Abstract
Nonalcoholic fatty liver disease is the most common chronic liver disease, which can progress to nonalcoholic steatohepatitis (NASH). Previous investigations demonstrated alterations in the expression and activity of hepatic drug transporters in NASH. Moreover, studies using rodent models of cholestasis suggest that compensatory changes in kidney transporter expression occur to facilitate renal excretion during states of hepatic stress; however, little information is currently known regarding extrahepatic regulation of drug transporters in NASH. The purpose of the current study was to investigate the possibility of renal drug transporter regulation in NASH across multiple experimental rodent models. Both rat and mouse NASH models were used in this investigation and include: the methionine and choline-deficient (MCD) diet, atherogenic diet, fa/fa rat, ob/ob and db/db mice. Histologic and pathologic evaluations confirmed that the MCD and atherogenic rats as well as the ob/ob and db/db mice all developed NASH. In contrast, the fa/fa rats did not develop NASH but did develop extensive renal injury compared with the other models. Renal mRNA and protein analyses of xenobiotic transporters suggest that compensatory changes occur in NASH to favor increased xenobiotic secretion. Specifically, both apical efflux and basolateral uptake transporters are induced, whereas apical uptake transporter expression is repressed. These results suggest that NASH may alter the expression and potentially function of renal drug transporters, thereby impacting drug elimination mechanisms in the kidney.
Introduction
Nonalcoholic fatty liver disease (NAFLD) represents a range of distinct liver histopathologies ranging from simple steatosis to the more advanced nonalcoholic steatohepatitis (NASH) (Lomonaco et al., 2013). Often regarded as the hepatic manifestation of the metabolic syndrome, NAFLD is frequently accompanied by metabolic comorbidities such as obesity, hypertension, dyslipidemia, and hyperglycemia (Ali and Cusi, 2009). Given its close association with the metabolic syndrome, the prevalence of NAFLD has quickly risen, making it the most common form of chronic liver disease in Western society (Ali and Cusi, 2009). Current estimates suggest that the worldwide prevalence of NAFLD ranges between 6–33% and as high as 50% in certain regions and ethnic populations, whereas NASH is predicted to affect 2.7–12.2% of the population (Lomonaco et al., 2013; Rahimi and Landaverde, 2013). Although the mechanisms are not entirely understood, it is generally well accepted that NASH pathogenesis involves several “hits,” such as inflammation and oxidative stress, that may act independently or in parallel to drive disease progression (Lomonaco et al., 2013; Rahimi and Landaverde, 2013).
The role of adipocytes has recently emerged as being instrumental in the development and propagation of inflammation in NASH. In obese states, fat-laden adipocytes are a primary source for the secretion of proinflammatory cytokines, including tumor necrosis factor α (TNF-α), interleukin (IL)-6, and IL-1β, which contribute to the increased systemic levels readily observed in NASH (Hotamisligil et al., 1993; Tilg, 2010; Kochi et al., 2014). The induction of these proinflammatory cytokines within adipose tissue may act in a paracrine fashion by targeting the liver and propagating the development of NASH pathogenesis (Tilg, 2010). Moreover, inflammatory mediators, particularly TNF-α, activate various intracellular signaling cascades and may contribute to the extensive dysregulation of hepatic gene expression profiles observed in the disease (Moylan et al., 2014).
A recent investigation concerning the global changes of hepatic gene expression profiles in NAFLD revealed extensive changes in the expression of drug metabolizing and membrane transporter genes in NASH (Lake et al., 2011). Indeed, NASH causes extensive alterations in the regulation of hepatic xenobiotic transporters of both human and rodent models, leading to the functional disruption of acetaminophen, ezetimibe, methotrexate, and arsenic disposition (Lickteig et al., 2007; Hardwick et al., 2011, 2012; Canet et al., 2012, 2014). A common observation in these studies is increased plasma retention coupled with decreased biliary elimination of xenobiotics, suggesting that patients with NASH may represent a unique population of individuals that are at higher risk of experiencing adverse drug reactions. However, little information is currently known regarding the regulation of xenobiotic transporters in distal sites during times of hepatic stress, which may further contribute to altered xenobiotic disposition in hepatic disease states.
In addition to the liver, the kidneys serve as important sites for xenobiotic excretion, and an estimated 30% of the top 200 prescribed drugs in 2010 are eliminated by renal clearance (Morrissey et al., 2013). Studies using experimental models of cholestasis have linked liver dysfunction with compensatory responses in the regulation of xenobiotic transporters in the kidneys. Particularly, liver-specific adaptations in xenobiotic transporters occur to restrict further hepatic exposure to toxic levels of bile acids, resulting in higher systemic levels of potentially toxic bile acids (Keppler, 2011). However, cholestatic stress also results in compensatory adaptations in the expression of renal efflux and uptake transporters that may function to facilitate the secretion of bile acids into the urine (Brandoni et al., 2006b; Slitt et al., 2007). A recent study confirmed the functional outcome of these adaptations by demonstrating increased cimetidine clearance as a result of kidney organic cation transporter (Oct) Oct2 induction in a rat model of cholestasis (Kurata et al., 2010). Together, these findings demonstrate the influence of liver dysfunction on kidney-specific handling of xenobiotics, which may impact their renal secretion.
The purpose of the current study was to investigate the effects of NASH on xenobiotic transporter expression in the kidneys. Several rodent models of NASH were used, and a comprehensive analysis of kidney mRNA and protein expression profiles across these models was performed. These studies contribute to the hypothesis that during times of hepatic stress, compensatory alterations in kidney function occur to limit the accumulation of endogenous and exogenous compounds in systemic circulation. Moreover, these findings will change the manner in which we investigate disease-mediated effects on xenobiotic disposition by confirming that physiologic adaptations occur in distal tissue sites, which can ultimately contribute to interindividual variability in response to xenobiotics.
Materials and Methods
Tris-HCl, EDTA, sodium chloride (NaCl), glycerol, potassium phosphate (KPO4), potassium chloride (KCl), sodium pyrophosphate (decahydrate), and Nonidet P-40 were obtained from Sigma-Aldrich (St. Louis, MO). Neutral buffered formalin (10%) was obtained from Fisher Scientific (Pittsburgh, PA).
Animals.
Eight- to ten-week-old male C57BL/6J, B6.Cg-Lep<ob>/J (ob/ob), and B6.BKS(D)-Lepr<db>/J (db/db) mice were obtained from Jackson Laboratories (Bar Harbor, ME). Eight- to ten-week-old male Sprague-Dawley and Crl:ZUC-Lepr<fa> fatty (fa/fa) rats were obtained from Charles River Laboratories (Wilmington, MA). All animals were acclimated in 12-hour light and dark cycles in a University of Arizona Association for Assessment and Accreditation of Laboratory Animal Care–certified animal facility for at least 1 week before initiation of experiments and were given access to standard chow and water ad libitum. Housing and experimental procedures were in accordance with National Institutes of Health guidelines for the care and use of experimental animals. To model NASH, C57BL/6J mice and Sprague-Dawley rats (N = 4) were fed either a methionine and choline-deficient (MCD) diet (Dyets, Inc., Bethlehem, PA) or an atherogenic diet (Research Diets Inc., New Brunswick, NJ) for 8 weeks. As a control, C57BL/6J mice (N = 4) and Sprague-Dawley rats (N = 4) were fed a methionine and choline resupplemented diet (Dyets, Inc.). The ob/ob (N = 4) and db/db (N = 4) mice were fed an MCD diet for 4 weeks to induce NASH. The fa/fa rats were provided a modified high-fat diet for 8 weeks (Dyets, Inc.). The models chosen in this study represent a good spectrum of dietary and genetically altered animal models that have varying pathophysiological and clinical characteristics of NASH. This allows for a more thorough and accurate analysis of the disease on renal transporter regulation. NASH pathology and liver membrane transporter expression was characterized previously (Canet et al., 2014; Supplemental Fig. 1). Moreover, a thorough review highlighting the important features of these models has been published, which the reader is encouraged to visit (Larter and Yeh, 2008).
Tissue Harvesting.
At the conclusion of dietary feeding, the animals were euthanized via CO2 asphyxiation. Liver and kidney slices for histolomorphologic examination were placed in 10% neutral-buffered formalin for 24 hours, followed by 70% ethanol. The remaining tissue was snap frozen in liquid nitrogen and stored at −80°C for future analyses.
Tissue Staining and Evaluations.
Paraffin-embedded kidney and liver sections were stained with hematoxylin and eosin at the University of Arizona Histology Core. Kidney sections were evaluated and scored for renal injury. Liver sections were injury scored according to a previously validated NASH scoring method (Kleiner et al., 2005), and the results were published previously (Canet et al., 2014).
RNA Purification.
Total RNA was extracted and isolated from rat and mouse kidney using RNAzol B reagent (Tel-Test Inc., Friendswood, TX) per the manufacturer's protocol. RNA concentrations were determined using ultraviolet spectrophotometry, and the integrity of the RNA was confirmed by ethidium bromide staining after agarose gel electrophoresis.
Branched DNA Analysis.
Branched DNA analysis was used to determine mRNA transcript levels of transporter genes. Specific oligonucleotide probes for multidrug resistance-associated protein (Mrp) Mrp2, Mrp4, Mdr1a, Bcrp, organic anion transporter (Oat) Oatp1a1, Oat1, Oat3, Oct1, and Oct2 were diluted in lysis buffer supplied by the Quantigene HV Signal Amplification Kit (Genospectra, Fremont, CA). Substrate solution, lysis buffer, capture hybridization buffer, amplifier, and label probe buffer used in the analysis were all obtained from the Quantigene Discovery Kit (Genospectra). The assay was performed in 96-well format with 10 µg of total RNA added to the capture hybridization buffer and 50 µl of the diluted probe set. The total RNA was then allowed to hybridize to the probe set overnight at 53°C. Hybridization steps were performed per the manufacturer's protocol the following day. Luminescence of the samples was measured with a Quantiplex 320 branched DNA luminometer interfaced with Quantiplex Data Management Software, version 5.02 (Bayer, Walpole, MA).
Protein Preparations.
Whole cell lysate preparations of mouse and rat kidney were prepared from ∼200 mg of tissue homogenized in NP40 buffer (20 mM Tris HCl, 137 mM NaCl, 10% glycerol, 1% Nonidet P-40, and 2 mM EDTA) with 1 Protease Inhibitor Cocktail Tablet (Roche, Indianapolis, IN) per 25 ml at 4°C. Homogenized tissue was then agitated at 4°C for 2 hours, centrifuged at 10,000g for 30 minutes, and the supernatant was transferred to a clean collection tube. Rat kidney crude membrane fractions were prepared from ∼100 mg of frozen tissue. Briefly, tissue was homogenized in homogenization buffer (100 mM, Tris HCl, pH 7.4) with added Protease Inhibitor Cocktail Tablet per 25 ml at 4°C. The resulting homogenate was centrifuged at 1500g for 10 minutes at 4°C, and the supernatant was collected into ultracentrifuge tubes and centrifuged at 100,000g for 70 minutes at 4°C. The resulting pellet was resuspended in 500 µl of homogenization buffer from above. Protein concentrations for both whole cell and microsomal fractions were determined using the Pierce BCA Protein Quantitation Assay (Thermo Scientific, Rockford, IL) per the manufacturer’s protocol, and all samples were stored at −80°C until further analysis.
Immunoblot Protein Analysis.
Whole cell lysate or membrane fraction proteins (50 μg/well, 20 µg/well, respectively) were prepared in Laemmli sample buffer (Bio-Rad Laboratories, Hercules, CA) with or without β-mercaptoethanol and heated at 37°C for 30 minutes before separation by SDS-PAGE on 7.5% gels. Resolved protein was transferred to polyvinylidene fluoride or nitrocellulose membranes for 70 minutes at 350 mA at 4°C. After transfer, the membranes were blocked in 5% nonfat dry milk diluted in phosphate-buffered saline-Tween 20 for 1 hour at room temperature. To determine relative protein levels the following primary antibodies were used: P-glycoprotein (P-gp), Oct1, Oatp1a1 (Santa Cruz Biotechnology, Santa Cruz, CA); Mrp2, Mrp4, Oat1 (Abcam, Cambridge, MA); Bcrp (Kamiya Biomedical Co., Seattle, WA). The blots were incubated with primary antibody overnight at 4°C with constant rocking. The following horseradish peroxidase-conjugated secondary antibodies were used: anti-rat, anti-rabbit, anti-mouse, and anti-goat (Santa Cruz Biotechnology). Quantification of relative protein expression was determined using image processing and analysis with Image J software (NIH, Bethesda, MD) and normalized to β-actin protein (whole cell lysate) (Santa Cruz Biotechnology) or pan-cadherin (microsomal fraction) (Abcam).
Statistical Analysis.
Data were analyzed using one-way analysis of variance to determine significant differences between model groups with a Tukey’s post hoc analysis. Histologic scores were rank-ordered before analysis via analysis of variance. A significance level of P ≤ 0.05 was used to determine experimental significance. All analyses were carried out using GraphPad Prism software Version 5 (GraphPad Software, Inc., La Jolla, CA).
Results
Rodent Kidney Pathology in NASH.
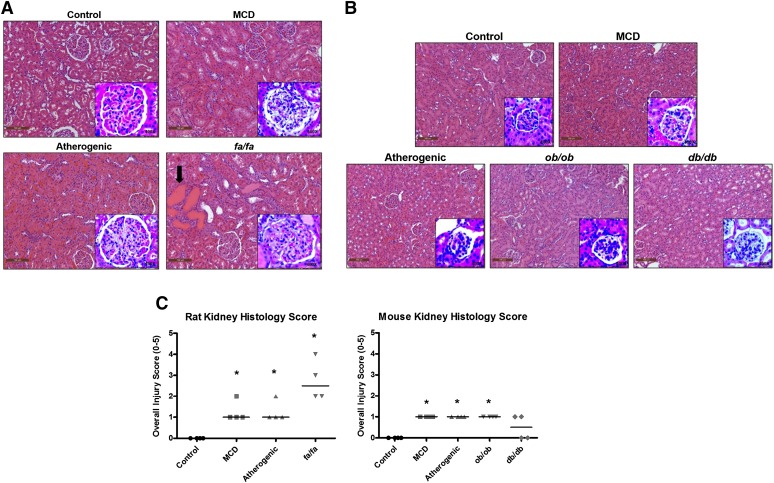
Histologic assessments were conducted to investigate the effect of NASH on renal morphology. The incidence and severity of kidney pathology (including tubular degeneration, necrosis and regeneration, parenchymal inflammation and glomerular mesangial expansion, and/or hypertrophy) are summarized in Table 1. Across the rat models, a significant increase in overall severity scores was observed in the MCD, atherogenic, and fa/fa animals (Fig. 1C). Glomerular changes (mesangial expansion and/or hypertrophy) were the most common observation(s) in MCD and atherogenic rats, whereas the fa/fa rats additionally developed parenchymal tubular changes (degeneration/necrosis/regeneration, protein casts) and parenchymal inflammation (Fig. 1A, arrow, inserts; Table 1). Similarly, glomerular finding were more common than tubular injury in the mouse models (Fig. 1B, inserts). All mouse groups had increased overall severity scores with the exception of the db/db mice (Fig. 1C and Table 1).
TABLE 1.
Kidney Pathology Scoring in NASH Models
Kidney pathology was assessed via the lesions described below. Values represent the median (range) of N = 4 animals and were rounded to next whole number (median of 1.5 is reported as 2). Scoring key: 0, none; 1, minimal (<10% affected); 2, mild (10-25% affected); 3, moderate (25-40% affected); 4, marked (40–-50% affected); 5, severe (>50% affected).
| Tubular Degeneration | Necrosis | Regeneration | Inflammation | Mesangial Expansion | Glomerular Hypertrophy | ||
|---|---|---|---|---|---|---|---|
| Rats | Control | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| MCD | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (1–2) | 0 (0) | |
| Athero | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (1–2) | 0 (0) | |
| fa/fa | 1 (1) | 1 (0–1) | 2 (1–2) | 1 (0–1) | 3 (2–4) | 1 (0–1) | |
| Mice | Control | 0 (0) | 0 | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| MCD | 0 (0–1) | 0 | 1 (0–1) | 0 (0) | 1 (1) | 0 (0–1) | |
| Athero | 0 (0) | 0 | 0 (0) | 0 (0) | 1 (1) | 0 (0–1) | |
| ob/ob | 0 (0) | 0 | 0 (0) | 0 (0) | 1 (1) | 0 (0–1) | |
| db/db | 0 (0) | 0 (0–1) | 0 (0) | 0 (0) | 1 (1) | 1 (0–1) |
Fig. 1.
Kidney histology in rodent NASH. Representative H&E-stained kidney sections from rat (A) and mouse (B) NASH models. A significant number of protein casts was present in the kidneys of fa/fa rats (2A; arrow). Images were taken at 20× magnification. . Higher magnification (100×) images of glomerular changes were captured and shown as an insert to each figure. (C) Pathologic scoring evaluations describing total kidney injury scores are shown. Horizontal bar represents the median of data (N = 4 animals) and *P ≤ 0.05 versus control within each group. Data were ranked-ordered before statistical analysis. Scoring key: 0, none; 1, minimal (<10% affected); 2, mild (10–25% affected); 3, moderate (25–40% affected); 4, marked (40–50% affected); 5, severe (>50% affected).
Kidney Transporter mRNA Expression in Rodent NASH. mRNA.
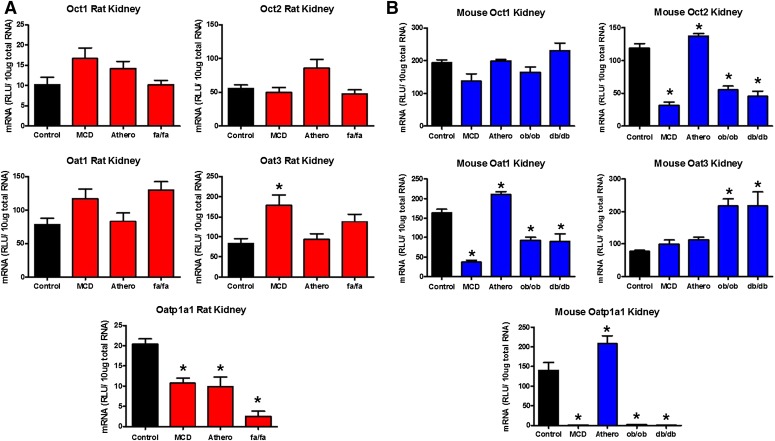
quantification analyses were conducted on renal transporter genes to determine the effect of NASH on their regulation (Figs. 2 and 3). The transporters chosen to be investigated in this study represent a group of important proteins that mediate the renal secretion of many xenobiotics, which include pharmaceuticals. Among the uptake transporters investigated in rats, no significant changes in gene expression were noted with the exception of Oat3, which was induced in rat MCD whereas Oatp1 was repressed in the MCD, atherogenic, and fa/fa rat models (Fig. 2A). In contrast, Oct2 and Oat1 were downregulated in the MCD, ob/ob, and db/db mice along with an induction seen in the atherogenic model (Fig. 2B). Like the rats, Oat3 was significantly induced in the ob/ob and db/db mouse models, whereas Oatp1 expression was repressed in the MCD, ob/ob, and db/db models and upregulated in the atherogenic mice (Fig. 2B).
Fig. 2.
Kidney uptake transporter mRNA expression in rodent NASH. mRNA expression of rat uptake (A) and mouse uptake (B) transporters in the kidneys of rodent NASH models via branched DNA gene analysis. The transporters investigated, with the exception of Oatp1a1, are localized on the basolateral membrane of renal proximal tubules and participate in the uptake of substrates from the peritubular capillaries into the proximal tubule cell for secretion into to lumen for urinary secretion. In contrast, Oatp1a1 is expressed on the apical membrane of the proximal tubule cell and is responsible for the uptake of substrates into the proximal tubule cell from the lumen filtrate in a process known as reabsorption. Data represent the mean ± S.E.M. from 4 animals. *P ≤ 0.05 versus control within each group. Athero, atherogenic.
Fig. 3.
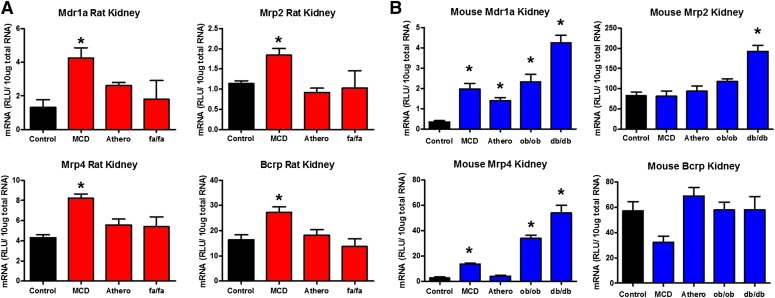
Kidney efflux transporter mRNA expression in rodent NASH. mRNA expression of rat efflux (A) and mouse efflux (B) transporters in the kidneys of rodent NASH models via bDNA gene analysis. The efflux transporters investigated are localized on the apical membrane of renal proximal tubule cells and participate in the efflux of substrates directly into the nephron lumen for urinary excretion. Data represent the mean ± S.E.M. from 4 animals. *P ≤ 0.05 versus control within each group. Athero, atherogenic.
In contrast to uptake transporters, efflux transporter mRNA was generally upregulated in the rodent models with confirmed NASH (Fig. 3, Table 2). Mrp2, Mrp4, Bcrp, and Mdr1a (P-gp) genes were all induced in the rat MCD model, whereas no changes were observed in the atherogenic and fa/fa rats (Fig. 3A). Similarly, Mrp2 was induced in the ob/ob mice, whereas Mdr1a expression was upregulated in the MCD, atherogenic, ob/ob, and db/db mouse models (Fig. 3B). No changes were observed in Bcrp expression; however, Mrp4 was induced in the kidney of MCD, ob/ob, and db/db mice (Fig. 3B).
TABLE 2.
Summary of mRNA and Protein Transporter Changes
Summary of the overall changes in mRNA and protein expression of renal membrane transporters across rodent models with NASH.
| Transporter | mRNA | Protein |
|---|---|---|
| Mrp2 | ↑ | ↑ |
| Mrp4 | ↑ | ↑ (MOUSE) |
| P-gp | ↑ | ↑ |
| Bcrp | ↑ (RAT) | NC |
| Oat1 | ↓ (MOUSE) | NC |
| Oat3 | ↑ | ND |
| Oct1 | NC | NC |
| Oct2 | ↓ (MOUSE) | ND |
| Oatp1a1 | ↓ | ↓ (MOUSE) |
NC, no change; ND, not done.
Kidney Transporter Protein Expression in Rodent NASH.
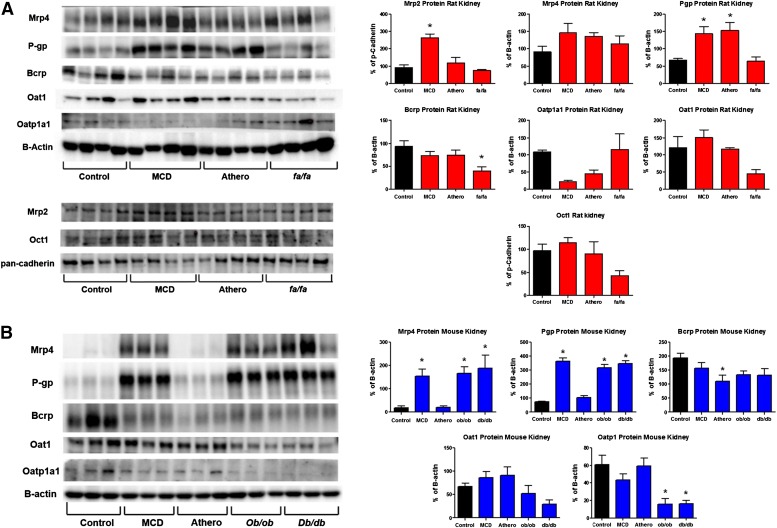
Protein expression of membrane transporters across rodent models was also investigated and shown in Fig. 4. Consistent with the mRNA analyses, protein expression of the renal efflux transporters Mrp2 and P-gp was induced in the MCD rats (Fig. 4A). In addition, P-gp expression was induced in the atherogenic rat model. Interestingly, protein expression of Bcrp was downregulated in the kidney of fa/fa rats (Fig. 4A). Oatp1a1, Oat1, and Oct1 renal uptake transporter expression was not significantly changed at the protein level in the rat models.
Fig. 4.
Kidney protein expression of membrane transporters in rodent NASH. Western blot analyses depicting rat (A) and mouse (B) transporter expression in the kidneys of rodent NASH models. Densitometry analysis and representative Western blots are shown. β-Actin was used a loading control for whole cell lysates, whereas pan-cadherin was used for crude membrane preparations. Rat Mrp2 and Oct1 protein were analyzed using crude membrane preparations, whereas the remainder (mouse and rat) were analyzed in whole cell fractions. Data represent the mean ± S.E.M. from 4 animals. *P ≤ 0.05 versus control within each group. Athero, atherogenic.
Similar to the rats, efflux transporter expression in the kidney was generally induced at the protein level in mice with NASH (Fig. 4B, Table 2). Specifically, Mrp4 and P-gp protein expression was induced in the mouse MCD, ob/ob, and db/db models. Bcrp expression did not change across models, except for atherogenic mice, which had a significant decrease in Bcrp protein expression in the kidney (Fig. 4B). In contrast, Oatp1 protein expression was significantly reduced in the mouse ob/ob and db/db models. No change in Oat1 expression was observed in the mice; however, it tended to decrease in the db/db model.
Discussion
The increase in adverse drug reaction incidents has become a significant health concern worldwide. In the United States, adverse drug reactions (ADRs) have become one of the top 10 causes of death, clearly highlighting the need for more effective pharmacovigilant practices within the healthcare industry (Wooten, 2010; Valente and Murray, 2011). Many ADRs can be attributed to interindividual variations in xenobiotic disposition, which may be preventable by identifying factors that contribute to these variations. In addition, recent advances in our understanding of the impact of disease states on xenobiotic pharmacokinetics have helped identify populations that may be at risk for developing ADRs. In particular, diseases that manifest in the liver have gained increased attention given the importance of the liver in mediating xenobiotic metabolism and disposition. Despite previous evidence demonstrating compensatory alterations in the kidney during cholestatic disease, our understanding of how NASH affects renal clearance is lacking and necessitates further investigation.
The purpose of this study was to determine the effects of NASH on the regulation of renal membrane transporters. The use of several models in these investigations allows for a more comprehensive profile that strengthens our findings and allows translating these findings to the human condition more feasible, considering it is currently unknown how the regulation of renal xenobiotic transporters is altered in human NASH. Our results demonstrate that the development of NASH causes significant alterations in the expression of several membrane transporters in the kidneys of various rodent models. In particular, we observe a coordinated upregulation of Mrp2, Mrp4, and P-gp, suggesting that during times of hepatic stress, the kidneys may compensate by facilitating the renal secretion of xenobiotics and endogenous substrates, such as bile acids, into the urine. Similar compensatory changes have been observed in other models of liver injury. Using bile duct-ligated mice, Slitt et al. (2007) demonstrated an induction of renal Mrp1–5 mRNA and a reduction in Oatp1a1 mRNA expression. Interestingly, our data also show a downregulation of renal uptake transporter Oatp1a1 expression in NASH, which facilitates the reabsorption of compounds from the renal tubule filtrate, suggesting an overall shift from renal reabsorption to renal secretion of organic anions in NASH. Furthermore, Mrp2 induction in the kidney has been observed in various rat models of cholestasis (Lee et al., 2001) as well as liver ischemia-reperfusion injury (Tanaka et al., 2008), which is consistent with our findings in the pathologically confirmed NASH models (MCD, ob/ob, and db/db rodents).
The induction of renal Mrp2 is a common observation across several cholestatic injury models. It is proposed that Mrp2 induction in the kidneys may serve as an alternative route of elimination of bile acids in situations in which liver function is compromised (Klaassen and Aleksunes, 2010). Similar to cholestasis, hepatic Mrp2 function is significantly reduced in NASH whereas Mrp4 is induced, leading to a functional shift in the disposition of xenobiotics from bile to plasma (Hardwick et al., 2012; Canet et al., 2014). The similarity in the changes to hepatic and renal transporter gene expression between cholestasis and NASH is suggestive of a common mechanism mediating these effects. Recent findings show that mice fed an MCD diet develop intrahepatic cholestasis, leading to increased plasma bile acid levels (Wu et al., 2014). Furthermore, Tanaka et al. (2002) demonstrated that Mrp2 expression is elevated after treatment of renal proximal tubule cells with conjugated bilirubin or human bile, suggesting that bile acids and/or bile constituents may directly regulate Mrp2 gene transcription, possibly through the nuclear receptors farnesoid X receptor and/or pregnane X receptor (Kast et al., 2002). Together, these results demonstrate that the upregulation of renal Mrp2 may partially be explained by direct exposure of bile acids to the kidneys due to the development of cholestasis secondary to NASH.
Similar to renal efflux transporters, the expression of Oat3 in the kidney is significantly elevated in rodents with NASH. In particular, we observe an induction of renal Oat3 in the rat MCD as well as the mouse ob/ob and db/db models. In contrast, our data suggest that Oat1 protein expression is not changed, although levels tend to decrease in the ob/ob and db/db models. This is in contrast to studies that suggest Oat1 and Oat3 are commonly coordinated together by various stressors and exogenous stressors (Jin et al., 2012; Ulu et al., 2012; Guo et al., 2013). However, consistent with our results, similar findings have been reported in experimental models of cholestasis. Chen et al. (2008) demonstrated an induction of Oat3 protein in the kidney, whereas Oat1 levels did not change in Eisai hyperbilirubinemic rats, which lack functional Mrp2 and serve as a cholestatic mode. However, Oat3 mRNA in Eisai hyperbilirubinemic rats did not change, whereas we observed an induction, suggesting an alternative mechanism of regulation in NASH. Several investigations have shown that members of the Oat transporter family, in particular Oat1 and Oat3, are posttranscriptionally regulated by intracellular phosphorylation events mediated by PKC and PKA. PKC activation causes a downregulation of Oat3-mediated uptake of estrone sulfate, whereas PKA activation leads to increased transport activity, suggesting that differential activation of protein kinase cascades may cause tissue- and disease-specific transporter regulation (Soodvilai et al., 2004). The effect of NASH on renal PKC and PKA regulation is not completely understood, and further investigations are needed to clarify the mechanistic role of renal Oat3 induction in NASH.
Liver damage sustained in NASH results in inflammation and oxidative stress, leading to hepatocellular damage. However, our results suggest that renal injury in the rodent models with NASH is minimal and lack significant evidence of inflammation and oxidative kidney injury. In contrast, the fa/fa rat model, which failed to develop pathologic NASH, had significant renal injury, and yet renal transporter expression in this rodent model did not vary significantly from control rats. Together, these findings suggest that direct renal injury is not a primary factor in membrane transporter regulation in NASH and that the minimal renal injury sustained in rodents with NASH may be due to liver-derived inflammation.
Alternatively, the pathologic disturbances that occur in the liver may secondarily affect distal tissue function through the release of cellular mediators that may act in a paracrine signaling fashion. Chronic liver inflammation observed in NASH is associated with increased systemic levels of proinflammatory cytokines such as TNF-α and IL-6 (Carter-Kent et al., 2008; Alaaeddine et al., 2012; Kochi et al., 2014). Moreover, several independent studies have shown a direct role of proinflammatory cytokines in mediating the regulation of membrane transporters. Treatment of primary hepatocytes with TNF-α and IL-6 results in marked alterations in membrane transporter expression (Le Vee et al., 2009b). Additionally, IL-1β exposure results in the downregulation of membrane transporters in human hepatocytes (Le Vee et al., 2008). Together, these findings suggest proinflammatory cytokines as a potential mediator in the regulation of hepatic and renal membrane transporters in NASH. Furthermore, TNF-α and IL-6 induction in NASH may regulate renal transporter expression and function in a paracrine fashion. It was recently shown that both TNF-α and IL-6 have differential effects on transporter expression across cell lines differing in tissue origin as well as different cell lines derived from the same tissue (Le Vee et al., 2009a; Mosaffa et al., 2009; Malekshah et al., 2011). Further investigation is needed to characterize the differential effects of proinflammatory cytokines on hepatic and renal xenobiotic transporter expression in NASH.
The altered regulation of renal transporters in disease states such as cholestasis has resulted in functional disturbances in xenobiotic disposition. Induction of Oct2 in the kidney after bile duct ligation has been shown to increase cimetidine clearance in rats (Kurata et al., 2010). Additionally, disturbances in the renal secretion of bromosulphophthalein and p-aminohippurate in rodent cholestatic models were linked to altered transporter function in the kidney (Brandoni et al., 2006a; Brandoni and Torres, 2009). However, information is lacking regarding the function of membrane transporters in the kidney during diseases such as NASH.
In conclusion, we demonstrated that rodent models of NASH pathology cause significant alterations to membrane transporter expression in the kidney. In particular, we observe a general induction of renal apical efflux transporters as well as the basolateral uptake transporter, Oat3, whereas Oatp1 expression is significantly downregulated. Together, these data suggest a coordinated regulation of renal membrane transporters in NASH that favors renal secretion. This may serve as an adaptive response mechanism that facilitates the elimination of xenobiotics and bile acids during times of hepatic stress. Furthermore, our data demonstrate that the manifestation of NASH fails to cause significant pathology in the kidney, suggesting that direct tissue injury is not responsible for the changes observed in transporter regulation. These findings highlight the importance of investigating the contribution of renal elimination mechanisms during hepatic disease states.
Supplementary Material
Abbreviations
- ADR
adverse drug reaction
- IL
interleukin
- MCD
methionine and choline deficient
- Mrp
multidrug resistance-associated protein
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
- Oat
organic anion transporter
- Oct
organic cation transporter
- P-gp
P-glycoprotein
- PK
protein kinase
- TNF-α
tumor necrosis factor α
Authorship Contributions
Participated in research design: Canet, Cherrington.
Conducted experiments: Canet, Hardwick, Lake, Dzierlenga, Clarke, Goedken.
Performed data analysis: Canet, Cherrington.
Wrote or contributed to the writing of the manuscript: Canet, Cherrington.
Footnotes
This work was supported by National Institutes of Health National Institute of Allergy and Infectious Diseases [Grant R01-AI083927], National Institute of Environmental Health Sciences [Grant P30-ES006694], National Institute of Child Health and Human Development [Grant R01-HD062489], National Institute of Environmental Health Science Toxicology Training Grant [ES007091], and The National Science Foundation of Arizona.
References
- Alaaeddine N, Sidaoui J, Hilal G, Serhal R, Abedelrahman A, Khoury S. (2012) TNF-α messenger ribonucleic acid (mRNA) in patients with nonalcoholic steatohepatitis. Eur Cytokine Netw 23:107–111. [DOI] [PubMed] [Google Scholar]
- Ali R, Cusi K. (2009) New diagnostic and treatment approaches in non-alcoholic fatty liver disease (NAFLD). Ann Med 41:265–278. [DOI] [PubMed] [Google Scholar]
- Brandoni A, Anzai N, Kanai Y, Endou H, Torres AM. (2006a) Renal elimination of p-aminohippurate (PAH) in response to three days of biliary obstruction in the rat. The role of OAT1 and OAT3. Biochim Biophys Acta 1762:673–682. [DOI] [PubMed] [Google Scholar]
- Brandoni A, Torres AM. (2009) Characterization of the mechanisms involved in the increased renal elimination of bromosulfophthalein during cholestasis: involvement of Oatp1. J Histochem Cytochem 57:449–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandoni A, Villar SR, Picena JC, Anzai N, Endou H, Torres AM. (2006b) Expression of rat renal cortical OAT1 and OAT3 in response to acute biliary obstruction. Hepatology 43:1092–1100. [DOI] [PubMed] [Google Scholar]
- Canet MJ, Hardwick RN, Lake AD, Dzierlenga AL, Clarke JD, Cherrington NJ. (2014) Modeling human nonalcoholic steatohepatitis-associated changes in drug transporter expression using experimental rodent models. Drug Metab Dispos 42:586–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canet MJ, Hardwick RN, Lake AD, Kopplin MJ, Scheffer GL, Klimecki WT, Gandolfi AJ, Cherrington NJ. (2012) Altered arsenic disposition in experimental nonalcoholic fatty liver disease. Drug Metab Dispos 40:1817–1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter-Kent C, Zein NN, Feldstein AE. (2008) Cytokines in the pathogenesis of fatty liver and disease progression to steatohepatitis: implications for treatment. Am J Gastroenterol 103:1036–1042. [DOI] [PubMed] [Google Scholar]
- Chen J, Terada T, Ogasawara K, Katsura T, Inui K. (2008) Adaptive responses of renal organic anion transporter 3 (OAT3) during cholestasis. Am J Physiol Renal Physiol 295:F247–F252. [DOI] [PubMed] [Google Scholar]
- Guo X, Meng Q, Liu Q, Wang C, Sun H, Peng J, Ma X, Kaku T, Liu K. (2013) JBP485 improves gentamicin-induced acute renal failure by regulating the expression and function of Oat1 and Oat3 in rats. Toxicol Appl Pharmacol 271:285–295. [DOI] [PubMed] [Google Scholar]
- Hardwick RN, Fisher CD, Canet MJ, Scheffer GL, Cherrington NJ. (2011) Variations in ATP-binding cassette transporter regulation during the progression of human nonalcoholic fatty liver disease. Drug Metab Dispos 39:2395–2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardwick RN, Fisher CD, Street SM, Canet MJ, Cherrington NJ. (2012) Molecular mechanism of altered ezetimibe disposition in nonalcoholic steatohepatitis. Drug Metab Dispos 40:450–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotamisligil GS, Shargill NS, Spiegelman BM. (1993) Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science 259:87–91. [DOI] [PubMed] [Google Scholar]
- Jin L, Kikuchi R, Saji T, Kusuhara H, Sugiyama Y. (2012) Regulation of tissue-specific expression of renal organic anion transporters by hepatocyte nuclear factor 1 α/β and DNA methylation. J Pharmacol Exp Ther 340:648–655. [DOI] [PubMed] [Google Scholar]
- Kast HR, Goodwin B, Tarr PT, Jones SA, Anisfeld AM, Stoltz CM, Tontonoz P, Kliewer S, Willson TM, Edwards PA. (2002) Regulation of multidrug resistance-associated protein 2 (ABCC2) by the nuclear receptors pregnane X receptor, farnesoid X-activated receptor, and constitutive androstane receptor. J Biol Chem 277:2908–2915. [DOI] [PubMed] [Google Scholar]
- Keppler D. (2011) Cholestasis and the role of basolateral efflux pumps. Z Gastroenterol 49:1553–1557. [DOI] [PubMed] [Google Scholar]
- Klaassen CD, Aleksunes LM. (2010) Xenobiotic, bile acid, and cholesterol transporters: function and regulation. Pharmacol Rev 62:1–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Ariba A, Yeh M, McCullough AJ, Sanyal AJ. (2005) Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 41:1313–1321. [DOI] [PubMed] [Google Scholar]
- Kochi T, Shimizu M, Terakura D, Baba A, Ohno T, Kubota M, Shirakami Y, Tsurumi H, Tanaka T, Moriwaki H. (2014) Non-alcoholic steatohepatitis and preneoplastic lesions develop in the liver of obese and hypertensive rats: suppressing effects of EGCG on the development of liver lesions. Cancer Lett 342:60–69. [DOI] [PubMed] [Google Scholar]
- Kurata T, Muraki Y, Mizutani H, Iwamoto T, Okuda M. (2010) Elevated systemic elimination of cimetidine in rats with acute biliary obstruction: the role of renal organic cation transporter OCT2. Drug Metab Pharmacokinet 25:328–334. [DOI] [PubMed] [Google Scholar]
- Lake AD, Novak P, Fisher CD, Jackson JP, Hardwick RN, Billheimer DD, Klimecki WT, Cherrington NJ. (2011) Analysis of global and absorption, distribution, metabolism, and elimination gene expression in the progressive stages of human nonalcoholic fatty liver disease. Drug Metab Dispos 39:1954–1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Vee M, Gripon P, Stieger B, Fardel O. (2008) Down-regulation of organic anion transporter expression in human hepatocytes exposed to the proinflammatory cytokine interleukin 1beta. Drug Metab Dispos 36:217–222. [DOI] [PubMed] [Google Scholar]
- Le Vee M, Lecureur V, Moreau A, Stieger B, Fardel O. (2009a) Differential regulation of drug transporter expression by hepatocyte growth factor in primary human hepatocytes. Drug Metab Dispos 37:2228–2235. [DOI] [PubMed] [Google Scholar]
- Le Vee M, Lecureur V, Stieger B, Fardel O. (2009b) Regulation of drug transporter expression in human hepatocytes exposed to the proinflammatory cytokines tumor necrosis factor-alpha or interleukin-6. Drug Metab Dispos 37:685–693. [DOI] [PubMed] [Google Scholar]
- Lee J, Azzaroli F, Wang L, Soroka CJ, Gigliozzi A, Setchell KD, Kramer W, Boyer JL. (2001) Adaptive regulation of bile salt transporters in kidney and liver in obstructive cholestasis in the rat. Gastroenterology 121:1473–1484. [DOI] [PubMed] [Google Scholar]
- Larter CZ, Yeh MM. (2008) Animal models of NASH: getting both pathology and metabolic context right. J Gastroenterol Hepatol 23:1635–1648. [DOI] [PubMed] [Google Scholar]
- Lickteig AJ, Fisher CD, Augustine LM, Aleksunes LM, Besselsen DG, Slitt AL, Manautou JE, Cherrington NJ. (2007) Efflux transporter expression and acetaminophen metabolite excretion are altered in rodent models of nonalcoholic fatty liver disease. Drug Metab Dispos 35:1970–1978. [DOI] [PubMed] [Google Scholar]
- Lomonaco R, Sunny NE, Bril F, Cusi K. (2013) Nonalcoholic fatty liver disease: current issues and novel treatment approaches. Drugs 73:1–14. [DOI] [PubMed] [Google Scholar]
- Malekshah OM, Bahrami AR, Afshari JT, Mosaffa F, Behravan J. (2011) Correlation between PXR and ABCG2 patterns of mRNA expression in a MCF7 breast carcinoma cell derivative upon induction by proinflammatory cytokines. DNA Cell Biol 30:25–31. [DOI] [PubMed] [Google Scholar]
- Morrissey KM, Stocker SL, Wittwer MB, Xu L, Giacomini KM. (2013) Renal transporters in drug development. Annu Rev Pharmacol Toxicol 53:503–529. [DOI] [PubMed] [Google Scholar]
- Mosaffa F, Lage H, Afshari JT, Behravan J. (2009) Interleukin-1 beta and tumor necrosis factor-alpha increase ABCG2 expression in MCF-7 breast carcinoma cell line and its mitoxantrone-resistant derivative, MCF-7/MX. Inflamm Res 58:669–676. [DOI] [PubMed] [Google Scholar]
- Moylan CA, Pang H, Dellinger A, Suzuki A, Garrett ME, Guy CD, Murphy SK, Ashley-Koch AE, Choi SS, Michelotti GA, et al. (2014) Hepatic gene expression profiles differentiate presymptomatic patients with mild versus severe nonalcoholic fatty liver disease. Hepatology 59:471–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahimi RS, Landaverde C. (2013) Nonalcoholic fatty liver disease and the metabolic syndrome: clinical implications and treatment. Nutr Clin Pract 28:40–51. [DOI] [PubMed] [Google Scholar]
- Slitt AL, Allen K, Morrone J, Aleksunes LM, Chen C, Maher JM, Manautou JE, Cherrington NJ, Klaassen CD. (2007) Regulation of transporter expression in mouse liver, kidney, and intestine during extrahepatic cholestasis. Biochim Biophys Acta 1768:637–647. [DOI] [PubMed] [Google Scholar]
- Soodvilai S, Chatsudthipong V, Evans KK, Wright SH, Dantzler WH. (2004) Acute regulation of OAT3-mediated estrone sulfate transport in isolated rabbit renal proximal tubules. Am J Physiol Renal Physiol 287:F1021–F1029. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Chen C, Maher JM, Klaassen CD. (2008) Ischemia-reperfusion of rat livers decreases liver and increases kidney multidrug resistance associated protein 2 (Mrp2). Toxicol Sci 101:171–178. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Kobayashi Y, Gabazza EC, Higuchi K, Kamisako T, Kuroda M, Takeuchi K, Iwasa M, Kaito M, Adachi Y. (2002) Increased renal expression of bilirubin glucuronide transporters in a rat model of obstructive jaundice. Am J Physiol Gastrointest Liver Physiol 282:G656–G662. [DOI] [PubMed] [Google Scholar]
- Tilg H. (2010) The role of cytokines in non-alcoholic fatty liver disease. Dig Dis 28:179–185. [DOI] [PubMed] [Google Scholar]
- Ulu R, Dogukan A, Tuzcu M, Gencoglu H, Ulas M, Ilhan N, Muqbil I, Mohammad RM, Kucuk O, Sahin K. (2012) Regulation of renal organic anion and cation transporters by thymoquinone in cisplatin induced kidney injury. Food Chem Toxicol 50:1675–1679. [DOI] [PubMed] [Google Scholar]
- Valente S, Murray LP. (2011) Creative strategies to improve patient safety: allergies and adverse drug reactions. J Nurses Staff Dev 27:E1–E5, quiz E6–E7. [DOI] [PubMed] [Google Scholar]
- Wooten JM. (2010) Adverse drug reactions: Part I. South Med J 103:1025–1028, quiz 1029. [DOI] [PubMed] [Google Scholar]
- Wu W, Liu X, Peng X, Xue R, Ji L, Shen X, Chen S, Gu J, Zhang S. (2014) Bile acids override steatosis in farnesoid X receptor deficient mice in a model of non-alcoholic steatohepatitis. Biochem Biophys Res Commun 448:50–55. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.