Abstract
There is ample experimental and clinical evidence that chemotherapies are more efficient if they succeed in (re)activating immune surveillance, hence triggering a long-term immune response against residual tumor cells. Most of the preclinical evidence supporting this notion has been obtained with transplantable cancers, for which it has been shown that chemotherapy-induced autophagy in cancer cells is mandatory for the recruitment of myeloid cells into the tumor bed and the subsequent T lymphocyte-mediated reduction in tumor growth. Here, we characterized the chemotherapeutic response of melanomas caused by 4-hydroxy-tamoxifen-induced expression of the Cre recombinase in melanocytes that results in the activation of oncogenic Braf together with the inactivation of the tumor suppressor Pten, as well as the optional inactivation of the essential autophagy gene Atg7. Systemic chemotherapy with the anthracycline Mitoxantrone (MTX) reduced the growth of autophagy-competent melanomas (genotype: BrafCa/+; Ptenfl/fl; Atg7+/+), yet failed to affect the progression of autophagy-deficient melanomas (genotype: BrafCa/+; Ptenfl/fl; Atg7fl/fl). The growth-inhibitory effect of MTX on autophagy-competent melanomas was abolished by the combined depletion of CD4+ or CD8+ T lymphocytes. In conclusion, it appears that the success of chemotherapy against “spontaneous,” genetically induced cancers is governed by the same rules as those applicable to transplantable tumors.
Keywords: anthracycline, autophagy, cancer
Introduction
A selected panel of chemotherapeutic agents is able to induce Immunogenic Cell Death (ICD) of malignant cells, thus converting the tumor into a therapeutic vaccine that elicits an anticancer immune response against residual cancer cells.1-4 Thus, anthracyclines,5-7 oxaliplatin,8,9 cyclophosphamide,10,11 cardiac glycosides,12,13 taxanes,14 vorinostat,15,16 ionizing radiation17-19 and photodynamic therapy20,21 can induce ICD. Apart from its cell biological characteristics (endoplasmic reticulum stress leading to the exposure of calreticulin on the cell surface, premortem autophagy allowing for ATP release during the blebbing phase of apoptosis, secondary necrosis resulting in the exodus of HMGB1 from cells and nuclei),22-26 ICD is usually monitored by 2 complementary in vivo experiments. First, mouse tumor cells are treated with the ICD inducer in vitro until ∼70% of the cells demonstrate signs of apoptosis, washed and injected subcutaneously in the absence of any adjuvant into histocompatible, immunocompentent mice. After one week the mice are challenged by a second injection of live tumor cells into the contralateral flank, and the absence of tumor growth is interpreted as the sign of a protective anticancer immune response elicited by dying tumor cells.27,28 Second, immunodeficient or immunocompetent mice bearing established cancers are treated with the ICD inducer in vivo. If the treatment is more efficient in the context of an intact immune system than in its absence, the result is interpreted to indicate that the therapeutic effect depends on the active contribution of the immune system.27,28
Defects affecting specifically the recognition of ICD-related danger-associated molecular patterns (DAMPs) by the host, as well as broader immune defects (such as the complete absence of dendritic cells or T cells), strongly reduce the efficacy of chemotherapy with ICD inducers.27,29,30 In addition, specific defects in tumor cells that lead to their incapacity to produce ICD-related DAMPs can cause therapeutic failure. Thus, cancer cells that are unable to mount a premortem autophagic response are unable to secrete ATP6,23,31,32 and hence have a reduced ability to attract myeloid cells into the tumor bed after chemotherapy.29,33 As a result, tumors from which essential autophagy genes (such as Atg5 or Atg7) have been deleted, can exhibit a reduced anticancer immune response and growth in response to chemotherapy with ICD inducers.6
Most of the experiments demonstrating the capacity of anticancer agents to induce ICD have been performed on transplantable cancer cell lines including CT26 colorectal carcinomas, EG7 and EL4 lymphomas, LLC lung adenocarcinomas, MCA205 fibrosarcomas and MC38 colon adenocarcinomas.27 Driven by the argument that “spontaneous” cancers induced by oncogenes (contrasting with transplantable tumors) would provide a more realistic setting for the evaluation of the immune contribution to chemotherapeutic effects,34,35 we decided to evaluate the therapeutic effects of the prototypic ICD inducer, mitoxantrone (MTX),7 on a mouse model of spontaneous Braf-driven melanoma. Here we reveal that melanomas only reduce their growth in response to MTX in the presence of a functional cellular immune system. Moreover, we show that melanomas lacking the essential autophagy gene Atg7 fail to reduce their growth after chemotherapy.
Results and Discussion
Autophagy-deficient BRAF-induced melanomas
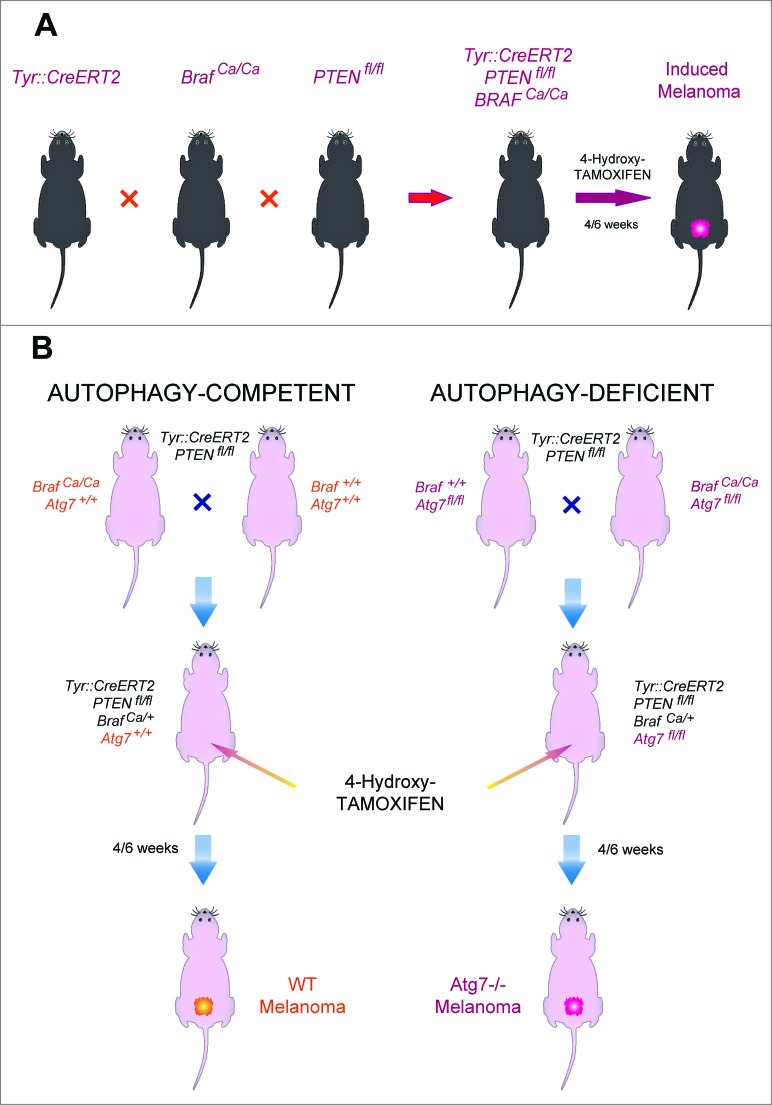
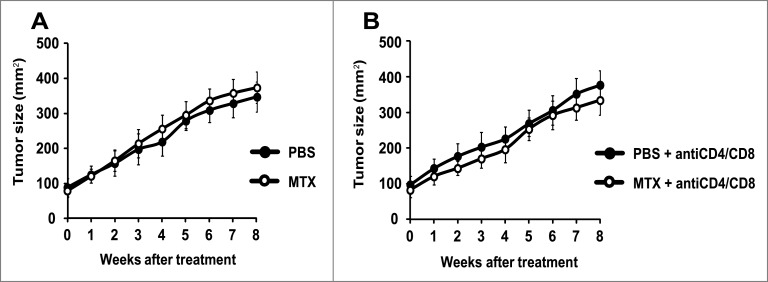
C57BL/6 mice bearing a tamoxifen-activable Cre recombinase transgene (i.e., a Cre recombinase fused to a G400V/M543A/L544A triple mutation of the human estrogen receptor ligand binding domain, yielding a Cre-ERT2 fusion protein) expressed under the control of the melanocyte-specific tyrosinase promoter/enhancer regions (yielding the Tyr::CreERT2 transgene) were crossed with mice bearing a constitutively active oncogene Braf (BrafCA) (in which Cre expression lead to oncogenic V600E Braf expression), and mice in which tumor suppressor gene Pten is floxed (PTENfl/fl) (and hence can be inactivated by Cre). Optionally, both alleles coding for the Atg7 gene were also floxed (Atg7fl/fl). In this constellation, painting the dorsal skin with 4-hydroxy-tamoxifen results in the nuclear expression of Cre in melanocytes. Cre then causes the activation of BrafCA, as well as the inactivation of Pten and optionally that of Atg7 in melanocytes (Fig. 1). As the result, the mice developed one or several melanomas in the tamoxifen-painted area that were autophagy-proficient (because they expressed Atg7) or autophagy deficient (because they lacked Atg7). Such tumors usually developed within 3–6 weeks after 4-hydroxy-tamoxifen treatment in 85–95% of the mice, irrespective of the status of the Atg7 gene (not shown) and proliferated in a linear fashion (Figs. 2A, 3A). Moreover, depletion of CD4+ and CD8+ cells from mice that were bearing established melanomas (with an approximate surface of 25 mm2) failed to affect tumor growth (Figs. 2B, 3B), suggesting that T lymphocyte-mediated immunosurveillance does not play any major role in the progression of such tumors, be they autophagy-competent or autophagy-deficient.
Figure 1.
Breeding strategy for the generation of an inducible melanoma mouse model. (A) Schematic representation of the breeding. Tyr::CreERT2 mice were repeatedly crossed with Ptenfl/fl, then BRafCA/CA and optionally Atg7fl/fl mice until the floxed loci were homozygous (A). Topical tamoxifen administration on the dorsal skin locally induced melanoma in Tyr::CreERT2;Ptenfl/fl;BRafCA/+;Atg7+/+ and Tyr::CreERT2;Ptenfl/fl;BRafCA/+;Atg7fl/fl mice (B).
Figure 2.
Failure of autophagy-deficient melanomas to respond to chemotherapy. Melanomas were induced by adding tamoxifen on the shaved back of mice with the Tyr::CreERT2;Ptenfl/fl;BRafCA/+;Atg7fl/fl genotype. Once the tumors had reached a surface of approximately 100 mm2 (day 0), melanomas were treated by intraperitoneal injections of mitoxantrone (MTX) or vehicle (PBS) every 2 weeks, starting on day 0. Tumor growth was monitored with a caliper for 8 weeks. To assess the impact of the cellular immune system, mice were treated i.p. with PBS (A) or a mix of αCD4 and αCD8 antibodies (B) at different times days −3, 0, 1, 3, 5, 8, 15, 22, 29, 36, 43, 50). Experiments were done on 15 mice per group. Results are reported as means ± SEM. *p < 0.05 (unpaired Student's t test).
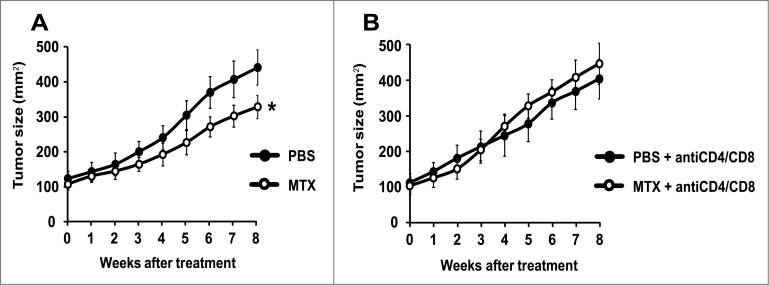
Figure 3.
Mitoxantrone reduces the growth of autophagy-competent melanomas in mice with an intact immune system. Once the melanomas from Tyr::CreERT2;Ptenfl/fl;BRafCA/+;Atg7+/+ mice had reached a surface of approximately 100 mm2 (day 0), they were treated by intraperitoneal injections of MTX or vehicle (PBS) every 2 weeks. Tumor growth was monitored by means of a caliper for 8 weeks. Additionally, to assess the involvement of the immune system, mice were treated i.p. with PBS (A) or a mix of αCD4 and αCD8 antibodies (B) at different times (days −3, 0, 1, 3, 5, 8, 15, 22, 29, 36, 43, 50). Experiments were done on groups of 15 mice. Results are reported as means ± SEM. *p < 0.05 (unpaired Student's t test).
Immune-dependent and autophagy-dependent chemotherapeutic responses of BRAF-induced melanomas
To evaluate the chemotherapeutic response, melanoma-bearing mice were biweekly treated by intraperitoneal injections of the prototypic ICD inducer mitoxantrone (MTX), a regime that had no major side effects on control mice (and hence failed to cause a significant weight loss or other manifest alterations in animal behavior). MTX failed to reduce the growth of Atg7-deficient melanomas (Fig. 2A), and this effect was not altered in conditions in which CD4+ and CD8+ lymphocytes were depleted (Fig. 2B). In contrast, MTX did reduce the growth of autophagy-competent (Atg7 expressing) melanomas, although this effect was partial (Fig. 3A). The relative success of this MTX-based chemotherapeutic regime was completely lost upon depletion of CD4+ and CD8+ cells (Fig. 3B), in line with idea that the anticancer effects of MTX against autophagy-competent melanomas rely on a cellular immune response.
Concluding Remarks
Here, we provide evidence that chemotherapy of a spontaneous (non-transplanted) model of melanoma involves the active contribution of the cellular immune system. This observation adds to previous reports demonstrating that anthracycline-based chemotherapies of carcinogen-induced skin tumors36 and transgene (MMTV-Neu) induced HER2-positive breast cancers37 are abolished upon depletion of CD8+ T cells. Altogether, these data underscore the critical importance of the anticancer immune response for the efficacy of chemotherapies, in line with extensive epidemiological evidence demonstrating that the pre-established immune infiltrate determines the long-term fate of cancer patients38-40 and influences the reduction of the tumor mass by neoadjuvant chemotherapy, as exemplified by the treatment of locally invasive breast cancer with anthracyclines.14,41-44
Beyond these confirmatory aspects, our results reveal for the first time that spontaneous cancers have a reduced ability to respond to MTX if their autophagic machinery has been disabled, presumably because they are unable to elicit natural or chemotherapy-induced immunosurveillance mechanisms.6,45 Reportedly, autophagy can be deficient in tumor cells,46-48 and it is hence tempting to speculate that the fraction of cancers that are autophagy-deficient may be refractory to conventional therapies. This possibility requires further preclinical and clinical exploration.
Material and Methods
Chemicals and antibodies
Mitoxantrone and 4-hydroxy-tamoxifen were purchased from Sigma-Aldrich (St Louis, MO USA). Specific antibodies for the elimination of CD4+ and CD8+ lymphocytes, respectively GK1.5 (BE0003-1) and 2.43 (BE0061), were purchased from BioXcell.
Animal experimentation
Mice were maintained in specific pathogen-free conditions, and experiments followed the Federation of European Laboratory Animal Science Association (FELASA) guidelines. Animal experiments were approved by the local Ethics Committee (CEEA IRCIV / IGR n°26, registered with the French Ministry of Research) and were in compliance with EU 63/2010 directive. Animals were used between 6 and 30 weeks of age and those bearing tumors exceeding 20–25% of the body mass were euthanatized.
Animal breeding was also performed using Institutional Animal Care and Use approved protocols, I10-064-8 “Use of mice for tumorigenicity,” which was approved by IACUC committee members at the University of Medicine and Dentistry of New Jersey (UMDNJ).
Mouse breeding and genotyping
Breeding was performed in the IGR or CINJ animal facilities. Tyr::CreERT2 and Ptenfl/fl mice were purchased from JAX (JAX012328 and JAX004597, respectively). BRafCA/CA mice were obtained from McMahon's laboratory.49 Atg7fl/fl mice were provided by Kamatsu's laboratory.50
By the age of 3 weeks, pup tails were partially cut for genotyping. DNA was extracted from using the DNAeasy Blood and Tissue Kit (Quiagen). DNA was then amplified by PCR in Micro-Amp 96-well reaction plate (Applied Biosystems), using REDExtract-N-Amp PCR ready Mix (Sigma). The primers used were 5′-GCG GTC TGG CAG TAA AAA CTA TC-3′, 5′-GTG AAA CAG CT TGC TGT CAC TT-3′, 5′-CTA GGC CAC AGA ATT GAA AGA TCT-3′ and 5′-GTA GGT GGA AAT TCT AGC ATC ATC C-3′ for Tyr-CRE, 5′-AAG CAC TCT GCG AAC TGA G-3′ and 5′-AAG TTT TTG AAG GCA AGA TGC-3′ for PTEN, 5′-TGA GTA TTT TTG TGG CAA CTG C-3′ and 5′-CTC TGC TGG GAA AGC GGC-3′ for BRAF, 5′-TGG CTG CTA CTT CTG CAA TGA TGT-3′ and 5′- CAG GAC AGA GAC CT CAG CTC CAC-3′ for ATG7 (JAX database).49,50 PCR products were finally loaded on agarose gels and revealed, as previously described (JAX database).
Induction and treatment of melanomas
Mice were shaved on their back. One day later, 1 μl 4-hydroxy-tamoxifen (5 mM in ethanol) was applied and dried on the mice skin. Melanomas appeared 3–6 weeks later. Such melanomas could be directly treated or cut into 1 mm3 pieces for further transplantation into the flank of syngenic mice. Once melanomas had reached a surface of ∼100 mm2, mice were treated i.p. either with PBS (day 0) or 5.17 mg/kg MTX (in 200 μl sterile water), and this treatment was repeated every 2 weeks. Additionally, mice were treated i.p. by PBS or a mix of anti-CD4/anti-CD8 (200 μL, 0.5 mg/mL) antibodies at different time (days -3, 0, 1, 3, 5, 8, 15, 22, 29, 36, 43, 50). The tumor surface was then monitored with a caliper every week for up to 8 weeks.
Funding Statement
GK is supported by the Ligue contre le Cancer (équipe labelisée); Agence National de la Recherche (ANR); Association pour la recherche sur le cancer (ARC); Cancéropôle Ile-de-France; Institut National du Cancer (INCa); Fondation Bettencourt-Schueller; Fondation de France; Fondation pour la Recherche Médicale (FRM); the European Commission (ArtForce); the European Research Council (ERC); the LabEx Immuno-Oncology; the SIRIC Stratified Oncology Cell DNA Repair and Tumor Immune Elimination (SOCRATE); the SIRIC Cancer Research and Personalized Medicine (CARPEM); and the Paris Alliance of Cancer Research Institutes (PACRI).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1. Galluzzi L, Senovilla L, Zitvogel L, Kroemer G. The secret ally: immunostimulation by anticancer drugs. Nat Rev Drug Discov 2012; 11:215-33; PMID: ; http://dx.doi.org/10.1038/nrd3626 [DOI] [PubMed] [Google Scholar]
- 2. Krysko DV, Garg AD, Kaczmarek A, Krysko O, Agostinis P, Vandenabeele P. Immunogenic cell death and DAMPs in cancer therapy. Nat Rev Cancer 2012; 12:860-75; PMID: ; http://dx.doi.org/10.1038/nrc3380 [DOI] [PubMed] [Google Scholar]
- 3. Zitvogel L, Galluzzi L, Smyth MJ, Kroemer G. Mechanism of action of conventional and targeted anticancer therapies: reinstating immunosurveillance. Immunity 2013; 39:74-88; PMID: ; http://dx.doi.org/10.1016/j.immuni.2013.06.014 [DOI] [PubMed] [Google Scholar]
- 4. Zitvogel L, Kepp O, Kroemer G. Immune parameters affecting the efficacy of chemotherapeutic regimens. Nat Rev Clin Oncol 2011; 8:151-60; PMID: ; http://dx.doi.org/10.1038/nrclinonc.2010.223 [DOI] [PubMed] [Google Scholar]
- 5. Casares N, Pequignot MO, Tesniere A, Ghiringhelli F, Roux S, Chaput N, Schmitt E, Hamai A, Hervas-Stubbs S, Obeid M, et al. Caspase-dependent immunogenicity of doxorubicin-induced tumor cell death. J Exp Med 2005; 202:1691-701; PMID: ; http://dx.doi.org/10.1084/jem.20050915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Michaud M, Martins I, Sukkurwala AQ, Adjemian S, Ma Y, Pellegatti P, Shen S, Kepp O, Scoazec M, Mignot G, et al. Autophagy-dependent anticancer immune responses induced by chemotherapeutic agents in mice. Science 2011; 334:1573-7; PMID: ; http://dx.doi.org/10.1126/science.1208347 [DOI] [PubMed] [Google Scholar]
- 7. Obeid M, Tesniere A, Ghiringhelli F, Fimia GM, Apetoh L, Perfettini JL, Castedo M, Mignot G, Panaretakis T, Casares N, et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med 2007; 13:54-61; PMID: ; http://dx.doi.org/10.1038/nm1523 [DOI] [PubMed] [Google Scholar]
- 8. Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A, Mignot G, Maiuri MC, Ullrich E, Saulnier P, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med 2007; 13:1050-9; PMID: ; http://dx.doi.org/10.1038/nm1622 [DOI] [PubMed] [Google Scholar]
- 9. Tesniere A, Schlemmer F, Boige V, Kepp O, Martins I, Ghiringhelli F, Aymeric L, Michaud M, Apetoh L, Barault L, et al. Immunogenic death of colon cancer cells treated with oxaliplatin. Oncogene 2009; 29:482-91; PMID: ; http://dx.doi.org/10.1038/onc.2009.356 [DOI] [PubMed] [Google Scholar]
- 10. Schiavoni G, Sistigu A, Valentini M, Mattei F, Sestili P, Spadaro F, Sanchez M, Lorenzi S, D’Urso MT, Belardelli F, et al. Cyclophosphamide synergizes with type I interferons through systemic dendritic cell reactivation and induction of immunogenic tumor apoptosis. Cancer Res 2010; 71:768-78; PMID: ; http://dx.doi.org/10.1158/0008-5472.CAN-10-2788 [DOI] [PubMed] [Google Scholar]
- 11. Viaud S, Saccheri F, Mignot G, Yamazaki T, Daillere R, Hannani D, Enot DP, Pfirschke C, Engblom C, Pittet MJ, et al. The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science 2013; 342:971-6; PMID: ; http://dx.doi.org/10.1126/science.1240537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Menger L, Vacchelli E, Adjemian S, Martins I, Ma Y, Shen S, Yamazaki T, Sukkurwala AQ, Michaud M, Mignot G, et al. Cardiac glycosides exert anticancer effects by inducing immunogenic cell death. Sci Transl Med 2012; 4:143ra99; PMID: ; http://dx.doi.org/10.1126/scitranslmed.3003807 [DOI] [PubMed] [Google Scholar]
- 13. Menger L, Vacchelli E, Kepp O, Eggermont A, Tartour E, Zitvogel L, Kroemer G, Galluzzi L. Trial watch: cardiac glycosides and cancer therapy. Oncoimmunology 2013; 2:e23082; PMID: ; http://dx.doi.org/10.4161/onci.23082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Senovilla L, Vitale I, Martins I, Tailler M, Pailleret C, Michaud M, Galluzzi L, Adjemian S, Kepp O, Niso-Santano M, et al. An immunosurveillance mechanism controls cancer cell ploidy. Science 2012; 337:1678-84; PMID: ; http://dx.doi.org/10.1126/science.1224922 [DOI] [PubMed] [Google Scholar]
- 15. Kepp O, Galluzzi L, Kroemer G. Immune effectors required for the therapeutic activity of vorinostat. Oncoimmunology 2014; 2:e27157; http://dx.doi.org/10.4161/onci.27157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. West AC, Mattarollo SR, Shortt J, Cluse LA, Christiansen AJ, Smyth MJ, Johnstone RW. An intact immune system is required for the anticancer activities of histone deacetylase inhibitors. Cancer Res 2013; 73:7265-76; PMID: ; http://dx.doi.org/10.1158/0008-5472.CAN-13-0890 [DOI] [PubMed] [Google Scholar]
- 17. Galluzzi L, Kepp O, Kroemer G. Immunogenic cell death in radiation therapy. Oncoimmunology 2014; 2:e26536; http://dx.doi.org/10.4161/onci.26536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ko A, Kanehisa A, Martins I, Senovilla L, Chargari C, Dugue D, Marino G, Kepp O, Michaud M, Perfettini JL, et al. Autophagy inhibition radiosensitizes in vitro, yet reduces radioresponses in vivo due to deficient immunogenic signalling. Cell Death Differ 2013; 21:92-9; PMID: ; http://dx.doi.org/10.1038/cdd.2013.124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Obeid M, Panaretakis T, Joza N, Tufi R, Tesniere A, van Endert P, Zitvogel L, Kroemer G. Calreticulin exposure is required for the immunogenicity of gamma-irradiation and UVC light-induced apoptosis. Cell Death Differ 2007; 14:1848-50; PMID: ; http://dx.doi.org/10.1038/sj.cdd.4402201 [DOI] [PubMed] [Google Scholar]
- 20. Garg AD, Krysko DV, Vandenabeele P, Agostinis P. The emergence of phox-ER stress induced immunogenic apoptosis. Oncoimmunology 2012; 1:786-8; PMID: ; http://dx.doi.org/10.4161/onci.19750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Garg AD, Krysko DV, Verfaillie T, Kaczmarek A, Ferreira GB, Marysael T, Rubio N, Firczuk M, Mathieu C, Roebroek AJ, et al. A novel pathway combining calreticulin exposure and ATP secretion in immunogenic cancer cell death. EMBO J 2012; 31:1062-79; PMID: ; http://dx.doi.org/10.1038/emboj.2011.497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Martins I, Kepp O, Schlemmer F, Adjemian S, Tailler M, Shen S, Michaud M, Menger L, Gdoura A, Tajeddine N, et al. Restoration of the immunogenicity of cisplatin-induced cancer cell death by endoplasmic reticulum stress. Oncogene 2010; 30:1147-58; PMID: ; http://dx.doi.org/10.1038/onc.2010.500 [DOI] [PubMed] [Google Scholar]
- 23. Martins I, Wang Y, Michaud M, Ma Y, Sukkurwala AQ, Shen S, Kepp O, Metivier D, Galluzzi L, Perfettini JL, et al. Molecular mechanisms of ATP secretion during immunogenic cell death. Cell Death Differ 2013; 21:79-91; PMID: ; http://dx.doi.org/10.1038/cdd.2013.75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Panaretakis T, Joza N, Modjtahedi N, Tesniere A, Vitale I, Durchschlag M, Fimia GM, Kepp O, Piacentini M, Froehlich KU, et al. The co-translocation of ERp57 and calreticulin determines the immunogenicity of cell death. Cell Death Differ 2008; 15:1499-509; PMID: ; http://dx.doi.org/10.1038/cdd.2008.67 [DOI] [PubMed] [Google Scholar]
- 25. Panaretakis T, Kepp O, Brockmeier U, Tesniere A, Bjorklund AC, Chapman DC, Durchschlag M, Joza N, Pierron G, van Endert P, et al. Mechanisms of pre-apoptotic calreticulin exposure in immunogenic cell death. EMBO J 2009; 28:578-90; PMID: ; http://dx.doi.org/10.1038/emboj.2009.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yamazaki T, Hannani D, Poirier-Colame V, Ladoire S, Locher C, Sistigu A, Prada N, Adjemian S, Catani JP, Freudenberg M, et al. Defective immunogenic cell death of HMGB1-deficient tumors: compensatory therapy with TLR4 agonists. Cell Death Differ 2013; 21:69-78; PMID: ; http://dx.doi.org/10.1038/cdd.2013.72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kroemer G, Galluzzi L, Kepp O, Zitvogel L. Immunogenic cell death in cancer therapy. Annu Rev Immunol 2012; 31:51-72; PMID: ; http://dx.doi.org/10.1146/annurev-immunol-032712-100008 [DOI] [PubMed] [Google Scholar]
- 28. Tesniere A, Apetoh L, Ghiringhelli F, Joza N, Panaretakis T, Kepp O, Schlemmer F, Zitvogel L, Kroemer G. Immunogenic cancer cell death: a key-lock paradigm. Curr Opin Immunol 2008; 20:504-11; PMID: ; http://dx.doi.org/10.1016/j.coi.2008.05.007 [DOI] [PubMed] [Google Scholar]
- 29. Ma Y, Adjemian S, Mattarollo SR, Yamazaki T, Aymeric L, Yang H, Portela Catani JP, Hannani D, Duret H, Steegh K, et al. Anticancer chemotherapy-induced intratumoral recruitment and differentiation of antigen-presenting cells. Immunity 2013; 38:729-41. [DOI] [PubMed] [Google Scholar]
- 30. Ma Y, Mattarollo SR, Adjemian S, Yang H, Aymeric L, Hannani D, Portela Catani JP, Duret H, Teng MW, Kepp O, et al. CCL2/CCR2-dependent recruitment of functional antigen-presenting cells into tumors upon chemotherapy. Cancer Res 2013; 74:436-45; PMID: ; http://dx.doi.org/10.1158/0008-5472.CAN-13-1265 [DOI] [PubMed] [Google Scholar]
- 31. Michaud M, Sukkurwala AQ, Martins I, Shen S, Zitvogel L, Kroemer G. Subversion of the chemotherapy-induced anticancer immune response by the ecto-ATPase CD39. Oncoimmunology 2012; 1:393-5; PMID: ; http://dx.doi.org/10.4161/onci.19070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wang Y, Martins I, Ma Y, Kepp O, Galluzzi L, Kroemer G. Autophagy-dependent ATP release from dying cells via lysosomal exocytosis. Autophagy 2013; 9:1624-5. [DOI] [PubMed] [Google Scholar]
- 33. Ma Y, Galluzzi L, Zitvogel L, Kroemer G.. Autophagy and cellular immune responses. Immunity 2013; 39:211-27; PMID: ; http://dx.doi.org/10.1016/j.immuni.2013.07.017 [DOI] [PubMed] [Google Scholar]
- 34. Ciampricotti M, Hau CS, Doornebal CW, Jonkers J, de Visser KE. Chemotherapy response of spontaneous mammary tumors is independent of the adaptive immune system. Nat Med 2012; 18:344-6; author reply 6; PMID: ; http://dx.doi.org/10.1038/nm.2652 [DOI] [PubMed] [Google Scholar]
- 35. Kroemer G, Galluzzi L, Zitvogel L. Immunological effects of chemotherapy in spontaneous breast cancers. Oncoimmunology 2014; 2:e27158; http://dx.doi.org/10.4161/onci.27158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mattarollo SR, Loi S, Duret H, Ma Y, Zitvogel L, Smyth MJ. Pivotal role of innate and adaptive immunity in anthracycline chemotherapy of established tumors. Cancer Res 2011; 71:4809-20; PMID: ; http://dx.doi.org/10.1158/0008-5472.CAN-11-0753 [DOI] [PubMed] [Google Scholar]
- 37. Hannesdottir L, Tymoszuk P, Parajuli N, Wasmer MH, Philipp S, Daschil N, Datta S, Koller JB, Tripp CH, Stoitzner P, et al. Lapatinib and doxorubicin enhance the Stat1-dependent antitumor immune response. Eur J Immunol 2013; 43:2718-29; PMID: ; http://dx.doi.org/10.1002/eji.201242505 [DOI] [PubMed] [Google Scholar]
- 38. Fridman WH, Galon J, Pages F, Tartour E, Sautes-Fridman C, Kroemer G. Prognostic and predictive impact of intra- and peritumoral immune infiltrates. Cancer Res 2011; 71:5601-5; PMID: ; http://dx.doi.org/10.1158/0008-5472.CAN-11-1316 [DOI] [PubMed] [Google Scholar]
- 39. Galon J, Angell HK, Bedognetti D, Marincola FM. The continuum of cancer immunosurveillance: prognostic, predictive, and mechanistic signatures. Immunity 2013; 39:11-26; PMID: ; http://dx.doi.org/10.1016/j.immuni.2013.07.008 [DOI] [PubMed] [Google Scholar]
- 40. Senovilla L, Vacchelli E, Galon J, Adjemian S, Eggermont A, Fridman WH, Sautes-Fridman C, Ma Y, Tartour E, Zitvogel L, et al. Trial watch: Prognostic and predictive value of the immune infiltrate in cancer. Oncoimmunology 2012; 1:1323-43; PMID: ; http://dx.doi.org/10.4161/onci.22009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Denkert C, Loibl S, Noske A, Roller M, Muller BM, Komor M, Budczies J, Darb-Esfahani S, Kronenwett R, Hanusch C, et al. Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J Clin Oncol 2009; 28:105-13; PMID: ; http://dx.doi.org/10.1200/JCO.2009.23.7370 [DOI] [PubMed] [Google Scholar]
- 42. Ladoire S, Mignot G, Dabakuyo S, Arnould L, Apetoh L, Rebe C, Coudert B, Martin F, Bizollon MH, Vanoli A, et al. In situ immune response after neoadjuvant chemotherapy for breast cancer predicts survival. J Pathol 2011; 224:389-400; PMID: ; http://dx.doi.org/10.1002/path.2866 [DOI] [PubMed] [Google Scholar]
- 43. Loi S. Tumor-infiltrating lymphocytes, breast cancer subtypes and therapeutic efficacy. Oncoimmunology 2013; 2:e24720; PMID: ; http://dx.doi.org/10.4161/onci.24720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Loi S, Sirtaine N, Piette F, Salgado R, Viale G, Van Eenoo F, Rouas G, Francis P, Crown JP, Hitre E, et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02-98. J Clin Oncol 2013; 31:860-7; PMID: ; http://dx.doi.org/10.1200/JCO.2011.41.0902 [DOI] [PubMed] [Google Scholar]
- 45. Rao S, Yang H, Penninger JM, Kroemer G. Autophagy in non-small cell lung carcinogenesis: A positive regulator of antitumor immunosurveillance. Autophagy 2014; 10:529-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Maiuri MC, Tasdemir E, Criollo A, Morselli E, Vicencio JM, Carnuccio R, Kroemer G. Control of autophagy by oncogenes and tumor suppressor genes. Cell Death Differ 2009; 16:87-93; PMID: ; http://dx.doi.org/10.1038/cdd.2008.131 [DOI] [PubMed] [Google Scholar]
- 47. Morselli E, Shen S, Ruckenstuhl C, Bauer MA, Marino G, Galluzzi L, Criollo A, Michaud M, Maiuri MC, Chano T, et al. p53 inhibits autophagy by interacting with the human ortholog of yeast Atg17, RB1CC1/FIP200. Cell Cycle 2011; 10:2763-9; PMID: ; http://dx.doi.org/10.4161/cc.10.16.16868 [DOI] [PubMed] [Google Scholar]
- 48. Shen S, Niso-Santano M, Adjemian S, Takehara T, Malik SA, Minoux H, Souquere S, Marino G, Lachkar S, Senovilla L, et al. Cytoplasmic STAT3 represses autophagy by inhibiting PKR activity. Mol Cell 2012; 48:667-80; PMID: ; http://dx.doi.org/10.1016/j.molcel.2012.09.013 [DOI] [PubMed] [Google Scholar]
- 49. Cartlidge RA, Thomas GR, Cagnol S, Jong KA, Molton SA, Finch AJ, McMahon M. Oncogenic BRAF(V600E) inhibits BIM expression to promote melanoma cell survival. Pigment Cell Melanoma Res 2008; 21:534-44; PMID: ; http://dx.doi.org/10.1111/j.1755-148X.2008.00491.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Komatsu M, Waguri S, Ueno T, Iwata J, Murata S, Tanida I, Ezaki J, Mizushima N, Ohsumi Y, Uchiyama Y, et al. Impairment of starvation-induced and constitutive autophagy in Atg7-deficient mice. J Cell Biol 2005; 169:425-34; PMID: ; http://dx.doi.org/10.1083/jcb.200412022 [DOI] [PMC free article] [PubMed] [Google Scholar]