Abstract
Notoginsenoside R1 (R1) is the main bioactive component in Panax notoginseng, an old herb medicine widely used in Asian countries in the treatment of microcirculatory diseases. However, little is known about the effect of R1 on inflammatory bowel disease (IBD). The present study demonstrated that R1 alleviated the severity of dextran sulfate sodium–induced colitis in mice by decreasing the activity of myeloperoxidase, the production of cytokines, the expression of proinflammatory genes, and the phosphorylation of IκB kinase, IκBα, and p65 in the colon. Further studies indicated that R1 dose-dependently activated human/mouse pregnane X receptor (PXR), a known target for decreasing inflammation in IBD, and upregulated the expression of genes involved in xenobiotic metabolism in colorectal cells and the colon. Ligand pocket–filling mutant (S247W/C284W or S247W/C284W/S208W) of the human PXR abrogated the effect of R1 on PXR activation. Time-resolved fluorescence resonance energy transfer PXR competitive binding assay confirmed R1 (ligand) binding affinity. In addition, PXR overexpression inhibited nuclear factor-κB (NF-κB)–luciferase activity, which was potentiated by R1 treatment. PXR knockdown by small interfering RNA demonstrated the necessity of PXR in R1-induced upregulation of the expression of xenobiotic-metabolizing enzymes and downregulation of NF-κB activity. Finally, the anti-inflammatory effect of R1 was confirmed in trinitrobenzene sulfonic acid–induced colitis in mice. These findings suggest that R1 attenuates experimental IBD possibly via the activation of intestinal PXR signaling.
Introduction
Inflammatory bowel disease (IBD), which includes ulcerative colitis (UC) and Crohn’s disease (CD), is a chronic inflammatory condition of the gastrointestinal tract. UC is limited to the colon, whereas CD can affect any part of the gastrointestinal tract from the mouth to the anus (Baumgart and Sandborn, 2007; Gupta et al., 2013). Conventional therapeutics for IBD have been associated with multiple adverse effects. New biologic therapies, including tumor necrosis factor-α (TNF-α) antagonists (e.g., infliximab, adalimumab, and golimumab) and integrin antagonists (e.g., vedolizumab and natalizumab), have profoundly influenced the management of IBD patients. However, there remain concerns about their potential side effects, tolerability, and high costs (Jobin, 2010; Gilroy and Allen, 2014).
Pregnane X receptor (PXR; NR1I2), one of the members of the nuclear receptor superfamily, is a xenobiotic/metabolic sensor that regulates the expression of drug-metabolizing enzymes and transporters involved in the clearance of many xenobiotic chemicals (Mani et al., 2013; Smutny et al., 2013). PXR has a bulky and flexible ligand-binding pocket that enables this receptor to accommodate structurally diverse ligands, including prescription drugs, natural products, dietary supplements, environmental pollutants, endogenous hormones, and bile acids (Zhang et al., 2008; Cheng et al., 2012). Ligand-activated PXR regulates the expression of a battery of genes, including phase I metabolic enzymes (CYP2B6, CYP2B9, CYP2C8, CYP2C9, CYP3A4, and CYP3A7), phase II metabolic enzymes [glutathione S-transferases (GSTs), UDP-glucuronosyltransferases (UGTs), and sulfotransferases], and transporters [multidrug resistance protein 1 (MDR1), MDR2, multidrug resistance–associated protein 2 (MRP2), and the organic anion transporter polypeptide 2]. These enzymes and transporters are capable of recognizing a variety of xenobiotics to promote their clearance/detoxification, which is thought to be critical for maintaining intestinal barrier integrity (Zhang et al., 2008; Mencarelli et al., 2010). Indeed, disruption of the intestinal barrier function is linked to IBD. Gene analysis studies have indicated that the mRNA expression of PXR and MDR1 is significantly reduced in the colons of UC patients (Langmann et al., 2004). The present study and others have demonstrated that PXR activation inhibits the nuclear factor-κB (NF-κB) pathway and alleviates the severity of experimental IBD (Cheng et al., 2010; Dou et al., 2012). In a dextran sodium sulfate (DSS)–induced IBD model, administration of pregnenolone-16α-carbonitrile (PCN), a rodent-specific PXR ligand, attenuates development of colitis by decreasing the expression of NF-κB target genes [monocyte chemotactic protein-1 (MCP-1), inducible NO synthase (iNOS), interleukin (IL)-1β, IL-6, and TNF-α] and increasing the expression of phase II enzymes (GSTa1, GSTm1, and GSTt1) and transporters (MDR1a and MRP2) (Shah et al., 2007). Thus, targeted activation of PXR in recent years has become a therapeutic strategy for IBD (Cheng et al., 2010; Mencarelli et al., 2010).
Notoginsenoside R1 (R1) is a characteristic constituent of Radix notoginseng, a well known herbal medicine widely used in Asian countries for treating microcirculatory disturbance-related diseases, such as cardiovascular disease, cerebral vascular disease, and liver dysfunction (Sun et al., 2007; Geng et al., 2010; Fan et al., 2012). R1 has multiple pharmacologic activities, including cardioprotective, neuroprotective, anti-inflammatory, and anticancer effects (Wang et al., 2009; Sun et al., 2013; Meng et al., 2014). However, thus far, no study has reported the effect of R1 on IBD. Therefore, in the current study, we used in vitro and in vivo models to investigate the effects of R1 on IBD and to uncover the possible underlying mechanisms mediated through PXR activation.
Materials and Methods
Cell lines, reagents, semiquantitative and real-time quantitative polymerase chain reaction (PCR), Western blot analysis, PXR-mediated NF-κB repression reporter assay, gene silencing, and time-resolved fluorescence resonance energy transfer (TR-FRET) assay details are mentioned in the Supplemental Methods.
Mice.
Eight-week-old female C57BL/6 mice (20 ± 2 g) were obtained from the Shanghai Laboratory Animal Center (Shanghai, China) and were housed in cages at room temperature (25 ± 2°C) with a 12-hour light/dark cycle. Standard mouse chow pellets and water were available ad libitum. The experiments were approved by the Animal Ethics Committee of Shanghai University of Traditional Chinese Medicine.
DSS and Trinitrobenzene Sulfonic Acid Colitis Models in Mice.
DSS or trinitrobenzene sulfonic acid (TNBS) colitis was induced in mice as described previously (Dou et al., 2012, 2013b). In brief, 4% (w/v) DSS (molecular mass 36–50 kDa; MP Biomedicals, Solon, OH) was administered in the drinking water (tap water) for 7 days, whereas control mice received tap water only (n = 10 mice in each group). In the TNBS model, food (but not water) was withdrawn overnight prior to TNBS (Sigma-Aldrich, St. Louis, MO) administration. Colitis was induced by slow and continuous intrarectal administration of 0.1 ml of 50% ethanol solution containing 2.5 mg of TNBS. Solvent alone (0.1 ml of 50% ethanol) was administered in the control group. Mice received a daily gavage of R1 (25 mg/kg) in 0.5% methylcellulose from day 1 to day 7. R1 dosage was selected based on the results of a previous report in which 25 mg/kg of R1 pretreatment blocked NF-κB activation and the subsequent myocardial inflammation and apoptotic responses in endotoxemic mice (Sun et al., 2013). Mice IBD studies were repeated again for independent confirmation of the initial data.
Assessment of Colitis.
The severity of diarrhea was monitored throughout the experimental period. The total length of the colon was measured after sacrifice of the mice under anesthesia. The distal colon was fixed in 10% buffered formalin, embedded in paraffin, and processed for routine H&E staining of sections. Histologic scoring was performed in a blinded fashion by two pathologists to obtain a combined score of inflammatory cell infiltration (score 0–3) and tissue damage (score 0–3), as described previously (Dou et al., 2013b).
Wild-Type/Mutant PXR Transactivation Reporter Assays.
HT-29 cells (1 × 106) in 100 μl of transfection buffer (Cell Line Nucleofector Kit V; Lonza AG, Walkersville, MD) were electroporated using the program W-017 according to the manufacturer’s recommendation (Lonza Nucleofector II instrument; Amaxa Biosystems, Gaithersburg, MD). For the PXR wild-type transactivation assay, the cells were transfected with 1 μg of CYP3A4-luciferase reporter combined with 0.1 μg of pRL-TK, and 0.5 μg of the plasmid expressing wild-type human PXR (pSG5-hPXR) or wild-type mouse PXR (pSG5-mPXR), as shown in schematic diagrams (see Fig. 3B). For the PXR mutant transactivation assay, the cells were transfected with 1 μg of MRP2-luciferase reporter combined with 0.1 μg of pRL-TK, and 0.5 μg of plasmid expressing the wild-type hPXR or the hPXR double mutant (S247W/C284W) or the hPXR triple mutant (S247W/C284W/S208W), as shown in schematic diagrams (see Fig. 6A). For detailed plasmid information, refer to our previous reports (Wang et al., 2008; Venkatesh et al., 2011). For the wild-type PXR transactivation assay, the cells were treated with R1 (0, 1, 10, or 25 μM), rifampicin (10 μM, for hPXR transactivation), or PCN (10 μM, for mPXR transactivation) for 24 hours. For mutant PXR transactivation assay, the cells were treated with R1 (0 or 25 μM) or rifampicin (10 μM) for 24 hours. The luciferase activity of the cell extracts was measured and expressed as the fold induction compared with that of the control cells, as previously described (Dou et al., 2012).
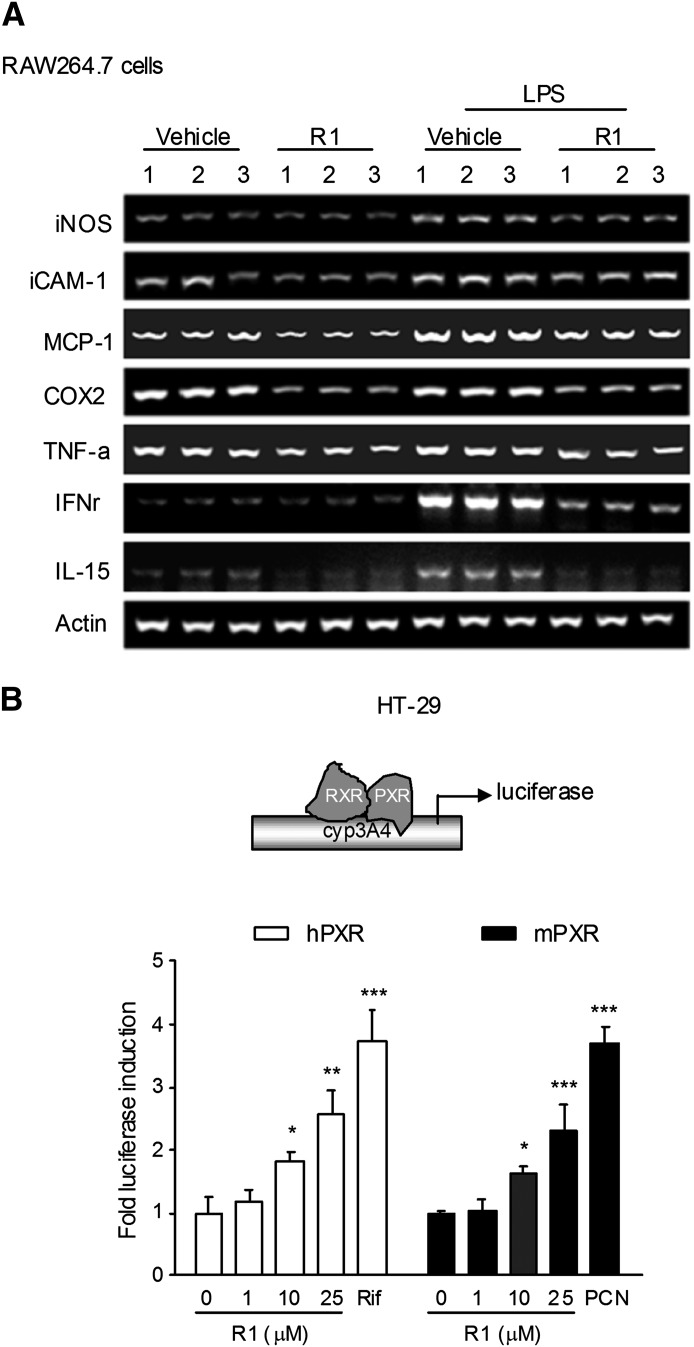
Fig. 3.
The effects of R1 on NF-κB target gene expression in vitro and on PXR activation. (A) The levels of the mRNA expression of NF-κB target genes in lipopolysaccharide (LPS)-stimulated RAW264.7 macrophages following R1 treatment (0 and 25 μM, for 48 hours) were detected by semiquantitative reverse-transcription PCR. The results are representative of three independent experiments. (B) Wild-type human/mouse PXR transactivation reporter assay. Transient transactivation assays were performed in HT-29 cells cotransfected with CYP3A4-reporter, pRL-TK, and pSG5-hPXR or pSG5-mouse PXR (mPXR). The cells were incubated with R1 (0, 1, 10, or 25 μM), rifampicin (Rif; 10 μM, for hPXR), or PCN (10 μM, for mPXR) for 24 hours. A standard dual-luciferase assay of the cell lysates was performed, and the results are expressed as the fold induction compared with that of the control cells. *P < 0.05; **P < 0.01; ***P < 0.001 versus vehicle-treated cells. COX2, cyclooxygenase 2; iCAM-1, intercellular adhesion molecule-1; IFNr, interferon-r.
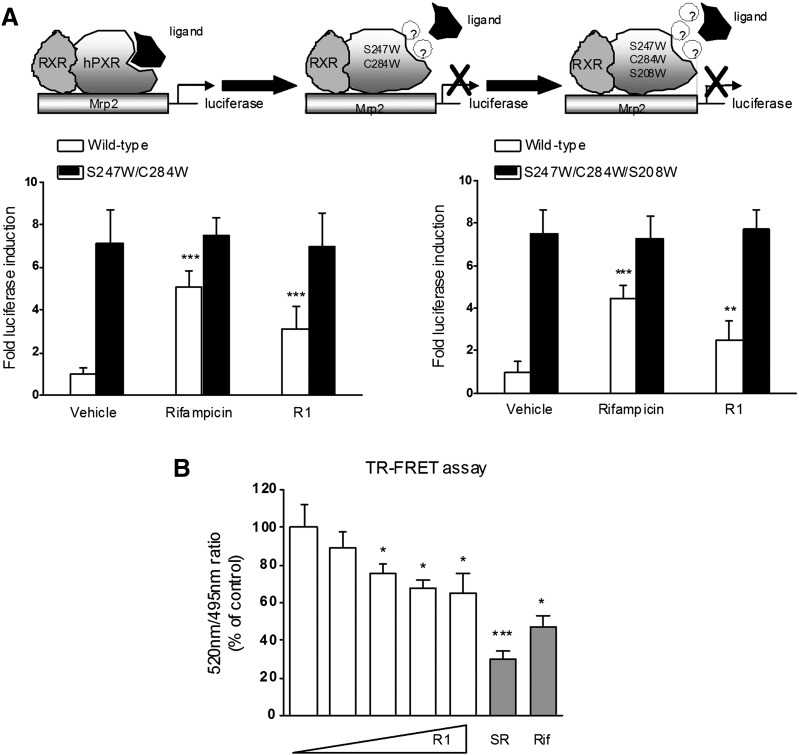
Fig. 6.
Effects of R1 on the transactivation of PXR mutants and TR-FRET assay. (A) Transient transcription assays were performed in HT-29 cells cotransfected with MRP2-luciferase reporter, pRL-TK, and the wild-type hPXR (pSG5-hPXR) or the hPXR double mutant (S247W/C284W) or the hPXR triple mutant (S247W/C284W/S208W). The cells were treated with R1 (0 and 25 μM) or rifampicin (Rif; 10 μM) for 24 hours. Luciferase activities were measured, and the results are expressed as the fold induction compared with that of the control cells. The results are presented as the means ± S.D. of three independent experiments. **P < 0.01; ***P < 0.001 versus vehicle-treated cells. (B) The LanthaScreen TR-FRET competitive binding assay was used to evaluate the ability of R1 (0, 12.5, 25, 50, and 100 µM) to interact with PXR in vitro. Rifampicin (10 µM) and SR12813 (SR; 1 µM) were included as positive control PXR ligands. The TR-FRET ratio was calculated by dividing the emission signal at 520 nm by that at 495 nm. The results are expressed as the means ± S.D. from a representative experiment performed in quadruplicate. *P < 0.05; ***P < 0.001 versus vehicle-treated cells. RXR, retinoid X receptor.
Measurement of Cytokines.
Colon segments were homogenized in ice-cold phosphate-buffered saline. The homogenates were centrifuged at 3000g for 10 minutes, and the supernatants were assayed for the determination of levels of TNF-α and IL-6, as described previously (Dou et al., 2013a). The level of each cytokine was evaluated using enzyme-linked immunosorbent assay kits according to the manufacturer’s protocols (R&D Systems, Minneapolis, MN), and the results are expressed in picograms per milligram of protein in each sample.
Myeloperoxidase Assay.
Tissue myeloperoxidase (MPO) activity, which is linearly related to neutrophil infiltration in inflamed tissue, was assayed to monitor the degree of inflammation. MPO activity in colon tissues was measured as previously described (Zhang et al., 2014), and the values were expressed as units per milligram of protein.
Statistical Analysis.
All of the data are expressed as means ± S.D. The differences between the groups were analyzed using one-way analysis of variance followed by the least significant difference post-hoc test. The statistical analyses were performed using the SPSS 16.0 software package (SPSS, Chicago, IL). P < 0.05 was considered statistically significant.
Results
R1 Ameliorated DSS-Induced Colitis.
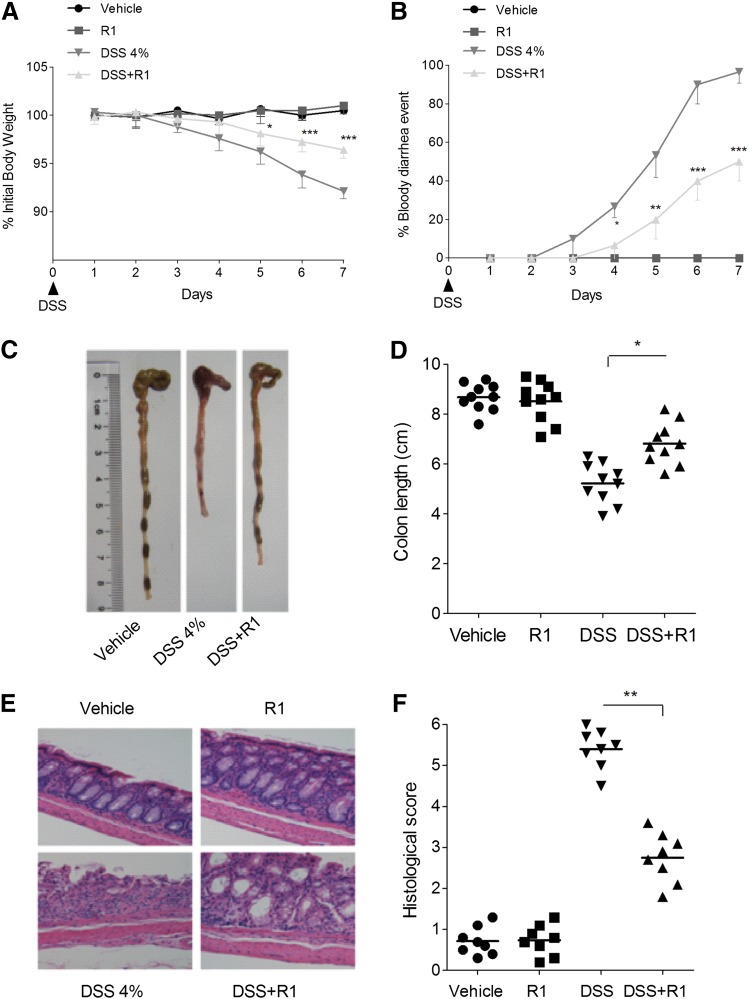
The DSS-induced colitis model is a well established chemical IBD model with clinical features resembling human UC (Neurath and Travis, 2012). R1 treatment significantly ameliorated the DSS-induced loss of body weight, bloody diarrhea, colon shortening, and histologic damage (Fig. 1). The activity of MPO, as well as the levels of TNF-α and IL-6, was markedly reduced by R1 treatment (Table 1). In addition, none of the mice that received R1 alone exhibited loss of body weight, diarrhea, colon shortening, or mucosal disruption at any point during the study.
Fig. 1.
R1 attenuated DSS-induced colitis in mice. (A) Changes in body weight following DSS induction of colitis. The data are plotted as a percentage of the original body weight. (B) The occurrence of bloody diarrhea. The data are plotted as a percentage of the total mice that had bloody diarrhea at different time points of DSS treatment. (C) Macroscopic observation of colon shortening. (D) Assessment of colon shortening. (E) Representative H&E-stained colon sections (magnification, 200×). (F) Histology scoring for H&E-stained colon sections. The data are expressed as the means ± S.D. of n = 10 mice in each group. *P < 0.05; **P < 0.01; ***P < 0.001 versus the DSS-treated group.
TABLE 1.
Effects of R1 on the activity of MPO and the levels of TNF-α and IL-6 in mice with DSS- or TNBS-induced colitis
Colon segments from mice (n = 6 per group) were excised and homogenized. The supernatants were assayed for the determination of the activity of MPO and the levels of TNF-α and IL-6 as described in Materials and Methods. Values are expressed as the mean ± S.D.
| Group | DSS |
TNBS |
||||
|---|---|---|---|---|---|---|
| TNF-α | IL-6 | MPO | TNF-α | IL-6 | MPO | |
| pg/mg protein | U/mg protein | pg/mg protein | U/mg protein | |||
| Vehicle | 14.6 ± 1.7 | 28.9 ± 2.3 | 3.7 ± 0.6 | 27.4 ± 1.2 | 39.7 ± 3.3 | 2.8 ± 0.2 |
| R1 | 16.96 ± 1.1 | 23.79 ± 4.1 | 3.2 ± 0.2 | 30.9 ± 3.6 | 35.8 ± 1.9 | 3.1 ± 0.3 |
| DSS/TNBS | 127.4 ± 8.5 | 214.3 ± 17.9 | 26.8 ± 2.2 | 86.6 ± 6.4 | 119.7 ± 7.9 | 15.3 ± 1.7 |
| DSS/TNBS + R1 | 61.5 ± 3.9** | 164.2 ± 16.6* | 11.4 ± 1.8** | 52.2 ± 4.1** | 86.3 ± 7.4* | 8.8 ± 0.7* |
P < 0.05; **P < 0.01 versus DSS- or TNBS-treated group.
R1 Inhibited NF-κB Activation and Downregulated NF-κB Target Gene Expression.
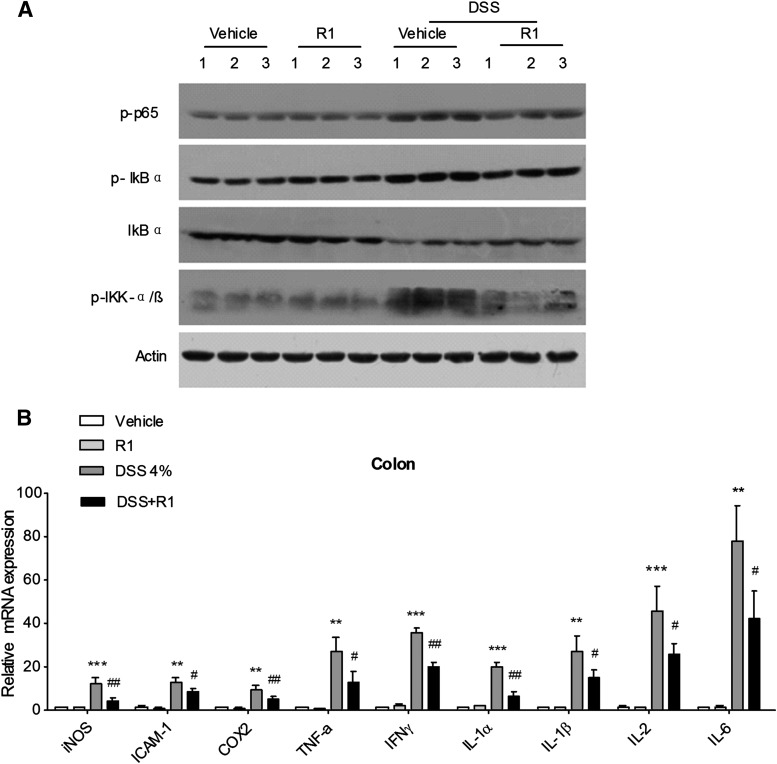
Intestinal NF-κB activation plays a central role in the pathogenesis of IBD (Atreya et al., 2008). Major steps for NF-κB activation involve the activation of IκB kinase (IKK)-α/β, which is followed by IκBα degradation and the subsequent p65/p50 nuclear translocation (Tak and Firestein, 2001). Using Western blot analysis, we observed a phosphorylation/activation of IKK-α/β and p65 in the colonic tissue of DSS-treated mice (Fig. 2A). IκBα phosphorylation and degradation were induced in mice with DSS-induced colitis. In contrast, administration of R1 obviously reduced the expression of phosphorylated p65 and phosphorylated IKK-α/β in DSS-induced colitis, and the phosphorylation/degradation of IκBα was also repressed. To determine the effect of R1 on the expression of NF-κB signaling genes, several proinflammatory cytokines and chemokines in the colon were evaluated using quantitative reverse-transcription PCR analysis. The increase in the levels of inflammatory mediators (intercellular adhesion molecule-1, cyclooxygenase 2, TNF-α, interferon-γ, iNOS, MCP-1, IL-1α, IL-1β, IL-2, and IL-6) after DSS treatment dramatically decreased in the mice that received R1 treatment (Fig. 2B). Next, we investigated whether R1 affects the expression of NF-κB target genes in RAW264.7 mouse macrophage cells using semiquantitative reverse-transcription PCR. The RAW264.7 macrophage cell line is a well established model system for inflammatory studies (Wu et al., 2011). In accordance with in vivo data, R1 treatment obviously decreased the expression of iNOS, intercellular adhesion molecule-1, MCP-1, cyclooxygenase 2, interferon-γ, TNF-α, and IL-15 in lipopolysaccharide-stimulated RAW264.7 cells (Fig. 3A).
Fig. 2.
The effects of R1 on NF-κB activation and target gene expression in vivo. (A) Total protein (40 μg) was extracted from colon samples (n = 6 per group) and examined for the expression of p-IKK-α/β, p-p65, p-IκBα, IκBα, and β-actin by Western blot analysis. The results are representative of three independent experiments. (B) The levels of the expression of NF-κB target genes in colon samples isolated from mice (n = 6 per group) were determined using quantitative reverse-transcription PCR. The levels of expression are normalized to that of β-actin, and each bar represents the mean ± S.D. of two independent experiments performed using samples in triplicate. **P < 0.01; ***P < 0.001 versus Vehicle-treated group. #P < 0.05; ##P < 0.01 versus DSS-treated group. COX2, cyclooxygenase 2; ICAM-1, intercellular adhesion molecule-1; IFNγ, interferon-γ.
R1 Activated Both Human and Mouse Wild-Type PXR.
In transient transfection gene reporter assays, R1 increased human or mouse PXR-mediated CYP3A4 promoter transcription in a concentration-dependent manner (Fig. 3B). In addition, rifampicin and PCN significantly activated human and mouse PXR, respectively.
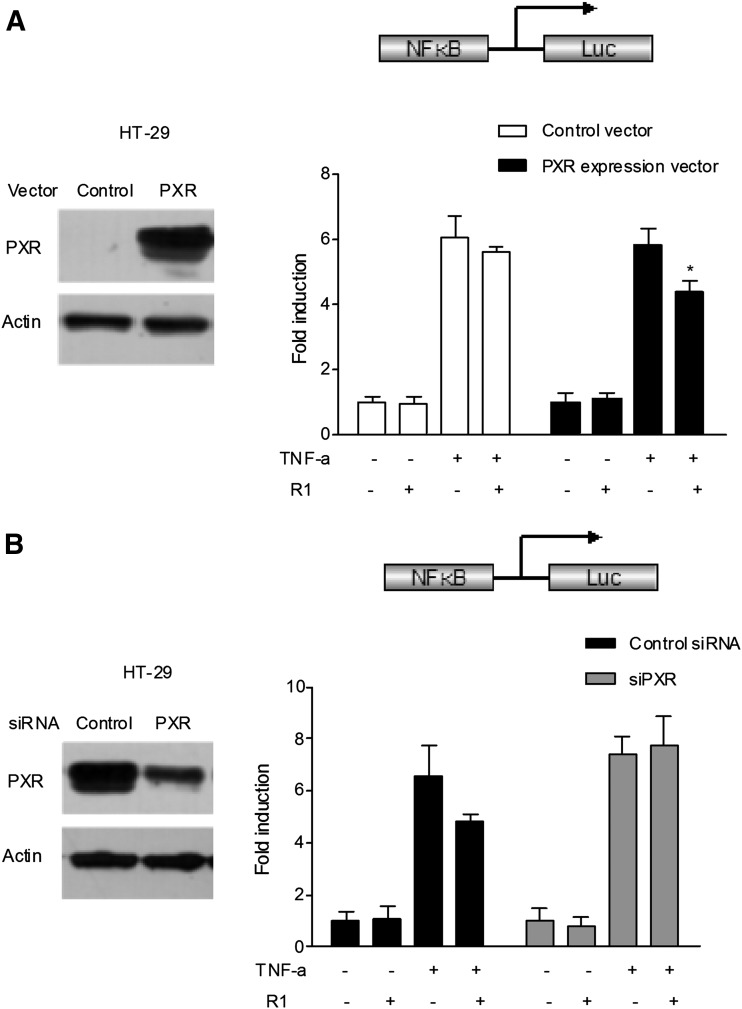
R1 Inhibited NF-κB Activity in a PXR-Dependent Manner.
To determine whether activation of PXR plays a direct role in the NF-κB repression by R1, a transient transfection reporter assay was performed. HT-29 cells have low or undetectable levels of constitutive human PXR expression (Dou et al., 2014). In cells transfected with the NF-κB–luciferase reporter and the PXR mock, treatment with the known NF-κB pathway activator TNF-α led to increased NF-κB activity; however, R1 had no effect on NF-κB activity in the absence of human PXR expression (Fig. 4A). In cells transfected with the NF-κB–luciferase reporter and the human PXR expression vector, TNF-α–stimulated NF-κB activity was significantly inhibited by R1 in the presence of human PXR expression. To further demonstrate that PXR is specifically involved in the suppression of NF-κB by R1, PXR small interfering RNA (siRNA) transfection was conducted in the aforementioned HT-29 cells, which had been previously transfected with the NF-κB–luciferase reporter and the human PXR expression plasmid. As expected, the regulatory effect of R1 on TNF-α–stimulated NF-κB activity was abrogated (Fig. 4B).
Fig. 4.
Role for PXR in R1-mediated NF-κB luciferase (Luc) inhibition. (A) HT-29 cells were electroporated with the pGL4.32[luc2P/NF-κB-RE/Hygro] reporter combined with pRL-TK and pSG5-hPXR or pSG5-mock. PXR overexpression was verified by Western blot analysis (left). After transfection, the cells were treated with TNF-α (20 ng/ml) alone or in combination with R1 (25 μM) for 24 hours. A standard dual-luciferase activity of the cell lysates was measured, and the results are expressed as the fold induction compared with that of the control cells (designated as 1). The results are presented as the means ± S.D. of three independent experiments. *P < 0.05 versus hPXR-transfected cells treated with TNF-α alone. (B) The human PXR gene was silenced by PXR siRNA transfection in the aforementioned HT-29 cells previously transfected with the pGL4.32[luc2P/NF-κB-RE/Hygro] reporter and the human PXR expression plasmid pSG5-hPXR. The depletion of PXR by siRNA transfection in HT-29 cells was verified using Western blot (left). After transfection, the cells were treated with TNF-α (20 ng/ml) alone or cotreated with R1 (25 μM) for 24 hours. A standard dual luciferase assay of the cell lysates was performed. The results are presented as the means ± S.D. of three independent experiments. *P < 0.05 versus control siRNA transfection samples treated with TNF-α alone.
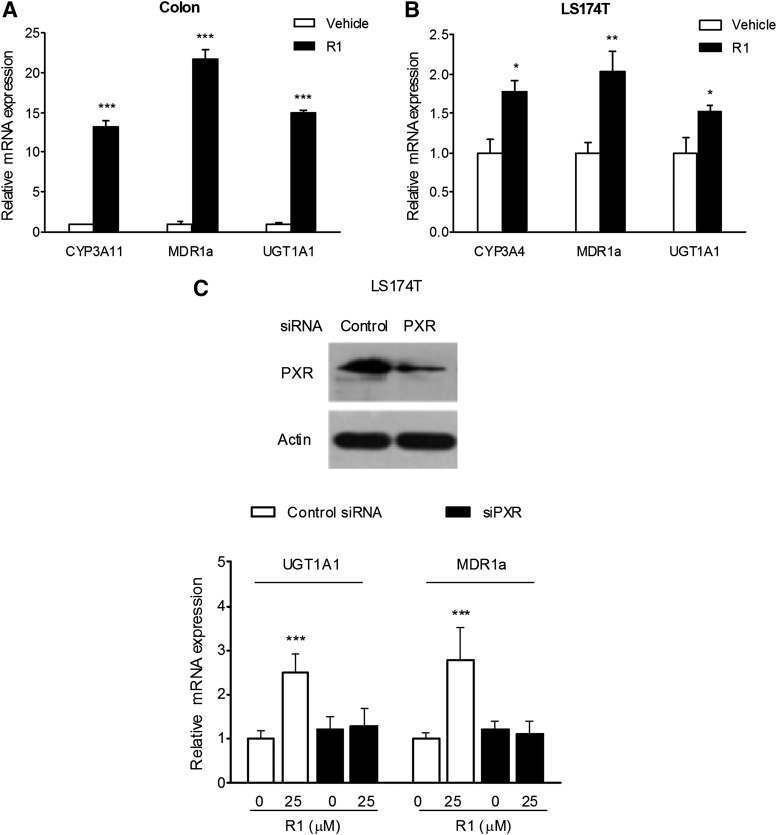
R1 Upregulated the Expression of Xenobiotic-Metabolizing Genes In Vitro and In Vivo.
PXR target genes are critical components in the intestinal barrier function against xenobiotics and bacteria (Langmann et al., 2004; Mencarelli et al., 2010). In the current study, we observed a robust upregulation of the expression of CYP3A11, UGT1A1, and MDR1a in the colons of mice that received 7 days of R1 treatment (Fig. 5A). Consistent with the in vivo results, an increase in the expression of CYP3A4, UGT1A1, and MDR1a was observed in LS174T cells exposed to R1 treatment (Fig. 5B).
Fig. 5.
The role of PXR in the R1-mediated upregulation of PXR target genes. Quantitative reverse-transcription PCR was performed to assess the mRNA expression of CYP3A11 (CYP3A4 for human), UGT1A1, and MDR1a in colon samples isolated from mice (n = 6 per group) orally fed 25 mg/kg R1 for 7 days (A) or in LS174T cells (n = 3) treated with 25 μM R1 for 48 hours (B). The levels of expression are normalized to that of β-actin, and each bar represents the mean ± S.D. of two independent experiments performed using samples in triplicate. *P < 0.05; **P < 0.01; ***P < 0.001 versus vehicle-treated group. (C) The depletion of PXR in LS174T cells using siRNA transfection was verified using Western blot (top). The mRNA expression of UGT1A1 and MDR1a was assessed using quantitative reverse-transcription PCR (bottom). The results are expressed as the means ± S.D. from triplicate samples of two independent experiments. ***P < 0.001 versus vehicle-treated cells transfected with control siRNA.
R1 Upregulated the Expression of Xenobiotic-Metabolizing Genes in a PXR-Dependent Manner.
To explore the mechanistic involvement of PXR in the modulation of the genes responsible for xenobiotic metabolism, the human PXR gene expression in LS174T colon adenocarcinoma cells was reduced by PXR siRNA transfection. As shown in Fig. 5C, the treatment of LS174T cells with an anti-PXR siRNA significantly decreased the expression of PXR. In addition, the regulatory effect of R1 on the mRNA expression of UGT1A1 and MDR1a was abrogated in cells transfected with anti-PXR siRNA.
Ligand Pocket–Filling Mutants of Human PXR Abolished R1-Mediated PXR Activation.
To provide evidence that R1 indeed binds to the ligand-binding pocket of the human PXR and subsequently activates the transcription of MRP2 promoter, we performed a transient transactivation reporter assay using the double-mutant (S247W/C284W) and triple-mutant (S247W/C284W/S208W) constructs of the human PXR ligand-binding pocket. The results showed that the double or triple mutants of PXR led to ligand-independent constitutive activation of the MRP2 promoter (Fig. 6A), which is consistent with the results of previous reports (Wang et al., 2008; Venkatesh et al., 2011). In addition, both rifampicin and R1 activated the wild-type human PXR, whereas none of them activated the double or triple mutant.
Confirmation of the PXR-Binding Properties by TR-FRET Assay.
To confirm the direct binding of R1 to the ligand-binding pocket of the human PXR, a LanthaScreen TR-FRET PXR competitive binding assay (Life Technologies, Grand Island, NY) was performed. R1 concentration-dependently decreased the TR-FRET emission ratio (Fig. 6B). In addition, both rifampicin and SR12813 tetraethyl 2-(3,5-di-tert-butyl-4-hydroxyphenyl)ethenyl-1,1-bisphosphonate) significantly decreased the TR-FRET ratio (Fig. 6B).
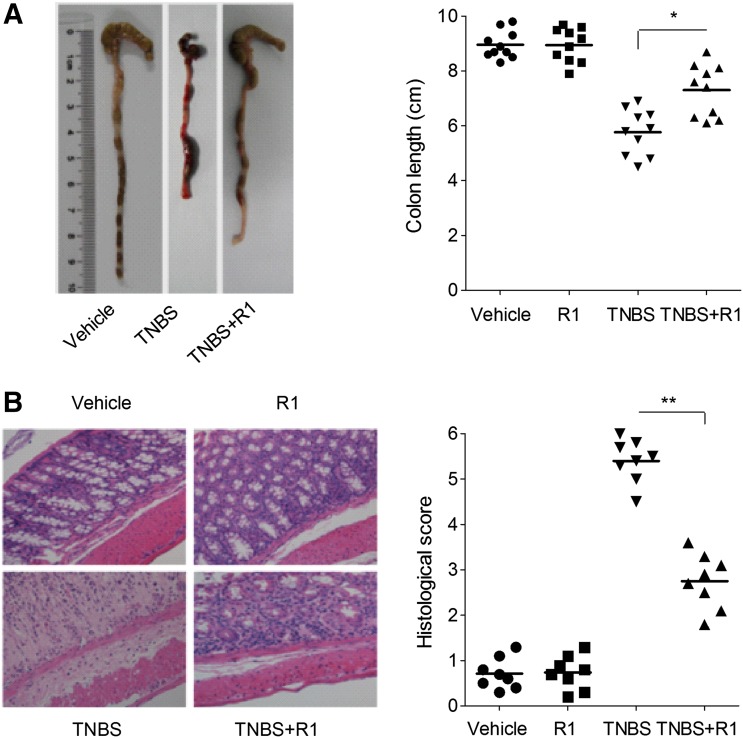
R1 Treatment Attenuated the Severity of TNBS-Induced Colitis.
Next, to determine whether R1 also had a therapeutic effect on another chemical model of colitis, we tested its effects on some parameters of colitis induced by the hapten TNBS, which constitutes a CD model (Neurath and Travis, 2012). The rectal administration of TNBS led to ulcers in the rectum, increased MPO activity, and upregulated TNF-α and IL-6 levels (Fig. 7; Table 1). Oral R1 administration significantly improved these parameters and ameliorated the histologic pathology. No significant changes were observed in vehicle or R1-treated normal mice.
Fig. 7.
R1 attenuated TNBS-induced colitis in mice. (A) Macroscopic observation and assessment of colon shortening (n = 10). (B) Representative H&E-stained colon sections (magnification, 200×) and histology scores. The results are expressed as the means ± S.D. of n = 10 mice in each group. *P < 0.05; **P < 0.01 versus TNBS-treated group.
Discussion
Chemically-induced colitis models have been developed and extensively used to elucidate the pathogenic mechanisms of IBD. One of the most widely used experimental models is developed by treating animals with DSS in drinking water for 6–10 days. The resulting inflammation generally affects the mucosal lining of the intestinal wall, with disease features that resemble human UC (Neurath and Travis, 2012). In the current study, colitis was induced by DSS treatment, and the exposure of mice to R1 ameliorated the disease hallmarks, such as body weight loss, bloody diarrhea, colon shortening, and histologic damage. To assess whether R1 would have similar effects on another chemical model of colitis, we tested its effects on some parameters of colitis induced by TNBS, which constitutes a CD model of transmucosal inflammation (Neurath and Travis, 2012). The results showed that the rectal administration of TNBS dissolved in ethanol induced severe colitis in mice, and that R1 treatment reduced the extent of histologic damage and colon shortening. Since no report about the effect of R1 on UC or CD has been available, our findings are unique in establishing the role of R1 in ameliorating the UC- or CD-like features in murine models of IBD. Notably, none of the mice that received R1 alone exhibited apparent body weight loss, diarrhea, colon shortening, or mucosal disruption throughout this study, indicating the relative safety of the R1 treatment.
Recent studies have shown that PXR is involved in the pathogenesis of IBD (Cheng et al., 2010, 2012). Loss of PXR function has been associated with intestinal inflammation in animal models (Cheng et al., 2010; Dou et al., 2012). Gene expression profiling of the colon tissues from UC and CD patients demonstrated a significant inhibition of PXR and its target genes (Shah et al., 2007). Several polymorphisms in the PXR locus have been proved to be associated with an increased susceptibility to IBD (Langmann et al., 2004; Dring et al., 2006). PXR has a bulky and flexible ligand-binding pocket, which enables it to accommodate a structurally promiscuous library of ligands (Tolson and Wang, 2010). Upon activation, PXR heterodimerizes with retinoid X receptor and binds to the response elements of PXR target genes. In the present study, we found that R1 dose-dependently activated human PXR, which is likely due to binding to the ligand-binding domain of PXR. It has long been known that ligand-activated PXR regulates the expression of biotransformation enzymes and transport proteins involved in the metabolism and elimination of harmful substances from the body (Langmann et al., 2004). The detoxification properties of PXR and its target genes are necessary to maintain the integrity of the intestinal epithelial barrier (Shah et al., 2007). In this study, we found that R1 upregulated the expression of CYP3A, UGT1A1, and MDR1a, and this effect was mediated by PXR. It was recently reported that the natural compound curcumin decreases the susceptibility of MDR1a−/− mice to spontaneous colonic inflammation through PXR activation (Nones et al., 2009). Our results support the notion that enhanced intestinal epithelial integrity via PXR-mediated induction of xenobiotic detoxification might contribute to the observed anti-inflammatory effects of R1, but further studies are required.
Although the exact etiology of IBD remains unknown, it has been generally accepted that the increase of proinflammatory cytokines, such as IL-1β and TNF-α, within colonic tissues plays an important role in the pathogenesis of IBD (Shah et al., 2007). Also, it has been demonstrated that all of these proinflammatory cytokine genes are transcriptionally regulated by NF-κB (Tak and Firestein, 2001). NF-κB can be activated by diverse stimuli, including proinflammatory cytokines, microbes and microbial products, and oxidative stress (Liu and Wang, 2011). Activation of NF-κB leads to the gene expression of proinflammatory cytokines and chemokines involved in the pathogenesis of IBD. The most abundant form of NF-κB in cells is the p50/p65 heterodimer, which is normally sequestered in the cytoplasm by binding to the inhibitory protein IκBα. Following various stimuli, IκBα is rapidly phosphorylated by IKK, ubiquitinated, and subsequently degraded by proteasome, allowing NF-κB p50/p65 to translocate to the nucleus, where it drives the expression of target genes (Liu and Wang, 2011). In our study, we found that R1 treatment not only decreased the proteins of phosphorylated IKK-α/β, phosphorylated IκBα, and phosphorylated p65, but also downregulated the proinflammatory gene expression, and reduced the activity of MPO and the accumulation of TNF-α and IL-6. Further studies indicated that R1 decreased the activity of NF-κB in a PXR-dependent manner. In fact, recent molecular and pharmacologic studies have revealed a mutual suppression between PXR and the NF-κB signaling pathway (Xie and Tian, 2006; Wahli, 2008). High NF-κB activity inhibits the activation of PXR (Gu et al., 2006), whereas PXR activation suppresses the activity of NF-κB and the expression of its target genes (Zhou et al., 2006).
IBD is associated with a considerable reduction in the quality of life of patients, and currently no curative treatment is available (van der Marel et al., 2011; Gupta et al., 2013). The use of medicinal plants or their active components is becoming an increasingly attractive approach for the management of IBD (Shin et al., 2009; Zhang et al., 2014). In the present study, as a part of our ongoing screening program to evaluate the anti-inflammatory potentials of natural compounds (Dou et al., 2013a; Zhang et al., 2014), we investigated the anti-inflammatory effects of R1 on DSS- or TNBS-induced colitis. To our knowledge, the present study is the first to demonstrate a beneficial effect of R1 on chemically induced IBD (UC- or CD-like). The anti-inflammatory effect of R1 might be associated with the activation of PXR signaling. The results support further evaluation of the therapeutic potential of R1 in human IBD.
Supplementary Material
Abbreviations
- CD
Crohn’s disease
- DSS
dextran sulfate sodium
- GST
glutathione S-transferase
- IBD
inflammatory bowel disease
- IKK
IκB kinase
- IL
interleukin
- iNOS
inducible NO synthase
- MCP-1
monocyte chemotactic protein-1
- MDR
multidrug resistance protein
- MPO
myeloperoxidase
- MRP2
multidrug resistance–associated protein 2
- NF-κB
nuclear factor-κB
- PCN
pregnenolone-16α-carbonitrile
- PCR
polymerase chain reaction
- PXR
pregnane X receptor
- R1
notoginsenoside R1
- siRNA
small interfering RNA
- SR12813
tetraethyl 2-(3,5-di-tert-butyl-4-hydroxyphenyl)ethenyl-1,1-bisphosphonate
- TNBS
trinitrobenzene sulfonic acid
- TNF-α
tumor necrosis factor-α
- TR-FRET
time-resolved fluorescence resonance energy transfer
- UC
ulcerative colitis
- UGT
UDP-glucuronosyltransferase
Authorship Contributions
Participated in research design: Z. Wang, Dou.
Conducted experiments: Zhang, Ding, Ren, Deng.
Contributed new reagents or analytic tools: B. Wang, Wei.
Performed data analysis: Zhang, Sun.
Wrote or contributed to the writing of the manuscript: Mani, Dou.
Footnotes
This work was supported by the National Natural Science Foundation of China [81273572, U1032604]; Natural Science Foundation of Shanghai [12ZR1431400]; Innovation Program of Shanghai Municipal Education Commission [13YZ043]; the National Institutes of Health National Cancer Institute [Grant R01-CA127231]; and Damon Runyon Foundation Clinical Investigator Award [CI 1502].
 This article has supplemental material available at jpet.aspetjournals.org.
This article has supplemental material available at jpet.aspetjournals.org.
References
- Atreya I, Atreya R, Neurath MF. (2008) NF-kappaB in inflammatory bowel disease. J Intern Med 263:591–596. [DOI] [PubMed] [Google Scholar]
- Baumgart DC, Sandborn WJ. (2007) Inflammatory bowel disease: clinical aspects and established and evolving therapies. Lancet 369:1641–1657. [DOI] [PubMed] [Google Scholar]
- Cheng J, Shah YM, Gonzalez FJ. (2012) Pregnane X receptor as a target for treatment of inflammatory bowel disorders. Trends Pharmacol Sci 33:323–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J, Shah YM, Ma X, Pang X, Tanaka T, Kodama T, Krausz KW, Gonzalez FJ. (2010) Therapeutic role of rifaximin in inflammatory bowel disease: clinical implication of human pregnane X receptor activation. J Pharmacol Exp Ther 335:32–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dou W, Mukherjee S, Li H, Venkatesh M, Wang H, Kortagere S, Peleg A, Chilimuri SS, Wang ZT, Feng Y, et al. (2012) Alleviation of gut inflammation by Cdx2/Pxr pathway in a mouse model of chemical colitis. PLoS ONE 7:e36075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dou W, Zhang J, Ren G, Ding L, Sun A, Deng C, Wu X, Wei X, Mani S, Wang Z. (2014) Mangiferin attenuates the symptoms of dextran sulfate sodium-induced colitis in mice via NF-κB and MAPK signaling inactivation. Int Immunopharmacol 23:170–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dou W, Zhang J, Sun A, Zhang E, Ding L, Mukherjee S, Wei X, Chou G, Wang ZT, Mani S. (2013a) Protective effect of naringenin against experimental colitis via suppression of Toll-like receptor 4/NF-κB signalling. Br J Nutr 110:599–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dou W, Zhang J, Zhang E, Sun A, Ding L, Chou G, Wang Z, Mani S. (2013b) Chrysin ameliorates chemically induced colitis in the mouse through modulation of a PXR/NF-κB signaling pathway. J Pharmacol Exp Ther 345:473–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dring MM, Goulding CA, Trimble VI, Keegan D, Ryan AW, Brophy KM, Smyth CM, Keeling PW, O’Donoghue D, O’Sullivan M, et al. (2006) The pregnane X receptor locus is associated with susceptibility to inflammatory bowel disease. Gastroenterology 130:341–348, quiz 592. [DOI] [PubMed] [Google Scholar]
- Fan JS, Liu DN, Huang G, Xu ZZ, Jia Y, Zhang HG, Li XH, He FT. (2012) Panax notoginseng saponins attenuate atherosclerosis via reciprocal regulation of lipid metabolism and inflammation by inducing liver X receptor alpha expression. J Ethnopharmacol 142:732–738. [DOI] [PubMed] [Google Scholar]
- Geng J, Peng W, Huang Y, Fan H, Li S. (2010) Ginsenoside-Rg1 from Panax notoginseng prevents hepatic fibrosis induced by thioacetamide in rats. Eur J Pharmacol 634:162–169. [DOI] [PubMed] [Google Scholar]
- Gilroy L, Allen PB. (2014) Is there a role for vedolizumab in the treatment of ulcerative colitis and Crohn’s disease? Clin Exp Gastroenterol 7:163–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu X, Ke S, Liu D, Sheng T, Thomas PE, Rabson AB, Gallo MA, Xie W, Tian Y. (2006) Role of NF-kappaB in regulation of PXR-mediated gene expression: a mechanism for the suppression of cytochrome P-450 3A4 by proinflammatory agents. J Biol Chem 281:17882–17889. [DOI] [PubMed] [Google Scholar]
- Gupta SC, Patchva S, Aggarwal BB. (2013) Therapeutic roles of curcumin: lessons learned from clinical trials. AAPS J 15:195–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobin C. (2010) Probiotics and ileitis: could augmentation of TNF/NFκB activity be the answer? Gut Microbes 1:196–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langmann T, Moehle C, Mauerer R, Scharl M, Liebisch G, Zahn A, Stremmel W, Schmitz G. (2004) Loss of detoxification in inflammatory bowel disease: dysregulation of pregnane X receptor target genes. Gastroenterology 127:26–40. [DOI] [PubMed] [Google Scholar]
- Liu X, Wang JM. (2011) Iridoid glycosides fraction of Folium syringae leaves modulates NF-κB signal pathway and intestinal epithelial cells apoptosis in experimental colitis. PLoS ONE 6:e24740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mani S, Dou W, Redinbo MR. (2013) PXR antagonists and implication in drug metabolism. Drug Metab Rev 45:60–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mencarelli A, Migliorati M, Barbanti M, Cipriani S, Palladino G, Distrutti E, Renga B, Fiorucci S. (2010) Pregnane-X-receptor mediates the anti-inflammatory activities of rifaximin on detoxification pathways in intestinal epithelial cells. Biochem Pharmacol 80:1700–1707. [DOI] [PubMed] [Google Scholar]
- Meng X, Sun G, Ye J, Xu H, Wang H, Sun X. (2014) Notoginsenoside R1-mediated neuroprotection involves estrogen receptor-dependent crosstalk between Akt and ERK1/2 pathways: a novel mechanism of Nrf2/ARE signaling activation. Free Radic Res 48:445–460. [DOI] [PubMed] [Google Scholar]
- Neurath MF, Travis SP. (2012) Mucosal healing in inflammatory bowel diseases: a systematic review. Gut 61:1619–1635. [DOI] [PubMed] [Google Scholar]
- Nones K, Dommels YE, Martell S, Butts C, McNabb WC, Park ZA, Zhu S, Hedderley D, Barnett MP, Roy NC. (2009) The effects of dietary curcumin and rutin on colonic inflammation and gene expression in multidrug resistance gene-deficient (mdr1a-/-) mice, a model of inflammatory bowel diseases. Br J Nutr 101:169–181. [DOI] [PubMed] [Google Scholar]
- Shah YM, Ma X, Morimura K, Kim I, Gonzalez FJ. (2007) Pregnane X receptor activation ameliorates DSS-induced inflammatory bowel disease via inhibition of NF-kappaB target gene expression. Am J Physiol Gastrointest Liver Physiol 292:G1114–G1122. [DOI] [PubMed] [Google Scholar]
- Shin EK, Kwon HS, Kim YH, Shin HK, Kim JK. (2009) Chrysin, a natural flavone, improves murine inflammatory bowel diseases. Biochem Biophys Res Commun 381:502–507. [DOI] [PubMed] [Google Scholar]
- Smutny T, Mani S, Pavek P. (2013) Post-translational and post-transcriptional modifications of pregnane X receptor (PXR) in regulation of the cytochrome P450 superfamily. Curr Drug Metab 14:1059–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun K, Wang CS, Guo J, Horie Y, Fang SP, Wang F, Liu YY, Liu LY, Yang JY, Fan JY, et al. (2007) Protective effects of ginsenoside Rb1, ginsenoside Rg1, and notoginsenoside R1 on lipopolysaccharide-induced microcirculatory disturbance in rat mesentery. Life Sci 81:509–518. [DOI] [PubMed] [Google Scholar]
- Sun B, Xiao J, Sun XB, Wu Y. (2013) Notoginsenoside R1 attenuates cardiac dysfunction in endotoxemic mice: an insight into oestrogen receptor activation and PI3K/Akt signalling. Br J Pharmacol 168:1758–1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tak PP, Firestein GS. (2001) NF-κB: a key role in inflammatory diseases. J Clin Invest 107:7–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolson AH, Wang H. (2010) Regulation of drug-metabolizing enzymes by xenobiotic receptors: PXR and CAR. Adv Drug Deliv Rev 62:1238–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Marel S, Majowicz A, van Deventer S, Petry H, Hommes DW, Ferreira V. (2011) Gene and cell therapy based treatment strategies for inflammatory bowel diseases. World J Gastrointest Pathophysiol 2:114–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatesh M, Wang H, Cayer J, Leroux M, Salvail D, Das B, Wrobel JE, Mani S. (2011) In vivo and in vitro characterization of a first-in-class novel azole analog that targets pregnane X receptor activation. Mol Pharmacol 80:124–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahli W. (2008) A gut feeling of the PXR, PPAR and NF-kappaB connection. J Intern Med 263:613–619. [DOI] [PubMed] [Google Scholar]
- Wang H, Li H, Moore LB, Johnson MD, Maglich JM, Goodwin B, Ittoop OR, Wisely B, Creech K, Parks DJ, et al. (2008) The phytoestrogen coumestrol is a naturally occurring antagonist of the human pregnane X receptor. Mol Endocrinol 22:838–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CZ, Xie JT, Fishbein A, Aung HH, He H, Mehendale SR, He TC, Du W, Yuan CS. (2009) Antiproliferative effects of different plant parts of Panax notoginseng on SW480 human colorectal cancer cells. Phytother Res 23:6–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu TY, Khor TO, Saw CL, Loh SC, Chen AI, Lim SS, Park JH, Cai L, Kong AN. (2011) Anti-inflammatory/Anti-oxidative stress activities and differential regulation of Nrf2-mediated genes by non-polar fractions of tea Chrysanthemum zawadskii and licorice Glycyrrhiza uralensis. AAPS J 13:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie W, Tian Y. (2006) Xenobiotic receptor meets NF-kappaB, a collision in the small bowel. Cell Metab 4:177–178. [DOI] [PubMed] [Google Scholar]
- Zhang J, Dou W, Zhang E, Sun A, Ding L, Wei X, Chou G, Mani S, Wang Z. (2014) Paeoniflorin abrogates DSS-induced colitis via a TLR4-dependent pathway. Am J Physiol Gastrointest Liver Physiol 306:G27–G36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Xie W, Krasowski MD. (2008) PXR: a xenobiotic receptor of diverse function implicated in pharmacogenetics. Pharmacogenomics 9:1695–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou C, Tabb MM, Nelson EL, Grün F, Verma S, Sadatrafiei A, Lin M, Mallick S, Forman BM, Thummel KE, et al. (2006) Mutual repression between steroid and xenobiotic receptor and NF-kappaB signaling pathways links xenobiotic metabolism and inflammation. J Clin Invest 116:2280–2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.