Abstract
Many phytopathogenic type III secretion effectors (T3Es) have been shown to target and suppress plant immune signaling, but perturbation of the plant immune system by T3Es can also elicit a plant response. XopX is a “core” Xanthomonas T3E that contributes to growth and symptom development during Xanthomonas euvesicatoria (Xe) infection of tomato, but its functional role is undefined. We tested the effect of XopX on several aspects of plant immune signaling. XopX promoted ethylene production and plant cell death (PCD) during Xe infection of susceptible tomato and in transient expression assays in Nicotiana benthamiana, which is consistent with its requirement for the development of Xe-induced disease symptoms. Additionally, although XopX suppressed flagellin-induced reactive oxygen species, it promoted the accumulation of pattern-triggered immunity (PTI) gene transcripts. Surprisingly, XopX co-expression with other PCD elicitors resulted in delayed PCD, suggesting antagonism between XopX-dependent PCD and other PCD pathways. However, we found no evidence that XopX contributed to the suppression of effector-triggered immunity during Xe-tomato interactions, suggesting that XopX’s primary virulence role is to modulate PTI. These results highlight the dual role of a core Xanthomonas T3E in simultaneously suppressing and activating plant defense responses.
INTRODUCTION
Phytopathogenic bacteria of the genus Xanthomonas cause disease in diverse crop systems worldwide. Xanthomonas euvesicatoria (Xe; formerly Xanthomonas campestris pathovar vesicatoria (Jones et al., 2004)) causes bacterial spot disease in tomato (Solanum lycopersicum) and pepper (Capsicum annuum) (Jones et al., 1998). During infection of susceptible plants, Xe infiltrates the apoplastic space of foliar tissue through natural openings or wounds and multiplies rapidly there. Xe utilizes type III secretion (T3S) to translocate effector proteins (T3Es) directly into the hosts’ cells (Buttner, 2012), and functional T3S is required for maximal Xe growth in its hosts (Bonas et al., 1991). Genomic analyses have identified nine “core” T3Es that are found in almost all sequenced Xanthomonas strains (Hajri et al., 2009; Moreira et al., 2010; Jalan et al., 2011; Potnis et al., 2011). The conservation of these core T3Es suggest that they serve a critical role in Xanthomonas pathology, and insight gained by the study of these core T3Es may aid in the development of disease mitigation strategies (Potnis et al., 2011; Dangl et al., 2013).
XopX is one such core T3E. It was originally identified in a screen of Xe genes that conferred the ability to cause plant cell death (PCD) in Nicotiana benthamiana to the non-PCD-eliciting bacterium Xanthomonas campestris pathovar (pv.) campestris (Xcc) strain 8004 (Metz et al., 2005). XopX is delivered into the plant cell by T3S and required for full virulence of Xe strain GM98-38 on tomato and pepper (Metz et al., 2005). Despite the ability of XopX to elicit PCD in N. benthamiana when delivered by bacteria, transgenic N. benthamiana expressing XopX are viable, more susceptible to Xanthamonas and Pseudomonas, and develop more severe symptoms during infection (Metz et al., 2005). This suggests that XopX elicitation of PCD is linked to its virulence activity rather than evidence of avirulence activity. Although XopX’s function in the plant cell during infection is unknown, its ortholog from Xanthomonas oryzae pv. oryzae (Xoo) was recently shown to suppress Xoo LipA-elicited callose deposition in rice (Sinha et al., 2013). This latter evidence suggests that XopX may contribute to bacterial virulence by suppressing plant immune signaling. Given that XopX is cytotoxic when expressed in yeast (Salomon et al., 2011), it is likely that XopX targets a broadly conserved eukaryotic cell process that is required for viability.
We are interested in identifying a specific role for XopX in Xe pathogenesis. Like many phytopathogenic bacteria, Xe maintains a hemibiotrophic lifestyle that requires the pathogen to suppress or evade plant defense responses but avoid killing its host at early stages of infection (Doidge, 1921). During infection, the detection of conserved microbe-associated molecular patterns (MAMPs), such as bacterial flagellin, by plant cell surface receptors elicits a limited plant defense response called pattern-triggered immunity (PTI) (Jones and Dangl, 2006). It is well known that phytopathogenic bacteria employ T3Es to suppress PTI (Boller and He, 2009). In response, plants exploit the pathogen’s requirement of a living host by activating PCD during effector-triggered immunity (ETI), an elevated defense response elicited when plant disease resistance proteins detect the presence or activity of specific T3Es (Spoel and Dong, 2012). However, T3Es can also suppress ETI (Jones and Dangl, 2006). The interaction between T3Es and the plant immune system is thus complex and multi-layered, and the specific combination of T3Es deployed by the pathogen is a critical determinant of the outcome of a plant-pathogen interaction.
In addition to its role during ETI in resistant plants, PCD leads to host tissue necrosis, which is a symptom and eventual outcome of disease caused by hemibiotrophic pathogens in susceptible plants. The regulation of this PCD (by host or pathogen) is not well understood (del Pozo et al., 2004; Cohn and Martin, 2005; Badel et al., 2006). In tomato, the phytohormones salicylic acid (SA) and ethylene (ET) are critical, positive regulators of PCD and symptom development that occurs during infection by Xe and Pseudomonas syringae pv. tomato strain DC3000 (Pst) (Lund et al., 1998; O'Donnell et al., 2001; O'Donnell et al., 2003; Cohn and Martin, 2005). Our previous work revealed that Xe employs the T3E XopD as a “tolerance factor” to suppress SA- and ET-dependent defense and symptom development in tomato (Kim et al., 2008; Kim et al., 2013). The T3E XopJ was also shown to suppress SA accumulation, resulting in the delay of tissue senescence during Xe infection of pepper (Ustun et al., 2013). By contrast, the T3Es AvrPto and AvrPtoB are responsible for activating ET production during Pst infection of tomato, which impacts symptom development (Cohn and Martin, 2005). Currently, it is not known whether individual Xe T3Es are also responsible for promoting ET and/or SA production.
In this study, we focused on assessing the role of XopX in regulating PCD, manipulating phytohormone signaling, and suppressing immunity during PTI and ETI. These three critical functions help to distinguish the role individual T3Es play within the context of a given T3E repertoire (Cunnac et al., 2009). We provide evidence that XopX contributes to Xe virulence by suppressing specific aspects of plant immunity (i.e., ROS accumulation), but simultaneously activates plant defense responses and PCD. A similar pattern of dual behavior (i.e., suppression of plant immunity coupled to activation of plant defense and PCD) was previously identified for the Pst core T3E AvrE1 and led to the model that plants can respond to T3E virulence function with a “default to death and defense” strategy (Badel et al., 2006; Lindeberg et al., 2012). Our results for XopX provide further support for this model and highlight important considerations for evaluating how individual T3Es contribute to the outcome of plant-microbe interactions.
RESULTS
Confirmation of XopX contribution to Xe virulence using Xe strain 85-10
The contribution of XopX to Xe growth in tomato and pepper was previously demonstrated using Xe strain GM98-38 (Metz et al., 2005). In order to take advantage of the genome sequence and annotation of Xe strain 85-10 during our study of XopX (Thieme et al., 2005), we used an Xe 85-10 strain with a genomic deletion in the xopX coding sequence (ΔxopX) (Sonnewald et al., 2012). Consistent with prior work, ΔxopX grew less and caused reduced leaf chlorosis and necrotic spots during infection of tomato culivar (cv.) VF36, which is susceptible to Xe 85-10 (Supplementary Fig. 1A–B). We genetically complemented ΔxopX by transforming it with a broad host range plasmid bearing the xopX locus from Xe 85-10 (pDSK519(xopX2623), Table 1). pDSK519(xopX2623) restored ΔxopX to wild-type levels of Xe growth and disease symptoms in infected tomato cv. VF36 leaves (Supplementary Fig. 1A–B). To confirm the contribution of XopX protein expression to Xe virulence, we generated XopX anti-sera for use in monitoring XopX protein levels in Xe cultures. Plasmid-borne xopX restored XopX protein expression to ΔxopX, although at higher levels than that detected for WT (Supplementary Fig. 1C). These results support the requirement of XopX for full Xe virulence (Metz et al., 2005) and allowed us to use Xe 85-10 (referred to from now on as WT) and ΔxopX for further study of XopX function.
Table 1.
Bacterial strains and plasmids used
| Strain or Plasmid | Relevant characteristics | Source or reference |
|---|---|---|
| Bacteria | ||
| Xanthomonas euvesicatoria | ||
| Wild-type | strain 85-10; RifR | (Minsavage et al., 1990; Thieme et al., 2005) |
| ΔxopX | strain 85-10; deletion in xopX; RifR | (Sonnewald et al., 2012) |
| ΔhrcV | strain 85-10; deletion in hrcV; RifR | (Rossier et al., 1999) |
| ΔavrRxv | strain 85-10; deletion in avrRxv; RifR | |
| ΔxopX ΔavrRxv | derived from ΔxopX; deletion in avrRxv; RifR | |
| Pseudomonas syringae pv. tomato | ||
| ΔhrcU | strain DC3000; deletion in hrcU; RifR | (Mudgett and Staskawicz, 1999) |
| Agrobacterium tumefaciens | ||
| C58C1, pCH32 | C58 with Ti plasmid cured; pCH32 is derivative of pCC113 with virE; RifR TetR | (Tai et al., 1999) |
| EHA105 GV2260 |
C58 with pEHA105 (pTiBo542ΔT-DNA); RifR C58 with pGV2260 (pTiB6S3ΔT-DNA); RifR |
(Hood et al., 1993) (Mcbride and Summerfelt, 1990) |
| Escherichia coli | ||
| DH5α | F− 80dlacZΔM15 Δ(lacZYA-argF)U169 endA1 deoR recA1 hsdR17(rK− mK+) phoA supE44 λ−thi-l gyrA96 relA1 | Life Technologies, Carlsbad, CA |
| HB101, pRK600 | F- Δ(gpt-proA)62leuB6glnV44 ara-14 galK2 lacY1 (mcrC-mrr) rpsL20 StrR) xyl-5 mtl-1 recA13 thi-1; pRK600 is CmpR derivative of RK2013 | (Kessler et al., 1992) |
| Acinetobacter | ||
| ADPWH_lux | Insertion of luxCDABE between salA and salR | (Huang et al., 2005) |
| Plasmids | ||
| pCR8/GW/TOPO | SpcR | Life Technologies, Carlsbad, CA |
| pJET1.2/blunt | AmpR | Thermo Scientific, Waltham, MA |
| pDEST17 | AmpR | Life Technologies, Carlsbad, CA |
| pDEST17(xopX) | pDEST17 with xopX fusion to N-terminal 6×His tag; AmpR | |
| pLVC18-RfC | Gateway destination suicide vector; TetR | (Roden et al., 2004) |
| pLVC18-RfC(ΔavrRxv) | pLVC18 derivative with fusion of genomic regions upstream and downstream of avrRxv; TetR | |
| pDSK519 | KanR | (Keen et al., 1988) |
| pDSK519(xopX2623) | pDSK519 with xopX including upstream and downstream regions; KanR | |
| pGWB6 | KanR | (Nakagawa et al., 2007) |
| pGWB6(xopX) | pGWB6 with xopX and 35S promoter; KanR | |
| pBTEX | KanR | (Frederick et al., 1998) |
| spBTEX(Pto; avrPto) | pBTEX with Pto and avrPto, both with 35S promoter; KanR | (Frederick et al., 1998) |
| pBTEX(Bax) | pBTEX with Bax and 35S promoter; KanR | G. Sessa |
| pER8 | SpcR | (Zuo et al., 2000) |
| pER8(SlMKKKa) | pER8 with SpcR | G. Sessa |
| pER8(SlMKK2DD) | SpcR | G. Sessa |
| pER8(SlMPK1) | SpcR | G. Sessa |
XopX is required for Xe-induced ET and PCD in susceptible tomato
Previous studies have shown that some Pseudomonas T3Es required for full pathogenicity also promote PCD (i.e., the functionally redundant T3E pairs AvrE1/HopM1 and AvrPto/AvrPtoB), possibly as a consequence of their virulence function (Cohn and Martin, 2005; Badel et al., 2006). Given that XopX is required for symptom development and tissue necrosis during Xe infection of tomato, we tested whether or not XopX is required for Xe-induced PCD in tomato by performing trypan blue staining of tomato leaves inoculated with Xe. High-titer (2 × 108 CFU mL−1) inoculation of Xe into susceptible tomato leaves results in PCD and tissue collapse 3–5 days post inoculation (dpi) (Fig. 1A). The extent of cell death can be visualized by the relative amount of trypan blue stain taken up by dead cells. As expected, tomato leaves inoculated with WT were heavily stained with trypan blue by 3 dpi (Fig. 1A). However, tomato leaves inoculated with ΔxopX had lower levels of staining (Fig. 1A), suggesting that XopX is required for Xe-induced PCD in tomato.
Fig. 1. XopX contribution to PCD and phytohormone accumulation during Xe infection of susceptible tomato.

Tomato cv. VF36 leaves were inoculated with 2 × 108 colony forming units (CFU) mL−1 of WT and ΔxopX in specific regions of the same leaflet (A) or whole leaflets were inoculated with 2 × 108 CFU mL−1 of WT, ΔxopX, ΔhrcV, or 1 mM MgCl2 (mock) (B–D). A) Leaflets were harvested 1, 2, or 3 dpi and photographed before (left insets) and after (right insets) staining with trypan blue to highlight regions of PCD. B) 4 leaf disks from each treatment were harvested at 1, 2, or 3 dpi, washed, and floated on 5 mL distilled water for 4 h with gentle shaking prior to measuring the conductivity of bathing water. Values are mean conductivity of bathing water (µS mm−2 leaf mL−1 bathing water) ± SD (n = 3 plants). C) Inoculated whole leaflets were harvested 48 hpi and placed into separate sealed vials with minimal damage. ET gas in headspace around leaflet was quantified by gas chromatography over the course of 1 h. Values are mean ET concentration (nL ET g−1 leaf h−1) ± SD (n = 4 plants). D) Leaf disks from inoculated leaflets were harvested 48 hpi and total (left) and free (right) SA were quantified using the Acinetobacter sp. ADPWH_lux biosensor. Values are mean total or free SA (µg SA g−1 leaf) ± SD (n = 4 plants). E) Tomato cv. VF36 leaves were inoculated with 2 × 108 CFU mL−1 of Xe WT, ΔxopX, or ΔhrcV. Leaf bacteria were enumerated for each treatment 0, 6, 24, and 48 hpi. Values are mean leaf bacteria (CFU cm−1 leaf) ± SD (n = 4). Different letters above bars in B–E indicate statistically significant differences as determined by two-way ANOVA and Tukey’s HSD, P < 0.05 (B, E) or one-way ANOVA and Tukey’s HSD, P < 0.05 (C, D). n.s. = no significant differences among treatments.
To quantify XopX’s contribution to Xe-induced PCD in tomato, we measured electrolyte leakage from leaves inoculated with 1 mM MgCl2 (mock) or 2 × 108 CFU mL−1 of WT, ΔxopX, or ΔhrcV, an Xe 85-10 mutant unable to secrete T3Es (Rossier et al., 1999). At 3 dpi, electrolyte leakage from leaves inoculated with WT was significantly higher than the mock treatment (Fig. 1B). By contrast, electrolyte leakage from ΔhrcV-inoculated leaves was similar to the mock treatment (Fig. 1B), indicating that the secretion of T3Es is required for Xe-induced PCD. Electrolyte leakage from ΔxopX-inoculated leaves was similar to electrolyte leakage from ΔhrcV-inoculated leaves and the mock treatment (Fig. 1B), suggesting that T3S of XopX causes PCD or that XopX is required for PCD caused by another T3E.
During both Xe and Pst infection of susceptible tomato, PCD and the resulting symptom development is positively regulated in part by host factors, such as the phytohormones ET and SA (Lund et al., 1998; O'Donnell et al., 2001; O'Donnell et al., 2003; del Pozo et al., 2004). In fact, specific Xe T3Es suppress ET and SA signaling to mitigate symptom development in susceptible plants and promote Xe growth (Kim et al., 2008; Kim et al., 2013; Ustun et al., 2013). Although ET and SA signaling are activated during ETI in resistant plants, less is known about how these phytohormones are activated by T3Es in susceptible plants (Bari and Jones, 2009). Previously, the elicitation of PCD by AvrPto and AvrPtoB was linked to their promotion of ET biosynthesis (Cohn and Martin, 2005). Because XopX is required for Xe-induced PCD (Fig. 1A–B), we next tested the role of XopX in promoting ET and SA production in susceptible tomato leaves. Consistent with previous reports (O'Donnell et al., 2001; O'Donnell et al., 2003; Kim et al., 2013), inoculation of 2 × 108 CFU mL−1 of WT significantly increased ET accumulation in tomato leaves compared to mock inoculation by 2 dpi (Fig. 1C). A low level of ET was detected in ΔhrcV- and mock-inoculated leaves (Fig. 1C), demonstrating that Xe-induced ET requires the secretion of T3Es. The ET level in ΔxopX-inoculated leaves was 26% of that in WT-inoculated leaves on average, indicating that XopX alone accounts for the majority of the ET accumulation induced by WT (Fig. 1C). However, the ET level in ΔhrcV-inoculated leaves was still significantly lower than that in ΔxopX-inoculated leaves (only 6% of WT), suggesting that other Xe T3Es contribute to ET accumulation, albeit to a lesser extent.
Total and free SA levels in WT-inoculated leaves were significantly higher than that in ΔhrcV- and mock-inoculated leaves (Fig. 1D), suggesting that SA accumulation during Xe infection is also primarily T3S-dependent. This accumulation was not dependent on XopX as SA levels in ΔxopX-inoculated leaves were similar to those in WT-inoculated leaves (Fig. 1D).
The results of our PCD and phytohormone analyses in Xe-inoculated tomato leaves therefore indicate that the primary tomato phenotypes associated with the presence of XopX within the plant cell are the production of ET (Fig. 1D,E) and the activation of PCD (Fig. 1A,B). We also quantified Xe growth in tomato leaves to account for the contribution of bacterial load to PCD and ET production during these experiments. Prior to the onset of PCD and tissue collapse at 3 dpi, WT and ΔxopX populations increased approximately 10-fold and were present at similar titers at 0, 6, and 24 hours post inoculation (hpi), although WT was significantly higher than ΔxopX at 48 hpi (Fig. 1E). By contrast, ΔhrcV grew less and was significantly lower than both WT and ΔxopX at 48 hpi (Fig. 1E). Because ΔxopX and ΔhrcV elicited similar PCD (Fig. 1B) and ET (Fig. 1C) phenotypes but had different growth patterns (Fig. 1E), bacterial titer alone does not explain the lack of PCD and ET elicited by the ΔxopX strain. These data therefore suggest that the difference in PCD and ET production between WT- and ΔxopX-inoculated leaves was primarily due to the presence of XopX. To confirm this hypothesis, we next expressed XopX in planta to determine whether XopX alone is sufficient for elicitation of PCD and ET.
Transient XopX expression promotes ET and PCD in N. benthamiana
The bacterial genetic approach for studying XopX described above may be complicated by the presence of other Xe T3Es as well as the growth defect of the ΔxopX strain. Therefore, to study XopX function in isolation, we transiently transformed N. benthamiana leaf cells to express XopX with an N-terminal GFP tag (GFP:XopX) and then monitored PCD and ET production. We chose N. benthamiana as the host because Agrobacterium-mediated transient transformation of N. benthamiana is more efficient than tomato. Consistent with the requirement of XopX for Xe-induced PCD in tomato (Fig. 1), transient expression of XopX in N. benthamiana was previously reported to cause PCD either on its own or in conjunction with an unidentified, T3S-dependent factor (Metz et al., 2005; Salomon et al., 2011). In our experiements, transient expression of GFP:XopX alone caused weak PCD in N. benthamiana leaves (Fig. 2A). Macroscopic PCD was usually not visible before 3–4 dpi, although trypan blue staining and electrolyte leakage measurements of regions expressing GFP:XopX (Fig. 2A–B) provided evidence of detectable PCD beginning 48 hpi.
Fig. 2. PCD and phytohormone accumulation in N. benthamiana leaves after transient expression of GFP:XopX.

A. tumefaciens strains bearing binary vectors for GFP or GFP:XopX expression were inoculated into N. benthamiana leaves at 4 × 108 CFU mL−1. A) Leaves were harvested at 3 days post inoculation (dpi) and photographed before (top) and after (bottom) staining with trypan blue to indicate regions of PCD. B) 4 leaf disks from each treatment were harvested 20 hours post inoculation (hpi), washed, and floated on 5 mL distilled water. Conductivity of bathing water was measured at 24 hpi and every subsequent 24 h for 5 days. Values are mean conductivity of bathing water (µS mm−2 leaf mL−1 bathing water) ± SD (n = 3 plants). C) Inoculated regions were harvested at 24 and 48 hpi and placed into separate sealed vials with minimal damage. Ethylene (ET) gas in headspace around leaf was quantified by gas chromatography over the course of 1 h. Values are mean ET concentration (nL ET g−1 leaf h−1) ± SD (n = 3 plants). D) Leaf disks were harvested 24 and 48 hpi and total (left) and free (right) salicylic acid (SA) was quantified using the Acinetobacter sp. ADPWH_lux biosensor. Values are mean total or free SA (µg SA g−1 leaf) ± SD (n = 3 plants). Asterisks above bars in B-D indicate statistically significant differences as determined by two-way ANOVA and Tukey’s HSD, P < 0.05.
Next we used this transient expression system to test whether the presence of XopX in the plant cell is sufficient to promote ET production. Transient expression of GFP:XopX, but not GFP, induced ET accumulation in N. benthamiana leaves (Fig. 2C) prior to the onset of XopX-induced PCD. Therefore, XopX is both required (Xe-inoculated tomato, Fig. 1D) and sufficient (transient expression in N. benthamiana) to elicit ET production prior to PCD. By contrast, total SA accumulation was similar in regions expressing GFP and GFP:XopX. Free SA accumulation was slightly less in regions expressing GFP:XopX than in those expressing GFP (Fig. 2D). This slight suppression of free SA accumulation might be a consequence of XopX-triggered ET production, since data from Arabidopsis thaliana suggests that ET signaling can antagonize SA signaling (Kunkel and Brooks, 2002).
XopX promotes PTI gene transcript accumulation but suppresses PTI-associated ROS
The finding that XopX promotes ET production and PCD is consistent with the requirement of XopX in promoting Xe symptom development, but it does not explain how XopX contributes to Xe growth. Although AvrPto and AvrPtoB were shown to elicit ET and PCD during Pst infection of tomato (Cohn and Martin, 2005), subsequent research has suggested that their primary role is suppression of plant immune signaling by plant cell receptor-kinases (Gohre et al., 2008; Xiang et al., 2008; Gimenez-Ibanez et al., 2009). Indeed, a primary function of phytopathogenic T3Es in promoting bacterial growth is the suppression of plant immune signaling at different nodes (Mukhtar et al., 2011). Because XopX is also required for full Xe growth in susceptible plants, we next sought to explicitly test whether XopX suppresses PTI signaling during Xe infection of susceptible tomato by monitoring the transcript levels of known PTI genes (i.e., SlPti5, SlWRKY28, SlLRR22, and SlGRAS2) in response to Xe with or without XopX. Previously, transcripts of these genes were found to increase in response to flagellin and infection by T3S mutants of Pst and Xe, and were suppressed by the Xe T3E, XopN (Kim et al., 2009; Nguyen et al., 2010). We used qPCR to quantify the transcript levels of these genes in tomato cv. VF36 leaves inoculated with 1 mM MgCl2 or a high titer (2 × 108 CFU mL−1) of WT, ΔxopX, or ΔhrcV at 6 and 12 hpi. As expected, ΔhrcV treatment induced transcript accumulation for all 4 PTI genes relative to the mock treatment and this induction was suppressed by WT treatment (Fig. 3A). SlPti5 and SlLRR22 were significantly lower in WT-inoculated leaves than in ΔhrcV-inoculated leaves, while SlWRKY28 and SlGRAS2 were slightly—though not significantly—lower in WT than ΔhrcV (Fig. 3A). Surprisingly, PTI gene transcripts tended to be lower in ΔxopX-inoculated leaves than in WT-inoculated leaves (Fig. 3A). A significant difference was observed for SlLRR22 (Fig. 3A). Thus, XopX appears to weakly promote, rather than suppress, a plant defense response in tomato, at least at the level of transcript accumulation.
Fig. 3. Effect of XopX on PTI responses in tomato and N. benthamiana.
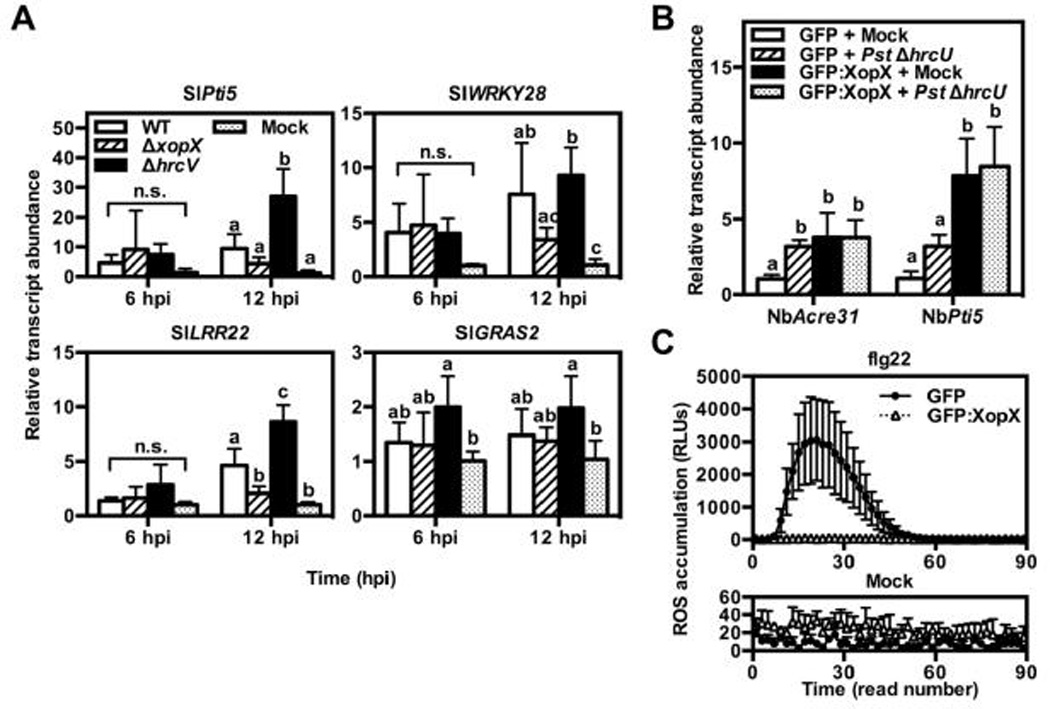
A) Tomato cv. VF36 leaves were inoculated with 2 × 108 CFU mL−1 of Xe wild-type, ΔxopX, ΔhrcV, or 1 mM MgCl2 (mock). Samples were harvested 6 and 12 hpi for RNA extraction and cDNA synthesis. Transcript levels for known tomato PTI gene markers (i.e., SlPti5, SlWRKY28, SlLRR22, SlGRAS2) were quantified by qPCR. Relative fold change in transcript abundance for each gene was calibrated to the average ΔCT values from the control treatment (Mock) with SlACTIN as the internal control (Livak and Schmittgen, 2001). Values are mean relative fold induction (2−ΔΔCT) ± SD (n = 4 plants). B) A. tumefaciens strains bearing binary vectors for GFP or GFP:XopX expression were inoculated into N. benthamiana leaves at 4 × 108 CFU mL−1. 24 hpi, intact leaves were inoculated with 1 mM MgCl2 (mock) or 2 × 108 CFU mL−1 of Pst ΔhrcU. Leaf tissue was harvested 6 hr after ΔhrcU treatment for RNA extraction and cDNA synthesis. Transcript levels for known PTI gene markers (i.e., NbAcre31 and NbPti5) were quantified by qPCR using the comparative CT method. Relative fold change in transcript abundance for each gene was calibrated to the average ΔCT values from the control treatment (EV + mock) with NbPP2A as the internal control. Values are mean relative fold induction (2−ΔΔCT) ± SD (n = 4 plants). C) GFP or GFP:XopX were expressed in N. benthamiana leaves as in (B). 24 hpi, 3 leaf disks were harvested and floated on distilled water in individual wells of a 96-well plate O/N under continuous light. Water was removed and replaced with horseradish peroxidase (HRP) solution with 200 nM flg22 (flg22, top) or without flg22 (mock, bottom). H2O2 production was quantified for 90 min after flg22 treatment using a plate reader luminometer. Each plate read lasted ~50 sec. Values for each read are mean relative light units produced ± SD (n = 4 plants). Different letters above bars in A–B indicate statistically significant differences as determined by two-way ANOVA and Tukey’s HSD, P < 0.05 (A) or one-way ANOVA and Tukey’s HSD, P < 0.05 (B). n.s. = no significant differences among treatments.
We next tested whether XopX is sufficient to suppress PTI elicited by Pst using transient expression of XopX in Pst-infected N. benthamiana leaves. We monitored XopX-dependent suppression of PTI prior to the onset of XopX-induced PCD in this system. Treatment of N. benthamiana leaves with T3S-deficient Pst (ΔhrcU) triggers transcript accumulation of the PTI genes NbAcre31 and NbPti5 (Nguyen et al., 2010). We treated N.benthamiana leaves transiently expressing GFP or GFP:XopX with 2 × 108 CFU mL−1 of a T3S-deficient Pst strain (ΔhrcU) and quantified PTI gene transcript accumulation. Pst ΔhrcU treatment triggered accumulation of NbAcre31 and NbPti5 transcripts compared to a mock treatment in leaf tissue expressing GFP, although for NbPti5 this increase was not statistically significant (Fig. 3B). Gene transcript levels were the same (NbAcre31) or higher (NbPti5) in the Pst ΔhrcU-inoculated leaf tissue expressing GFP:XopX compared to that expressing GFP (Fig. 3B). However, GFP:XopX expression alone triggered accumulation of these transcripts, and Pst ΔhrcU treatment did not have a further effect on their accumulation (Fig. 3B). XopX induction of PTI gene expression in N. benthamiana is consistent with our results using Xe in tomato and further suggests that XopX itself promotes defense in planta.
Finally, we sought to test the effect of XopX on extracellular reactive oxygen species (ROS) production, a non-transcriptional PTI response. Application of flg22, a 22-amino acid peptide derived from flagellin, to N. benthamiana leaf tissue elicits the rapid accumulation of extracellular ROS which, as in A. thaliana, requires a plasma membrane-localized NAPDH oxidase, NbRbohD (Segonzac et al., 2011). We treated leaf disks transiently expressing GFP or GFP:XopX with 200 nM flg22 and quantified the resulting ROS burst. By contrast to PTI transcript accumulation, GFP:XopX but not GFP alone was sufficient to prevent flg22-induced ROS burst (Fig. 3C). This confirms that XopX presence in the plant cell suppresses at least one PTI output. This suppression activity is, however, contradictory to the activation of plant defense gene transcription by XopX.
XopX interferes with PCD induced by Pto-AvrPto, MAPK signaling, or Bax
Although there is an overlap in the signaling components and outputs involved in PTI and ETI (Tsuda and Katagiri, 2010; Thomma et al., 2011), the ability to suppress both PTI and ETI appears to be restricted to a subset of phytopathogenic T3Es (Guo et al., 2009; Feng et al., 2012). Such dual activity suggests that these T3Es either target a regulatory node common to both pathways or have multiple plant targets or suppression activities. We therefore assessed the versatility of XopX in suppressing plant immune responses by testing its ability to suppress ETI. In N. benthamiana, transient co-expression of the Pst T3E AvrPto and its cognate R protein, Pto, initiates ETI signaling that culminates in PCD (Oh and Martin, 2011a). We co-expressed Pto-AvrPto in N. benthamiana with either GFP or GFP:XopX and monitored PCD. Surprisngly, despite the fact that XopX induces weak PCD (Fig. 2A,B), GFP:XopX delayed PCD activated by Pto-AvrPto (Fig. 4, left).
Fig. 4. Transient co-expression of GFP:XopX and PCD elicitors in N. benthamiana.

A. tumefaciens strains bearing binary vectors for GFP or GFP:XopX expression were co-inoculated into N. benthamiana leaves with strains bearing binary vectors for expression of PCD elicitors: Pto-AvrPto, MAPK signaling components, or Bax. A) Representative images of co-inoculations 3 dpi (Pto-AvrPto and Bax) or 5 dpi (MAPK components; 4 days after estradiol treatment). Circle indicates region of inoculation and color of circle indicates amount of tissue collapse (magenta = full, blue = partial, yellow = none/limited). B) Summary of tissue collapse scores for treatments shown in (A). Bars indicate percentage of replicates (n = 18) in each category of tissue collapse using the same color scale as in (A). Asterisks over bars indicate statistically significant difference in distribution of replicates among tissue collapse categories between GFP and GFP:XopX co-inoculations with each PCD elicitor as determined by Wilcoxon signed-rank test, P < 0.05. Final A. tumefaciens concentrations in Pto-AvrPto co-inoculations were 2 × 108 CFU mL−1 for all strains, in MAPK component co-inoculations were 2 × 108 CFU mL−1 (GFP, GFP:XopX, SlMPK1, Empty) or 1 × 108 CFU mL−1 (SlMAPKKKa, SlMKK2DD), and in Bax co-inoculations were 4 × 108 CFU mL−1 for all strains. MAPK component constructs were driven by estradiol-inducible promoters and estradiol was applied by spraying 24 hpi. All other constructs were driven by 35S promoter.
We next sought to exploit knowledge of the Pto-AvrPto ETI signaling pathway to determine the signaling node at which XopX may be acting (Oh and Martin, 2011a). In N. benthamiana, NbMAPKKKα, its downstream substrate NbMKK2, and the MAPKs NbSIPK and NbWIPK are required for PCD activated by Pto recognition of AvrPto (Oh and Martin, 2011a). Additionally, transient expression of SlMAPKKKα or the constitutively active version of SlMKK2 (SlMKK2DD) both activate PCD (del Pozo et al., 2004; Oh and Martin, 2011b), as does transient expression of a tomato MAPK, SlMPK1 (Fig. 4), which acts downstream in this pathway (Pedley and Martin, 2004). To determine the effect of XopX expression on MAPK signaling in N. benthamiana, we co-expressed estradiol-inducible constructs of the tomato orthologs of each of the MAPK components with either GFP or GFP:XopX. In all cases, GFP:XopX delayed PCD caused by induction of the MAPK components (Fig. 4, middle).
Previously, the ability of Pseudomonas T3Es (including AvrPtoB) to suppress ETI was linked to general PCD suppression activity, including PCD elicited by transgenic expression of the mammalian apoptotic protein, Bax (Abramovitch et al., 2003; Jamir et al., 2004). To test the ability of XopX to suppress or antagonize PCD generally, we co-expressed the mammalian apoptotic protein Bax with either GFP or GFP:XopX. Again, GFP:XopX co-expression with Bax delayed Bax-induced PCD (Fig. 4, right).
Activation of the NtMEK2 (the tobacco ortholog of SlMKK2) elicits ROS accumulation upstream of PCD, an effect also linked to Bax-elicted PCD (Kawai-Yamada et al., 2004; Liu et al., 2007). Therefore, we next monitored PCD-associated ROS accumulation by cytological staining of H2O2 with 3,3’-Diaminobenzidine (DAB). Indeed, GFP:XopX co-expression with NtMEK2DD in N. benthamiana resulted in reduced DAB staining compared to co-expression with GFP (Fig. 5A), suggesting that XopX acts upstream of ROS accumulation to suppress PCD.
Fig. 5. ROS accumulation and protein levels during GFP:XopX co-expression with MAPK signaling components in N. benthamiana.

A) A. tumefaciens strains bearing GFP and GFP:XopX constructs were mixed to a final concentration of 2 × 108 CFU mL−1 with A. tumefaciens GV2260 pER8(NtMEK2DD). Estradiol was applied by spraying 24 hr after infiltration. 10 hr after estradiol application, prior to the onset of macroscope tissue collapse, infiltrated leaf tissue was vacuum-infiltrated with DAB solution. Leaf tissue was de-stained overnight in ethanol and imaged under a dissecting microscope. ROS accumulation is indicated by dark brown staining. B) A. tumifaciens strains bearing binary vectors for GFP or GFP:XopX expression were co-inoculated into N. benthamiana leaves with strains bearing binary vectors for expression of MAPK signaling components. Final A. tumefaciens concentrations were 2 × 108 CFU mL−1 (GFP, GFP:XopX, SlMPK1, Empty) or 1 × 108 CFU mL−1 (SlMAPKKKa, SlMKK2DD). MAPK component constructs were driven by estradiol-inducible promoters and estradiol was applied by spraying 24 hpi. Leaf tissue was harvested 10 hr after estradiol treatment for protein extraction. Protein from leaf extracts was separated by SDS-PAGE and subjected to immunoblotting with anti-HA or anti-GFP primary antibodies followed by HRP-conjugated secondary antibodies. Asterisks at right of blots indicate protein band corresponding to the labelled protein in each lane.
We also analyzed whether XopX could suppress PCD by affecting the stability or abundance of PCD elicitors. Immunoblot analysis of HA-tagged MAPK components resulted in detectable protein levels in both GFP and GFP:XopX expressing regions (Fig. 5B). Interestingly, all MAPK component protein levels were consistently lower in leaf tissue expressing GFP:XopX than in leaf tissue expressing GFP, correlating with reduced PCD in those regions (Fig. 5B). Notably, reduction of MAPK component protein levels occurred prior to tissue collapse and had a negative correlation with the level of PCD (i.e., XopX reduced protein accumulation and PCD), suggesting that this effect was not due to PCD per se. Furthermore, XopX did not affect total protein levels, as determined by Ponceau staining of immunoblots and similar levels of non-specific bands in immunoblots for both GFP and GFP:XopX samples (Fig. 5B). These data suggest that XopX alters the steady state levels of immune-related or highly expressed proteins specifically.
XopX does not contribute to ETI suppression in resistant tomato
Because XopX interfered with the activity of a broad array of PCD elicitors in N. benthamiana, we hypothesized that XopX might interfere with ETI elicited by Xe in resistant tomato. We therefore tested whether resistant tomato exhibited increased ETI in response to Xe lacking xopX. The tomato cv. Hawaii 7998 is resistant to WT and this resistance is triggered by the T3E AvrRxv (Whalen et al., 1993). We generated unmarked deletions of avrRxv in WT and ΔxopX (ΔavrRxv and ΔxopXΔavrRxv, respectively). Then we monitored growth of these Xe strains in Hawaii 7998 and the susceptible tomato cv. Hawaii 7981. ΔxopX growth was slightly reduced compared to WT in Hawaii 7998 leaves at 3 dpi, but this defect was transient and not significant (Fig. 6A). By contrast, ΔxopX growth was significantly lower than WT in Hawaii 7981 leaves (Fig. 6A). ΔavrRxv growth was higher than WT in Hawaii 7998, but lower in Hawaii 7981 (Fig. 6A), suggesting a virulence role for AvrRxv that has not been previously reported (Whalen et al., 1993; Bonshtien et al., 2005). ΔxopXΔavrRxv growth was significantly lower than ΔavrRxv in both Hawaii 7998 and 7981, further demonstrating that XopX virulence activity is independent of AvrRxv in both genetic backgrounds (Fig. 6A).
Fig. 6. Analysis of XopX effect on AvrRxv- and AvrBs2-dependent ETI responses in tomato.
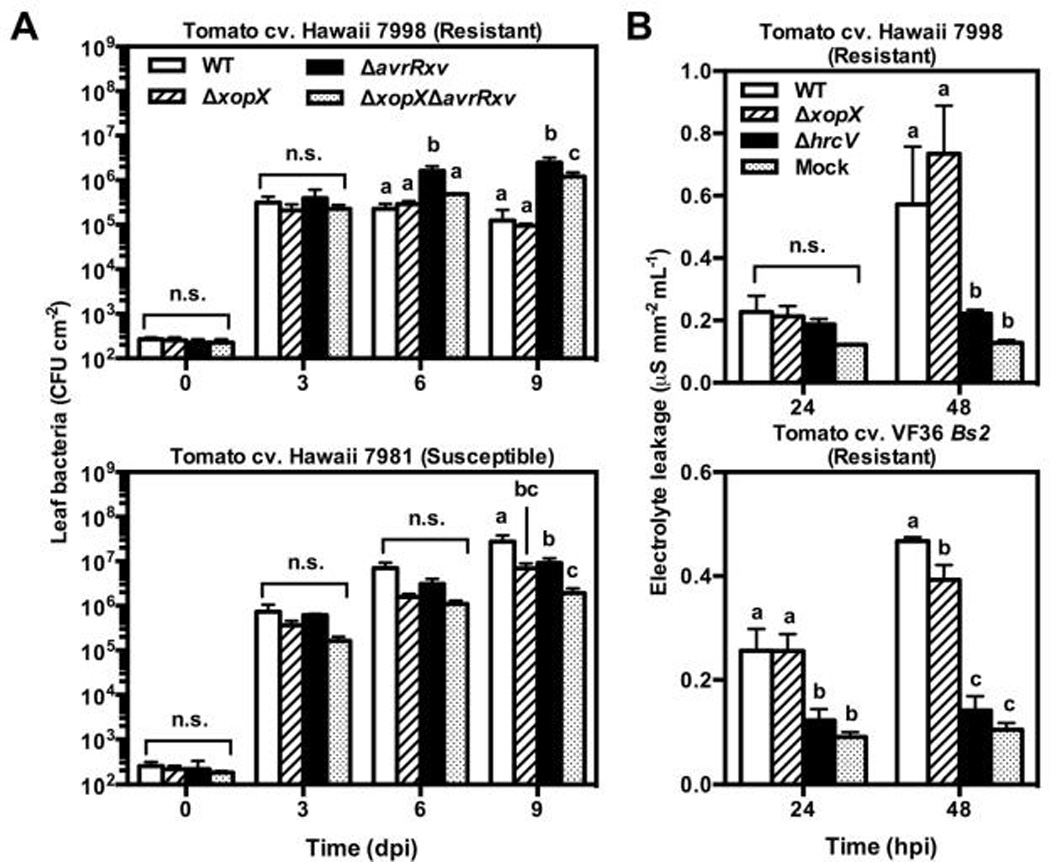
A) Tomato cv. Hawaii 7998 (resistant; top) or 7981 susceptible; bottom) leaves were inoculated with 2 × 104 CFU mL−1 of Xe WT, ΔxopX, ΔavrRxv, or ΔxopX ΔavrRxv. Leaf bacteria were enumerated for each treatment 0, 3, 6, and 9 dpi. Values are mean leaf bacteria (CFU cm−1 leaf) ± SD n = 3). B) Tomato cv. Hawaii 7998 (top) or VF36 Bs2 (bottom) leaves were inoculated with 2 × 108 CFU mL−1 of Xe WT, ΔxopX, or ΔhrcV. 4 leaf disks were harvested per sample at 24 and 48 hpi, rinsed, and floated on 3 mL distilled water for 2 h with gentle shaking and the conductivity of the bathing water was measured. Values are mean percent electrolyte leakage (µS mm−2 leaf mL−1 bathing water) ± SD (n = 3 plants). Different letters above bars indicate statistically significant differences within each time point as determined by two-way ANOVA and Tukey’s HSD, P < 0.05.
When inoculated into Hawaii 7998 leaves at high titer, Xe triggers rapid PCD, which is characteristic of ETI (Whalen et al., 1993). We inoculated WT, ΔxopX, or ΔhrcV into Hawaii 7998 leaves at 2 × 108 CFU mL−1 and quantified electrolyte leakage from the leaves (Fig. 6B). Leaves inoculated with WT or ΔxopX had similar levels of electrolyte leakage, which were significantly higher than ΔhrcV (Fig. 6B). This suggests that XopX is not able to suppress AvrRxv-elicited ETI in tomato. To confirm that the lack of ETI suppression by XopX was not specific to AvrRxv ETI, we repeated this experiment in transgenic tomato cv. VF36 expressing the pepper resistance gene Bs2, which recognizes avrBs2 from WT (Tai et al., 1999). As in Hawaii 7998, WT elicited high levels of electrolyte leakage (significantly higher than ΔhrcV) when inoculated at high titer into tomato cv. VF36 Bs2 leaves (Fig. 6B). Electrolyte leakage from ΔxopX-inoculated leaves was similar to that from WT-inoculated leaves at 24 hpi and slightly lower at 48 hpi (Fig. 6B), indicating that XopX does not suppress AvrBs2 ETI, and may in fact contribute to PCD associated with AvrBs2 ETI. These data suggest that, although transient expression of XopX in N. benthamiana interferes with PCD, Xe-delivered XopX is not a general suppressor of ETI in tomato.
DISCUSSION
We have identified a role for the Xanthomonas core T3E XopX in modulating plant immune signaling, including suppression of flagellin-induced ROS production and interference with rapid PCD elicited by Pto detection of AvrPto, activation of MAPK signaling, and the general cell death elicitor, Bax. However, we also found that XopX itself promotes plant defense gene transcription, ET production, and PCD during Xe infection of tomato and when transiently expressed in N. benthamiana. This complex combination of suppression and activation of immunity-related plant responses raises intriguing parallels with well-studied Pst T3Es, especially the core T3Es AvrE1 and HopM1 (Badel et al., 2006) and potent PTI suppressors AvrPto and AvrPtoB (Cohn and Martin, 2005). Our results therefore provide a useful starting point for contextualizing XopX function.
The plant immune system features multiple nodes that positively regulate immune signaling but negatively regulate each other; thus, perturbation of one node by a T3E may lead to hyperactivation of another (Sato et al., 2010). As recently noted (Kadota et al., 2014), null mutations in certain A. thaliana PTI signaling components display overactive immune responses, including increased PTI gene expression (Petersen et al., 2000; Nishimura et al., 2003; He et al., 2007; Kemmerling et al., 2007; Zhang et al., 2010; Kadota et al., 2014), leading to the hypothesis that severe perturbation of specific PTI signaling components is detected by the plant (Segonzac and Zipfel, 2011).
Suppression of flg22-elicited ROS by XopX suggests that it interferes with plant immune signaling at an early stage. However, XopX promotes— rather than suppresses—PTI-elicited gene expression, indicating that it does not suppress PTI signaling to all downstream outputs. It is known that flg22-elicited PTI gene expression is independent of NbRbohD-mediated ROS production in N. benthamiana (Segonzac et al., 2011). Our data is thus consistent with a model whereby XopX functions to target a specific node of plant immunity (i.e., NbRbohD-produced ROS). This targeting would not result in the suppression of PTI gene expression. Rather, perturbation of the node targeted by XopX would result in increased PTI gene transcription.
The idea that T3E perturbation of plant immunity itself leads to a plant defense response was put forward as a model to explain why the functionally redundant Pst T3Es AvrE1 and HopM1 suppress immunity but also cause R-gene-independent PCD across a range of plant species (Badel et al., 2006). In this “default to death and defense” model (Lindeberg et al., 2012), AvrE1/HopM1 suppress plant immunity by interfering with a critical plant cell process: vesicle trafficking (Nomura et al., 2006; Ham et al., 2009). The plant cell responds to this perturbation of a critical cell process by initiating, in this case, a PCD response. The fact that AvrE1/HopM1 elicit PCD in many plants suggests that this process is not mediated by R protein detection of a T3E, but is instead a general response to T3E virulence activity (Badel et al., 2006; Lindeberg et al., 2012). XopX activation of PTI gene transcription, ET production, and PCD is consistent with this model. Notably, XopX is also cytotoxic in yeast (Salomon et al., 2011), which suggests that—although the cellular pathways leading to yeast cell death and PCD may differ—XopX may indeed target a basic cell process common among eukaryotes.
Whether it is suppressed by the pathogen or just ineffective in halting pathogen growth in susceptible plants, the “default to death and defense” model implies that the PCD that underlies symptom develeopment may in fact be a plant defense response to T3Es. Consistent with plant regulation of PCD in susceptible interactions (i.e., through the phytohormones ET and SA), SlMAPKKKα (a positive regulator of ETI) is also required for PCD during Pst infection of tomato (del Pozo et al., 2004). Previously, Pst T3Es AvrPto and AvrPtoB were shown to promote ET biosynthesis and subsequent PCD during Pst infection of tomato (Cohn and Martin, 2005), although the direct mechanism by which these T3Es contribute to bacterial virulence appears to be through the inactivation of plant receptor-like kinases (Gohre et al., 2008; Xiang et al., 2008; Gimenez-Ibanez et al., 2009). Elicitation of defense-associated phytohormone signaling and PCD may then be a side effect of AvrPto/AvrPtoB virulence activitiy—perhaps another example of the plant “default to death and defense” response—which hemibiotrphic pathogens must control in order to achieve maximum virulence. Indeed, Xe T3Es XopD and XopJ suppress ET- and/or SA-dependent symptom development during late stages of infection to increase bacterial pathogenesis (Kim et al., 2008; Kim et al., 2013; Ustun et al., 2013).
We cannot rule out the possibility that XopX-elicited PCD is due to weak detection by an R-protein, although this seems unlikely given the correlation between PCD and bacterial virulence. XopX homologs are found in almost every sequenced Xanthomonas strain, and variation among XopX homologs at the amino acid sequence level correlates with Xanthomonas phylogeny. This suggests that XopX is an ancient Xanthomonas T3E and that it has evolved along with host-specific traits that distinguish Xanthomonas strains. It is not clear why XopX from Xe confers PCD-eliciting activity on N. benthamiana leaves in response to Xcc (Metz et al., 2005), the genome of which encodes two XopX homologs. Future comparative studies of XopX homologs will help to distinguish whether variation in XopX is due to evolved virulence function or escape from host detection.
Because transient expression of XopX itself elicits PCD, we were surprised to find that XopX interfered with ETI and PCD elicited by Pto-AvrPto, MAPK signaling components, and Bax. Antagonism between PCD-eliciting T3Es has been previously described. For example, AvrRpt2-elicited ETI was shown to interfere with AvrRpm1-elicited ETI in A. thaliana (Reuber and Ausubel, 1996; Ritter and Dangl, 1996). This genetic relationship was later explained by the fact that prior AvrRpt2 proteolysis of guardee protein RIN4 prevents phosphorylation of RIN4 by AvrRpm1 (Mackey et al., 2002; Axtell and Staskawicz, 2003; Mackey et al., 2003; Kim et al., 2005). XopX antagonism of a wide range of PCD elicitors is consistent with XopX targeting of a basic cell process required by the PCD pathways tested here. However, our results suggest that XopX is not a general suppressor of known ETI pathways when delivered by Xe in tomato (Fig. 6). These findings suggest that suppression of plant immunity by transient expression of T3Es should be interpreted with caution, as results may be affected by different levels of protein expression, biological differences between plant species (i.e., N. benthamiana and tomato), and/or the relative strength of different immune signaling pathways.
In conclusion, the study of highly conserved, core T3Es sheds light on the principal virulence strategies used by plant pathogens. Our results add XopX to the class of T3Es (along with AvrE1 and HopM1) that activate the plant “default to death and defense” response. Further elucidating the function of XopX and other core T3Es in this emerging T3E class will be important for uncovering the mechanisms that regulate both plant defense and symptom development during plant-microbe interactions.
MATERIALS AND METHODS
Bacterial culture and transformation
Detailed descriptions of all bacterial strains and plasmids used are provided in Table 1. Escherichia coli strains were cultured with lysogeny broth (LB; pH 7.0, 1% tryptone; 0.5% yeast extract; 171 mM NaCl) at 37°C, Xanthomonas euvesicatoria (Xe) strain 85-10 (and derivative strains) with nutrient yeast glycerol (NYG; pH 7.0, 0.5% peptone; 0.3% yeast extract; 2% glycerol; (Turner et al., 1984)) or hrp-inducing medium (XVM2; pH 6.7, 20 mM NaCl, 10 mM (NH4)2SO4, 5 mM MgSO4, 1 mM CaCl2, 0.16 mM KH2PO4, 0.32 mM K2HPO4, 0.01 mM FeSO4, 10 mM fructose, 10 mM sucrose, 0.03% casamino acid; (Wengelnik et al., 1996)) at 28°C, Pseudomonas syringae pathovar tomato strain DC3000 (Pst) ΔhrcU with NYG at 28°C, and Agrobacterium tumefaciens strains with LB at 28°C, with appropriate antibiotics. LB and NYG were supplemented with 1.5% w/v agar (LA and NYGA, respectively) for bacterial growth on plates. Antibiotics were used at the following final concentrations: carbenicillin (50 Pg mL−1), chloramphenicol (25 Pg mL−1), cyclohexamide (50 Pg mL−1), kanamycin (50 Pg mL−1), rifampicin (100 Pg mL−1), spectinomycin (50 Pg mL−1), tetracycline (5 Pg mL−1 for A. tumefaciens, 10 Pg mL−1 for Xe). Plasmids were introduced into E. coli and A. tumefaciens by heat shock transformation and into Xe by tri-parental mating using E. coli HB101, pRK600 (Kessler et al., 1992).
Plant materials
Seeds from N. benthamiana and tomato (Solanum lycopersicum) cultivars VF36, Hawaii 7998, Hawaii 7981, and VF36 Bs2 were germinated in soil at room temperature under 16 h light. After germination, tomato plants were grown in a greenhouse (16 h light, 25–28°C) and N. benthamiana in a growth chamber (16 h light, 25°C). For experiments, 4–6 wk-old tomato and 6–8 wk-old N. benthamiana plants were used.
Genetic complementation of ΔxopX
The xopX locus from Xe 85-10 (including 387 bp upstream and 355 bp downstream of the xopX coding sequence) was cloned by PCR using the primer pair RK2-1/WS2-12 (Table 2) and ligated into pCR8 (Life Technologies, Carlsbad, CA), creating pCR8(xopX2623). The xopX locus from pCR8(xopX2623) was then sub-cloned into pDSK519 by digesting pCR8(xopX2623) with EcoRI to obtain the xopX fragment and then ligating this fragment into the EcoRI site of pDSK519, creating pDSK519(xopX2623). pDSK519(xopX2623) was then introduced into ΔxopX by tri-parental mating and selection of exconjugates with kanamycin.
Table 2.
Primers used
| Target | Primer name and sequence | Reference |
|---|---|---|
| For cloning xopX | ||
| xopX | CA359: ATGGAGATCAAGAAACAGCAAACC CA360: GGACGAAGGCACAGTGCTGGCTGCGGCCT |
|
| xopX2623 | RK2-1: CAATGCGCTGCAACGACGCCTG WS2–12: ACAAGGTCAAGGAAGGCAGCGG |
|
| For deleting avrRxv | ||
| avrRxv upstream | WS1–40: TTGCCCTTGATGCCGCCGTT JG767: GAAGCTTAAGGATCCATATTTATTAGATCGCGCTAATCG |
|
| avrRxv downstream | JG768: GGATCCGACAATTACCAAATATTATTTACTTTCC WS1–41: GAAGCTTATTGCCGATGCCGATGCTGG |
|
| For qPCR | ||
| SlACTIN | JG234: GAGCGTGGTTACTCGTTCA JG136: CTAATATCCACGTCACATTTCAT |
(Kim et al., 2008) |
| SlPti5 | CA305: ATTCGCGATTCGGCTAGACATGGT CA306: AGTAGTGCCTTAGCACCTCGCATT |
(Kim et al., 2009; Nguyen et al., 2010) |
| SlLRR22 | CA293: AAGATTGGAGGTTGCCATTGGAGC CA294: ATCGCGATGAATGATCGGTGGAGT |
(Kim et al., 2009; Nguyen et al., 2010) |
| SlGRAS2 | CA295: TAATCCAAGGGATGAGCTTCT CA296: CCACCAACGTGACCACCTT |
(Kim et al., 2009; Nguyen et al., 2010) |
| SlWRKY28 | CA301: ACAGATGCAGCTACCTCATCCTCA CA302: GTGCTCAAAGCCTCATGGTTCTTG |
(Kim et al., 2009; Nguyen et al., 2010) |
| NbPP2A | WS252:GACCCTGATGTTGATGTTCGCT WS253: GAGGGATTTGAAGAGAGATTTC |
(Nguyen et al., 2010) |
| NbAcre31 | WS258: AATTCGGCCATCGTGATCTTGGTC WS259: GAGAAACTGGGATTGCCTGAAGGA |
(Nguyen et al., 2010) |
| NbPti5 | WS256: CCTCCAAGTTTGAGCTCGGATAGT WS257: CCAAGAAATTCTCCATGCACTCTGTC |
(Nguyen et al., 2010) |
Restriction sites are underlined.
Xe growth in tomato leaves
Xe strains grown for 48 h on NYGA were re-suspended in 1 mM MgCl2 and diluted to 1 × 105 colony forming units (CFU) mL−1 (for low titer inoculation in tomato cv. VF36), 2 × 108 CFU mL−1 (for high titer inoculation in tomato cv. VF36), or 2 × 104 CFU mL−1 (for low titer inoculation in tomato cv. Hawaii 7998 and Hawaii 7981). Xe suspensions were infiltrated into the apoplastic space of tomato leaves using a needleless syringe. To quantify Xe bacterial population in leaf tissue, 0.5 cm2 of leaf tissue was harvested at 0, 3, 6, and 9 dpi, ground completely in 1 mL of 1 mM MgCl2, serially diluted in 1 mM MgCl2, and plated on NYGA with approprate antibiotics. Xe CFUs on plates were counted after 48 h incubation at 28°C.
Preparation of Xe culture for protein expression
Individual Xe colonies were isolated on NYGA and cultured in NYG for 24 h at 28°C with shaking. Cellular pellets were collected by centrifugation (8,000 × g, 5 min, RT), re-suspended in 1 mM MgCl2, collected again by centrifugation, and re-suspended in NYG or XVM2 at a final titer of 4 × 108 CFU mL−1. Cultures were incubated for 2–4 h at 28°C with shaking. OD600nm of final cultures was measured using a spectrophotometer and cellular pellets were collected by centrifugation (16,000 × g, 2 min, RT).
Agrobacterium-mediated transient protein expression in N. benthamiana
A. tumefaciens grown 24–48 h on LA plates were re-suspended in LB. Pellets were collected by centrifugation (8,000 × g, 2 min, RT) and re-suspended after removing supernatant in Agrobacterium Induction Medium (10 mM MES, pH 5.6, 10 mM MgCl2, 150 PM acetosyringone). For expression of GFP or GFP:XopX alone, A. tumefaciens was resuspended at a final titer of 4 × 108 CFU mL−1. Final A. tumefaciens concentrations in Pto-AvrPto co-inoculations were 2 × 108 CFU mL−1 for all strains. Final A. tumefaciens concentrations in MAPK component co-inoculations were: 2 × 108 CFU mL−1 (GFP, GFP:XopX, SlMPK1, Empty) or 1 × 108 CFU mL−1 (SlMAPKKKα, SlMKK2DD). Final A. tumefaciens concentrations in Bax co-inoculations were 4 × 108 CFU mL−1 for all strains. Descriptions and references for all strains and plasmids used are provided in Table 1. Briefly, GFP and GFP:XopX constructs were driven by the 35S promoter and borne on binary vector pGWB6 in A. tumefaciens C58C1(pCH32). Pto-AvrPto was driven by the 35S promoter and borne on pBTEX in A. tumefaciens EHA105. MAPK components have a C-terminal HA tag and were driven by an estradiol-inducible promoter and borne on pER8 in A. tumefaciens GV2260. Bax was driven by the 35S promoter and borne on pBTEX in A. tumefaciens GV2260.
Final A. tumefaciens suspensions were syringe infiltrated into N. benthamiana leaves using a needleless syringe. For estradiol-inducible constructs, 5 PM 17-β estradiol with 0.05% Tween-20 was applied by spraying leaves 24 hr after initial A. tumefaciens infiltration. Infiltrated plants were placed under continuous light at room temperature for the duration of all experiments.
Analysis of protein expression
Protein levels from Xe cultures and transient expression in N. benthamiana were analyzed by immunoblot essentially as previously described (Mudgett and Staskawicz, 1999). For Xe cultures, collected cellular pellets were denatured in urea sample buffer (8 M urea, 15% β-mercaptoethanol, 60 mM Tris pH 6.8, 2% sodium dodecyl sulfate, 11.8% glycerol, 0.0013% bromophenol blue, 25 nL mL−1 protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO)) using 100 PL per 1 OD600nm unit (1 mL of Xe culture at 1 OD600nm = 1 OD600nm unit). For N. benthamiana, leaf tissue was completely disrupted in urea sample buffer. Total protein was separated by SDS-PAGE and transferred to nitrocellulose membrane. Protein was detected by ECL Plus (GE Healthcare, Buckinghamshire, United Kingdom) after blotting membranes with rabbit anti-XopX antisera (Covance, Denver, PA), mouse anti-HA antibody (Covance, Berkeley, CA), or mouse anti-GFP antibody (Clontech, Mountain View, CA) followed by horseradish-peroxidase-conjugated secondary antibodies (Bio-Rad, Hercules, CA). The anti-XopX antisera were generated against recombinant, full-length XopX with an N-terminal sexta-histidine tag expressed in E. coli off of pDEST17(xopX), batch purified with nickel-NTA resin under denaturing conditions (100 mM NaH2PO4, 10 mM Tris HCl, 8 M urea; pH 8.0 for lysis, pH 6.3 for wash, pH 4.5 for elution; 10 mM imidazole for lysis, 20 mM imidazole for wash/elution), and separated by SDS-PAGE.
Trypan blue staining for PCD
Sample preparation prior to trypan blue staining in N. benthamiana and tomato experiments differed slightly. For N. benthamiana, transient expression of GFP or GFP:XopX was performed in different regions of the same leaf using 4 × 108 CFU mL−1 of A. tumefaciens. The whole leaf was harvested at 3 dpi, photographed, and subjected to trypan blue staining. For tomato, Xe WT and ΔxopX were inoculated into different regions of the same leaf at 2 × 108 CFU mL−1. Whole leaves were harvest at 1, 2, and 3 dpi, photographed, and subjected to trypan blue staining. Trypan blue staining was performed as described (van Wees, 2008), with slight modifications. Whole leaves were completely submerged in 50 mL of staining solution (67% ethanol, 8.3% lactic acid, 8.3% phenol, 8.3% glycerol, 2.5 mg mL−1 trypan blue), boiled for 5 min, and incubated overnight (O/N) at RT. Leaves were transferred to de-staining solution (67% ethanol, 8.3% lactic acid, 8.3% phenol, 8.3% glycerol) O/N at RT with gentle shaking. Leaves were washed in ethanol, dried briefly, and photographed. Regions of dark blue indicate uptake of trypan blue by dead and dying cells.
Electrolyte leakage quantification
Electrolyte leakage quantification in N. benthamiana and tomato experiments differed slightly. For N. benthamiana, transient expression of GFP or GFP:XopX was performed in different regions of the same leaf using 4 × 108 CFU mL−1 of A. tumefaciens. Four leaf disks (12 mm diameter) were sampled from each inoculation region at 20 hpi, rinsed in distilled water, and floated on 5 mL of distilled water in a 6 well tissue plate under continuous light. 24 h after initial A. tumefaciens inoculation, conductivity of the bathing water was measured using a conductivity meter, and repeated every subsequent 24 h. Mean conductivity ± SD from 3 plants is reported for each treatment. For tomato, Xe strains were inoculated into different leaflets of the same leaf at 2 × 108 CFU mL−1. Leaf disks (4 × 12 mm diameter for cv. VF36, 5 × 8 mm diameter for cv. Hawaii 7998 and VF36 Bs2) were sampled at indicated times (24, 48, and 72 hpi for cv. VF36; 16, 24 hpi for cv. Hawaii 7998 and VF36 Bs2), rinsed in distilled water, and floated on 5 mL (cv. VF36) or 3 mL (cv. Hawaii 7998 and VF36 Bs2) of distilled water for 4 h with gentle shaking. Conductivity of the bathing water was then measured. Mean conductivity ± SD from 3 plants is reported for each treatment.
ET quantification
Sample preparation prior to ET quantification in N. benthamiana and tomato experiments differed slightly. For N. benthamiana, transient expression of GFP or GFP:XopX was performed on 2 halves of the same leaf, divided by the mid-vein, using 4 × 108 CFU mL−1 of A. tumefaciens. Whole leaves were excised and GFP and GFP:XopX expressing halves were separated by cutting along the midvein. For tomato, Xe strains were inoculated into different leaflets of the same leaf at 2 × 108 CFU mL−1. Leaf halves (N. benthamiana) or leaflets (tomato) were weighed, placed in a glass tube capped with a Suba-Seal septa stopper (Sigma-Aldrich, St. Louis, MO), and incubated for 1 h at 25°C. A 1 mL gas sample was injected into a gas chromatograph and ET levels were quantified. Mean ET ± SD from 3 (N. benthamiana) or 4 (tomato) plants is reported for each treatment.
SA quantification
SA quantification was performed as described (Defraia et al., 2008), with slight modifications. Sample preparation prior to SA quantification in N. benthamiana and tomato experiments differed slightly. For N. benthamiana, transient expression of GFP or GFP:XopX was performed on different regions of the same leaf using 4 × 108 CFU mL−1 of A. tumefaciens. For tomato, Xe strains were inoculated into different leaflets of the same leaf at 2 × 108 CFU mL−1. Inoculated leaf tissue (50 mg) was excised and homogenized in 500 PL of 0.1 M acetate buffer, pH 5.6. Samples were centrifuged (16,000 × g, 15 min, 4°C), 100 PL of supernatant was transferred to a new tube for free SA quantification, and 50 PL of supernatant was incubated with 5 PL of 0.4 U PL−1 β-glucosidase (Sigma-Aldrich, St. Louis, MO) for 90 min at 37°C for total SA quantification. 60 PL of LB, 20 PL of extract, and 50 PL of Acinetobacter sp. ADPWH_lux (Huang et al., 2005) were added to each well of a black 96-well plate at 4 × 108 CFU mL−1. The plate was incubated at 37°C for 60 min and then luminescence was measured using a plate reader with luminometer function. To generate a standard curve, 1 PL of SA standard solutions were diluted 10-fold in untreated leaf extract from N. benthamiana or tomato cv. Moneymaker nahG (Brading et al., 2000). 5 PL of each standard was added to 60 PL of LB and 50 PL of Acinetobacter sp. ADPWH_lux (4 × 108 CFU mL−1) and luminescence measured at the same time as experimental samples. Mean SA (free or total) ± SD from 3 (N. benthamiana) or 4 (tomato) plants is reported for each treatment.
Quantification of plant gene transcript abundance by RT-qPCR
Sample preparation prior to transcript quantification in N. benthamiana and tomato experiments differed slightly. For N. benthamiana, transient expression of GFP or GFP:XopX was performed on different regions of the same leaf using 4 × 108 CFU mL−1 of A. tumefaciens. 24 hpi, GFP and GFP:XopX expressing regions were inoculated with 1 mM MgCl2 (mock) or 2 × 108 CFU mL−1 Pst ΔhrcU. For tomato, Xe strains were inoculated into different leaflets of the same leaf at 2 × 108 CFU mL−1. Leaf tissue was excised and total RNA was extracted using TRI Reagent (Life Technologies, Carlsbad, CA). 5 Pg of RNA was used for cDNA synthesis with poly-T primers and Maxima Reverse Transcriptase (Thermo Scientific, Waltham, MA).
Quantitative PCR of cDNA was performed on MJ Opticon 2 (Bio-Rad, Hercules, CA) using SYBR Green reporter (Thermo Scientific, Waltham, MA) with gene-specific primers (Table 2). Relative fold changes in gene transcript abundance were determined by the comparative CT method (Livak and Schmittgen, 2001). CT values were determined by the average of 2 technical replicates per gene per sample. Relative fold change for each gene was calibrated to the average ΔCT values from mock (1 mM MgCl2) treatments with NbPP2A or SlACTIN used as the internal control. Mean 2−ΔΔCT values ± SD for 4 plants are reported.
Quantification of flg22-elicited reactive oxygen species
Transient expression of GFP or GFP:XopX was performed on different regions of the same leaf using 4 × 108 CFU mL−1 of A. tumefaciens. At 24 hpi, 3 leaf disks (4 mm diameter) were excised from each inoculated region and floated in 200 PL of sterile distilled water O/N in a 96-well plate. To initiate the assay, water was removed and 100 PL of solution (34 Pg mL−1 luminol, 20 Pg mL−1 horseradish peroxidase) with 200 nM flg22 (flg22) or without flg22 (mock) was added to each well. Using an automatic plate reader with luminometer function, luminescence from individual leaf disks was measured as relative light units (RLUs) and recorded at regular intervals for approximately 90 min starting immediately after addition of solution. The time interval between plate reads was approximately 50 sec. For each sample, RLUs measured at the indicated time point were averaged for 3 technical replicates from each biological replicate. Mean RLU ± SD for 4 plants is reported.
Scoring PCD phenotype in N. benthamiana after XopX co-expression with PCD elicitors
N. benthamiana leaves were co-inoculated with two A. tumefaciens strains to express GFP or GFP:XopX with PCD elicitors Pto-AvrPto, SlMAPKKα, SlMKK2DD, SlMPK1, Bax, or appropriate empty vectors. For each experiment, co-inoculations were performed with the first 3 fully expanded leaves from each of 6 plants. For estradiol-inducible MAPK component constructs, 5 PM 17-β estradiol with 0.05% Tween-20 was applied by spraying leaves 24 hr after initial A. tumefaciens infiltration. Inoculated leaf tissue was monitored for 3–5 days and each inoculated region was qualitatively scored for the amount of tissue collapse (0–10% collapse = None/Limited, 10–85% collapse = Partial, >85% collapse = Full). The percent of co-inoculated regions with each level of tissue collapse at 3 (Pto-AvrPto, Bax) or 5 (MAPK components) dpi was compared between GFP and GFP:XopX treatments for each PCD elicitor.
DAB staining of N. benthamiana leaves
N. benthamiana leaves were co-inoculated with two A. tumefaciens strains to express GFP or GFP:XopX with NtMEK2DD. To induce NtMEK2 DD expression, 5 PM 17-β estradiol with 0.05% Tween-20 was applied by spraying leaves 24 hr after initial A. tumefaciens infiltration. 10 h after estradiol treatment, inoculated regions were excised, submerged in staining solution (1 mg mL−1 DAB, 10 mM K2HPO4, 0.05% Tween-20), subjected to vacuum for 5 min, and incubated in the dark at RT for 4 hr. Leaf chlorophyll was cleared by floating the leaf in ethanol with gentle shaking for several days. Cellular accumulation of H2O2 was imaged using a light microscope under 10× magnification.
Genomic deletion of avrRxv in Xe 85-10 and ΔxopX
The genomic region (1,567 bp) directly upstream of the avrRxv coding sequence was amplified by PCR with a 3’ BamHI::HindIII extension (primer pair WS01–40/JG767) and ligated into pCR8 (Life Technologies, Carlsbad, CA). The genomic region (1,594 bp) directly downstream of avrRxv was amplified as a BamHI-HindIII fragment (primer pair JG768/WS01–41) and blunt-end ligated into pJET1.2 (Thermo Scientific, Waltham, MA). The BamHI-HindIII downstream fragment was then sub-cloned into the BamHI::HindIII site of the pCR8 vector containing the upstream fragment, generating the ΔavrRxv construct. The ΔavrRxv construct was then recombined using LR Clonase II (Life Technologies, Carlsbad, CA) into a Gateway-compatible version of the suicide vector pLVC18-Rfc, generating pLVC18-Rfc(ΔavrRxv). pLVC18-Rfc(ΔavrRxv) was then introduced into Xe 85-10 and ΔxopX by tri-parental mating. Single-crossover exconjugates were first selected on tetracycline NYGA and then grown in NYG without tetracycline selection with several sub-culturing steps. Cultures were diluted and plated on NYGA without tetracycline. Individual colonies were replica-plated with and without tetracycline selection to identify tetracycline-sensitive double-crossover ΔavrRxv mutants. Deletion of avrRxv coding sequence in individual mutants was confirmed by colony PCR and by loss of the ability of mutants to induce the hypersensitive response in tomato cv. Hawaii 7998.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Mica Soriano for technical assistance, Sophia Sonnewald for providing ΔxopX, Guido Sessa for providing useful discussion, A. tumefaciens strains, and Hawaii 7998 and 7981 seeds, members of the Mudgett Laboratory for feedback on this manuscript, and the laboratories of Dominique Bergmann, Or Gozani, Sharon Long, Justin Sonnenburg, Virginia Walbot for scientific discussions and/or use of equipment. WS is supported by USDA NIFA Grant 2012-67011-19669. MBM is supported by NIH Grant 2 R01 GM068886-06A1.
LITERATURE CITED
- Abramovitch RB, Kim YJ, Chen S, Dickman MB, Martin GB. Pseudomonas type III effector AvrPtoB induces plant disease susceptibility by inhibition of host programmed cell death. EMBO J. 2003;22:60–69. doi: 10.1093/emboj/cdg006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axtell MJ, Staskawicz BJ. Initiation of RPS2-specified disease resistance in Arabidopsis is coupled to the AvrRpt2-directed elimination of RIN4. Cell. 2003;112:369–377. doi: 10.1016/s0092-8674(03)00036-9. [DOI] [PubMed] [Google Scholar]
- Badel JL, Shimizu R, Oh HS, Collmer A. A Pseudomonas syringae pv. tomato avrE1/hopM1 mutant is severely reduced in growth and lesion formation in tomato. Mol. Plant-Microbe Interact. 2006;19:99–111. doi: 10.1094/MPMI-19-0099. [DOI] [PubMed] [Google Scholar]
- Bari R, Jones JD. Role of plant hormones in plant defence responses. Plant Mol. Biol. 2009;69:473–488. doi: 10.1007/s11103-008-9435-0. [DOI] [PubMed] [Google Scholar]
- Boller T, He SY. Innate immunity in plants: an arms race between pattern recognition receptors in plants and effectors in microbial pathogens. Science. 2009;324:742–744. doi: 10.1126/science.1171647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonas U, Schulte R, Fenselau S, Minsavage GV, Staskawicz BJ, Stall RE. Isolation of a gene cluster from Xanthomonas campestris pv. vesicatoria that determines pathogenicity and the hypersensitive response on pepper and tomato. Mol. Plant-Microbe Interact. 1991;4:81–88. [Google Scholar]
- Bonshtien A, Lev A, Gibly A, Debbie P, Avni A, Sessa G. Molecular properties of the Xanthomonas AvrRxv effector and global transcriptional changes determined by its expression in resistant tomato plants. Mol. Plant-Microbe Interact. 2005;18:300–310. doi: 10.1094/MPMI-18-0300. [DOI] [PubMed] [Google Scholar]
- Brading PA, Hammond-Kosack KE, Parr A, Jones JD. Salicylic acid is not required for Cf-2- and Cf-9-dependent resistance of tomato to Cladosporium fulvum. Plant J. 2000;23:305–318. doi: 10.1046/j.1365-313x.2000.00778.x. [DOI] [PubMed] [Google Scholar]
- Buttner D. Protein export according to schedule: architecture, assembly, and regulation of type III secretion systems from plant- and animal-pathogenic bacteria. Microbiol. Mol. Biol. Rev. 2012;76:262–310. doi: 10.1128/MMBR.05017-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn JR, Martin GB. Pseudomonas syringae pv. tomato type III effectors AvrPto and AvrPtoB promote ethylene-dependent cell death in tomato. Plant J. 2005;44:139–154. doi: 10.1111/j.1365-313X.2005.02516.x. [DOI] [PubMed] [Google Scholar]
- Cunnac S, Lindeberg M, Collmer A. Pseudomonas syringae type III secretion system effectors: repertoires in search of functions. Curr. Opin. Microbiol. 2009;12:53–60. doi: 10.1016/j.mib.2008.12.003. [DOI] [PubMed] [Google Scholar]
- Dangl JL, Horvath DM, Staskawicz BJ. Pivoting the plant immune system from dissection to deployment. Science. 2013;341:746–751. doi: 10.1126/science.1236011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Defraia CT, Schmelz EA, Mou Z. A rapid biosensor-based method for quantification of free and glucose-conjugated salicylic acid. Plant Methods. 2008;4:28. doi: 10.1186/1746-4811-4-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Pozo O, Pedley KF, Martin GB. MAPKKKalpha is a positive regulator of cell death associated with both plant immunity and disease. EMBO J. 2004;23:3072–3082. doi: 10.1038/sj.emboj.7600283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doidge EM. A tomato canker. Ann. Appl. Biol. 1921;7:407–430. [Google Scholar]
- Feng F, Yang F, Rong W, Wu X, Zhang J, Chen S, He C, Zhou JM. A Xanthomonas uridine 5'-monophosphate transferase inhibits plant immune kinases. Nature. 2012;485:114–118. doi: 10.1038/nature10962. [DOI] [PubMed] [Google Scholar]
- Frederick RD, Thilmony RL, Sessa G, Martin GB. Recognition specificity for the bacterial avirulence protein AvrPto is determined by Thr-204 in the activation loop of the tomato Pto kinase. Mol. Cell. 1998;2:241–245. doi: 10.1016/s1097-2765(00)80134-3. [DOI] [PubMed] [Google Scholar]
- Gimenez-Ibanez S, Hann DR, Ntoukakis V, Petutschnig E, Lipka V, Rathjen JP. AvrPtoB targets the LysM receptor kinase CERK1 to promote bacterial virulence on plants. Curr. Biol. 2009;19:423–429. doi: 10.1016/j.cub.2009.01.054. [DOI] [PubMed] [Google Scholar]
- Gohre V, Spallek T, Haweker H, Mersmann S, Mentzel T, Boller T, de Torres M, Mansfield JW, Robatzek S. Plant pattern-recognition receptor FLS2 is directed for degradation by the bacterial ubiquitin ligase AvrPtoB. Curr. Biol. 2008;18:1824–1832. doi: 10.1016/j.cub.2008.10.063. [DOI] [PubMed] [Google Scholar]
- Guo M, Tian F, Wamboldt Y, Alfano JR. The majority of the type III effector inventory of Pseudomonas syringae pv. tomato DC3000 can suppress plant immunity. Mol. Plant-Microbe Interact. 2009;22:1069–1080. doi: 10.1094/MPMI-22-9-1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajri A, Brin C, Hunault G, Lardeux F, Lemaire C, Manceau C, Boureau T, Poussier S. A "repertoire for repertoire" hypothesis: repertoires of type three effectors are candidate determinants of host specificity in Xanthomonas. PLOS ONE. 2009;4:e6632. doi: 10.1371/journal.pone.0006632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ham JH, Majerczak DR, Nomura K, Mecey C, Uribe F, He SY, Mackey D, Coplin DL. Multiple activities of the plant pathogen type III effector proteins WtsE and AvrE require WxxxE motifs. Mol. Plant-Microbe Interact. 2009;22:703–712. doi: 10.1094/MPMI-22-6-0703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He K, Gou X, Yuan T, Lin H, Asami T, Yoshida S, Russell SD, Li J. BAK1 and BKK1 regulate brassinosteroid-dependent growth and brassinosteroid-independent cell-death pathways. Curr. Biol. 2007;17:1109–1115. doi: 10.1016/j.cub.2007.05.036. [DOI] [PubMed] [Google Scholar]
- Hood EE, Gelvin SB, Melchers LS, Hoekema A. New Agrobacterium helper plasmids for gene transfer to plants. Transgenic Res. 1993;2:208–218. [Google Scholar]
- Huang WE, Wang H, Zheng H, Huang L, Singer AC, Thompson I, Whiteley AS. Chromosomally located gene fusions constructed in Acinetobacter sp. ADP1 for the detection of salicylate. Environ. Microbiol. 2005;7:1339–1348. doi: 10.1111/j.1462-5822.2005.00821.x. [DOI] [PubMed] [Google Scholar]
- Jalan N, Aritua V, Kumar D, Yu F, Jones JB, Graham JH, Setubal JC, Wang N. Comparative genomic analysis of Xanthomonas axonopodis pv. citrumelo F1, which causes citrus bacterial spot disease, and related strains provides insights into virulence and host specificity. J. Bacteriol. 2011;193:6342–6357. doi: 10.1128/JB.05777-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamir Y, Guo M, Oh HS, Petnicki-Ocwieja T, Chen S, Tang X, Dickman MB, Collmer A, Alfano JR. Identification of Pseudomonas syringae type III effectors that can suppress programmed cell death in plants and yeast. Plant J. 2004;37:554–565. doi: 10.1046/j.1365-313x.2003.01982.x. [DOI] [PubMed] [Google Scholar]
- Jones JB, Stall RE, Bouzar H. Diversity among xanthomonads pathogenic on pepper and tomato. Annu. Rev. Phytopathol. 1998;36:41–58. doi: 10.1146/annurev.phyto.36.1.41. [DOI] [PubMed] [Google Scholar]
- Jones JB, Lacy GH, Bouzar H, Stall RE, Schaad NW. Reclassification of the xanthomonads associated with bacterial spot disease of tomato and pepper. Syst. Appl. Microbiol. 2004;27:755–762. doi: 10.1078/0723202042369884. [DOI] [PubMed] [Google Scholar]
- Jones JD, Dangl JL. The plant immune system. Nature. 2006;444:323–329. doi: 10.1038/nature05286. [DOI] [PubMed] [Google Scholar]
- Kadota Y, Sklenar J, Derbyshire P, Stransfeld L, Asai S, Ntoukakis V, Jones JD, Shirasu K, Menke F, Jones A, Zipfel C. Direct regulation of the NADPH oxidase RBOHD by the PRR-associated kinase BIK1 during plant immunity. Mol. Cell. 2014;54:43–55. doi: 10.1016/j.molcel.2014.02.021. [DOI] [PubMed] [Google Scholar]
- Kawai-Yamada M, Ohori Y, Uchimiya H. Dissection of Arabidopsis Bax inhibitor-1 suppressing Bax-, hydrogen peroxide-, and salicylic acid-induced cell death. Plant Cell. 2004;16:21–32. doi: 10.1105/tpc.014613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keen NT, Tamaki S, Kobayashi D, Trollinger D. Improved broad-host-range plasmids for DNA cloning in gram-negative bacteria. Gene. 1988;70:191–197. doi: 10.1016/0378-1119(88)90117-5. [DOI] [PubMed] [Google Scholar]
- Kemmerling B, Schwedt A, Rodriguez P, Mazzotta S, Frank M, Qamar SA, Mengiste T, Betsuyaku S, Parker JE, Mussig C, Thomma BP, Albrecht C, de Vries SC, Hirt H, Nurnberger T. The BRI1-associated kinase 1, BAK1, has a brassinolide-independent role in plant cell-death control. Curr. Biol. 2007;17:1116–1122. doi: 10.1016/j.cub.2007.05.046. [DOI] [PubMed] [Google Scholar]
- Kessler B, de Lorenzo V, Timmis KN. A general system to integrate lacZ fusions into the chromosomes of gram-negative eubacteria: regulation of the Pm promoter of the TOL plasmid studied with all controlling elements in monocopy. Mol. Gen. Genet. 1992;233:293–301. doi: 10.1007/BF00587591. [DOI] [PubMed] [Google Scholar]
- Kim HS, Desveaux D, Singer AU, Patel P, Sondek J, Dangl JL. The Pseudomonas syringae effector AvrRpt2 cleaves its C-terminally acylated target, RIN4, from Arabidopsis membranes to block RPM1 activation. Proc. Natl. Acad. Sci. U.S.A. 2005;102:6496–6501. doi: 10.1073/pnas.0500792102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JG, Stork W, Mudgett MB. Xanthomonas type III effector XopD desumoylates tomato transcription factor SlERF4 to suppress ethylene responses and promote pathogen growth. Cell Host Microbe. 2013;13:143–154. doi: 10.1016/j.chom.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JG, Taylor KW, Hotson A, Keegan M, Schmelz EA, Mudgett MB. XopD SUMO protease affects host transcription, promotes pathogen growth, and delays symptom development in Xanthomonas-infected tomato leaves. Plant Cell. 2008;20:1915–1929. doi: 10.1105/tpc.108.058529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JG, Li X, Roden JA, Taylor KW, Aakre CD, Su B, Lalonde S, Kirik A, Chen Y, Baranage G, McLane H, Martin GB, Mudgett MB. Xanthomonas T3S effector XopN suppresses PAMP-triggered immunity and interacts with a tomato atypical receptor-like kinase and TFT1. Plant Cell. 2009;21:1305–1323. doi: 10.1105/tpc.108.063123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel BN, Brooks DM. Cross talk between signaling pathways in pathogen defense. Curr. Opin. Plant Biol. 2002;5:325–331. doi: 10.1016/s1369-5266(02)00275-3. [DOI] [PubMed] [Google Scholar]
- Lindeberg M, Cunnac S, Collmer A. Pseudomonas syringae type III effector repertoires: last words in endless arguments. Trends Microbiol. 2012;20:199–208. doi: 10.1016/j.tim.2012.01.003. [DOI] [PubMed] [Google Scholar]
- Liu Y, Ren D, Pike S, Pallardy S, Gassmann W, Zhang S. Chloroplast-generated reactive oxygen species are involved in hypersensitive response-like cell death mediated by a mitogen-activated protein kinase cascade. Plant J. 2007;51:941–954. doi: 10.1111/j.1365-313X.2007.03191.x. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lund ST, Stall RE, Klee HJ. Ethylene regulates the susceptible response to pathogen infection in tomato. Plant Cell. 1998;10:371–382. doi: 10.1105/tpc.10.3.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackey D, Holt BF, 3rd, Wiig A, Dangl JL. RIN4 interacts with Pseudomonas syringae type III effector molecules and is required for RPM1-mediated resistance in Arabidopsis. Cell. 2002;108:743–754. doi: 10.1016/s0092-8674(02)00661-x. [DOI] [PubMed] [Google Scholar]
- Mackey D, Belkhadir Y, Alonso JM, Ecker JR, Dangl JL. Arabidopsis RIN4 is a target of the type III virulence effector AvrRpt2 and modulates RPS2-mediated resistance. Cell. 2003;112:379–389. doi: 10.1016/s0092-8674(03)00040-0. [DOI] [PubMed] [Google Scholar]
- Mcbride KE, Summerfelt KR. Improved Binary Vectors for Agrobacterium-Mediated Plant Transformation. Plant Mol. Biol. 1990;14:269–276. doi: 10.1007/BF00018567. [DOI] [PubMed] [Google Scholar]
- Metz M, Dahlbeck D, Morales CQ, Al Sady B, Clark ET, Staskawicz BJ. The conserved Xanthomonas campestris pv. vesicatoria effector protein XopX is a virulence factor and suppresses host defense in Nicotiana benthamiana. Plant J. 2005;41:801–814. doi: 10.1111/j.1365-313X.2005.02338.x. [DOI] [PubMed] [Google Scholar]
- Minsavage GV, Dahlbeck D, Whalen MC, Kearney B, Bonas U, Staskawicz BJ, Stall RE. Gene-for-gene relationships specifying disease resistance in Xanthomonas campestris pv. vesicatoria-pepper interactions. Mol. Plant-Microbe Interact. 1990;3:41–47. [Google Scholar]
- Moreira LM, Almeida NF, Jr, Potnis N, Digiampietri LA, Adi SS, Bortolossi JC, da Silva AC, da Silva AM, de Moraes FE, de Oliveira JC, de Souza RF, Facincani AP, Ferraz AL, Ferro MI, Furlan LR, Gimenez DF, Jones JB, Kitajima EW, Laia ML, Leite RP, Jr, Nishiyama MY, Rodrigues Neto J, Nociti LA, Norman DJ, Ostroski EH, Pereira HA, Jr, Staskawicz BJ, Tezza RI, Ferro JA, Vinatzer BA, Setubal JC. Novel insights into the genomic basis of citrus canker based on the genome sequences of two strains of Xanthomonas fuscans subsp. aurantifolii. BMC Genomics. 2010;11:238. doi: 10.1186/1471-2164-11-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudgett MB, Staskawicz BJ. Characterization of the Pseudomonas syringae pv. tomato AvrRpt2 protein: demonstration of secretion and processing during bacterial pathogenesis. Mol. Microbiol. 1999;32:927–941. doi: 10.1046/j.1365-2958.1999.01403.x. [DOI] [PubMed] [Google Scholar]
- Mukhtar MS, Carvunis AR, Dreze M, Epple P, Steinbrenner J, Moore J, Tasan M, Galli M, Hao T, Nishimura MT, Pevzner SJ, Donovan SE, Ghamsari L, Santhanam B, Romero V, Poulin MM, Gebreab F, Gutierrez BJ, Tam S, Monachello D, Boxem M, Harbort CJ, McDonald N, Gai L, Chen H, He Y, Vandenhaute J, Roth FP, Hill DE, Ecker JR, Vidal M, Beynon J, Braun P, Dangl JL. Independently evolved virulence effectors converge onto hubs in a plant immune system network. Science. 2011;333:596–601. doi: 10.1126/science.1203659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa T, Kurose T, Hino T, Tanaka K, Kawamukai M, Niwa Y, Toyooka K, Matsuoka K, Jinbo T, Kimura T. Development of series of gateway binary vectors, pGWBs, for realizing efficient construction of fusion genes for plant transformation. J. Biosci. Bioeng. 2007;104:34–41. doi: 10.1263/jbb.104.34. [DOI] [PubMed] [Google Scholar]
- Nguyen HP, Chakravarthy S, Velasquez AC, McLane HL, Zeng L, Nakayashiki H, Park DH, Collmer A, Martin GB. Methods to study PAMP-triggered immunity using tomato and Nicotiana benthamiana. Mol. Plant-Microbe Interact. 2010;23:991–999. doi: 10.1094/MPMI-23-8-0991. [DOI] [PubMed] [Google Scholar]
- Nishimura MT, Stein M, Hou BH, Vogel JP, Edwards H, Somerville SC. Loss of a callose synthase results in salicylic acid-dependent disease resistance. Science. 2003;301:969–972. doi: 10.1126/science.1086716. [DOI] [PubMed] [Google Scholar]
- Nomura K, Debroy S, Lee YH, Pumplin N, Jones J, He SY. A bacterial virulence protein suppresses host innate immunity to cause plant disease. Science. 2006;313:220–223. doi: 10.1126/science.1129523. [DOI] [PubMed] [Google Scholar]
- O'Donnell PJ, Jones JB, Antoine FR, Ciardi J, Klee HJ. Ethylene-dependent salicylic acid regulates an expanded cell death response to a plant pathogen. Plant J. 2001;25:315–323. doi: 10.1046/j.1365-313x.2001.00968.x. [DOI] [PubMed] [Google Scholar]
- O'Donnell PJ, Schmelz E, Block A, Miersch O, Wasternack C, Jones JB, Klee HJ. Multiple hormones act sequentially to mediate a susceptible tomato pathogen defense response. Plant Physiol. 2003;133:1181–1189. doi: 10.1104/pp.103.030379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh CS, Martin GB. Effector-triggered immunity mediated by the Pto kinase. Trends Plant Sci. 2011a;16:132–140. doi: 10.1016/j.tplants.2010.11.001. [DOI] [PubMed] [Google Scholar]
- Oh CS, Martin GB. Tomato 14-3-3 protein TFT7 interacts with a MAP kinase kinase to regulate immunity-associated programmed cell death mediated by diverse disease resistance proteins. J. Biol. Chem. 2011b;286:14129–14136. doi: 10.1074/jbc.M111.225086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedley KF, Martin GB. Identification of MAPKs and their possible MAPK kinase activators involved in the Pto-mediated defense response of tomato. J. Biol. Chem. 2004;279:49229–49235. doi: 10.1074/jbc.M410323200. [DOI] [PubMed] [Google Scholar]
- Petersen M, Brodersen P, Naested H, Andreasson E, Lindhart U, Johansen B, Nielsen HB, Lacy M, Austin MJ, Parker JE, Sharma SB, Klessig DF, Martienssen R, Mattsson O, Jensen AB, Mundy J. Arabidopsis MAP Kinase 4 negatively regulates systemic acquired resistance. Cell. 2000;103:1111–1120. doi: 10.1016/s0092-8674(00)00213-0. [DOI] [PubMed] [Google Scholar]
- Potnis N, Krasileva K, Chow V, Almeida NF, Patil PB, Ryan RP, Sharlach M, Behlau F, Dow JM, Momol M, White FF, Preston JF, Vinatzer BA, Koebnik R, Setubal JC, Norman DJ, Staskawicz BJ, Jones JB. Comparative genomics reveals diversity among xanthomonads infecting tomato and pepper. BMC Genomics. 2011;12:146. doi: 10.1186/1471-2164-12-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuber TL, Ausubel FM. Isolation of Arabidopsis genes that differentiate between resistance responses mediated by the RPS2 and RPM1 disease resistance genes. Plant Cell. 1996;8:241–249. doi: 10.1105/tpc.8.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritter C, Dangl JL. Interference between two specific pathogen recognition events mediated by distinct plant disease resistance genes. Plant Cell. 1996;8:251–257. doi: 10.1105/tpc.8.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roden JA, Belt B, Ross JB, Tachibana T, Vargas J, Mudgett MB. A genetic screen to isolate type III effectors translocated into pepper cells during Xanthomonas infection. Proc. Natl. Acad. Sci. U.S.A. 2004;101:16624–16629. doi: 10.1073/pnas.0407383101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossier O, Wengelnik K, Hahn K, Bonas U. The Xanthomonas Hrp type III system secretes proteins from plant and mammalian bacterial pathogens. Proc. Natl. Acad. Sci. U.S.A. 1999;96:9368–9373. doi: 10.1073/pnas.96.16.9368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomon D, Dar D, Sreeramulu S, Sessa G. Expression of Xanthomonas campestris pv. vesicatoria type III effectors in yeast affects cell growth and viability. Mol. Plant-Microbe Interact. 2011;24:305–314. doi: 10.1094/MPMI-09-10-0196. [DOI] [PubMed] [Google Scholar]
- Sato M, Tsuda K, Wang L, Coller J, Watanabe Y, Glazebrook J, Katagiri F. Network modeling reveals prevalent negative regulatory relationships between signaling sectors in Arabidopsis immune signaling. PLoS Path. 2010;6:e1001011. doi: 10.1371/journal.ppat.1001011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segonzac C, Zipfel C. Activation of plant pattern-recognition receptors by bacteria. Curr. Opin. Microbiol. 2011;14:54–61. doi: 10.1016/j.mib.2010.12.005. [DOI] [PubMed] [Google Scholar]
- Segonzac C, Feike D, Gimenez-Ibanez S, Hann DR, Zipfel C, Rathjen JP. Hierarchy and roles of pathogen-associated molecular pattern-induced responses in Nicotiana benthamiana. Plant Physiol. 2011;156:687–699. doi: 10.1104/pp.110.171249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha D, Gupta MK, Patel HK, Ranjan A, Sonti RV. Cell wall degrading enzyme induced rice innate immune responses are suppressed by the type 3 secretion system effectors XopN, XopQ, XopX and XopZ of Xanthomonas oryzae pv. oryzae. PLOS ONE. 2013;8:e75867. doi: 10.1371/journal.pone.0075867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnewald S, Priller JP, Schuster J, Glickmann E, Hajirezaei MR, Siebig S, Mudgett MB, Sonnewald U. Regulation of cell wall-bound invertase in pepper leaves by Xanthomonas campestris pv. vesicatoria type three effectors. PLOS ONE. 2012;7:e51763. doi: 10.1371/journal.pone.0051763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spoel SH, Dong X. How do plants achieve immunity? Defence without specialized immune cells. Nat. Rev. Immunol. 2012;12:89–100. doi: 10.1038/nri3141. [DOI] [PubMed] [Google Scholar]
- Tai TH, Dahlbeck D, Clark ET, Gajiwala P, Pasion R, Whalen MC, Stall RE, Staskawicz BJ. Expression of the Bs2 pepper gene confers resistance to bacterial spot disease in tomato. Proc. Natl. Acad. Sci. U.S.A. 1999;96:14153–14158. doi: 10.1073/pnas.96.24.14153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thieme F, Koebnik R, Bekel T, Berger C, Boch J, Buttner D, Caldana C, Gaigalat L, Goesmann A, Kay S, Kirchner O, Lanz C, Linke B, McHardy AC, Meyer F, Mittenhuber G, Nies DH, Niesbach-Klosgen U, Patschkowski T, Ruckert C, Rupp O, Schneiker S, Schuster SC, Vorholter FJ, Weber E, Puhler A, Bonas U, Bartels D, Kaiser O. Insights into genome plasticity and pathogenicity of the plant pathogenic bacterium Xanthomonas campestris pv. vesicatoria revealed by the complete genome sequence. J. Bacteriol. 2005;187:7254–7266. doi: 10.1128/JB.187.21.7254-7266.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomma BP, Nurnberger T, Joosten MH. Of PAMPs and effectors: the blurred PTI-ETI dichotomy. Plant Cell. 2011;23:4–15. doi: 10.1105/tpc.110.082602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda K, Katagiri F. Comparing signaling mechanisms engaged in pattern-triggered and effector-triggered immunity. Curr. Opin. Plant Biol. 2010;13:459–465. doi: 10.1016/j.pbi.2010.04.006. [DOI] [PubMed] [Google Scholar]
- Turner P, Barber C, Daniels M. Behavior of the transposons Tn5 and Tn7 in Xanthomonas campestris pv. campestris. Mol. Gen. Genet. 1984;195:101–107. [Google Scholar]
- Ustun S, Bartetzko V, Bornke F. The Xanthomonas campestris type III effector XopJ targets the host cell proteasome to suppress salicylic-acid mediated plant defence. PLoS Path. 2013;9:e1003427. doi: 10.1371/journal.ppat.1003427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wees S. Phenotypic analysis of Arabidopsis mutants: trypan blue stain for fungi, oomycetes, and dead plant cells. CSH Protoc. 2008;2008 doi: 10.1101/pdb.prot4982. pdb prot4982. [DOI] [PubMed] [Google Scholar]
- Wengelnik K, VandenAckerveken G, Bonas U. HrpG, a key hrp regulatory protein of Xanthomonas campestris pv. vesicatoria is homologous to two-component response regulators. Mol. Plant-Microbe Interact. 1996;9:704–712. doi: 10.1094/mpmi-9-0704. [DOI] [PubMed] [Google Scholar]
- Whalen MC, Wang JF, Carland FM, Heiskell ME, Dahlbeck D, Minsavage GV, Jones JB, Scott JW, Stall RE, Staskawicz BJ. Avirulence gene avrRxv from Xanthomonas campestris pv. vesicatoria specifies resistance on tomato line Hawaii 7998. Mol. Plant-Microbe Interact. 1993;6:616–627. doi: 10.1094/mpmi-6-616. [DOI] [PubMed] [Google Scholar]
- Xiang T, Zong N, Zou Y, Wu Y, Zhang J, Xing W, Li Y, Tang X, Zhu L, Chai J, Zhou JM. Pseudomonas syringae effector AvrPto blocks innate immunity by targeting receptor kinases. Curr. Biol. 2008;18:74–80. doi: 10.1016/j.cub.2007.12.020. [DOI] [PubMed] [Google Scholar]
- Zhang J, Li W, Xiang T, Liu Z, Laluk K, Ding X, Zou Y, Gao M, Zhang X, Chen S, Mengiste T, Zhang Y, Zhou JM. Receptor-like cytoplasmic kinases integrate signaling from multiple plant immune receptors and are targeted by a Pseudomonas syringae effector. Cell Host Microbe. 2010;7:290–301. doi: 10.1016/j.chom.2010.03.007. [DOI] [PubMed] [Google Scholar]
- Zuo JR, Niu QW, Chua NH. An estrogen receptor-based transactivator XVE mediates highly inducible gene expression in transgenic plants. Plant J. 2000;24:265–273. doi: 10.1046/j.1365-313x.2000.00868.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


