD risk factors in this cohort (7.1% with diabetes, 32.7% with hypertension, 46.7% with hypercholesterolemia) is very similar to that of women in the U.S. population (1). Additionally, when we examined the association between healthy lifestyle and clinical CVD risk factors within racial subgroups, the PAR% was similar for Hispanic and Asian women and higher for African-American women, although confidence intervals for these estimates were wide and overlapping due to limited power. The healthy lifestyle factors examined in this study were self-reported; however, this method of data collection has been validated for many factors, including physical activity (9), diet (8,32), alcohol (33), and weight (34). Furthermore, measurement error would be non-differential with respect to subsequent disease status and likely results in an underestimate of the true effect. Hypertension and hypercholesterolemia were also self-reported, possibly resulting in misclassification of these outcomes. However, reporting of hypertension and hypercholesterolemia has previously been found to be fairly reliable in this and a similar population (35,36) with 94% of self-reported cases of hypertension and 86% of self-reported cases of hypercholesterolemia confirmed by medical records.
CONCLUSION
In conclusion, a healthy lifestyle was associated with significant reductions in the incidence of CHD and clinical CVD risk factors, including diabetes, hypertension, and hypercholesterolemia, in this population of young women at baseline. Adhering to a healthy lifestyle similar to that used here for CHD prevention in young women has been found to be associated with a lower risk of several diseases in middle-aged and older women, including CHD (37,38), stroke (39), sudden cardiac death (SCD) (40), diabetes (41), and cancer (38,42). Thus, promoting adherence to a healthy lifestyle has the potential to not only substantially reduce the burden of CHD and CVD-related conditions, but could be a simple, but important, strategy to lower overall morbidity and premature death in young and middle-aged women. Primordial prevention provides enormous potential for future reductions in CHD rates in young women.
PERSPECTIVES
Competency in Medical Knowledge
Preventing the development of clinical risk factors through a healthy lifestyle can reduce incidence of diabetes, hypertension, and hypercholesterolemia and reduce the incidence of coronary artery disease in young women, just as a healthy lifestyle can prevent coronary disease in women with these risk factors.
Translational Outlook
More work is needed to identify the most effective strategies to encourage patients to adopt or maintain a healthy lifestyle.
Acknowledgments
Funding/Support: This study was supported by National Institutes of Health grants UM1 CA176726 and R01 CA050385. Dr. Chomistek was supported by an institutional training grant (DK007703) from the National Institute of Diabetes and Digestive and Kidney Diseases.
Role of sponsor: The sponsors were not involved in the design and conduct of the study; collection, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
ABBREVIATIONS
- BMI
body mass index
- CHD
coronary heart disease
- CVD
cardiovascular disease
- CI
confidence interval
- HR
hazard ratio
- MET
metabolic equivalent task
- NHS2
Nurses’ Health Study 2
APPENDIX
Appendix Table 1.
Hazard ratios (95% CI) of coronary heart disease (CHD) by optimal lifestyle factor status, stratified by presence of clinical CVD risk factors (diabetes, hypertension, hypercholesterolemia).
|
|
|||||
|---|---|---|---|---|---|
| No clinical risk factors (n = 138 CHD cases) | One or more clinical risk factors (n = 318 CHD cases) | ||||
|
| |||||
| Lifestyle Factor | Definition of optimal | MV-adjusted HR* | PAR, % | MV-adjusted HR* | PAR, % |
| Smoking | Not currently smoking | 0.35 (0.23, 0.54) | 14.6 (5.4, 23.5) | 0.27 (0.21, 0.35) | 21.2 (14.6, 27.6) |
| Physical activity | ≥ 2.5 hours/week | 0.68 (0.47, 0.98) | 18.8 (−2.0, 38.0) | 0.73 (0.56, 0.94) | 20.2 (4.7, 34.7) |
| AHEI-2010 Score | AHEI score ≥ 47 | 0.66 (0.46, 0.96) | 21.0 (0.1, 40.1) | 0.72 (0.57, 0.92) | 18.7 (4.7, 31.9) |
| BMI | 18.5 – 24.9 kg/m2 | 0.76 (0.54, 1.07) | 13.8 (−2.7, 29.6) | 0.67 (0.51, 0.87) | 27.1 (10.9, 41.8) |
| Alcohol | >0 – 14.9 g/day | 0.72 (0.51, 1.00) | 15.3 (−0.9, 30.6) | 0.80 (0.64, 1.01) | 11.6 (0.1, 22.7) |
| All 5 factors | ----- | 0.09 (0.02, 0.29) | 64.9 (24.6, 86.1) | 0.08 (0.03, 0.22) | 71.1 (36.3, 88.5) |
The models were stratified by age (in months) and time period and included parental history of MI before 60 years of age, aspirin use, menopausal status, postmenopausal hormone use, parity, and oral contraceptive use. All healthy lifestyle factors were included simultaneously in the same model.
Appendix Table 2.
Risk of diagnosis with diabetes, hypertension, or hypercholesterolemia separately by optimal lifestyle factor status.
|
|
|||||||
|---|---|---|---|---|---|---|---|
| Diabetes | Hypertension | Hypercholesterolemia | |||||
|
| |||||||
| Lifestyle Factor |
Definition of optimal |
MV-adjusted HR* |
PAR, % | MV-adjusted HR* |
PAR, % | MV-adjusted HR* |
PAR, % |
| Smoking | Not currently smoking | 0.97 (0.85, 1.11) | 0.5 (−0.8, 1.7) | 0.99 (0.94, 1.05) | 0.2 (−0.3, 0.7) | 0.91 (0.87, 0.95) | 1.2 (0.7, 1.7) |
| Physical activity | ≥ 2.5 hrs/wk | 0.60 (0.55, 0.65) | 29.8 (25.2, 34.3) | 0.88 (0.85, 0.90) | 8.1 (6.2, 10.0) | 0.88 (0.86, 0.91) | 7.5 (5.8, 9.1) |
| AHEI-2010 score | AHEI score ≥ 47 | 0.77 (0.70, 0.83) | 13.9 (9.3, 18.5) | 0.89 (0.86, 0.92) | 6.2 (4.3, 8.1) | 0.92 (0.90, 0.95) | 3.9 (2.2, 5.7) |
| BMI | 18.5 – 24.9 kg/m2 | 0.08 (0.07, 0.09) | 85.2 (82.8, 87.3) | 0.37 (0.36, 0.39) | 44.8 (43.2, 46.3) | 0.66 (0.64, 0.68) | 19.5 (18.1, 20.9) |
| Alcohol | >0 – 14.9 g/day | 0.71 (0.66, 0.77) | 16.7 (13.0, 20.4) | 0.87 (0.85, 0.90) | 6.4 (4.9, 7.8) | 0.94 (0.91, 0.96) | 2.9 (1.6, 4.2) |
| T.V. watching | ≤ 7 hrs/wk | 0.84 (0.77, 0.91) | 11.0 (6.6, 15.4) | 0.90 (0.88, 0.93) | 6.1 (4.4, 7.8) | 0.90 (0.88, 0.93) | 6.0 (4.5, 7.5) |
| All 6 factors | ----- | 0.02 (0.01, 0.04) | 92.9 (87.6, 96.0) | 0.24 (0.20, 0.28) | 57.0 (52.8, 60.9) | 0.39 (0.33, 0.45) | 40.0 (35.5, 44.3) |
The models were stratified by age (in months) and time period and included parental history of MI before 60 years of age, aspirin use, menopausal status, postmenopausal hormone use, parity, and oral contraceptive use. All healthy lifestyle factors were included simultaneously in the same model.
Footnotes
Relationship with Industry: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics--2013 update: a report from the American Heart Association. Circulation. 2013;127:e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ford ES, Capewell S. Coronary heart disease mortality among young adults in the U.S. from 1980 through 2002: concealed leveling of mortality rates. J Am Coll Cardiol. 2007;50:2128–32. doi: 10.1016/j.jacc.2007.05.056. [DOI] [PubMed] [Google Scholar]
- 3.Ford ES. Trends in predicted 10-year risk of coronary heart disease and cardiovascular disease among U.S. adults from 1999 to 2010. J Am Coll Cardiol. 2013;61:2249–52. doi: 10.1016/j.jacc.2013.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang Q, Cogswell ME, Flanders WD, et al. Trends in cardiovascular health metrics and associations with all-cause and CVD mortality among US adults. JAMA. 2012;307:1273–83. doi: 10.1001/jama.2012.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Daviglus ML, Stamler J, Pirzada A, et al. Favorable cardiovascular risk profile in young women and long-term risk of cardiovascular and all-cause mortality. JAMA. 2004;292:1588–92. doi: 10.1001/jama.292.13.1588. [DOI] [PubMed] [Google Scholar]
- 6.Liu K, Daviglus ML, Loria CM, et al. Healthy lifestyle through young adulthood and the presence of low cardiovascular disease risk profile in middle age: the Coronary Artery Risk Development in (Young) Adults (CARDIA) study. Circulation. 2012;125:996–1004. doi: 10.1161/CIRCULATIONAHA.111.060681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weintraub WS, Daniels SR, Burke LE, et al. Value of primordial and primary prevention for cardiovascular disease: a policy statement from the American Heart Association. Circulation. 2011;124:967–90. doi: 10.1161/CIR.0b013e3182285a81. [DOI] [PubMed] [Google Scholar]
- 8.Willett WC, Sampson L, Stampfer MJ, et al. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol. 1985;122:51–65. doi: 10.1093/oxfordjournals.aje.a114086. [DOI] [PubMed] [Google Scholar]
- 9.Wolf AM, Hunter DJ, Colditz GA, et al. Reproducibility and validity of a self-administered physical activity questionnaire. Int J Epidemiol. 1994;23:991–9. doi: 10.1093/ije/23.5.991. [DOI] [PubMed] [Google Scholar]
- 10.Ainsworth BE, Haskell WL, Leon AS, et al. Compendium of physical activities: classification of energy costs of human physical activities. Med Sci Sports Exerc. 1993;25:71–80. doi: 10.1249/00005768-199301000-00011. [DOI] [PubMed] [Google Scholar]
- 11.U.S. Department of Agriculture. Composition of Foods-- Raw, Processed, and Prepared: Agricultural Handbook. 8. Washington D.C: US Govt Printing Offices; 1963. [Google Scholar]
- 12.Hu FB, Stampfer MJ, Rimm E, et al. Dietary fat and coronary heart disease: a comparison of approaches for adjusting for total energy intake and modeling repeated dietary measurements. Am J Epidemiol. 1999;149:531–40. doi: 10.1093/oxfordjournals.aje.a009849. [DOI] [PubMed] [Google Scholar]
- 13.Physical Activity Guidelines Advisory Committee. Physical Activity Guidelines Committee Report, 2008. U.S. Department of Health and Human Services; Washington, D.C: 2008. p. 683. [Google Scholar]
- 14.Wijndaele K, Brage S, Besson H, et al. Television viewing and incident cardiovascular disease: prospective associations and mediation analysis in the EPIC Norfolk Study. PloS one. 2011;6:e20058. doi: 10.1371/journal.pone.0020058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Obesity: preventing and managing the global epidemic: report of a WHO consultation on obesity. Geneva: World Health Organization; 1998. [PubMed] [Google Scholar]
- 16.U.S. Department of Agriculture and U.S. Department of Health and Human Services. Dietary Guidelines for Americans, 2010. 7. Washington D.C: U.S. Government Printing Office; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chiuve SE, Fung TT, Rimm EB, et al. Alternative dietary indices both strongly predict risk of chronic disease. J Nutr. 2012;142:1009–18. doi: 10.3945/jn.111.157222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McCullough ML, Feskanich D, Stampfer MJ, et al. Diet quality and major chronic disease risk in men and women: moving toward improved dietary guidance. Am J Clin Nutr. 2002;76:1261–71. doi: 10.1093/ajcn/76.6.1261. [DOI] [PubMed] [Google Scholar]
- 19.Rose GABH, Gillum R, Prineas RJ. Cardiovascular Survey Methods: WHO Monograph Series No. 56. Geneva, Switzerland: World Health Organization; 1982. pp. 162–165. [PubMed] [Google Scholar]
- 20.Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1997;20:1183–97. doi: 10.2337/diacare.20.7.1183. [DOI] [PubMed] [Google Scholar]
- 21.Flint AJ, Hu FB, Glynn RJ, et al. Whole grains and incident hypertension in men. The Am J Clin Nutr. 2009;90:493–8. doi: 10.3945/ajcn.2009.27460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med. 1989;8:551–61. doi: 10.1002/sim.4780080504. [DOI] [PubMed] [Google Scholar]
- 23.Spiegelman D, Hertzmark E, Wand HC. Point and interval estimates of partial population attributable risks in cohort studies: examples and software. Cancer Causes Control. 2007;18:571–9. doi: 10.1007/s10552-006-0090-y. [DOI] [PubMed] [Google Scholar]
- 24.Rosenberg L, Palmer JR, Rao RS, Shapiro S. Low-dose oral contraceptive use and the risk of myocardial infarction. Arch Intern Med. 2001;161:1065–70. doi: 10.1001/archinte.161.8.1065. [DOI] [PubMed] [Google Scholar]
- 25.La Vecchia C, Franceschi S, Decarli A, Pampallona S, Tognoni G. Risk factors for myocardial infarction in young women. Am J Epidemiol. 1987;125:832–43. doi: 10.1093/oxfordjournals.aje.a114599. [DOI] [PubMed] [Google Scholar]
- 26.Rosenberg L, Slone D, Shapiro S, Kaufman DW, Miettinen OS, Stolley PD. Alcoholic beverages and myocardial infarction in young women. Am J Public Health. 1981;71:82–5. doi: 10.2105/ajph.71.1.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Palmer JR, Rosenberg L, Rao RS, Shapiro S. Coffee consumption and myocardial infarction in women. Am J Epidemiol. 1995;141:724–31. doi: 10.1093/oxfordjournals.aje.a117494. [DOI] [PubMed] [Google Scholar]
- 28.Katsi V, Marketou M, Vlachopoulos C, et al. Impact of arterial hypertension on the eye. Curr Hypertens Rep. 2012;14:581–90. doi: 10.1007/s11906-012-0283-6. [DOI] [PubMed] [Google Scholar]
- 29.Stitt AW, Lois N, Medina RJ, Adamson P, Curtis TM. Advances in our understanding of diabetic retinopathy. Clin Sci. 2013;125:1–17. doi: 10.1042/CS20120588. [DOI] [PubMed] [Google Scholar]
- 30.Hozawa A, Folsom AR, Sharrett AR, Chambless LE. Absolute and attributable risks of cardiovascular disease incidence in relation to optimal and borderline risk factors: comparison of African American with white subjects--Atherosclerosis Risk in Communities Study. Arch Intern Med. 2007;167:573–9. doi: 10.1001/archinte.167.6.573. [DOI] [PubMed] [Google Scholar]
- 31.Chiuve SE, McCullough ML, Sacks FM, Rimm EB. Healthy lifestyle factors in the primary prevention of coronary heart disease among men: benefits among users and nonusers of lipid-lowering and antihypertensive medications. Circulation. 2006;114:160–7. doi: 10.1161/CIRCULATIONAHA.106.621417. [DOI] [PubMed] [Google Scholar]
- 32.Feskanich D, Rimm EB, Giovannucci EL, et al. Reproducibility and validity of food intake measurements from a semiquantitative food frequency questionnaire. J Am Diet Assoc. 1993;93:790–6. doi: 10.1016/0002-8223(93)91754-e. [DOI] [PubMed] [Google Scholar]
- 33.Giovannucci E, Colditz G, Stampfer MJ, et al. The assessment of alcohol consumption by a simple self-administered questionnaire. Am J Epidemiol. 1991;133:810–7. doi: 10.1093/oxfordjournals.aje.a115960. [DOI] [PubMed] [Google Scholar]
- 34.Grobbee DE, Rimm EB, Giovannucci E, Colditz G, Stampfer M, Willett W. Coffee, caffeine, and cardiovascular disease in men. N Engl J Med. 1990;323:1026–32. doi: 10.1056/NEJM199010113231504. [DOI] [PubMed] [Google Scholar]
- 35.Colditz GA, Martin P, Stampfer MJ, et al. Validation of questionnaire information on risk factors and disease outcomes in a prospective cohort study of women. Am J Epidemiol. 1986;123:894–900. doi: 10.1093/oxfordjournals.aje.a114319. [DOI] [PubMed] [Google Scholar]
- 36.Forman JP, Curhan GC, Taylor EN. Plasma 25-hydroxyvitamin D levels and risk of incident hypertension among young women. Hypertension. 2008;52:828–32. doi: 10.1161/HYPERTENSIONAHA.108.117630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stampfer MJ, Hu FB, Manson JE, Rimm EB, Willett WC. Primary prevention of coronary heart disease in women through diet and lifestyle. N Engl J Med. 2000;343:16–22. doi: 10.1056/NEJM200007063430103. [DOI] [PubMed] [Google Scholar]
- 38.Knoops KT, de Groot LC, Kromhout D, et al. Mediterranean diet, lifestyle factors, and 10-year mortality in elderly European men and women: the HALE project. JAMA. 2004;292:1433–9. doi: 10.1001/jama.292.12.1433. [DOI] [PubMed] [Google Scholar]
- 39.Chiuve SE, Rexrode KM, Spiegelman D, et al. Primary prevention of stroke by healthy lifestyle. Circulation. 2008;118:947–54. doi: 10.1161/CIRCULATIONAHA.108.781062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chiuve SE, Fung TT, Rexrode KM, et al. Adherence to a low-risk, healthy lifestyle and risk of sudden cardiac death among women. JAMA. 2011;306:62–9. doi: 10.1001/jama.2011.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hu FB, Manson JE, Stampfer MJ, et al. Diet, lifestyle, and the risk of type 2 diabetes mellitus in women. N Engl J Med. 2001;345:790–7. doi: 10.1056/NEJMoa010492. [DOI] [PubMed] [Google Scholar]
- 42.Ford ES, Bergmann MM, Kroger J, Schienkiewitz A, Weikert C, Boeing H. Healthy living is the best revenge: findings from the European Prospective Investigation Into Cancer and Nutrition-Potsdam study. Arch Intern Med. 2009;169:1355–62. doi: 10.1001/archinternmed.2009.237. [DOI] [PubMed] [Google Scholar]