Abstract
The acetylation of histone proteins in the core of DNA regulates gene expression, including those affecting mitochondria. Both histone acetylation and mitochondrial deficit have been implicated in neuronal damage associated with drinking problems. Many alcoholics will repeat unsuccessful attempts at abstaining, developing a pattern of repeated drinking and withdrawal. We investigated whether aberrant histone acetylation contributes to mitochondrial and cellular damage induced by repeated ethanol withdrawal (EW). We also investigated whether this effect of histone acetylation involves let-7f, a small noncoding RNA (microRNA). Male rats received two cycles of an ethanol/control diet (7.5%, 4 weeks) and withdrawal. Their prefrontal cortex was collected to measure the mitochondrial respiration and histone acetylation using extracellular flux (XF) real-time respirometry and gold immunostaining, respectively. Separately, HT22 (mouse hippocampal) cells received two cycles of ethanol exposure (100 mM, 20 hours) and withdrawal. Trichostatin A (TSA) as a histone acetylation promoter and let-7f antagomir were applied during withdrawal. The mitochondrial respiration, let-7f level, and cell viability were assessed using XF respirometry, quantitative polymerase chain reaction, TaqMan let-7f primers, and a calcein-acetoxymethyl assay, respectively. Repeated ethanol withdrawn rats showed a more than 2-fold increase in histone acetylation, accompanied by mitochondrial respiratory suppression. EW-induced mitochondrial respiratory suppression was exacerbated by TSA treatment in a manner that was attenuated by let-7f antagomir cotreatment. TSA treatment did not alter the increasing effect of EW on the let-7f level but dramatically exacerbated the cell death induced by EW. These data suggest that the multiple episodes of withdrawal from chronic ethanol impede mitochondrial and cellular integrity through upregulating histone acetylation, independent of or additively with let-7f.
Introduction
Long-term heavy drinking often results in alcoholism of which symptoms include craving for drinking, loss of self-control, organ damage, and withdrawal syndromes. Withdrawal syndromes such as anxiety and seizure can occur upon the abrupt termination of long-term heavy drinking. Many alcoholics repeat drinking and withdrawal, risking brain damage and premature death (George et al., 1990; Becker and Hale 1993; National Institute on Alcohol Abuse and Alcoholism, 1995; Krystal et al., 1997; Stephens et al., 2001). The premature death occurs even after complete abstinence (Marmot et al., 1981; Holahan et al., 2010). However, the exact mechanism underlying this problem and a preventative strategy remain obscure.
Mitochondria are the major source of cellular energy, critically affecting cell death and survival decision. Damaged mitochondria are unable to provide the ATP necessary for ATP-synthesizing enzyme and glutamate metabolism, provoking excitotoxic stress (Jamme et al., 1995; Eisenberg et al., 2000; Fighera et al., 2006). Mitochondria from ethanol withdrawn (EW) rats show oxidized mitochondrial proteins (Jung et al., 2008) and electron transfer deficit. In inner mitochondrial membranes, electrons are transferred across the four enzyme complexes (I–IV) for mitochondrial respiration and ATP synthesis. Of importance, cytochrome c oxidase (CcO) is the terminal enzyme complex (IV) that consumes most of mitochondrial O2 to help produce ATP. Because of its critical role in ATP production, damage to CcO inevitably impedes mitochondrial function, provoking aberrant neurotransmission accompanied by ATP loss (Sánchez-Prieto and González, 1988). Our previous study demonstrated that EW damages CcO, in turn suppressing mitochondrial respiration (Jung et al., 2011, 2012).
Histones, a protein family located in the core of the DNA, wrap and pack DNA inside the small nucleus. Recent studies have discovered that modification of histones, such as histone acetylation, plays an important role in gene expression, including the genes that regulate mitochondrial function (Wagner and Payne, 2011). Histone acetylation is regulated by a balance between histone acetyltransferase and histone deacetylase (Kwon et al., 2002). Histone acetyltransferases transfer acetyl moiety to lysine residues within histones, which enables transcriptional regulatory proteins to assess chromatin for gene activation (Kuo and Allis, 1998; Struhl, 1998; Peterson and Laniel, 2004). Histone deacetylases catalyze the removal of acetyl groups in histone (Kuo and Allis, 1998), inhibiting gene expression. Ethanol has been reported to increase histone acetylation. Treatment of hepatocytes with ethanol for 24 hours increases histone acetylation (Park et al., 2003). Ethanol is metabolized to acetaldehyde and subsequently to acetate. Acetate is converted to acetyl-coenzyme A (Ugarte and Iturriaga, 1976), which is required by histone acetyltransferase to acetylate histone (Breen et al., 1971; Smith et al., 1987). On the other hand, an increase or a decrease in histone acetylation has been reported in the brain of EW rats (Pandey et al., 2008; Pascual et al., 2012). These studies show that aberrant histone acetylation may mediate ethanol consumption or EW-related central nervous system disorders, making them important drug targets to manage alcoholism.
MicroRNA are a class of small, 18–23 nucleotide, noncoding RNA that largely downregulate gene expression through base pairing the 3′-untranslated region of (Djuranovic et al., 2012). A single microRNA can target hundreds of mRNA, affecting a variety of biologic as well as pathologic processes, including those associated with ethanol (Sathyan et al., 2007; Pietrzykowski et al., 2008; Tang et al., 2008; Miranda et al., 2010). A particular microRNA, let-7f, belongs to the let-7 family and has been associated with ethanol and mitochondria. Increased let-7f levels occur in the brain of postmortem human alcoholics (Lewohl et al., 2011) and in alcoholic rats (Jung and Metzger, 2014). Also, let-7f is highly expressed in human mitochondria (Sripada et al., 2012) and in cells whose death depends on mitochondria compared with mitochondria-independent cells (Shell et al., 2007).
As such, both histone acetylation and let-7f are implicated in the effect of ethanol and EW on mitochondria. However, it is unknown whether histone acetylation adversely influences mitochondria challenged by repeated EW and whether let-7f is involved in this process. To this end, we investigated whether histone acetylation plays a role in mitochondrial damage through let-7f under the condition of repeated EW. We used the model of repeated drinking/ethanol exposure and withdrawal because it mimics the drinking pattern of many alcoholics who repeat unsuccessful attempts at abstaining (George et al., 1990; Becker and Hale 1993; National Institute on Alcohol Abuse and Alcoholism, 1995; Krystal et al., 1997; Stephens et al., 2001). We report that repeated EW increases histone acetylation in a manner that suppresses mitochondrial respiration and cell survival. This effect of histone acetylation appears to occur independent of, or additively with, let-7f.
Materials and Methods
Major analytic reagents were purchased from Qiagen (Valencia, CA), Sigma-Aldrich (St. Louis, MO), Santa Cruz Biotechnology (Santa Cruz, CA), Mitosciences (Eugene, OR), and Seahorse Bioscience (North Billerica, MA). Reagents for histone acetylation assay were from Zymo Research (Orange, CA), Roche Diagnostic Corporation (Indianapolis, IN), and Bio-Rad Laboratories (Hercules, CA). Diet ingredients were obtained from Research Organics (Cleveland, OH) or MP Biomedicals (Irvine, CA). HT22 cells, a murine hippocampal cell line, were the generous gift of Dr. David Schubert (Salk Institute, San Diego, CA).
Animal Experimental Protocols.
Male Sprague-Dawley rats, aged 3 months, were housed individually at 22–25°C and 55% humidity, with ad libitum access to water and a 12-hour light/dark cycle. All animal experimentation was conducted in accordance with the Guide for the Care and Use of Laboratory Animals (National Research Council, 1996) and was approved by the University of North Texas Health Science Center Animal Care and Use Committee.
Ethanol Program.
Rats (seven rats/group) were assigned to two groups such that one group received an ethanol program consisting of two cycles of a liquid diet containing 7.5% (v/v) ethanol for 4 weeks followed by withdrawal for 2 weeks. Another group (control diet group) was fed a liquid diet with dextrin isocalorically substituted for ethanol. The concentration of ethanol was gradually increased to 7.5% during the first week of the ethanol program, as described in our previous study (Jung et al., 2011). The physical appearance and body weights were monitored daily. We did not include a chow pellet group to compare the effect of a dextrin and a chow pellet diet on study end points. We have repeatedly observed that there is no significant difference between a dextrin diet versus a regular chow diet in vital signs, including physical appearance and body weights (Supplemental Fig. 1). Animals were fed chow pellets during withdrawal periods.
For an in vitro ethanol program, HT22 cells (mouse hippocampal cell line) were used, as they have shown a consistent effect of EW on mitochondria and cell viability in our previous studies (Jung et al., 2011). HT22 cells were cultured in flasks until they reached 70% confluence, according to a method established by Perez et al. (2005). The cells were then subjected to the ethanol program consisting of two cycles of ethanol exposure (0 or 100 mM, 20 hours) and withdrawal (4 hours). 2-Deoxyglucose (2-DG; glycolysis inhibitor), trichostatin A (TSA; histone deacetylase inhibitor, histone acetylation promoter), or let-7f antagomir (inhibitor) were applied to HT22 cells during the first and second EW phases or during the corresponding time period of control cells.
Brain Tissue Extraction.
Rats were humanely sacrificed using the combination of xylazine (20 mg/kg i.p.) and ketamine (100 mg/kg i.p.), and the whole brain was harvested at the end of the ethanol program. The prefrontal cortex from each rat was used to measure mitochondrial respiration. A separate set of rats that received the same ethanol program was anesthetized and perfused intracardially with saline followed by 4% paraformaldehyde in phosphate-buffered saline (PBS). These fixed brain samples were used for gold immunolabeling to measure histone acetylation.
Gold Immunolabeling of Acetylated Histones Proteins.
Rats were deeply anesthetized with pentobarbital (50 mg/kg) and then perfused intracardially with saline, followed by 4% ice-cold paraformaldehyde fixative. After fixation, the brain sample was soaked in 10% sucrose, followed by 20% and then 30% sucrose (prepared in 0.1 M PBS, pH 7.4). The brain sample was then frozen, sectioned (20 µm) using a cryostat, and treated with the antibody for acetylated histones H3 (Lys 9) (Millipore, Billerica, MA) for gold immunolabeling, as described previously elsewhere (Pandey et al., 2006). Histones are a protein family, consisting of histones 1 through 5. We chose histone 3 (H3) because it is implicated in both ethanol (Pandey et al., 2008) and mitochondria (McBrian et al., 2013). The quantification of gold-immunolabeled proteins was performed using the Image Analysis System (Loats Associates, Westminster, MD) connected to a light microscope that calculated the number of immunogold particles/µ2 in the prefrontal cortex at high objective magnification (100×). The threshold of each image was set up in such a way that an area without staining gave zero counts. Under this condition, immunogold particles in the defined areas of five adjacent brain sections of each rat were counted, and the values were averaged for each rat and group.
Mitochondrial Respiration (Mitochondrial O2 Consumption Rate).
Mitochondrial O2 consumption rate was measured according to the manufacturer’s instruction (Seahorse Bioscience). For brain mitochondrial respiration, the prefrontal cortex was obtained from rats at the end of the ethanol diet program. Immediately thereafter, mitochondria were isolated by conventional differential centrifugation as described in our previous study (Jung et al., 2011). Mitochondria were then diluted with an extracellular flux (XF) assay solution (KCL, KH2PO4, MgCL2, HEPES, EGTA, and bovine serum albumin), and transferred into an XF24 analyzer (Seahorse Bioscience) cell culture plate. The plate was centrifuged (4°C, at 3000g), added with succinate (substrate), incubated (37°C), and loaded into the XF24 respirometer. For cell mitochondrial respiration, HT22 cells (800 cells/well) were seeded into each well of a XF24 cell plate, cultured, and subjected to the aforementioned ethanol program. The cell plate was then placed on an O2 sensor cartridge and inserted to the XF respirometer. TSA (0 or 400 nM) and/or let-7f antagomir (0 or 50 nM) was applied to HT22 cells during EW phases.
For both brain and HT22 cell samples, compounds that affect mitochondrial respiration were used to characterize the effect of repeated EW on mitochondrial respiration. Those compounds were ADP (1 mM), oligomycin (2 µM, ATP synthesis inhibitor), let-7f antagomir (50 nM, acute treatment), NaN3 (CcO inhibitor, 1 mM), or FCCP (carbonilcyanide p-triflouromethoxy phenylhydrazone) (uncoupler, 0.6 µM). They were added to an XF24 cell plate in a sequential order immediately after the XF24 respirometer read a basal O2 consumption rate. Data were normalized based on mitochondrial contents or cell numbers in each well.
Quantification of let-7f.
The level of let-7f was measured in prefrontal cortex and in HT22 cells at the end of the ethanol program. Total RNA was isolated from brain tissues or cells using RNeasy kit (Qiagen) and quantified using Agilent 2100 bioanalyzer (Agilent Technologies Inc., Santa Clara, CA). RNA was then reverse-transcribed to cDNA using TaqMan let-7f primers (for forward, 5′-GAAAGAGATTGATGTTT ATTTTAGAAG-3′; for reverse, 5′-AATTCACCTAAATTTATAATATCCTCT-3′) and the miScript reverse-transcription kit (Qiagen). Quantitative polymerase chain reaction reactions were performed as follows: 50°C for 2 minutes, 95°C for 10 minutes, followed by 40 cycles of 95°C for 15 seconds and 60°C for 1 minute. Cycle threshold (Ct) values were calculated with SDS software v.2.3 (Applied Biosystems, Foster City, CA) using automatic baseline settings and a threshold of 0.2. The comparative Ct method was used to calculate the relative microRNA expression. The Ct value of an internal control gene (U6) was measured and subtracted from the corresponding Ct value for let-7f gene to calculate the ∆Ct value.
Calcein-Acetoxymethyl Ester Viability Assay.
Cell viability was quantified using the membrane-permeant calcein-acetoxymethyl (calcein-AM) ester dye (Invitrogen, Carlsbad, CA). HT22 cells received the aforementioned ethanol program. The cells were then treated with calcein-AM for 30 minutes. TSA (0 or 400 nM) with or without a CcO inhibitor (NaN3, 5 µM) or let-7f antagomir (0 or 50 nM) was applied to cells during EW phases. After the removal of the medium from the 96-well plates, the cells were rinsed once with PBS and incubated in a solution of 2.5 µM calcein-AM in PBS. Twenty minutes later, we determined the fluorescence using a BioTek FL600 microplate reader (BioTek Instruments, Winooski, VT) with an excitation/emission filter set at 495/515 nm. Cell culture wells treated with methanol served as blanks. The results, obtained in relative fluorescent units, were expressed as the percentage of nonethanol, control media values.
Pearson Correlation Assay.
The physiologic relationship between mitochondrial respiration, histone acetylation, and let-7f was assessed using the prefrontal cortex obtained from rat study and Pearson correlation statistical assay. To do this, the level of let-7f was measured in the same rats that were used to measure histone acetylation, and mitochondrial respiration. The Pearson correlation coefficient (r), a degree of correlation between two independent variables, was computed between two variables: mitochondrial respiration versus histone acetylation, mitochondrial respiration versus let-7f, or histone acetylation versus let-7f. Results were presented as r and P values.
Statistical Analysis.
Student’s t test was used to analyze the data of histone acetylation. One- or two-way analysis of variance was used to analyze the data of one factor (basal mitochondrial respiration, let-7f level, or cell viability) or two factors [mitochondrial respiration with drug treatment (ethanol treatment × drug treatment)]. Analysis of variance was followed by a post hoc Tukey’s test to identify a specific difference between groups. Values were expressed as mean ± S.E.M. P < 0.05 was considered statistically significant.
Results
Characterization of the Mitochondrial Respiration of Ethanol Withdrawn Rat.
We characterized the mitochondrial respiration of EW rats using compounds that affect mitochondrial respiration (Fig. 1). The basal mitochondrial respiration without any drug treatment is lower in EW rats than control diet rats (P = 0.002). For control rats, the addition of ADP to mitochondria stimulates mitochondrial respiration as ADP is converted to ATP as a coupling reaction between mitochondrial respiration and ATP synthesis (P = 0.05 versus basal mitochondrial respiration). The addition of ATP synthase inhibitor oligomycin to mitochondria of control diet rats decreases mitochondrial respiration (P = 0.001 versus mitochondrial respiration stimulated by ADP treatment). FCCP uncouples mitochondrial respiration from ATP generation by dissipating the electrochemical gradient (membrane potential). FCCP treatment to mitochondria normally maximizes mitochondrial respiration to maintain electrochemical gradient across mitochondrial membranes as seen in the mitochondria of control diet rats (P < 0.0001 versus mitochondrial respiration suppressed by oligomycin treatment). By contrast, mitochondria from EW rats show a blunt response to the addition of ADP, oligomycin, or FCCP. The addition of CcO inhibitor (NaN3) inhibits the mitochondrial respiration of both control and EW rats (P < 0.002 versus mitochondrial respiration after FCCP treatment). These data suggest that the coupling function is not efficient in EW rats. They also indicate that EW impedes the maximal respiratory capacity of mitochondria in response to ATP demand.
Fig. 1.
Characterization of the mitochondrial respiration of EW rats. Young adult male rats received an ethanol diet program consisting of two cycles of an ethanol/control diet for 4 weeks and withdrawal for 2 weeks. At the end of the ethanol program, rats were humanely sacrificed, and their prefrontal cortex was collected to measure mitochondrial respiration using an XF respirometer. ADP (1 mM), oligomycin (ATP synthase inhibitor, 2 µM), FCCP (uncoupler, 0.6 µM), and NaN3 (CcO inhibitor, 5 µM) were added to mitochondria in a sequential order immediately after the basal respiration had been measured. For control rats, the addition of ADP increased mitochondrial respiration, which was subsequently decreased and then increased back by oligomycin and FCCP addition, respectively. EW rats show lower basal mitochondrial respiration (P = 0.002) and a lack of response to ADP, oligomycin, or FCCP compared with control diet rats. CcO inhibitor (NaN3) inhibited the mitochondrial respiration of both control and EW rats (P < 0.002). The time zero on the x-axis indicates the time when brain samples were loaded to the XF respirometer. Depicted values are mean ± S.E.M., n = 6 or 7 rats/group. Some S.E.M. values are not shown because they were small.
Effects of 2-DG (Glycolysis Inhibitor) on Mitochondrial Respiration.
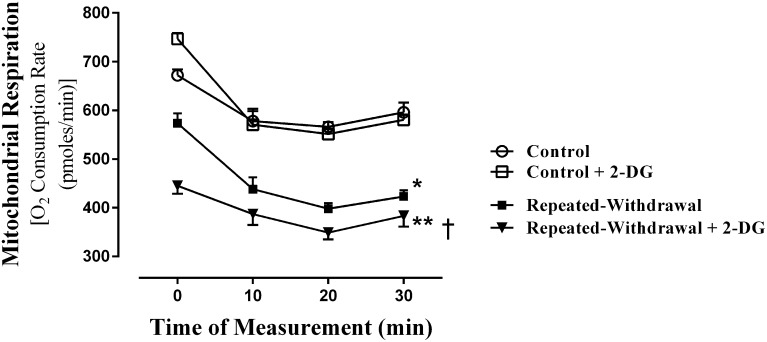
For most mammalian cells, the majority of cellular energy (38 ATP) is supplied from mitochondrial oxidative phosphorylation, and minimal ATP is supplied from glucose breakdown (glycolysis) (2 ATP) that takes place in cytoplasm. This difference in ATP production between mitochondrial oxidative phosphorylation and glycolysis is dramatically reduced in a disease condition, such as cancer (Nakashima et al., 1984). The 2-DG is phosphorylated by hexokinase, an enzyme that catalyzes the first step of glycolysis. This phosphorylated molecule (2-DG-6P) cannot be metabolized, thereby blocking the further process of glycolysis. Using 2-DG, we compared the extent of glycolysis-driven mitochondrial respiration between control and EW HT22 cells. Although 2-DG treatment does not significantly alter the mitochondrial respiration of control cells, it exacerbates (P < 0.001) the already suppressed (P < 0.002) mitochondrial respiration of EW cells (Fig. 2). This indicates that mitochondria from EW cells heavily rely on glycolysis.
Fig. 2.
Effects of 2-DG (glycolysis inhibitor) on mitochondrial respiration. HT22 cells were plated in 24-well microplate and subjected to an ethanol program consisting of two cycles of ethanol exposure (0 or 100 mM) for 20 hours and withdrawal for 4 hours. We added 2-DG during the EW phases. At the end of the program, the microplate was placed on the O2 sensor cartridge and inserted into the XF respirometer. The basal O2 consumption rate was measured at four time points. Compared with control cells, EW cells showed lower mitochondrial respiration in a manner that was further lowered by 2-DG treatment. *P < 0.002 or **P < 0.001 versus control cells. †P < 0.005 versus cells of repeated EW, n = 6 wells/each condition. Depicted values are mean ± S.E.M.
Effects of Repeated EW on Histone Acetylation.
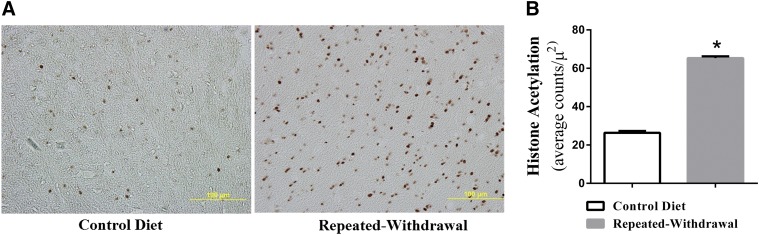
Previous investigations have shown a decrease or an increase in histone acetylation under the condition of EW. When measured 2 weeks after last ethanol feeding, we found a more than 2-fold increase (P < 0.0001) in histone acetylation in the prefrontal cortex of EW rats compared with control diet rats (Fig. 3). These results indicate that repeated EW from chronic ethanol feeding provokes the upregulation of histone acetylation in this brain area.
Fig. 3.
Effects of repeated EW on histone acetylation. Young adult male rats received an ethanol diet program consisting of two cycles of an ethanol/control diet for 4 weeks and withdrawal for 2 weeks. At the end of the ethanol program, rats were deeply anesthetized and subjected to the process of brain perfusion and fixation. Brains were sectioned and treated with the antibody against acetylated histone H3. Gold-immunolabeled protein was quantified using the Image Analysis System connected to a light microscope that calculated the number of immunogold particles/µ2 area of a defined prefrontal cortex area at high objective magnification (100×). Immunogold particles shown as dark deposits indicate acetylated histones (H3) (A). They are statistically significantly more populated in EW rats than control diet rats (B). *P < 0.0001 versus control diet rats. Depicted values are mean ± S.E.M., n = 5 slides/rat for four rats in each group.
Effects of Histone Acetylation on Mitochondrial Respiration.
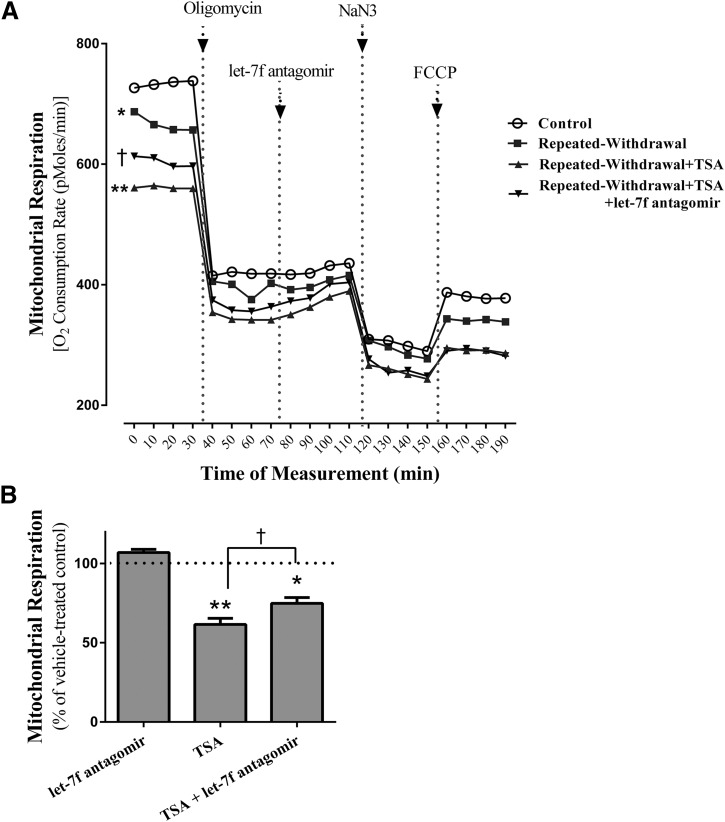
Numerous studies have used histone deacetylase inhibitor TSA to determine the involvement of histone acetylation in brain disorders associated with ethanol (Pascual et al., 2012; You et al., 2014). Using TSA, we determined whether histone acetylation is an upstream mediator that triggers EW-induced mitochondrial respiratory damage. We measured the mitochondrial respiration of EW cells in the presence or absence of TSA and/or let-7f antagomir. Repeated EW suppresses mitochondrial respiration (P < 0.01) in a manner that is further suppressed by TSA treatment (P < 0.002), most notably during the basal mitochondrial respiration (Fig. 4A). This effect of TSA was attenuated by the let-7f antagomir (P < 0.05) that was applied in both phases of EW (total 8 hours). However, when let-7f antagomir was acutely applied (marked with an arrow in Fig. 4A), it failed to improve mitochondrial respiration.
Fig. 4.
Effects of histone acetylation on mitochondrial respiration. HT22 cells (800 cells/well) were plated in 24-well microplates and subjected to an ethanol program consisting of two cycles of ethanol exposure (0 or 100 mM) for 20 hours and withdrawal for 4 hours. TSA and/or let-7f antagomir were added during both phases of EW. The microplate was then placed on the O2 sensor cartridge and inserted into the XF respirometry. The mitochondrial respiration was assessed by measuring the O2 consumption rate. Immediately after the basal O2 consumption rate had been read, we added oligomycin (2 µM), let-7f antagomir (50 nM), NaN3 (CcO inhibitor, 5 µM), and FCCP (0.6 µM) to cells in a sequential order. Compared with a control condition, repeated EW suppresses basal mitochondrial respiration (*P < 0.01) and further does so in the presence of TSA (**P < 0.002) (A). This effect of TSA is attenuated after let-7f antagomir cotreatment during the EW phases (†P < 0.05). However, acutely applied let-7f antagomir (marked with arrow in this figure) fails to alter mitochondrial respiration. For the effect of TSA on control cells (B), only basal respiration data are presented as a representative case. TSA decreased (**P < 0.001) the basal mitochondrial respiration of control cells in a manner that was attenuated by let-7f antagomir cotreatment (†P = 0.02). Depicted values are the mean for 16 wells/group; some S.E.M. are omitted for figure clarity. *P < 0.001; **P < 0.0001 versus control cells treated with vehicle at 100%.
CcO inhibitor (NaN3) treatment suppressed the mitochondrial respiration of both EW and control cells (P < 0.01). FCCP that was applied to cells subsequent to the CcO inhibitor treatment failed to maximize mitochondrial respiration. This suggests that mitochondria cannot reach maximal respiration after CcO is damaged. These data indicate that EW-induced histone acetylation impedes basal mitochondrial respiration and maximal respiratory capacity.
For the effect of TSA on the basal respiration of control cells (Fig. 4B), TSA treatment also decreased (P < 0.001) mitochondrial respiration in a manner that was attenuated by let-7f antagomir cotreatment (P = 0.02).
Effects of Histone Acetylation on the Level of let-7f.
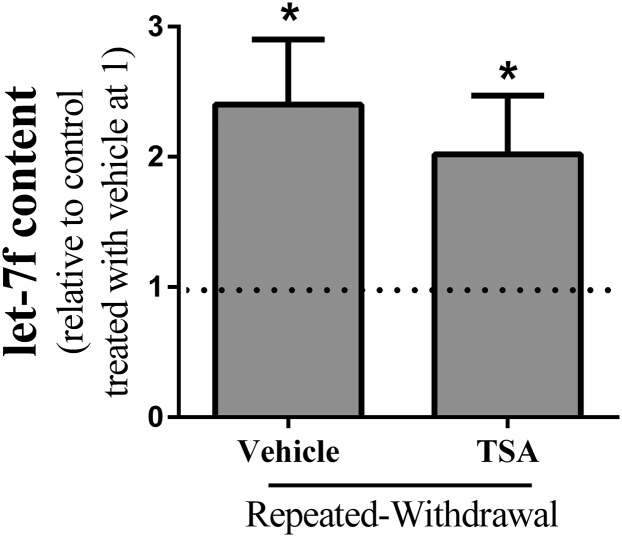
We determined whether EW-induced histone acetylation increases the level of let-7f. As we previously reported elsewhere (Jung and Metzger, 2014), repeated EW increases the level of let-7f (P < 0.001) (Fig. 5). However, TSA treatment during EW does not alter the level of let-7f in EW cells or control cells (data not shown). Given this, the aforementioned decreasing effect of TSA on mitochondrial respiration is unlikely through the effect of histone acetylation on let-7f.
Fig. 5.
Effects of histone acetylation on the level of let-7f. HT22 cells were subjected to an ethanol program consisting of two cycles of ethanol exposure (0 or 100 mM) for 20 hours and withdrawal for 4 hours. TSA was applied during both EW phases. At the end of the program, the total RNA were isolated and reverse-transcribed to cDNA using TaqMan let-7f primers and the miScript reverse-transcription kit. Quantitative PCR was performed to quantify the level of let-7f. An internal control gene (U6) was also measured and subtracted from the corresponding Ct value for let-7f gene to calculate the ∆Ct value. Compared with control cells without TSA treatment, EW cells showed an increase in the level of let-7f (*P < 0.001). This effect of EW was not altered by TSA treatment. Depicted are the mean ± S.E.M. for 6 wells/group. *P < 0.001 versus control cells without TSA treatment at 1 with dash line.
Effects of Histone Acetylation on Cell Viability.
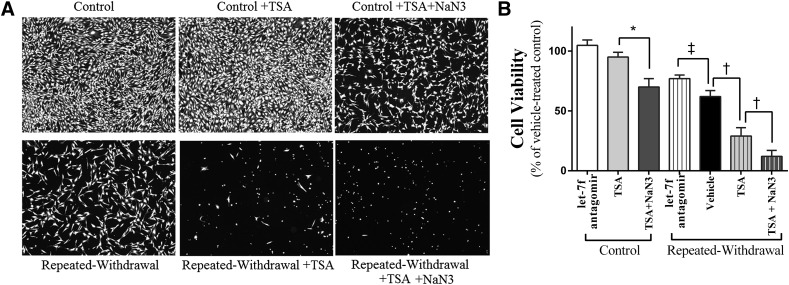
The cellular consequences of EW-induced histone acetylation were tested by assessing cell viability using a calcein-AM assay. The viability of control cells was not altered by TSA treatment but decreased by the combination of TSA with CcO inhibitor NaN3 treatment (P < 0.01). EW suppresses cell viability, and this effect was strikingly exacerbated by TSA treatment (P < 0.001) (Fig. 6). The cotreatment of TSA and NaN3 virtually left no live (spindle shape) EW cells. When let-7f antagomir was applied to ethanol-free control cells or EW cells, it tended to increase the cell viability of control cells or statistically significantly (P < 0.01) increased that of EW cells. These data indicate that CcO inhibition creates a milieu favorable for the adverse effect of TSA on cell survival. Notice that the viability of control cells treated with the combination of TSA with CcO inhibitor resembles that of vehicle-treated EW cells. Collectively, this raises a possibility that cell death induced by EW is attributed to the combined effect of histone acetylation upregulation, CcO damage, and excessive let-7f level.
Fig. 6.
Effects of histone acetylation on cell viability. HT22 cells were subjected to an ethanol program consisting of two cycles of ethanol exposure (0 or 100 mM) for 20 hours and withdrawal for 4 hours. TSA (0 or 400 nM) with or without NaN3 (0 or 5 µM), and let-7f antagomir (50 nM) alone were applied during EW phases or the corresponding time period of control cells. At the end of the program, cell viability was assessed using a calcein-AM assay. Microscopic cell populations were assessed using a fluorescence microscope at the objective magnification of 100× (A). Fully confluent, healthy cells with spindle-shaped morphology are shown in control cells. The viability of control cells was not altered by TSA treatment alone but decreased with the combination of TSA with the CcO inhibitor NaN3 (*P < 0.01). Healthy cells with spindle-shaped morphology were much less populated, hardly shown, or did not exist in EW cells treated with vehicle, TSA, or TSA + NaN3, respectfully. Except for control cells treated with TSA or let-7f antagomir, all treatment groups showed statistically significantly lower cell viability (P < 0.01) than the control cells treated with vehicle at dash line (statistical symbols of this difference are omitted for figure clarity); *P < 0.01 versus control cells treated with TSA; †P < 0.005 versus EW cells treated with vehicle or TSA + NaN3; ‡P < 0.01 versus EW cells treated with let-7f antagomir. Depicted values are mean ± S.E.M. for 16 wells/group.
Physiologic Relationship between Mitochondrial Respiration, Histone Acetylation, and let-7f.
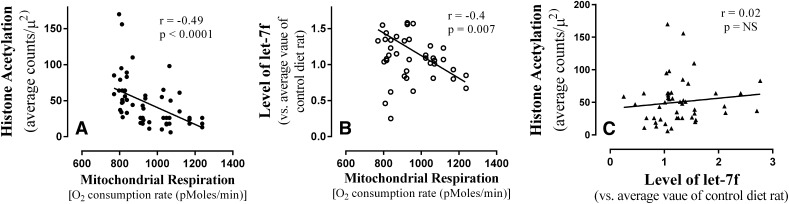
Pearson correlation assay indicated that there is a statistically significant inverse relationship between the level of mitochondrial respiration versus histone acetylation (r = −0.49, P < 0.0001) or let-7f (r = −0.40, P = 0.007) such that rats with a higher level of histone acetylation or let-7f showed a lower level of mitochondrial respiration (Fig. 7). However, no statistically significant correlation was found between the level of histone acetylation or let-7f among these rats. These data suggest that EW-induced upregulation of histone acetylation contributes to mitochondrial respiratory suppression independently or indirectly from the effect of let-7f.
Fig. 7.
The relationship between changes in mitochondrial respiration, let-7f, and histone acetylation. The levels of mitochondrial respiration, let-7f level, and histone acetylation were measured in the prefrontal cortex of rats using the method of XF respirometry, gold immunolabeling of acetylated histones proteins, and TaqMan let-7f primers, respectively. The data were analyzed using a Pearson correlation statistical assay to determine a physiologic relationship between changes in the three variables. The degree of mitochondrial respiration was inversely correlated with that of let-7f (r = −0.4, P = 0.007) and histone acetylation (r = −0.49, P < 0.0001), but no correlation was found between the level of let-7f and histone acetylation. Depicted are multiple data points from 14 rats.
Discussion
It is a common phenomenon that human alcoholics undergo multiple cycles of heavy drinking and withdrawal, and this increases the risk of brain damage (Ballenger and Post, 1978; Brown et al., 1988). Using the model of repeated ethanol consumption and withdrawal, our study has demonstrated that repeated EW from lengthy and heavy ethanol consumption increases histone acetylation in the brain of rats, accompanied by mitochondrial respiratory deficit. The mitochondrial deficit is exacerbated by TSA treatment but attenuated by the cotreatment with let-7f antagomir. These findings suggest that the multiple episodes of chronic ethanol consumption and withdrawal damage mitochondrial integrity through mechanisms involving the upregulation of histone acetylation and let-7f.
We first characterized the effect of repeated EW on mitochondrial respiration using compounds that affect mitochondria. Due to the coupling reaction with ATP generation, mitochondrial respiration is normally increased by ADP or uncoupler FCCP, and decreased by oligomycin treatment. However, none of the ADP, oligomycin, and FCCP treatments could alter mitochondrial respiration under repeated EW conditions, indicating an inefficient coupling function. The failure of ADP to stimulate mitochondrial respiration may also indicate that EW depletes ATP, and thus mitochondrial respiration becomes ATP independent. Instead, mitochondrial respiration during EW may be influenced by a second messenger molecule, cAMP. This view is based on a report that aberrant signaling mechanism associated with cAMP mediates EW-induced anxiety (Pandey et al., 2003). A CcO inhibitor decreased mitochondrial respiration in both control and EW rats, indicating the essential role of CcO in mitochondrial respiration. When mitochondrial ATP generation is malfunctioning, a less efficient way of ATP generation (glycolysis) is increased. The glycolysis inhibitor 2-DG significantly inhibited the mitochondrial respiration of EW cells, suggesting that EW cells heavily rely on glycolysis for ATP generation. These data demonstrate that mitochondria from EW rats undergo a respiratory and energetic crisis.
The acetylation of lysine residues of histone proteins regulates gene expression, including the genes affecting mitochondria (Wagner and Payne, 2011). The oncogene c-Myc contributes to histone acetylation by increasing the mitochondrial synthesis of acetyl-coenzyme A, which provides an acetyl moiety for histone acetylation (Morrish et al., 2010). The increased histone acetylation may activate genes of mitochondrial apoptotic molecules (Zhang et al., 2004), promoting apoptotic damage to mitochondria. Studies using histone deacetylase inhibitors (promoting histone acetylation) support this idea such that apicidin induces cytochrome c leakage, activating apoptotic caspases in human leukemia cells (Kwon et al., 2002). Suberic bishydroxamate induces the entry of apoptotic Bax to mitochondria (Zhang et al., 2004), triggering mitochondrial damage. We have demonstrated that repeated EW provokes the leakage of mitochondrial cytochrome c in male rats (Ju et al., 2012) and the collapse of mitochondrial membrane potential in HT22 cells (Jung et al., 2009). These studies suggest that aberrant histone acetylation has a potential to impede mitochondrial integrity (Bernhard et al., 1999; Burgess et al., 2001) through factors involving mitochondrial apoptosis.
Previous investigations of the effect of EW on histone acetylation have yielded controversial results. A decrease in histone acetylation occurs in the amygdaloid of EW rats (Pandey et al., 2008). TSA treatment attenuates EW-induced anxiety in rats (You et al., 2014), suggesting a protective effect of histone acetylation. More relevant to our current study, Pascual et al. (2012) observed that repeated ethanol feeding and withdrawal increased histone acetylation in the prefrontal cortex of adolescent rats but not in adult rats. By comparison, we observed a more than 2-fold increase in histone acetylation in EW adult rats (Fig. 1). Both our and Pascual’s studies used a program of repeated ethanol feeding and withdrawal, but our ethanol program was much longer (total 12 weeks) than theirs (2 weeks). This suggests that repeated EW from lengthy ethanol consumption may be more prone to increasing histone acetylation than that from short-term ethanol exposure. It is also possible that histone acetylation was initially decreased during the acute phase of EW and later increased as a rebound response in our study. Alternatively, ethanol-induced increase in histone acetylation (Park et al., 2003) might have persisted throughout the late phase of EW. Taken together, one can argue that the effects of repeated EW on histone acetylation depend on multiple factors, including brain region, the length of ethanol feeding, and an early versus late phase of EW.
We have recently demonstrated that EW-induced mitochondrial respiratory deficit is attenuated by let-7f antagomir treatment, suggesting that let-7f upregulation contributes to the mitochondrial deficit (Jung and Metzger, 2014). Increased let-7f has been shown in the postmortem brain of human alcoholics (Lewohl et al., 2011) and in alcoholic rats (Jung and Metzger, 2014). In particular, let-7f localizes in human mitochondria (Sripada et al., 2012) and inhibits CcO protein expression (Jung and Metzger, 2014). We thus tested whether let-7f mediates TSA’s inhibition of mitochondrial respiration. Although let-7f antagomir treatment during a few hours of EW attenuated the inhibiting effect of TSA on mitochondrial respiration, acutely applied let-7f antagomir failed to do so. This might reflect a slow genomic process. If TSA and let-7f act at the genomic level, this may be a slow process where a change in gene expression takes effect at the level of mitochondria.
We subsequently determined whether histone acetylation induced by TSA treatment increases let-7f level in HT22 cells. TSA treatment did not alter the level of let-7f either in control or EW cells (Fig. 5), suggesting no direct interaction between histone acetylation and let-7f. Presumably, histone acetylation and let-7f suppress mitochondrial respiration through pathways independent of each other. In support of this view, no correlation was found between the level of histone acetylation and let-7f (Fig. 7) in the rat brain. By comparison, a strong inverse correlation was observed between the levels of mitochondrial respiration versus histone acetylation or let-7f. These in vivo correlation data are consistent with the data of cell mitochondrial respiration, which was decreased and increased by TSA and let-7f antagomir treatment, respectively. This suggests that our cell model reflects a physiologic aspect at the level of mitochondrial respiration, histone acetylation, or let-7f.
Although histone acetylation largely activates gene expression, it also inhibits the expression such that histone deacetylase inhibitor phenylbutyrate represses CcO gene in flies (Kang et al., 2002). This raises a possibility that histone acetylation inhibits the CcO gene, thereby inhibiting mitochondrial respiration independently or additively with the inhibiting effect of let-7f on mitochondrial respiration. Notice that mitochondrial respiration after FCCP treatment is even lower than the basal level when FCCP is added to mitochondria subsequent to NaN3 (CcO inhibitor) (Fig. 4A). When CcO is not functioning, mitochondria may lose their ability to maximize respiration in response to FCCP. Given these results, complex serial and parallel mechanisms may simultaneously affect mitochondria through factors associated with histone acetylation, let-7f, and CcO upon repeated EW.
We finally determined a cellular consequence of an increase in histone acetylation by assessing the effect of TSA on cell viability. Studies on the effect of TSA on cell viability have yielded mixed results. TSA treatment protects rat cerebellar neurons from glutamate excitotoxicity (Leng and Chuang, 2006), controversially suppresses cell survival, and increases the apoptosis of dopaminergic neuronal cells (Wang et al., 2009). Histone deacetylase inhibitor suberic bishydroxamate increases mitochondrial membrane permeability and releases mitochondrial apoptogenic proteins in melanoma cells (Saito et al., 1999; Zhang et al., 2004). Of interest, the viability of control cells is not altered by TSA treatment but decreased by the combination of TSA and CcO inhibitor. Despite that histone acetylation induced by TSA suppresses mitochondrial respiration, control cells are resistant to the adverse effect of TSA on cell survival, perhaps through intact endogenous defense mechanisms. However, when CcO is damaged, it may create a mitochondrial milieu favorable for TSA’s cytotoxicity, overriding the defense mechanisms. In EW cells, CcO deficit (Jung et al., 2011) and histone acetylation are already occurring even in the absence of a CcO inhibitor or TSA treatment. Therefore, it is not surprising that the viability of vehicle-treated EW cells resembles that of control cells cotreated with a CcO inhibitor and TSA. Further insult to EW cells with TSA and/or CcO inhibitor virtually leaves few or no live cells.
In conclusion, our study provides empirical evidence that multiple withdrawals from lengthy and heavy drinking have a potential to activate histone acetylation and result in adverse downstream effects on mitochondrial and cellular homeostasis. Our findings may shed a new mechanistic insight into the role of histone acetylation and mitochondria for managing brain disorders associated with repeated unsuccessful attempts at abstaining.
Supplementary Material
Acknowledgments
The authors thank Anne-Marie Brun for technical support for this work.
Abbreviations
- calcein-AM
calcein-acetoxymethyl
- CcO
cytochrome c oxidase
- Ct
cycle threshold
- 2-DG
2-deoxyglucose
- EW
ethanol withdrawal/withdrawn
- FCCP
carbonilcyanide p-triflouromethoxy phenylhydrazone
- PBS
phosphate-buffered saline
- TSA
trichostatin A
- XF
extracellular flux
Authorship Contributions
Participated in research design: Jung.
Conducted experiments: Jung, Metzger.
Performed data analysis: Jung, Metzger.
Wrote or contributed to the writing of the manuscript: Jung, Metzger.
Footnotes
This work was supported by grants from the National Institutes of Health National Institute on Alcohol Abuse and Alcoholism [Grant AA018747]; and University of North Texas Health Science Center Institute for Aging and Alzheimer’s Disease (Grants IAADR-002 and IAADR-2014-001).
 This article has supplemental material available at jpet.aspetjournals.org.
This article has supplemental material available at jpet.aspetjournals.org.
References
- Ballenger JC, Post RM. (1978) Kindling as a model for alcohol withdrawal syndromes. Br J Psychiatry 133:1–14. [DOI] [PubMed] [Google Scholar]
- Becker HC, Hale RL. (1993) Repeated episodes of ethanol withdrawal potentiate the severity of subsequent withdrawal seizures: an animal model of alcohol withdrawal “kindling”. Alcohol Clin Exp Res 17:94–98. [DOI] [PubMed] [Google Scholar]
- Bernhard D, Ausserlechner MJ, Tonko M, Löffler M, Hartmann BL, Csordas A, Kofler R. (1999) Apoptosis induced by the histone deacetylase inhibitor sodium butyrate in human leukemic lymphoblasts. FASEB J 13:1991–2001. [DOI] [PubMed] [Google Scholar]
- Breen KJ, Shaw J, Levinson JD, Schenker S. (1971) The acute effect of alcohol on hepatic coenzyme A and acetyl CoA concentrations. Proc Soc Exp Biol Med 138:1096–1100. [DOI] [PubMed] [Google Scholar]
- Brown ME, Anton RF, Malcolm R, Ballenger JC. (1988) Alcohol detoxification and withdrawal seizures: clinical support for a kindling hypothesis. Biol Psychiatry 23:507–514. [DOI] [PubMed] [Google Scholar]
- Burgess AJ, Pavey S, Warrener R, Hunter LJ, Piva TJ, Musgrove EA, Saunders N, Parsons PG, Gabrielli BG. (2001) Up-regulation of p21(WAF1/CIP1) by histone deacetylase inhibitors reduces their cytotoxicity. Mol Pharmacol 60:828–837. [PubMed] [Google Scholar]
- Djuranovic S, Nahvi A, Green R. (2012) miRNA-mediated gene silencing by translational repression followed by mRNA deadenylation and decay. Science 336:237–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg D, Gill HS, Pfluegl GM, Rotstein SH. (2000) Structure-function relationships of glutamine synthetases. Biochim Biophys Acta 1477:122–145. [DOI] [PubMed] [Google Scholar]
- Fighera MR, Royes LF, Furian AF, Oliveira MS, Fiorenza NG, Frussa-Filho R, Petry JC, Coelho RC, Mello CF. (2006) GM1 ganglioside prevents seizures, Na+,K+-ATPase activity inhibition and oxidative stress induced by glutaric acid and pentylenetetrazole. Neurobiol Dis 22:611–623. [DOI] [PubMed] [Google Scholar]
- George DT, Nutt DJ, Dwyer BA, Linnoila M. (1990) Alcoholism and panic disorder: is the comorbidity more than coincidence? Acta Psychiatr Scand 81:97–107. [DOI] [PubMed] [Google Scholar]
- Holahan CJ, Schutte KK, Brennan PL, Holahan CK, Moos BS, Moos RH. (2010) Late-life alcohol consumption and 20-year mortality. Alcohol Clin Exp Res 34:1961–1971. [DOI] [PubMed] [Google Scholar]
- Jamme I, Petit E, Divoux D, Gerbi A, Maixent JM, Nouvelot A. (1995) Modulation of mouse cerebral Na+,K(+)-ATPase activity by oxygen free radicals. Neuroreport 7:333–337. [PubMed] [Google Scholar]
- Ju X, Mallet RT, Downey HF, Metzger DB, Jung ME. (2012) Intermittent hypoxia conditioning protects mitochondrial cytochrome c oxidase of rat cerebellum from ethanol withdrawal stress. J Appl Physiol (1985) 112:1706–1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung ME, Ju X, Metzger DB, Simpkins JW. (2012) Ethanol withdrawal hastens the aging of cytochrome c oxidase. Neurobiol Aging 33:618e621–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung ME, Ju X, Simpkins JW, Metzger DB, Yan LJ, Wen Y. (2011) Ethanol withdrawal acts as an age-specific stressor to activate cerebellar p38 kinase. Neurobiol Aging 32:2266–2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung ME, Metzger DB. (2014) MicroRNA let-7f mediates mitochondrial respiratory deficit induced by repeated ethanol exposure and withdrawal in HT22 cells. Int Neuropsychiatr Dis J 2:303–315. [Google Scholar]
- Jung ME, Wilson AM, Ju X, Wen Y, Metzger DB, Simpkins JW. (2009) Ethanol withdrawal provokes opening of the mitochondrial membrane permeability transition pore in an estrogen-preventable manner. J Pharmacol Exp Ther 328:692–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung ME, Yan LJ, Forster MJ, Simpkins JW. (2008) Ethanol withdrawal provokes mitochondrial injury in an estrogen preventable manner. J Bioenerg Biomembr 40:35–44. [DOI] [PubMed] [Google Scholar]
- Kang HL, Benzer S, Min KT. (2002) Life extension in Drosophila by feeding a drug. Proc Natl Acad Sci USA 99:838–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystal JH, Webb E, Grillon C, Cooney N, Casal L, Morgan CA, 3rd, Southwick SM, Davis M, Charney DS. (1997) Evidence of acoustic startle hyperreflexia in recently detoxified early onset male alcoholics: modulation by yohimbine and m-chlorophenylpiperazine (mCPP). Psychopharmacology (Berl) 131:207–215. [DOI] [PubMed] [Google Scholar]
- Kuo MH, Allis CD. (1998) Roles of histone acetyltransferases and deacetylases in gene regulation. BioEssays 20:615–626. [DOI] [PubMed] [Google Scholar]
- Kwon SH, Ahn SH, Kim YK, Bae GU, Yoon JW, Hong S, Lee HY, Lee YW, Lee HW, Han JW. (2002) Apicidin, a histone deacetylase inhibitor, induces apoptosis and Fas/Fas ligand expression in human acute promyelocytic leukemia cells. J Biol Chem 277:2073–2080. [DOI] [PubMed] [Google Scholar]
- Leng Y, Chuang DM. (2006) Endogenous alpha-synuclein is induced by valproic acid through histone deacetylase inhibition and participates in neuroprotection against glutamate-induced excitotoxicity. J Neurosci 26:7502–7512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewohl JM, Nunez YO, Dodd PR, Tiwari GR, Harris RA, Mayfield RD. (2011) Up-regulation of microRNAs in brain of human alcoholics. Alcohol Clin Exp Res 35:1928–1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmot MG, Rose G, Shipley MJ, Thomas BJ. (1981) Alcohol and mortality: a U-shaped curve. Lancet 1:580–583. [DOI] [PubMed] [Google Scholar]
- McBrian MA, Behbahan IS, Ferrari R, Su T, Huang TW, Li K, Hong CS, Christofk HR, Vogelauer M, Seligson DB, et al. (2013) Histone acetylation regulates intracellular pH. Mol Cell 49:310–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda RC, Pietrzykowski AZ, Tang Y, Sathyan P, Mayfield D, Keshavarzian A, Sampson W, Hereld D. (2010) MicroRNAs: master regulators of ethanol abuse and toxicity? Alcohol Clin Exp Res 34:575–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrish F, Noonan J, Perez-Olsen C, Gafken PR, Fitzgibbon M, Kelleher J, VanGilst M, Hockenbery D. (2010) Myc-dependent mitochondrial generation of acetyl-CoA contributes to fatty acid biosynthesis and histone acetylation during cell cycle entry. J Biol Chem 285:36267–36274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima RA, Paggi MG, Pedersen PL. (1984) Contributions of glycolysis and oxidative phosphorylation to adenosine 5′-triphosphate production in AS-30D hepatoma cells. Cancer Res 44:5702–5706. [PubMed] [Google Scholar]
- National Institute on Alcohol Abuse and Alcoholism (1995) The Physicians’ Guide to Helping Patients with Alcohol Problems. National Institute on Alcohol Abuse and Alcoholism, Rockville, MD. [Google Scholar]
- National Research Council (1996) Guide for the Care and Use of Laboratory Animals. 7th ed. National Academies Press, Washington, DC. [Google Scholar]
- Pandey SC, Roy A, Zhang H. (2003) The decreased phosphorylation of cyclic adenosine monophosphate (cAMP) response element binding (CREB) protein in the central amygdala acts as a molecular substrate for anxiety related to ethanol withdrawal in rats. Alcohol Clin Exp Res 27:396–409. [DOI] [PubMed] [Google Scholar]
- Pandey SC, Ugale R, Zhang H, Tang L, Prakash A. (2008) Brain chromatin remodeling: a novel mechanism of alcoholism. J Neurosci 28:3729–3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey SC, Zhang H, Roy A, Misra K. (2006) Central and medial amygdaloid brain-derived neurotrophic factor signaling plays a critical role in alcohol-drinking and anxiety-like behaviors. J Neurosci 26:8320–8331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park PH, Miller R, Shukla SD. (2003) Acetylation of histone H3 at lysine 9 by ethanol in rat hepatocytes. Biochem Biophys Res Commun 306:501–504. [DOI] [PubMed] [Google Scholar]
- Pascual M, Do Couto BR, Alfonso-Loeches S, Aguilar MA, Rodriguez-Arias M, Guerri C. (2012) Changes in histone acetylation in the prefrontal cortex of ethanol-exposed adolescent rats are associated with ethanol-induced place conditioning. Neuropharmacology 62:2309–2319. [DOI] [PubMed] [Google Scholar]
- Perez E, Liu R, Yang SH, Cai ZY, Covey DF, Simpkins JW. (2005) Neuroprotective effects of an estratriene analog are estrogen receptor independent in vitro and in vivo. Brain Res 1038:216–222. [DOI] [PubMed] [Google Scholar]
- Peterson CL, Laniel MA. (2004) Histones and histone modifications. Curr Biol 14:R546–R551. [DOI] [PubMed] [Google Scholar]
- Pietrzykowski AZ, Friesen RM, Martin GE, Puig SI, Nowak CL, Wynne PM, Siegelmann HT, Treistman SN. (2008) Posttranscriptional regulation of BK channel splice variant stability by miR-9 underlies neuroadaptation to alcohol. Neuron 59:274–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito A, Yamashita T, Mariko Y, Nosaka Y, Tsuchiya K, Ando T, Suzuki T, Tsuruo T, Nakanishi O. (1999) A synthetic inhibitor of histone deacetylase, MS-27-275, with marked in vivo antitumor activity against human tumors. Proc Natl Acad Sci USA 96:4592–4597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Prieto J, González P. (1988) Occurrence of a large Ca2+-independent release of glutamate during anoxia in isolated nerve terminals (synaptosomes). J Neurochem 50:1322–1324. [DOI] [PubMed] [Google Scholar]
- Sathyan P, Golden HB, Miranda RC. (2007) Competing interactions between micro-RNAs determine neural progenitor survival and proliferation after ethanol exposure: evidence from an ex vivo model of the fetal cerebral cortical neuroepithelium. J Neurosci 27:8546–8557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shell S, Park SM, Radjabi AR, Schickel R, Kistner EO, Jewell DA, Feig C, Lengyel E, Peter ME. (2007) Let-7 expression defines two differentiation stages of cancer. Proc Natl Acad Sci USA 104:11400–11405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CM, Israel BC, Iannucci J, Marino KA. (1987) Possible role of acetyl-CoA in the inhibition of CoA biosynthesis by ethanol in rats. J Nutr 117:452–459. [DOI] [PubMed] [Google Scholar]
- Sripada L, Tomar D, Prajapati P, Singh R, Singh AK, Singh R. (2012) Systematic analysis of small RNAs associated with human mitochondria by deep sequencing: detailed analysis of mitochondrial associated miRNA. PLoS ONE 7:e44873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens DN, Brown G, Duka T, Ripley TL. (2001) Impaired fear conditioning but enhanced seizure sensitivity in rats given repeated experience of withdrawal from alcohol. Eur J Neurosci 14:2023–2031. [DOI] [PubMed] [Google Scholar]
- Struhl K. (1998) Histone acetylation and transcriptional regulatory mechanisms. Genes Dev 12:599–606. [DOI] [PubMed] [Google Scholar]
- Tang R, Yu B, Zhang K, Chen D. (2008) Effects of supplementing two levels of magnesium aspartate and transportation stress on pork quality and gene expression of micro-calpain and calpastatin of finishing pigs. Arch Anim Nutr 62:415–425. [DOI] [PubMed] [Google Scholar]
- Ugarte G, Iturriaga H. (1976) Metabolic pathways of alcohol in the liver. Front Gastrointest Res 2:150–193. [DOI] [PubMed] [Google Scholar]
- Wagner GR, Payne RM. (2011) Mitochondrial acetylation and diseases of aging. J Aging Res 2011:234875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Wang X, Liu L, Wang X. (2009) HDAC inhibitor trichostatin A-inhibited survival of dopaminergic neuronal cells. Neurosci Lett 467:212–216. [DOI] [PubMed] [Google Scholar]
- You C, Zhang H, Sakharkar AJ, Teppen T, Pandey SC. (2014) Reversal of deficits in dendritic spines, BDNF and Arc expression in the amygdala during alcohol dependence by HDAC inhibitor treatment. Int J Neuropsychopharmacol 17:313–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XD, Gillespie SK, Borrow JM, Hersey P. (2004) The histone deacetylase inhibitor suberic bishydroxamate regulates the expression of multiple apoptotic mediators and induces mitochondria-dependent apoptosis of melanoma cells. Mol Cancer Ther 3:425–435. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.