Abstract
Background
High-dose thiotepa, busulfan, and cyclophosphamide (TBC) with autologous stem cell transplantation (ASCT) has been used in patients with central nervous system (CNS) involvement by non-Hodgkin lymphoma (NHL). Despite limited penetration into the CNS, rituximab (R) is active in primary CNS NHL. We therefore combined high-dose R with TBC for ASCT in patients with CNS NHL.
Methods
We conducted a single-arm phase II trial using high-dose rituximab with cytarabine for stem cell mobilization, followed by high-dose rituximab combined with TBC (R-TBC) for ASCT. Doses of R at 1000 mg/m2 were given on days 1 and 8 of mobilization and on days −9 and −2 of TBC. The primary endpoint was efficacy.
Results
Thirty patients were enrolled. Eighteen patients had primary CNS NHL (12 CR/PR1, 6 CR/PR2) and 12 patients had secondary CNS (5 CR/PR1, 7 CR/PR2 or beyond) lymphoma. All patients were in a partial or complete remission. 29 patients proceeded to R-TBC ASCT. Two patients developed significant neurotoxicity. The 100-day non-relapse mortality rate was 0% and one patient died from non-relapse causes 5 months after ASCT. For all patients, at a median follow-up of 24 months (range, 12-40), the estimated 2-year PFS is 81% (95% CI, 59%-92%) and 2-year OS is 93% (95% CI, 76%-98%). There have been no relapses or deaths in the 18 patients with primary CNS lymphoma.
Conclusion
For patients with CNS involvement by B-cell NHL, R-TBC ASCT shows encouraging activity and merits further study, especially in patients with primary CNS NHL.
INTRODUCTION
Historically, involvement of the central nervous system (CNS) by non-Hodgkin lymphoma (NHL) has carried a poor prognosis. In patients with primary CNS lymphoma (PCNSL), modern regimens including chemotherapy agents with high levels of CNS penetration (typically using a backbone of systemic high-dose methotrexate, MTX) and combined modality therapy have significantly improved upon the previously poor response rates and prognosis.1-5 Despite this success, relapse rates remained high with only 20-30% of patients achieving a durable long-term remission after high-dose MTX therapy. The inclusion of whole brain radiotherapy (WBRT) improved short-term outcomes, but has also been associated with significant rates of neurotoxicity particularly in patients over the age of 60. Recently, investigators have added consolidation chemotherapy using other agents such as cytarabine and etoposide7 or used reduced-dose WBRT8 with promising results.
Secondary CNS lymphoma (SCNSL), defined as either synchronous CNS involvement in a patient with systemic NHL or as a site of recurrence in a patient with a history of systemic NHL, is also a difficult therapeutic problem. Historically, the mainstay of therapy for secondary CNS NHL has been systemic or intrathecal chemotherapy and/or WBRT. In several large series, SCNSL has a median survival on the order of 2.5 to 4 months with 1-year survival rates of only ~25%.9-11 In the largest international case series of 113 patients with exclusively brain parenchymal relapses, systemic chemotherapy, particularly with regimens containing high-dose MTX, emerged as the only treatment modality suggesting improved overall survival, although relapses occurred in 68% of patients at a median time to progression of 1 year, and only 10% of patients attained a durable remission.12
Given these poor outcomes with conventional chemotherapy in PCNSL and SCNSL, intensification of treatment with high-dose chemotherapy and autologous stem cell transplantation (ASCT) in PCNSL and SCNSL has been explored.13-24 One high-dose chemotherapy regimen which has been used for patients with CNS lymphoma is the combination of thiotepa, busulfan, and cyclophosphamide (TBC), which was first reported by investigators in France. Pharmacokinetic studies have shown all three agents to have significant penetration of the blood brain barrier with thiotepa and busulfan achieving CSF levels of over 80% of plasma concentrations. We have previously reported our institutions’ experience with TBC ASCT for this population, showing compelling safety and efficacy.31 Addition of the anti-CD20 monoclonal antibody rituximab has become the standard of care for CD20 expressing systemic NHL, and also PCNSL. Despite a high molecular weight of 145 kD, rituximab appears to be able to penetrate into the CSF in small amounts, relative to systemic levels. Given this limited penetration but compelling single-agent activity observed in PCNSL, we explored if adding several high-doses of rituximab during high-dose cytarabine stem cell mobilization and TBC ASCT could increase levels of rituximab in the CNS and improve disease control.
PATIENTS AND METHODS
Patients
This study was approved by the Institutional Review Board at the Dana-Farber Harvard Cancer Center (DFHCC) and conducted at Dana-Farber Brigham and Women's Cancer Center and Massachusetts General Hospital Cancer Center. Informed consent was obtained from all subjects. All patients met institutional standard criteria for ASCT including Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1, total bilirubin < 2.0 mg/dL, creatinine < 2.0 mg/dL, a diffusing capacity of lungs for carbon monoxide (DLCO) corrected for hemoglobin of > 50% of predicted valued corrected for hemoglobin, and a cardiac left ventricular ejection fraction of > 45%. The CNS disease status at the time of ASCT was identified by MRI and/or CSF analysis (if applicable) as first or subsequent remission and either partial (PR) or complete response (CR). Complete response was defined as no evidence of contrast enhancing tumor on imaging and negative CSF analysis when available with assessments defined using modifications to the standard MacDonald criteria.34
Regimen
Mobilization
For autologous stem cell mobilization, patients received cytarabine at 3000 mg/m2 IV q12h x 4 doses on days 1 and 2 along with rituximab at 1000 mg/m2 IV on days 1 and 8. Actual body weight was used for rituximab dose calculations. For patients ≥ 60 years of age, the cytarabine dose was reduced to 2000 mg/m2 to avoid cerebellar toxicity (the lesser value of actual (ABW) or ideal body weight [IBW]). GCSF was started on day 4 at 10 mcg/kg SC daily rounded to the nearest pre-filled syringe dose and continued until adequate stem cell collection had been performed. For autologous stem cell collection, patients underwent large volume leukapheresis according to standard institutional procedures and progenitor cells were then cryopreserved based on validated institutional methods. A minimum of 2 x 106 CD34+ cells / kg were required to be collected before proceeding to ASCT.
High-Dose Chemotherapy and ASCT
Within 4 weeks of successful stem cell collection, participants were admitted for R-TBC ASCT. Mucositis prophylaxis with palifermin prior to conditioning and after ASCT was recommended but not mandated. Beginning on day −9 and through day −7 each patient was treated with thiotepa (250 mg/m2 IV per day, lesser value of ABW or IBW). On days −6 to −4 patients received busulfan (0.8 mg/kg IV every 6 hours for 12 doses, lesser value of ABW or IBW with use of adjusted IBW for obese patients), and on days −3 and −2 cyclophosphamide (60 mg/kg IV per day, lesser value of ABW or IBW) was given with mesna (15 mg/kg four times per day) for uroprotection. For patients ≥ 60 years of age, the busulfan dose was decreased to 0.6 mg/kg/dose (25% dose reduction). High-dose rituximab at 1000 mg/m2 IV was given on day −9 (after starting thiotepa) and day −2 (after completion of cyclophosphamide). Autologous stem cell infusion was performed on day 0 per standard institutional practice. Filgrastim (5 mcg/kg IV or SC once per day) was started on day +1 and continued until neutrophil recovery. Busulfan-related seizure prophylaxis was given with levetiracetam on days −6 to −3. Patients also received ursodiol (300 mg three times per day) and infectious prophylaxis against HSV/VZV and PCP. Neutrophil engraftment was defined as the first of two consecutive days with an absolute neutrophil count greater than 500 cells per mm3. Platelet recovery was defined as the first of two consecutive days with an unsupported platelet count greater than 20,000 per mm3.
Rituximab levels
In order to assess rituximab penetration into the CNS, rituximab levels were measured from both serum and cerebral spinal fluid (CSF) via lumbar puncture. Serum levels were measured before the first dose of high-dose rituximab during mobilization high-dose cytarabine / rituximab therapy and then concurrently with the CSF level (24-36 hours after infusion of rituximab was complete). Rituximab measurements were performed at Covance Bioanalytical Services, Chantilly, VA.
Statistical Methods
The study was designed to target an alternative 1-year progression-free survival (PFS) rate of 50% vs. a historical control rate of 30%. Patient safety was closely monitored during the trial with stopping rules for excessive toxicity or excessive 100 day progression/mortality rate incorporated into the study design. Data was locked at March 13, 2014 with a median follow up of 24 months for the 26 known survivors. The primary endpoint 1-year PFS rate was reported as a binary endpoint. Overall survival (OS) and PFS were also reported as time-to-event outcomes. OS was calculated from the date of transplant to death from any cause. PFS was calculated from the date of transplant to the date of disease progression or death from any cause. Patients who were alive without relapse or progression were censored at the time of last contact. Secondary endpoints included overall survival and non-relapse mortality (NRM). Overall survival (OS) was calculated from the date of transplant to death from any cause. Non-relapse mortality was defined as any death without evidence of relapse and estimated with relapse or death from relapse as competing risks.
RESULTS
Patient Characteristics
Thirty patients (17 men and 13 women) with CNS involvement by B-NHL were enrolled on the trial. 18 patients had PCNSL and 12 had SCNSL. Clinical characteristics for all patients are reported in Table 1. The median age at the time of enrollment was 58 (range, 24-74). All cases of PCNSL were diffuse large B-cell lymphoma by histology and included one case of Epstein Barr virus associated post-transplant lymphoproliferative disease (EBV-PTLD) in a recipient of a prior kidney transplant. In patients with SCNSL, histologies included DLBCL (n=9), CLL (n=2), and follicular lymphoma (n=1). In the patients with CLL and follicular lymphoma, CNS involvement was with these original histologies and not from disease transformation to a higher grade NHL.
Table 1.
Clinical characteristics (n=30)
| PCNSL (n = 18) | SCNSL (n = 12) | |
|---|---|---|
| Age at ASCT, median years (range) | 54 (24,69) | 63 (53,74) |
| Gender, male / female | 7 / 11 | 6 / 6 |
| Histology | ||
| Diffuse Large B-cell Lymphoma (%) | 18 (100) | 9 (75) |
| CLL / SLL (%) | 0 (0) | 2 (17) |
| Follicular NHL (%) | 0 (0) | 1 (8) |
| Prior Treatment History | ||
| High-dose IV MTX (%) | 18 (100) | 11 (92) |
| High-dose Cytarabine (%) | 2 (11) | 1 (8) |
| Rituximab (IV) (%) | 18 (100) | 12 (100) |
| WBRT (%) | 0 (0) | 2 (17) |
| Other XRT (%) | 1 (6) | 7 (58) |
| Response at enrollment | ||
| CR1 / PR1 | 12 (67) | 5 (42) |
| CR2 / PR2 or beyond | 6 (33) | 7 (58) |
| Time from diagnosis to ASCT, median months (range) | 6.8 (4.8, 42.1) | 23.2 (6.7, 130.3) |
| CD34+ cells / kg infused, median (range)* | 18.7 (2.3, 34.9) | 5.9 (2.1, 27.4) |
Values expressed are × 106 / kg of recipient actual body weight
Regarding the 18 patients with PCNSL, 11 were in CR1, 1 in PR1, 5 in CR2, and 1 in PR2 at the time of enrollment. All patients had been treated at some point during their course with high-dose intravenous methotrexate based therapy (range 3.5-8 gm/m2 per dose) as shown in Table 1. None of the patients with PCNSL had received WBRT. Of the 12 patients with SCNSL, one patient had refractory disease and achieved a temporary response after salvage infusional etoposide / high-dose cytarabine (EA) chemotherapy, but then progressed rapidly after high-dose cytarabine mobilization chemotherapy and did not undergo R-TBC ASCT on study. In the other 11 patients with SCNSL, 5 were in CR1 and 6 were in CR2 or beyond at the time of enrollment. Nine of the 11 patients had been treated with high-dose IV methotrexate based therapy and 2 had been treated with WBRT, while 7 patients had received localized radiation therapy to involved non-CNS fields. All 30 patients had been previously treated with standard dose (375 mg/m2/dose) intravenous rituximab as part of their induction or salvage treatments.
Treatment-related Toxicity
No significant toxicities were observed with high-dose rituximab – cytarabine mobilization chemotherapy except for expected neutropenia and thrombocytopenia. All patients tolerated infusions of high-dose rituximab without complications. All patients developed expected grade 4 neutropenia and thrombocytopenia during inpatient hospitalization for R-TBC ASCT. The most common toxicities observed during the ASCT hospitalization were diarrhea and mucositis as has been previously described with TBC. In terms of infectious complications, there were 9 documented bacterial infections, all of which were treated with resolution. One patient developed high-grade candidemia and also experienced cytomegalovirus (CMV) reactivation necessitating treatment. Two patients developed significant neurotoxicity. The first was a patient with SCNSL who had previously been treated with WBRT and subsequent HD-MTX. He had cognitive impairment prior to trial entry; during the ASCT hospitalization he developed delirium, and continued to have cognitive decline and repeated aspiration episodes. He died of complications related to cognitive decline 5 months after ASCT. The other patient developed thrombotic microangiopathy involving the CNS with seizures, in the setting of high-grade candidemia. She eventually recovered to hospital discharge. The 100-day non-relapse mortality rate was 0% with one death from non-relapse causes to date as mentioned above.
Outcomes
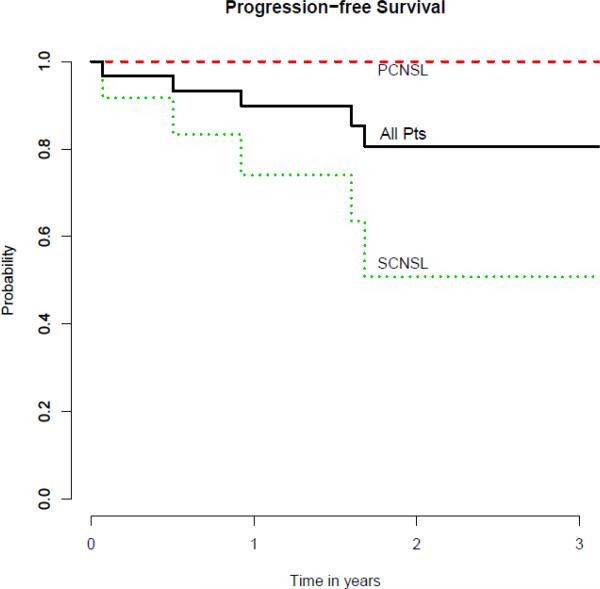
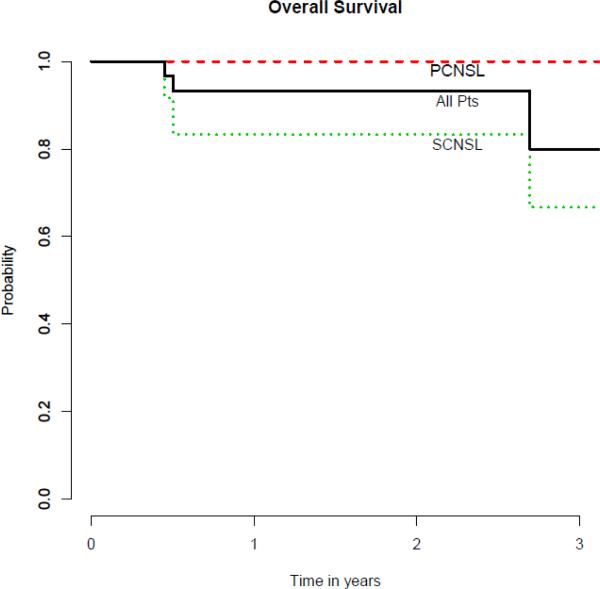
One patient with SCNSL was lost to follow-up at 6 months after ASCT. Median follow-up for the remaining surviving patients is 24 months with a range of 12-40. All of the 29 patients who underwent ASCT engrafted successfully. The median days to neutrophil engraftment and platelet recovery were 9 (range 8-12) and 11 (range 8-40), respectively. The median length of inpatient hospitalization stay for ASCT was 23 days (range 18-56). At first restaging performed at 3 months after ASCT, all 29 patients were in CR. The progression-free survival and overall survival curves are shown in Figure 1. For the entire cohort of patients (n=30), if the patient who was lost to follow-up was treated as an event, the estimated 2-year PFS and OS were 81% (95% CI, 59%-92%) and 93% (95% CI, 76%-98%), respectively. Among patients with SCNSL, 3 experienced disease relapse after ASCT with 2 being systemic disease and 1 in the CNS. The 2 cases of systemic relapse were both in patients in CR2 or beyond whereas the one case of CNS relapse was in a patient with SCNSL who was treated in CR1. For all patients with SCNSL, the estimated 2-year PFS and OS were 51% (95% CI, 18%-77%) and 83% (95% CI, 48%-96%), respectively. For the 18 patients with PCNSL, no patients have experienced death or relapse thus far.
Figure 1. Progression-Free Survival (n=30).
PCNSL = primary CNS lymphoma; SCNSL = secondary CNS lymphoma
CSF Rituximab Levels
Rituximab levels were measured in plasma and cerebral spinal fluid (CSF) from 8 patients on the first day of high-dose R-cytarabine mobilization therapy. Serum levels were measured prior to and after rituximab whereas CSF levels were measured after administration. Results are shown in Table 3. The median CSF concentration of rituximab was 347 ng/ml with a range of < 250 to 1310 ng/ml. The median CSF / post-infusion serum ratio was 0.09% with a range of 0 to 0.375%.
Table 3.
CSF and Serum Rituximab Levels (n=8)
| Patient | Pre* (ng/ml) | Post (ng/ml) | CSF (ng/ml) | CSF/Post (%) |
|---|---|---|---|---|
| 15 | -- | 410,000 | 343 | 0.084 |
| 21 | 36,400 | 375,000 | 498 | 0.133 |
| 22 | 47,500 | 367,000 | 351 | 0.096 |
| 25 | < 500 | 334,000 | 276 | 0.083 |
| 26 | -- | 373,000 | 440 | 0.118 |
| 27 | 31,400 | 394,000 | < 250** | not calculated+ |
| 28 | -- | 649,000 | 272 | 0.042 |
| 30 | 36,900 | 349,000 | 1310 | 0.375 |
Levels detected in “Pre” serum samples likely from previous treatment with rituximab
Level was less than minimum detectable limit
Not calculated given that CSF level was less than detectable limit
DISCUSSION
Primary and secondary CNS lymphomas are devastating diseases with a historically poor prognosis. High-dose chemotherapy with autologous stem cell transplantation in first or subsequent remission has shown promise in selected patients. We previously reported our experience with ASCT with TBC conditioning in patients with CNS involvement by NHL, illustrating favorable safety and preliminary efficacy.31 In the current study, we attempted to improve upon standard TBC conditioning with the addition of high-dose IV rituximab given during both high-dose cytarabine based mobilization chemotherapy and TBC conditioning prior to ASCT, in hopes that higher concentrations of rituximab in the CNS could reduce the risk of disease relapse. Our results illustrate the safety of such an approach and compelling efficacy, especially in the 18 patients with primary CNS lymphoma, none of whom have died or relapsed to date.
There have been several retrospective and prospective single-arm studies of ASCT in primary or secondary CNS lymphoma.13-24 For SCNSL, the EBMT (European Group for Blood and Marrow Transplantation) analyzed 62 patients with secondary CNS involvement and suggested an equivalent prognosis after ASCT compared to patients without CNS involvement as long as the CNS disease was well controlled. Several single institution series have also demonstrated encouraging results employing ASCT in this setting. Recently, Maziarz et al conducted a large analysis involving 151 patients with a history of CNS NHL using data from the Center for International Bone Marrow Transplant Research (CIBMTR) registry and showed comparable long-term outcomes compared to 4,688 patients with NHL and without CNS involvement who underwent ASCT.38
In primary CNS lymphoma, early studies involving ASCT were mainly in relapsed / refractory patients with a significant percentage of patients experiencing neurological toxicity. Soussain et al. recently described their large retrospective experience with TBC ASCT in 79 patients under the age of 65 with relapsed / refractory PCNSL or primary intra-ocular NHL. Their results showed encouraging safety and efficacy especially for patients who were chemosensitive to salvage treatment and in remission at the time of ASCT.35 More recently, ASCT has been increasingly used as consolidation in first remission for PCNSL and is now the subject of current prospective randomized clinical trials, comparing ASCT to other approaches for consolidation. Kasenda et al. have described the largest series (n=34) of PCNSL patients undergoing ASCT in CR1 with the longest follow-up (median 120 months) to date, illustrating a 5-year event-free survival of 79% in patients who achieved CR1 with initial methotrexate- based chemotherapy followed by thiotepa / BCNU ASCT.40 Interestingly, in their series, there were 12 total relapses with half of such relapses occurring 5 years or later after ASCT, suggesting that the use of ASCT in PCNSL may be changing the natural history of the disease.
The data presented in our study adds to the growing literature showing excellent safety and efficacy of high-dose chemotherapy with ASCT based approach for patients with both primary and secondary involvement of the CNS by NHL. Specifically, it reinforces the uncommon occurrence of cognitive decline with a treatment strategy comprised of high-dose methotrexate, high-dose cytarabine, and high-dose chemotherapy with ASCT and omission of WBRT entirely. Although formal prospective neuropsychiatric assessments were not performed, there were only two cases of significant neurologic toxicity with only one case of late cognitive decline to date. This occurred in a patient who had previously been treated with WBRT, which is not surprising given the well-described association of WBRT with an increased risk of neurotoxicity. Ongoing efforts are investigating if a lower dose of WBRT can decrease the risk of neurotoxicity. The event rate of neurologic toxicity in our study is comparable to other publications of TBC in PCNSL and in non-TBC high-dose regimens given to patients who have not previously received WBRT. It is noteworthy that the only 3 cases of disease relapse witnessed on this trial were in patients with SCNSL, while all 18 patients with PCNSL, including 6 in PR2/CR2, remain alive, in remission, and without neurotoxicity. While our cohort is relatively small in sample size, these results are fairly compelling for PCNSL.
The rationale behind the inclusion of high-dose IV rituximab was based on the premise that IV rituximab has limited penetration past the blood brain barrier, yet has been shown to be effective even as a single agent given IV in patients with PCNSL.32 One previous study in patients with multiple sclerosis suggested that approximately 0.1-0.2% of systemic rituximab concentrations are achieved in the CNS.42 Using samples taken from voluntary lumbar punctures performed during cytarabine mobilization, our data confirms that CSF concentrations of rituximab are indeed roughly 0.1% of those achieved in the serum of patients after an initial high IV dose. While it is not clear whether levels measured in the CSF represent the optimal surrogate for CNS penetration of a drug or biologic, there is no other feasible tissue to sample and subject to routine measurement. Given such limited penetration of rituximab past the blood brain barrier, the achievement of any incrementally higher levels in the CNS may potentially make a difference therapeutically. In the future, another method to potentially increase the concentration of rituximab in the CNS could be to treat with high systemic doses concurrently with administration of intraventricular rituximab which has recently been shown to be safe and effective.43 In addition, to better measure intraparenchymal concentrations, radiolableled rituximab could be used as has been preliminary done.
In summary, in patients with either primary or secondary CNS lymphoma, high-dose intravenous rituximab added to high-dose cytarabine mobilization followed by high-dose rituximab with TBC ASCT is safe, has low rates of toxicity, and appears to be efficacious. Given the results, especially in the 18 patients with primary CNS lymphoma with no observed relapses to date, this treatment program comprised of high-dose R-cytarabine with R-TBC ASCT merits further study. Certainly, more follow-up is needed to determine if the addition of high-dose rituximab results in improvements in long-term PFS and OS compared to TBC alone or other ASCT regimens, specifically the combination of BCNU with thiotepa, which is a widely used regimen in this setting. Importantly, we await the results of the ongoing prospective randomized studies aimed at defining the place for ASCT in consolidation for patients with PCNSL in hopes of improving outcomes for those undergoing ASCT by eventually randomizing patients to high-dose rituximab with high-dose chemotherapy vs. high-dose chemotherapy alone.
Precis.
Using high-dose systemic rituximab may allow higher concentrations to penetrate the central nervous system. The authors present a phase II trial combining high-dose rituximab with standard autologous stem cell transplantation, showing compelling results in patients with CNS involvement by non-Hodgkin lymphoma.
Figure 2. Overall Surival (n=30).
PCNSL = primary CNS lymphoma; SCNSL = secondary CNS lymphoma
Table 2.
ASCT Outcomes and Toxicities (n=29)
| Event | Median (range) |
|---|---|
| Days to neutrophil engraftment | 9 (8,12) |
| Days to platelet engraftment | 11 (8,40) |
| Hospital stay in days | 23 (18,56) |
| Infection | N (%) |
| Bacterial | 9 (31) |
| Fungal | 1 (3) |
| Viral | 1 (3) |
| Engraftment syndrome | 6 (21) |
| Veno-occlusive disease (VOD) | 0 (0) |
| Cardiac toxicity | 0 (0) |
| Hemorrhagic cystitis | 0 (0) |
| DAH / IPS | 0 (0) |
| CNS toxicity | 2 (7) |
| 100-day NRM | 0 (0) |
| 1-year NRM | 1 (3) |
| Therapy-related MDS or AML | 0 (0) |
Abbreviations: ANC= Absolute neutrophil count; CNS=Central nervous system; MDS = myelodysplastic syndrome; AML = acute myeloid leukemia; NRM = non-relapse mortality
Neutrophil engraftment was defined by ANC > 500 on two consecutive days
Platelet engraftment was defined by platelets > 20,000 / ul on two consecutive days unsupported by transfusions
Footnotes
Disclosures: Dr. Chen and Armand report receiving funding to conduct this and other clinical trials from Otsuka Pharmaceuticals, Inc. Rituximab for this clinical trial was provided by Genentech, Inc. SL's work is partially supported by NIH grant CA 006516. The remaining authors have no relevant conflicts of interest to disclose. Preliminary results of this study were presented in abstract form at the 2013 American Society of Blood and Marrow Transplantation Tandem Meetings, Salt Lake City, Utah. February 13-17, 2013.
References
- 1.Batchelor T, Carson K, O'Neill A, et al. Treatment of primary CNS lymphoma with methotrexate and deferred radiotherapy: a report of NABTT 96-07. J Clin Oncol. 2003;21:1044–9. doi: 10.1200/JCO.2003.03.036. [DOI] [PubMed] [Google Scholar]
- 2.DeAngelis LM, Seiferheld W, Schold SC, Fisher B, Schultz CJ. Combination chemotherapy and radiotherapy for primary central nervous system lymphoma: Radiation Therapy Oncology Group Study 93-10. J Clin Oncol. 2002;20:4643–8. doi: 10.1200/JCO.2002.11.013. [DOI] [PubMed] [Google Scholar]
- 3.Gerstner ER, Carson KA, Grossman SA, Batchelor TT. Long-term outcome in PCNSL patients treated with high-dose methotrexate and deferred radiation. Neurology. 2008;70:401–2. doi: 10.1212/01.wnl.0000300671.37279.0e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Poortmans PM, Kluin-Nelemans HC, Haaxma-Reiche H, et al. High-dose methotrexate-based chemotherapy followed by consolidating radiotherapy in non-AIDS-related primary central nervous system lymphoma: European Organization for Research and Treatment of Cancer Lymphoma Group Phase II Trial 20962. J Clin Oncol. 2003;21:4483–8. doi: 10.1200/JCO.2003.03.108. [DOI] [PubMed] [Google Scholar]
- 5.Abrey LE, DeAngelis LM, Yahalom J. Long-term survival in primary CNS lymphoma. J Clin Oncol. 1998;16:859–63. doi: 10.1200/JCO.1998.16.3.859. [DOI] [PubMed] [Google Scholar]
- 6.Harder H, Holtel H, Bromberg JE, et al. Cognitive status and quality of life after treatment for primary CNS lymphoma. Neurology. 2004;62:544–7. doi: 10.1212/wnl.62.4.544. [DOI] [PubMed] [Google Scholar]
- 7.Rubenstein JL, Hsi ED, Johnson JL, et al. Intensive chemotherapy and immunotherapy in patients with newly diagnosed primary CNS lymphoma: CALGB 50202 (Alliance 50202). J Clin Oncol. 2013;31:3061–8. doi: 10.1200/JCO.2012.46.9957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morris PG, Correa DD, Yahalom J, et al. Rituximab, methotrexate, procarbazine, and vincristine followed by consolidation reduced-dose whole-brain radiotherapy and cytarabine in newly diagnosed primary CNS lymphoma: final results and long-term outcome. J Clin Oncol. 2013;31:3971–9. doi: 10.1200/JCO.2013.50.4910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boehme V, Schmitz N, Zeynalova S, Loeffler M, Pfreundschuh M. CNS events in elderly patients with aggressive lymphoma treated with modern chemotherapy (CHOP-14) with or without rituximab: an analysis of patients treated in the RICOVER-60 trial of the German High-Grade Non-Hodgkin Lymphoma Study Group (DSHNHL). Blood. 2009;113:3896–902. doi: 10.1182/blood-2008-10-182253. [DOI] [PubMed] [Google Scholar]
- 10.Boehme V, Zeynalova S, Kloess M, et al. Incidence and risk factors of central nervous system recurrence in aggressive lymphoma--a survey of 1693 patients treated in protocols of the German High-Grade Non-Hodgkin's Lymphoma Study Group (DSHNHL). Ann Oncol. 2007;18:149–57. doi: 10.1093/annonc/mdl327. [DOI] [PubMed] [Google Scholar]
- 11.van Besien K, Ha CS, Murphy S, et al. Risk factors, treatment, and outcome of central nervous system recurrence in adults with intermediate-grade and immunoblastic lymphoma. Blood. 1998;91:1178–84. [PubMed] [Google Scholar]
- 12.Doolittle ND, Abrey LE, Shenkier TN, et al. Brain parenchyma involvement as isolated central nervous system relapse of systemic non-Hodgkin lymphoma: an International Primary CNS Lymphoma Collaborative Group report. Blood. 2008;111:1085–93. doi: 10.1182/blood-2007-07-101402. [DOI] [PubMed] [Google Scholar]
- 13.Cheng T, Forsyth P, Chaudhry A, et al. High-dose thiotepa, busulfan, cyclophosphamide and ASCT without whole-brain radiotherapy for poor prognosis primary CNS lymphoma. Bone Marrow Transplant. 2003;31:679–85. doi: 10.1038/sj.bmt.1703917. [DOI] [PubMed] [Google Scholar]
- 14.Alvarnas JC, Negrin RS, Horning SJ, et al. High-dose therapy with hematopoietic cell transplantation for patients with central nervous system involvement by non-Hodgkin's lymphoma. Biol Blood Marrow Transplant. 2000;6:352–8. doi: 10.1016/s1083-8791(00)70060-7. [DOI] [PubMed] [Google Scholar]
- 15.van Besien K, Przepiorka D, Mehra R, et al. Impact of preexisting CNS involvement on the outcome of bone marrow transplantation in adult hematologic malignancies. J Clin Oncol. 1996;14:3036–42. doi: 10.1200/JCO.1996.14.11.3036. [DOI] [PubMed] [Google Scholar]
- 16.Williams CD, Pearce R, Taghipour G, Green ES, Philip T, Goldstone AH. Autologous bone marrow transplantation for patients with non-Hodgkin's lymphoma and CNS involvement: those transplanted with active CNS disease have a poor outcome--a report by the European Bone Marrow Transplant Lymphoma Registry. J Clin Oncol. 1994;12:2415–22. doi: 10.1200/JCO.1994.12.11.2415. [DOI] [PubMed] [Google Scholar]
- 17.Abrey LE, Moskowitz CH, Mason WP, et al. Intensive methotrexate and cytarabine followed by high-dose chemotherapy with autologous stem-cell rescue in patients with newly diagnosed primary CNS lymphoma: an intent-to-treat analysis. J Clin Oncol. 2003;21:4151–6. doi: 10.1200/JCO.2003.05.024. [DOI] [PubMed] [Google Scholar]
- 18.Colombat P, Lemevel A, Bertrand P, et al. High-dose chemotherapy with autologous stem cell transplantation as first-line therapy for primary CNS lymphoma in patients younger than 60 years: a multicenter phase II study of the GOELAMS group. Bone Marrow Transplant. 2006;38:417–20. doi: 10.1038/sj.bmt.1705452. [DOI] [PubMed] [Google Scholar]
- 19.Illerhaus G, Marks R, Ihorst G, et al. High-dose chemotherapy with autologous stem-cell transplantation and hyperfractionated radiotherapy as first-line treatment of primary CNS lymphoma. J Clin Oncol. 2006;24:3865–70. doi: 10.1200/JCO.2006.06.2117. [DOI] [PubMed] [Google Scholar]
- 20.Illerhaus G, Marks R, Muller F, et al. High-dose methotrexate combined with procarbazine and CCNU for primary CNS lymphoma in the elderly: results of a prospective pilot and phase II study. Ann Oncol. 2009;20:319–25. doi: 10.1093/annonc/mdn628. [DOI] [PubMed] [Google Scholar]
- 21.Illerhaus G, Muller F, Feuerhake F, Schafer AO, Ostertag C, Finke J. High-dose chemotherapy and autologous stem-cell transplantation without consolidating radiotherapy as first-line treatment for primary lymphoma of the central nervous system. Haematologica. 2008;93:147–8. doi: 10.3324/haematol.11771. [DOI] [PubMed] [Google Scholar]
- 22.Soussain C, Hoang-Xuan K, Taillandier L, et al. Intensive chemotherapy followed by hematopoietic stem-cell rescue for refractory and recurrent primary CNS and intraocular lymphoma: Societe Francaise de Greffe de Moelle Osseuse-Therapie Cellulaire. J Clin Oncol. 2008;26:2512–8. doi: 10.1200/JCO.2007.13.5533. [DOI] [PubMed] [Google Scholar]
- 23.Soussain C, Suzan F, Hoang-Xuan K, et al. Results of intensive chemotherapy followed by hematopoietic stem-cell rescue in 22 patients with refractory or recurrent primary CNS lymphoma or intraocular lymphoma. J Clin Oncol. 2001;19:742–9. doi: 10.1200/JCO.2001.19.3.742. [DOI] [PubMed] [Google Scholar]
- 24.Montemurro M, Kiefer T, Schuler F, et al. Primary central nervous system lymphoma treated with high-dose methotrexate, high-dose busulfan/thiotepa, autologous stem-cell transplantation and response-adapted whole-brain radiotherapy: results of the multicenter Ostdeutsche Studiengruppe Hamato-Onkologie OSHO-53 phase II study. Ann Oncol. 2007;18:665–71. doi: 10.1093/annonc/mdl458. [DOI] [PubMed] [Google Scholar]
- 25.Egorin MJ, Akman SR, Gutierrez PL. Plasma pharmacokinetics and tissue distribution of thiotepa in mice. Cancer Treat Rep. 1984;68:1265–8. [PubMed] [Google Scholar]
- 26.Heideman RL, Cole DE, Balis F, et al. Phase I and pharmacokinetic evaluation of thiotepa in the cerebrospinal fluid and plasma of pediatric patients: evidence for dose-dependent plasma clearance of thiotepa. Cancer Res. 1989;49:736–41. [PubMed] [Google Scholar]
- 27.Vassal G, Gouyette A, Hartmann O, Pico JL, Lemerle J. Pharmacokinetics of high-dose busulfan in children. Cancer Chemother Pharmacol. 1989;24:386–90. doi: 10.1007/BF00257448. [DOI] [PubMed] [Google Scholar]
- 28.Hassan M, Oberg G, Ericson K, et al. In vivo distribution of [11C]-busulfan in cynomolgus monkey and in the brain of a human patient. Cancer Chemother Pharmacol. 1992;30:81–5. doi: 10.1007/BF00686397. [DOI] [PubMed] [Google Scholar]
- 29.Hassan M, Ehrsson H, Smedmyr B, et al. Cerebrospinal fluid and plasma concentrations of busulfan during high-dose therapy. Bone Marrow Transplant. 1989;4:113–4. [PubMed] [Google Scholar]
- 30.Wiebe VJ, Smith BR, DeGregorio MW, Rappeport JM. Pharmacology of agents used in bone marrow transplant conditioning regimens. Crit Rev Oncol Hematol. 1992;13:241–70. doi: 10.1016/1040-8428(92)90092-5. [DOI] [PubMed] [Google Scholar]
- 31.Cote GM, Hochberg EP, Muzikansky A, et al. Autologous stem cell transplantation with thiotepa, busulfan, and cyclophosphamide (TBC) conditioning in patients with CNS involvement by non-Hodgkin lymphoma. Biol Blood Marrow Transplant. 2012;18:76–83. doi: 10.1016/j.bbmt.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 32.Batchelor TT, Grossman SA, Mikkelsen T, Ye X, Desideri S, Lesser GJ. Rituximab monotherapy for patients with recurrent primary CNS lymphoma. Neurology. 2011;76:929–30. doi: 10.1212/WNL.0b013e31820f2d94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Birnbaum T, Stadler EA, von Baumgarten L, Straube A. Rituximab significantly improves complete response rate in patients with primary CNS lymphoma. Journal of neuro-oncology. 2012;109:285–91. doi: 10.1007/s11060-012-0891-7. [DOI] [PubMed] [Google Scholar]
- 34.Macdonald DR, Cascino TL, Schold SC, Jr., Cairncross JG. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8:1277–80. doi: 10.1200/JCO.1990.8.7.1277. [DOI] [PubMed] [Google Scholar]
- 35.Soussain C, Choquet S, Fourme E, et al. Intensive chemotherapy with thiotepa, busulfan and cyclophosphamide and hematopoietic stem cell rescue in relapsed or refractory primary central nervous system lymphoma and intraocular lymphoma: a retrospective study of 79 cases. Haematologica. 2012;97:1751–6. doi: 10.3324/haematol.2011.060434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kasamon YL, Jones RJ, Piantadosi S, et al. High-dose therapy and blood or marrow transplantation for non-Hodgkin lymphoma with central nervous system involvement. Biol Blood Marrow Transplant. 2005;11:93–100. doi: 10.1016/j.bbmt.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 37.Korfel A, Elter T, Thiel E, et al. Phase II study of central nervous system (CNS)-directed chemotherapy including high-dose chemotherapy with autologous stem cell transplantation for CNS relapse of aggressive lymphomas. Haematologica. 2013;98:364–70. doi: 10.3324/haematol.2012.077917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maziarz RT, Wang Z, Zhang MJ, et al. Autologous haematopoietic cell transplantation for non-Hodgkin lymphoma with secondary CNS involvement. British journal of haematology. 2013;162:648–56. doi: 10.1111/bjh.12451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brevet M, Garidi R, Gruson B, Royer B, Vaida I, Damaj G. First-line autologous stem cell transplantation in primary CNS lymphoma. Eur J Haematol. 2005;75:288–92. doi: 10.1111/j.1600-0609.2005.00508.x. [DOI] [PubMed] [Google Scholar]
- 40.Kasenda B, Schorb E, Fritsch K, Finke J, Illerhaus G. Prognosis after high-dose chemotherapy followed by autologous stem-cell transplantation as first-line treatment in primary CNS lymphoma--a long-term follow-up study. Ann Oncol. 2012;23:2670–5. doi: 10.1093/annonc/mds059. [DOI] [PubMed] [Google Scholar]
- 41.Shah GD, Yahalom J, Correa DD, et al. Combined immunochemotherapy with reduced whole-brain radiotherapy for newly diagnosed primary CNS lymphoma. J Clin Oncol. 2007;25:4730–5. doi: 10.1200/JCO.2007.12.5062. [DOI] [PubMed] [Google Scholar]
- 42.Petereit HF, Rubbert-Roth A. Rituximab levels in cerebrospinal fluid of patients with neurological autoimmune disorders. Multiple sclerosis. 2009;15:189–92. doi: 10.1177/1352458508098268. [DOI] [PubMed] [Google Scholar]
- 43.Rubenstein JL, Li J, Chen L, et al. Multicenter phase 1 trial of intraventricular immunochemotherapy in recurrent CNS lymphoma. Blood. 2013;121:745–51. doi: 10.1182/blood-2012-07-440974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Doolittle ND, Jahnke K, Belanger R, et al. Potential of chemo-immunotherapy and radioimmunotherapy in relapsed primary central nervous system (CNS) lymphoma. Leuk Lymphoma. 2007;48:1712–20. doi: 10.1080/10428190701493902. [DOI] [PubMed] [Google Scholar]
- 45.Muldoon LL, Lewin SJ, Dosa E, et al. Imaging and therapy with rituximab anti-CD20 immunotherapy in an animal model of central nervous system lymphoma. Clinical cancer research : an official journal of the American Association for Cancer Research. 2011;17:2207–15. doi: 10.1158/1078-0432.CCR-10-2923. [DOI] [PMC free article] [PubMed] [Google Scholar]