Abstract
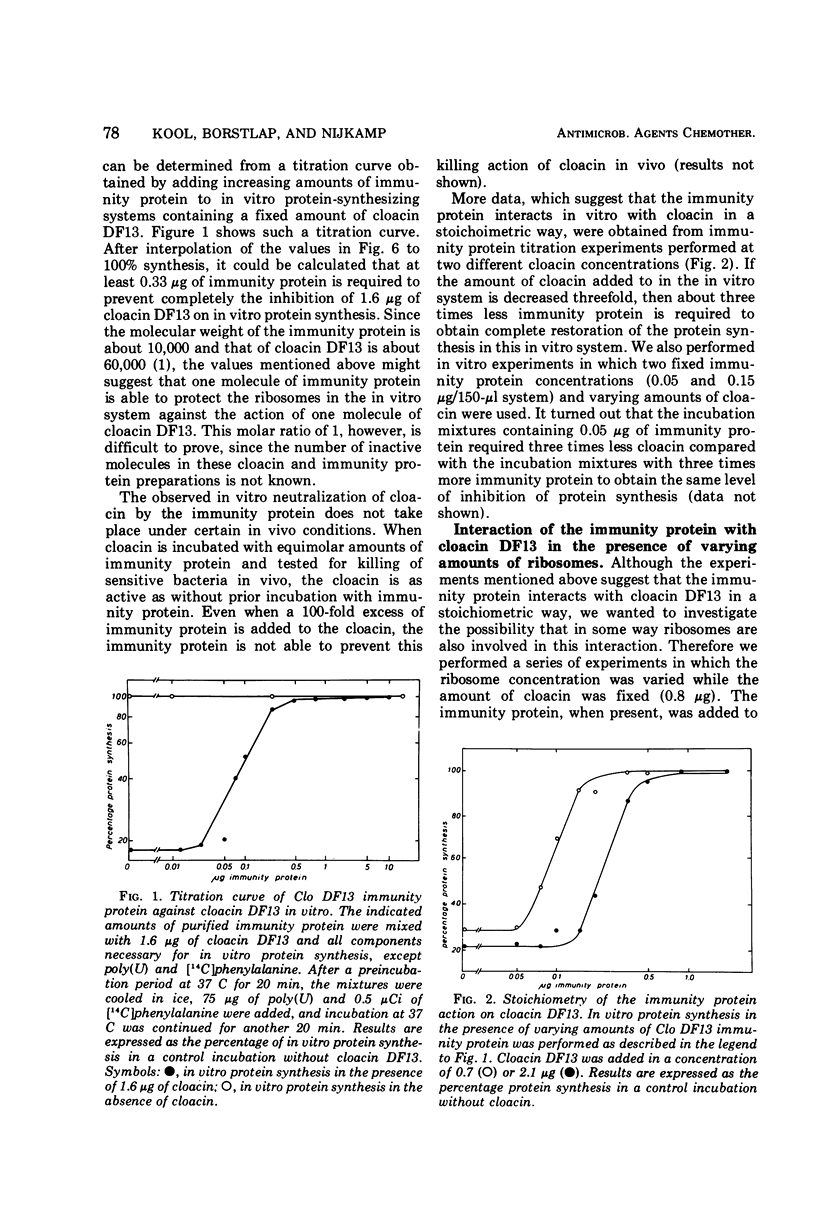
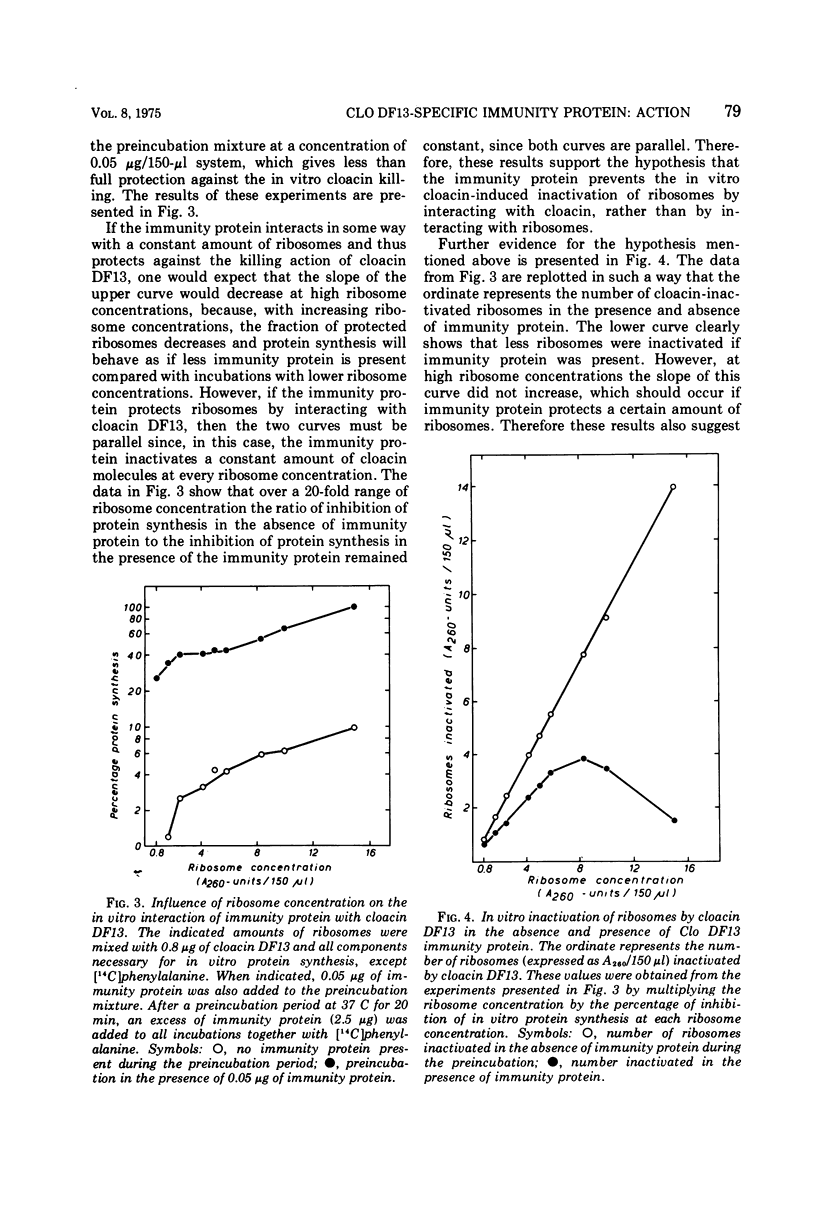
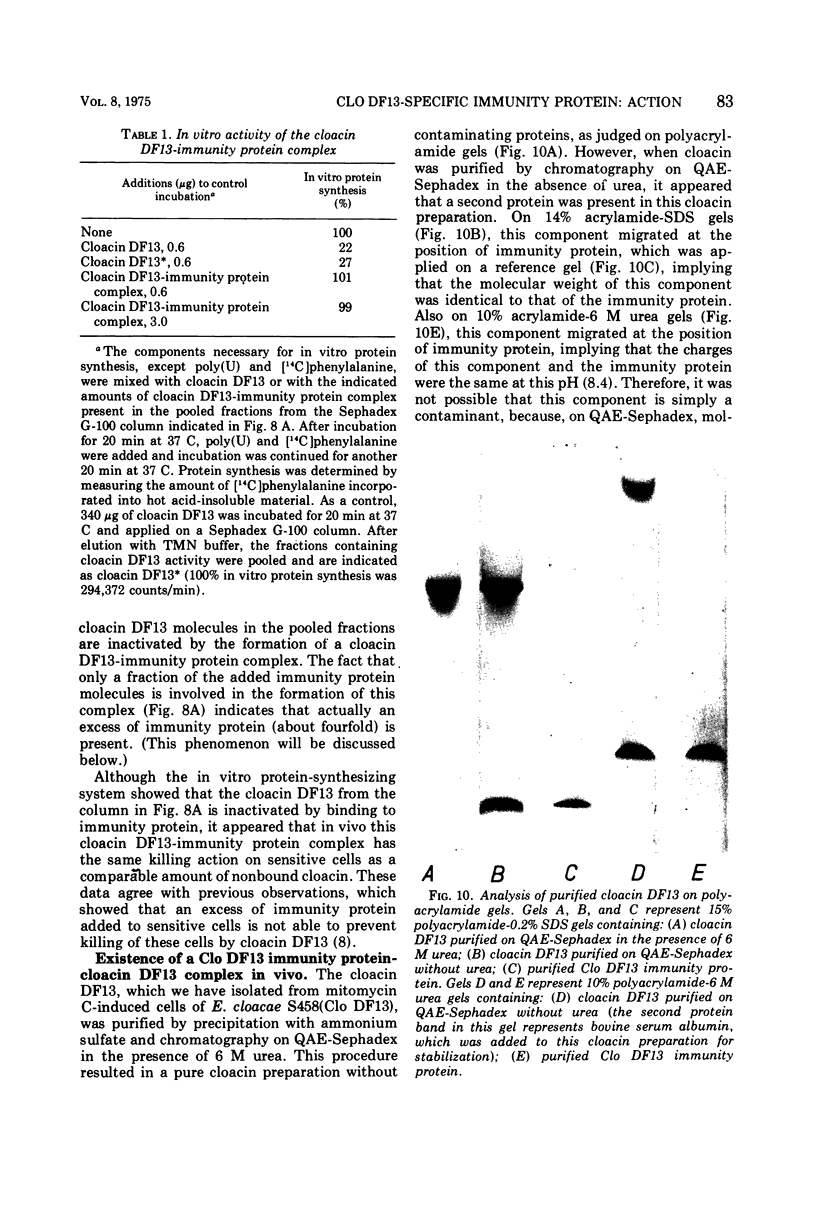
The Clo DF13 plasmid-specific immunity protein is able to prevent the inhibitory effect of cloacin DF13 on in vitro protein synthesis. We have shown, by gel filtration, that direct binding of the Clo DF13 immunity protein to cloacin occurs in vitro. This cloacin DF13-immunity protein complex is rather stable, and the cloacin present in the complex is no longer able to cause inhibition of in vitro protein synthesis. The binding of immunity protein to cloacin DF13 is rather specific because the Clo DF13 immunity protein does not bind to in vitro inactive cloacin and binds very poorly to the closely related bacteriocin colicin E3. Furthermore, we present data which strongly suggest that in vitro at least a fourfold excess of immunity protein is required to ensure that every cloacin molecule is inactivated by cloacin-immunity protein complex formation. Only a fraction (an about equimolar amount) of the immunity protein molecules, however, actually binds to cloacin DF13. The existence of an immunity protein-cloacin complex in vivo was concluded from the observation that cloacin, purified by chromatography on diethyl-(2-hydroxypropyl)-aminoethyl Sephadex in the absence of urea, contains an about equimolar amount of a second protein which comigrates with immunity protein on sodium dodecyl sulfate-polyacrylamide and urea-polyacrylamide gels. In an in vitro protein-synthesizing system, this component appeared to behave identical to the Clo DF13 immunity protein. The purified immunity protein-containing cloacin was at least 80 times less active in inhibiting in vitro protein synthesis, compared to cloacin, free of immunity protein. These data imply that few, if any, cloacin DF13 molecules are present in cloacinogenic cells as active, free cloacin molecules.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Herschman H. R., Helinski D. R. Purification and characterization of colicin E2 and colicin E3. J Biol Chem. 1967 Nov 25;242(22):5360–5368. [PubMed] [Google Scholar]
- Jakes K. S., Zinder N. D. Highly purified colicin E3 contains immunity protein. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3380–3384. doi: 10.1073/pnas.71.9.3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kool A. J., Nijkamp H. J. Isolation and characterization of a copy mutant of the bacteriocinogenic plasmid Clo DF13. J Bacteriol. 1974 Nov;120(2):569–578. doi: 10.1128/jb.120.2.569-578.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kool A. J., Nijkamp H. J. Isolation and characterization of immunity temperature sensitive mutants of Enterobacter cloacae harbouring the cloacinogenic factor DF13. Mol Gen Genet. 1972;114(4):312–324. doi: 10.1007/BF00267500. [DOI] [PubMed] [Google Scholar]
- Kool A. J., Pols C., Nijkamp H. J. Bacteriocinogenic Clo DF13 minicells of Escherichia coli synthesize a protein that accounts for immunity to bacteriocin Clo DF13: purification and characterization of the immunity protein. Antimicrob Agents Chemother. 1975 Jul;8(1):67–75. doi: 10.1128/aac.8.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kool A. J., Pranger M., Nijkamp H. J. Proteins synthesized by a non-induced bacteriocinogenic factor in minicells of Escherichia coli. Mol Gen Genet. 1972;115(4):314–323. doi: 10.1007/BF00333170. [DOI] [PubMed] [Google Scholar]
- Kool A. J., van Zeben M. S., Nijkamp H. J. Identification of messenger ribonucleic acids and proteins synthesized by the bacteriocinogenic factor Clo DF13 in purified minicells of Escherichia coli. J Bacteriol. 1974 Apr;118(1):213–224. doi: 10.1128/jb.118.1.213-224.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidikaro J., Nomura M. E3 immunity substance. A protein from e3-colicinogenic cells that accounts for their immunity to colicin E3. J Biol Chem. 1974 Jan 25;249(2):445–453. [PubMed] [Google Scholar]
- Spear P. G., Roizman B. Proteins specified by herpes simplex virus. V. Purification and structural proteins of the herpesvirion. J Virol. 1972 Jan;9(1):143–159. doi: 10.1128/jvi.9.1.143-159.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tieze G. A., Stouthamer A. H., Jansz H. S., Zandberg J., van Bruggen E. F. A bacteriocinogenic factor of Enterobacter cloacae. Mol Gen Genet. 1969;106(1):48–65. [PubMed] [Google Scholar]
- Veltkamp E., Barendsen W., Nijkamp H. J. Influence of protein and ribonucleic acid synthesis on the replication of the bacteriocinogenic factor Clo DF13 in Escherichia coli cells and minicells. J Bacteriol. 1974 Apr;118(1):165–174. doi: 10.1128/jb.118.1.165-174.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- de Graaf F. K., Goedvolk-de Groot L. E., Stouthamer A. H. Purification of a bacteriocin produced by Enterobacter cloacae DF 13. Biochim Biophys Acta. 1970 Dec 22;221(3):566–575. doi: 10.1016/0005-2795(70)90228-x. [DOI] [PubMed] [Google Scholar]
- de Graaf F. K., Klaasen-Boor P. Purification and characterization of the cloacin DF13 immunity protein. FEBS Lett. 1974 Apr 1;40(2):293–296. doi: 10.1016/0014-5793(74)80247-4. [DOI] [PubMed] [Google Scholar]
- de Graaf F. K., Niekus H. G., Klootwijk J. Inactivation of bacterial ribosomes in vivo and in vitro by cloacin DF13. FEBS Lett. 1973 Sep 1;35(1):161–165. doi: 10.1016/0014-5793(73)80601-5. [DOI] [PubMed] [Google Scholar]



