Abstract
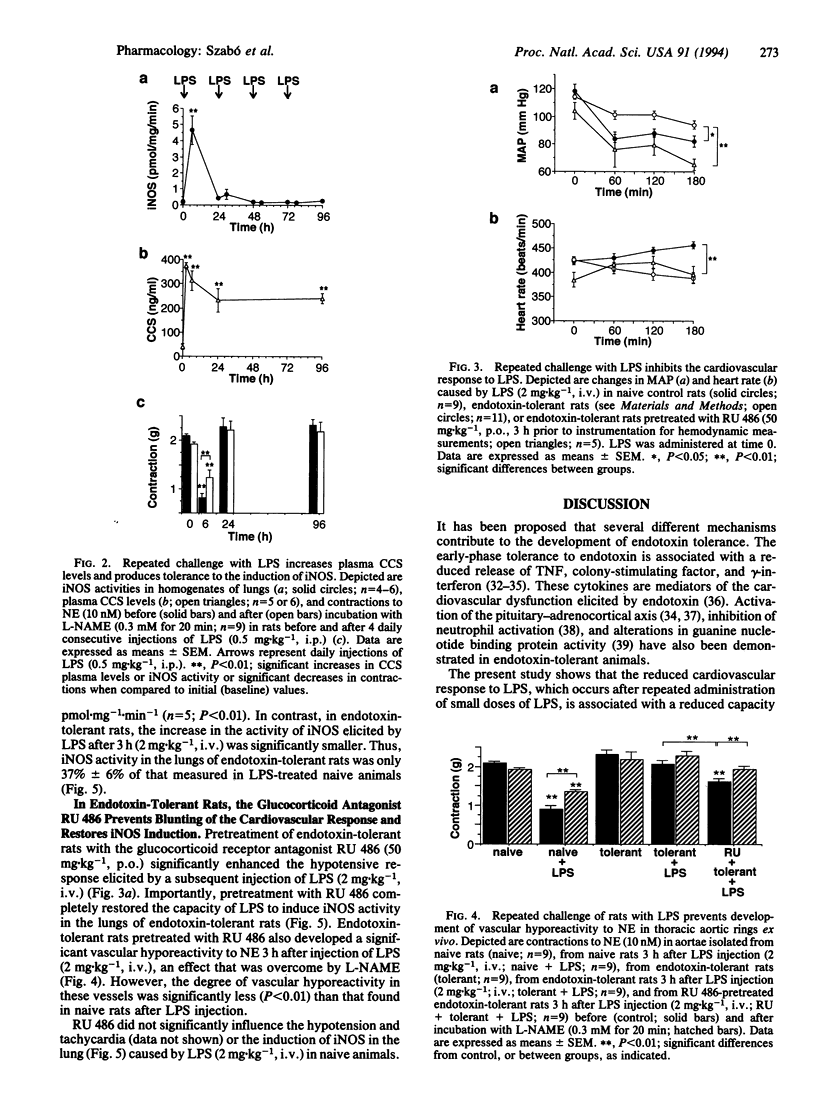
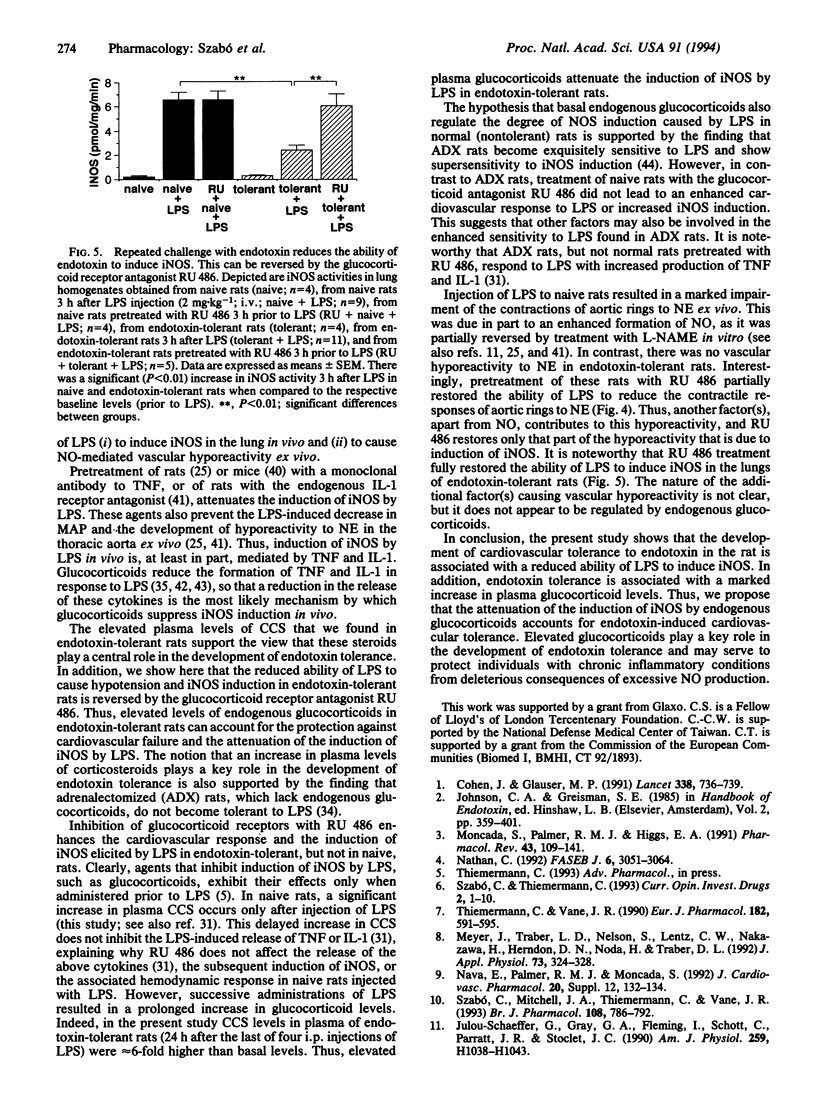
An enhanced formation of nitric oxide (NO) due to induction of a calcium-independent (inducible) NO synthase (iNOS) contributes importantly to the cardiovascular failure caused by bacterial endotoxin. Repeated challenges with small doses of endotoxin result in tolerance to both peripheral vascular failure and death caused by subsequent injection of a higher dose of endotoxin. Here we investigate whether tolerance to endotoxin is associated with a lack of induction of iNOS in vivo and whether endogenous glucocorticoids play a role in the development of endotoxin tolerance. In anesthetized rats, i.v. administration of Escherichia coli endotoxin [lipopolysaccharide (LPS); 2 mg.kg-1] resulted in a prolonged decrease in mean arterial blood pressure (MAP) and hyporeactivity to the contractile responses elicited by norepinephrine (NE; 10 nM) in aortic rings ex vivo. Hyporeactivity to NE was partially reversed by NG-nitro-L-arginine methyl ester (0.3 mM) in vitro, suggesting that an enhanced formation of NO contributes to this hyporeactivity. There was a substantial increase in the activity of iNOS in the lung 3 h after i.v. injection of LPS (0.2 +/- 0.1 to 6.6 +/- 0.6 pmol.mg-1.min-1; n = 5; P < 0.01). Rats injected i.p. with LPS (0.5 mg.kg-1) for 4 consecutive days became tolerant to an i.v. injection of LPS (2 mg.kg-1) in that both hypotension and vascular hyporeactivity to NE were significantly attenuated. Moreover, in these endotoxin-tolerant rats, the induction of iNOS by LPS in the lung was attenuated by 63% +/- 6%. Injection of LPS caused a 9-fold increase in plasma corticosterone (CCS) levels within 2 h and CCS levels remained significantly elevated 6 and 24 h after LPS. Animals rendered tolerant to endotoxin by administration of a low dose of LPS (0.5 mg.kg-1, i.p.) for 4 days still had a 6-fold increase in plasma CCS levels 24 h after the last injection of LPS. When endotoxin-tolerant rats were treated with the glucocorticoid receptor antagonist RU 486 (50 mg.kg-1, p.o. 3 h prior to LPS), there was a restoration of the effects of LPS (2 mg.kg-1, i.v.) in causing hypotension, vascular hyporeactivity to NE, and iNOS induction in the lung. However, in control rats RU 486 enhanced neither the decrease in MAP nor the induction of iNOS in response to LPS (2 mg.kg-1, i.v.). Thus, cardiovascular tolerance to endotoxin is accompanied and explained by reduced induction of iNOS in vivo due to the elevation of endogenous glucocorticoid levels.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barroso-Aranda J., Schmid-Schönbein G. W., Zweifach B. W., Mathison J. C. Polymorphonuclear neutrophil contribution to induced tolerance to bacterial lipopolysaccharide. Circ Res. 1991 Nov;69(5):1196–1206. doi: 10.1161/01.res.69.5.1196. [DOI] [PubMed] [Google Scholar]
- Besedovsky H., del Rey A., Sorkin E., Dinarello C. A. Immunoregulatory feedback between interleukin-1 and glucocorticoid hormones. Science. 1986 Aug 8;233(4764):652–654. doi: 10.1126/science.3014662. [DOI] [PubMed] [Google Scholar]
- Beutler B., Krochin N., Milsark I. W., Luedke C., Cerami A. Control of cachectin (tumor necrosis factor) synthesis: mechanisms of endotoxin resistance. Science. 1986 May 23;232(4753):977–980. doi: 10.1126/science.3754653. [DOI] [PubMed] [Google Scholar]
- Billiau A., Vandekerckhove F. Cytokines and their interactions with other inflammatory mediators in the pathogenesis of sepsis and septic shock. Eur J Clin Invest. 1991 Dec;21(6):559–573. doi: 10.1111/j.1365-2362.1991.tb01410.x. [DOI] [PubMed] [Google Scholar]
- Bogdan C., Vodovotz Y., Paik J., Xie Q. W., Nathan C. Traces of bacterial lipopolysaccharide suppress IFN-gamma-induced nitric oxide synthase gene expression in primary mouse macrophages. J Immunol. 1993 Jul 1;151(1):301–309. [PubMed] [Google Scholar]
- Brady A. J., Poole-Wilson P. A., Harding S. E., Warren J. B. Nitric oxide production within cardiac myocytes reduces their contractility in endotoxemia. Am J Physiol. 1992 Dec;263(6 Pt 2):H1963–H1966. doi: 10.1152/ajpheart.1992.263.6.H1963. [DOI] [PubMed] [Google Scholar]
- Coffee K. A., Halushka P. V., Ashton S. H., Tempel G. E., Wise W. C., Cook J. A. Endotoxin tolerance is associated with altered GTP-binding protein function. J Appl Physiol (1985) 1992 Sep;73(3):1008–1013. doi: 10.1152/jappl.1992.73.3.1008. [DOI] [PubMed] [Google Scholar]
- Cohen J., Glauser M. P. Septic shock: treatment. Lancet. 1991 Sep 21;338(8769):736–739. doi: 10.1016/0140-6736(91)91453-2. [DOI] [PubMed] [Google Scholar]
- Evans G. F., Zuckerman S. H. Glucocorticoid-dependent and -independent mechanisms involved in lipopolysaccharide tolerance. Eur J Immunol. 1991 Sep;21(9):1973–1979. doi: 10.1002/eji.1830210902. [DOI] [PubMed] [Google Scholar]
- Finkel M. S., Oddis C. V., Jacob T. D., Watkins S. C., Hattler B. G., Simmons R. L. Negative inotropic effects of cytokines on the heart mediated by nitric oxide. Science. 1992 Jul 17;257(5068):387–389. doi: 10.1126/science.1631560. [DOI] [PubMed] [Google Scholar]
- Geng Y., Hansson G. K., Holme E. Interferon-gamma and tumor necrosis factor synergize to induce nitric oxide production and inhibit mitochondrial respiration in vascular smooth muscle cells. Circ Res. 1992 Nov;71(5):1268–1276. doi: 10.1161/01.res.71.5.1268. [DOI] [PubMed] [Google Scholar]
- Green S. J., Chen T. Y., Crawford R. M., Nacy C. A., Morrison D. C., Meltzer M. S. Cytotoxic activity and production of toxic nitrogen oxides by macrophages treated with IFN-gamma and monoclonal antibodies against the 73-kDa lipopolysaccharide receptor. J Immunol. 1992 Sep 15;149(6):2069–2075. [PubMed] [Google Scholar]
- Julou-Schaeffer G., Gray G. A., Fleming I., Schott C., Parratt J. R., Stoclet J. C. Loss of vascular responsiveness induced by endotoxin involves L-arginine pathway. Am J Physiol. 1990 Oct;259(4 Pt 2):H1038–H1043. doi: 10.1152/ajpheart.1990.259.4.H1038. [DOI] [PubMed] [Google Scholar]
- Knowles R. G., Salter M., Brooks S. L., Moncada S. Anti-inflammatory glucocorticoids inhibit the induction by endotoxin of nitric oxide synthase in the lung, liver and aorta of the rat. Biochem Biophys Res Commun. 1990 Nov 15;172(3):1042–1048. doi: 10.1016/0006-291x(90)91551-3. [DOI] [PubMed] [Google Scholar]
- Lepe-Zuniga J. L., Klostergaard J. Tolerance to endotoxin in vitro: independent regulation of interleukin-1, tumor necrosis factor and interferon alpha production during in vitro differentiation of human monocytes. Lymphokine Res. 1990 Fall;9(3):309–319. [PubMed] [Google Scholar]
- Meyer J., Traber L. D., Nelson S., Lentz C. W., Nakazawa H., Herndon D. N., Noda H., Traber D. L. Reversal of hyperdynamic response to continuous endotoxin administration by inhibition of NO synthesis. J Appl Physiol (1985) 1992 Jul;73(1):324–328. doi: 10.1152/jappl.1992.73.1.324. [DOI] [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Higgs E. A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991 Jun;43(2):109–142. [PubMed] [Google Scholar]
- Moore P. K., al-Swayeh O. A., Chong N. W., Evans R. A., Gibson A. L-NG-nitro arginine (L-NOARG), a novel, L-arginine-reversible inhibitor of endothelium-dependent vasodilatation in vitro. Br J Pharmacol. 1990 Feb;99(2):408–412. doi: 10.1111/j.1476-5381.1990.tb14717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. Nitric oxide as a secretory product of mammalian cells. FASEB J. 1992 Sep;6(12):3051–3064. [PubMed] [Google Scholar]
- Peers S. H., Moon D., Flower R. J. Reversal of the anti-inflammatory effects of dexamethasone by the glucocorticoid antagonist RU 38486. Biochem Pharmacol. 1988 Feb 1;37(3):556–557. doi: 10.1016/0006-2952(88)90230-4. [DOI] [PubMed] [Google Scholar]
- Perretti M., Duncan G. S., Flower R. J., Peers S. H. Serum corticosterone, interleukin-1 and tumour necrosis factor in rat experimental endotoxaemia: comparison between Lewis and Wistar strains. Br J Pharmacol. 1993 Oct;110(2):868–874. doi: 10.1111/j.1476-5381.1993.tb13893.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perretti M., Mugridge K. G., Wallace J. L., Parente L. Reduction of aspirin-induced gastric damage in rats by interleukin-1 beta: possible involvement of endogenous corticosteroids. J Pharmacol Exp Ther. 1992 Jun;261(3):1238–1247. [PubMed] [Google Scholar]
- Radomski M. W., Palmer R. M., Moncada S. Glucocorticoids inhibit the expression of an inducible, but not the constitutive, nitric oxide synthase in vascular endothelial cells. Proc Natl Acad Sci U S A. 1990 Dec;87(24):10043–10047. doi: 10.1073/pnas.87.24.10043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Cantu L., Rode H. N., Christou N. V. Endotoxin tolerance is associated with reduced secretion of tumor necrosis factor. Arch Surg. 1989 Dec;124(12):1432–1436. doi: 10.1001/archsurg.1989.01410120082016. [DOI] [PubMed] [Google Scholar]
- Schulz R., Nava E., Moncada S. Induction and potential biological relevance of a Ca(2+)-independent nitric oxide synthase in the myocardium. Br J Pharmacol. 1992 Mar;105(3):575–580. doi: 10.1111/j.1476-5381.1992.tb09021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severn A., Xu D., Doyle J., Leal L. M., O'Donnell C. A., Brett S. J., Moss D. W., Liew F. Y. Pre-exposure of murine macrophages to lipopolysaccharide inhibits the induction of nitric oxide synthase and reduces leishmanicidal activity. Eur J Immunol. 1993 Jul;23(7):1711–1714. doi: 10.1002/eji.1830230747. [DOI] [PubMed] [Google Scholar]
- Stuehr D. J., Nathan C. F. Nitric oxide. A macrophage product responsible for cytostasis and respiratory inhibition in tumor target cells. J Exp Med. 1989 May 1;169(5):1543–1555. doi: 10.1084/jem.169.5.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabó C., Mitchell J. A., Gross S. S., Thiemermann C., Vane J. R. Nifedipine inhibits the induction of nitric oxide synthase by bacterial lipopolysaccharide. J Pharmacol Exp Ther. 1993 May;265(2):674–680. [PubMed] [Google Scholar]
- Szabó C., Mitchell J. A., Thiemermann C., Vane J. R. Nitric oxide-mediated hyporeactivity to noradrenaline precedes the induction of nitric oxide synthase in endotoxin shock. Br J Pharmacol. 1993 Mar;108(3):786–792. doi: 10.1111/j.1476-5381.1993.tb12879.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabó C., Thiemermann C., Vane J. R. Inhibition of the production of nitric oxide and vasodilator prostaglandins attenuates the cardiovascular response to bacterial endotoxin in adrenalectomized rats. Proc Biol Sci. 1993 Sep 22;253(1338):233–238. doi: 10.1098/rspb.1993.0108. [DOI] [PubMed] [Google Scholar]
- Thiemermann C., Mustafa M., Mester P. A., Mitchell J. A., Hecker M., Vane J. R. Inhibition of the release of endothelium-derived relaxing factor in vitro and in vivo by dipeptides containing NG-nitro-L-arginine. Br J Pharmacol. 1991 Sep;104(1):31–38. doi: 10.1111/j.1476-5381.1991.tb12380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiemermann C., Szabó C., Mitchell J. A., Vane J. R. Vascular hyporeactivity to vasoconstrictor agents and hemodynamic decompensation in hemorrhagic shock is mediated by nitric oxide. Proc Natl Acad Sci U S A. 1993 Jan 1;90(1):267–271. doi: 10.1073/pnas.90.1.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiemermann C., Vane J. Inhibition of nitric oxide synthesis reduces the hypotension induced by bacterial lipopolysaccharides in the rat in vivo. Eur J Pharmacol. 1990 Jul 17;182(3):591–595. doi: 10.1016/0014-2999(90)90062-b. [DOI] [PubMed] [Google Scholar]
- Thiemermann C., Wu C. C., Szabó C., Perretti M., Vane J. R. Role of tumour necrosis factor in the induction of nitric oxide synthase in a rat model of endotoxin shock. Br J Pharmacol. 1993 Sep;110(1):177–182. doi: 10.1111/j.1476-5381.1993.tb13789.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virca G. D., Kim S. Y., Glaser K. B., Ulevitch R. J. Lipopolysaccharide induces hyporesponsiveness to its own action in RAW 264.7 cells. J Biol Chem. 1989 Dec 25;264(36):21951–21956. [PubMed] [Google Scholar]
- Wright C. E., Rees D. D., Moncada S. Protective and pathological roles of nitric oxide in endotoxin shock. Cardiovasc Res. 1992 Jan;26(1):48–57. doi: 10.1093/cvr/26.1.48. [DOI] [PubMed] [Google Scholar]
- Zuckerman S. H., Evans G. F., Butler L. D. Endotoxin tolerance: independent regulation of interleukin-1 and tumor necrosis factor expression. Infect Immun. 1991 Aug;59(8):2774–2780. doi: 10.1128/iai.59.8.2774-2780.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckerman S. H., Qureshi N. In vivo inhibition of lipopolysaccharide-induced lethality and tumor necrosis factor synthesis by Rhodobacter sphaeroides diphosphoryl lipid A is dependent on corticosterone induction. Infect Immun. 1992 Jul;60(7):2581–2587. doi: 10.1128/iai.60.7.2581-2587.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckerman S. H., Shellhaas J., Butler L. D. Differential regulation of lipopolysaccharide-induced interleukin 1 and tumor necrosis factor synthesis: effects of endogenous and exogenous glucocorticoids and the role of the pituitary-adrenal axis. Eur J Immunol. 1989 Feb;19(2):301–305. doi: 10.1002/eji.1830190213. [DOI] [PubMed] [Google Scholar]


