Abstract
cAMP plays a critical role in regulating migration of various cancers. This role is context dependent and is determined by which of the two main cAMP sensors is at play: cAMP-dependent protein kinase or exchange protein directly activated by cAMP (EPAC). Recently, we have shown that the cAMP sensor protein EPAC1 promotes invasion/migration of pancreatic ductal adenocarcinoma (PDA) in vitro. In this study, we investigated the role of EPAC1 in invasion and metastasis of PDA in vivo, and evaluated the therapeutic potential of EPAC inhibitors as antimetastasis agents for this neoplasm. We employed an orthotopic metastatic mouse model in which the PDA cells MIA PaCa-2 were injected into the pancreas of athymic nude mice, and their local and distant spread was monitored by in vivo imaging and histologic evaluation of the number of metastatic foci in the liver. Either genetic suppression of EPAC1 or its pharmacologic inhibition with 3-(5-tert-butyl-isoxazol-3-yl)-2-[(3-chloro-phenyl)-hydrazono]-3-oxo-propionitrile, an EPAC-specific antagonist recently identified in our laboratory, decreased invasion and metastasis of the PDA cells. Mechanistically, EPAC1 promotes activation and trafficking of integrin β1, which plays an essential role in PDA migration and metastasis. Our data show that EPAC1 facilitates metastasis of PDA cells and EPAC1 might be a potential novel therapeutic target for developing antimetastasis agents for PDA.
Introduction
Pancreatic ductal adenocarcinoma (PDA) is one of the leading causes of cancer-related deaths worldwide and has a poor prognosis with ∼95% mortality rate (Kamangar et al., 2006; Raimondi et al., 2009). In addition to late diagnosis, the biggest factors behind such a dismal outlook are the aggressive nature and high metastatic potential of PDA (Keleg et al., 2003). The chemoradiation therapy currently approved for the treatment of this neoplasm has minimal efficacy, and, although surgical resection when possible can be curative, recurrence with distant metastases happens in the majority of patients (Cress et al., 2006; Winter et al., 2006). One of the biggest hurdles to developing effective therapeutic strategies is our incomplete understanding of the genetic and biochemical alterations that govern the progression of this cancer from well-differentiated intraepithelial neoplasia to highly metastatic and poorly differentiated PDA (Hezel et al., 2006).
The second messenger, cAMP, plays a critical role in regulating migration of various cell types, including cancer cells. However, this role is complex and depends on tissue type as well as the cAMP sensor transducing the signal (Burdyga et al., 2013). cAMP signals are mediated by two main protein families, cAMP-dependent protein kinase and exchange protein directly activated by cAMP (EPAC) (de Rooij et al., 1998; Kawasaki et al., 1998). The latter is composed of two isoforms, EPAC1 and 2, which primarily act as guanine nucleotide exchange factors that activate the small GTPases, Rap1 and Rap2 (de Rooij et al., 1998; Kawasaki et al., 1998). These small GTPases in turn mediate the vast majority of EPAC responses, which span a wide array of biologic functions, including the regulation of cell adhesion and migration in different cellular contexts (Enserink et al., 2004; Carmona et al., 2008).
It has been reported that EPAC1 is overexpressed in human PDA tissue compared with the surrounding normal pancreatic tissue (Lorenz et al., 2008). A number of studies have shown that EPAC1 plays an important role in regulating cancer migration, but this role appears to be context dependent. In melanoma, there is consensus that EPAC1 enhances metastasis, as has been shown in several in vitro and in vivo studies (Baljinnyam et al., 2010, 2011, 2014). In prostate cancer, some studies suggest that EPAC1 promotes migration and proliferation (Bailey et al., 2009; Misra and Pizzo, 2009, 2012), whereas others suggest an inhibitory role for the EPAC-selective activator 8-(4-chlorophenylthio)-2′-O-methyladenosine-3′, 5′-cyclic monophosphate (007) (Grandoch et al., 2009). However, it has been argued that it is actually 007’s indirect activation of cAMP-dependent protein kinase, rather than activation of EPAC1, that resulted in inhibition of migration and proliferation of prostate cancer (Menon et al., 2012). In ovarian cancer, EPAC1 seems to have promigratory effects in some cell lines (Ovcar3) (Rangarajan et al., 2003) and antimigratory effects in others (ES-2) (Bastian et al., 2009). To improve the development of antimetastasis therapeutic strategies, it is of paramount importance that we determine the role of EPAC1 in the context of each cancer and elucidate the mechanism by which it boosts or attenuates migration in each case.
We have recently shown that EPAC1 enhances migration/invasion of the two pancreatic cancer cell lines AsPC-1 and PANC-1 in vitro, without affecting their proliferation (Almahariq et al., 2013), a finding that was corroborated by a latter report (Burdyga et al., 2013). In this study, we investigated EPAC1’s role in the invasion and metastasis of PDA, and the potential of EPAC inhibitors as antimetastatic agents using an orthotopic metastatic PDA mouse model. We report in this work that genetic suppression and pharmacologic inhibition of EPAC1 reduce PDA metastasis. In addition, our results suggest that EPAC1 mediates Migration/invasion of PDA through regulation of integrin β1 (Itgβ1) activation and trafficking. The findings of this study have significant clinical implications, as they identify EPAC1 as a potential therapeutic target for preventing pancreatic cancer metastasis.
Materials and Methods
Cell Lines.
The pancreatic ductal adenocarcinoma cell lines AsPC1, PANC-1, and MIA PaCa-2, obtained from American Type Culture Collection (Manassas, VA), were maintained in glutamine containing RPMI 1640 medium (Thermo Scientific HyClone, Logan, UT) supplemented with 10% fetal bovine serum (Invitrogen, Grand Island, NY) at 37°C under 5% CO2. Stable cell lines with suppressed EPAC1 expression (Epac1-KD) or cells expressing firefly luciferase were generated using MISSION TRC (Sigma-Aldrich, St. Louis, MO) lentiviral-based short hairpin RNA (shRNA) or a lentiviral-based pGL4 luciferase reporter (Promega, Madison, WI), respectively, according to the manufacturer’s instructions. Two different clones were used to suppress EPAC1, C32 and C28. A nontargeting shRNA clone (Ctrl) was used as a control for the Epac1-knockdown experiments.
Small-Molecule Agonists/Antagonists.
8-(4-chlorophenylthio)-2′-O-methyladenosine-3′,5′-cyclic monophosphate, acetoxymethyl ester (007-AM), is a selective EPAC agonist (Vliem et al., 2008; Chepurny et al., 2009) (BioLog Life Science Institute, Bremen, Germany). Bisindolylmaleimide I (BIM I), 2,6-diamino-N-([1-(1-oxotridecyl)-2-piperidinyl] methyl) hexanamide (NPC 15437), and 3-[1-[3-(dimethylamino)propyl]-5-methoxy-1H-indol-3-yl]-4-(1H-indol-3-yl)-1H-pyrrole-2,5-dione (Gö 6983) are selective protein kinase C (PKC) antagonists (Toullec et al., 1991; Sullivan et al., 1992; Gschwendt et al., 1996) (Santa Cruz, Dallas, TX). The 3-(5-tert-butyl-isoxazol-3-yl)-2-[(3-chloro-phenyl)-hydrazono]-3-oxo-propionitrile (ESI-09) is a highly selective EPAC inhibitor recently identified in our laboratory (Almahariq et al., 2013) (BioLog Life Science Institute).
Antibodies.
The following antibodies were used for Western blotting: EPAC1 (4155), EPAC2 (4156), Itgβ1 (9699), Na+/K+ ATPase (3010), and ACTIN (4968) (Cell Signaling Technology, Danvers, MA). For fluorescence-activated cell sorter (FACS) analysis, the following antibodies were used: fluorescein isothiocyanate (FITC)–conjugated integrin β1 clone 12G10 (Abcam, Cambridge, MA) and FITC-conjugated Itgβ1 clone K-20 (Santa Cruz).
Animals.
Animal experiments were conducted in 6- to 8-week-old female athymic BALB/c nu/nu mice (Charles River Laboratories, Wilmington, MA). Use of all animals was in accordance with the guidelines of the University of Texas Medical Branch Institutional Animal Care and Use Committee.
Rap1 Activation Assay.
Cells were treated with 10 µM 007-AM for 10 minutes with or without pretreatment with 5 µM ESI-09 for 5 minutes. The activation of Rap1 was examined using an active Rap1 (GTP-bound) pull-down assay, as described previously (Mei et al., 2002).
Cell Viability Assay.
Cells were incubated for 24 hours at 37°C under 5% CO2 in the presence of 007-AM (10 µM), ESI-09 (5 µM), BIM I (1 µM), Gö 6983 (1 µM), or NPC 15437 (1 µM), and viability was measured by an alamarBlue cell viability assay (Life Technologies, Carlsbad, CA).
Luciferase Activity.
Luciferase-transduced MIA PaCa-2 cells were transfected with Ctrl- or Epac1-specific shRNA (Epac1-KD). Cells were then lysed, and luciferase activity was determined by a Luciferase Assay System assay (Promega). Bioluminescence was measured with a LmaxII 384 microplate reader (Molecular Devices, Sunnyvale, CA).
Transwell Migration/Invasion Assay.
The top chambers of 8-micron inserts (Corning, Tewksbury, MA) were coated with BD Matrigel Basement Membrane Matrix (50 µg/ml) (BD Biosciences, San Jose, CA). Cells were starved of serum for 24 hours, detached with 0.25% trypsin-EDTA, and pretreated with the EPAC agonist 007-AM (10 µM) alone or in combination with the EPAC inhibitor ESI-09 (5 µM) or PKC inhibitors BIM I (1 µM), NPC 15437 (1 µM), or Gö 6983 (1 µM) for 10 minutes in serum-free RPMI/0.25% bovine serum albumin (BSA). The bottom chamber was filled with RPMI/4% fetal bovine serum (FBS) and the same combination of drugs used for pretreatment. The cells (2× 105) were then added to the top chamber and incubated at 37°C under 5% CO2 for 20 hours. At the end of the incubation period, cells remaining inside the insert were removed, and the rest were fixed in methanol and stained with crystal violet. The numbers of migrated cells were counted in four different fields.
Wound-Healing Assay.
Cells were grown to 95–100% confluency and starved of serum for 24 hours before a scratch wound was made. The cells were treated with 007-AM (10 µM) alone or with ESI-09 (5 µM) or BIM I (1 µM) in serum-free RPMI for 30 minutes before adding 10% FBS. The cells were then incubated at 37°C under 5% CO2. The wound was imaged at 0 and 24 hours. Healing rate was determined by calculating the percentage of wound closure according to the following equation: % wound closure = (initial wound width − wound width 24 hours post-treatment)/initial wound width × 100. To make the results comparable across all assays, the widths of the initial wounds were all normalized to a 1-mm distance.
Surface Protein Isolation.
MIA PaCa-2 cells were seeded on a fibronectin matrix (Sigma-Aldrich), starved of serum for 24 hours, and treated with 007-AM (10 µM) alone or with ESI-09 (5 µM) or BIM I (1 µM) in serum-free RPMI for 45 minutes before surface proteins were biotinylated with EZ-link sulfo-NHS-SS-biotin (Pierce, Rockford, IL) and isolated according to the manufacturer’s instructions. Briefly, 0.25 mg/ml EZ-link sulfo-NHS-SS-biotin was added to the cells and incubated at 4°C for 30 minutes. Biotinylation was quenched, and the cells were harvested and centrifuged at 500g for 3 minutes. Cells were solubilized with the kit’s lysis buffer containing the protease inhibitor phenylmethanesulfonyl fluoride (Sigma-Aldrich) and incubated on ice for 30 minutes. The samples were centrifuged at 10,000g for 2 minutes at 4°C, and the supernatant containing biotinylated membrane proteins was incubated with NeutrAvidin gel slurry for 60 minutes at room temperature. Then surface proteins were eluted from the column with elution buffer containing 50 mM dithiothreitol. Approximately 15 µg biotinylated protein was separated by SDS-PAGE and transferred to a polyvinylidenedifluoride membrane, and surface Itgβ1 was probed by Western blotting. The plasma membrane protein Na+/K+ ATPase was used as a loading control. To obtain total Itgβ1, cells were treated as described for surface Itgβ1 isolation and lysed with SDS lysis buffer (2% SDS, 10% glycerol, 60 mM Tris, pH 6.8).
Flow Cytometry Analysis of Activation and Cell Surface Expression of Itgβ1.
MIA PaCa-2 cells were starved of serum for 24 hours and detached with 0.25% trypsin-EDTA for 5 minutes. Cells were then treated with 007-AM (10 µM) alone or in combination with ESI-09 (5 µM) or NPC 15437 (1 µM) in serum-free RPMI/0.25% BSA and incubated for 15 minutes at 37°C under 5% CO2. Cells were fixed in 4% paraformaldehyde for 12 minutes, and then active Itgβ1 was stained with 12G10 (1:100), which only recognizes the active conformation of Itgβ1 (Byron et al., 2009), and total Itgβ1 with K20 (1:5) for 30 minutes at 4°C in phosphate-buffered saline (PBS) containing 3% BSA. The samples were analyzed by FACS (FACSCalibur; BD Biosciences). The level of active Itgβ1 was determined by normalizing the mean fluorescence intensity of active Itgβ1 staining to total Itgβ1 mean fluorescence intensity.
Immunofluorescence Staining of Cell Surface Itgβ1.
Wild-type or Epac1-KD AsPC-1 and PANC1 cells were seeded on fibronectin-coated microscope slide cover slips and starved of serum overnight. Then cells were treated with vehicle dimethylsulfoxide or the EPAC inhibitor ESI-09 (5 µM) for ∼45 minutes and fixed with paraformaldehyde (∼12 minutes), followed by staining for total Itgβ1 with the FITC-conjugate antibody K20 (1:4) in nonpermeabilizing buffer for 2 hours at room temperature. Then nuclei were stained with the dye 4′,6′-diamidino-2-phenylindole, and cells were mounted on a microscope slide and visualized with an Olympus BX51 immunofluorescence microscope. Fluorescence intensity for Itgβ1 and cell nuclei was determined in four random fields for each sample using the SimplePCI6 imaging software. For comparison of different samples, fluorescence intensity of Itgβ1 was normalized to the fluorescence intensity of the nuclei from the same imaging field.
Orthotopic Mouse Model and In Vivo Imaging.
MIA PaCa-2 cells stably expressing firefly luciferase and transfected with nontargeting (Ctrl) or Epac1-specific shRNA-C32 (Epac1-KD) were grown to 80–90% confluency and detached with 0.25% trypsin-EDTA, washed with RPMI/10% FBS, and suspended in ice-cold PBS/Matrigel (1:1). Mice were anesthetized with isoflurane inhalation to effect. A small nose< >cone was used to maintain anesthesia during the procedure. A small incision (∼10 mm) was made through the skin overlying the spleen/pancreas. The spleen/pancreas were exteriorized, and MIA PaCa-2 was injected into the parenchyma of the pancreas (50 µl PBS/Matrigel suspension containing 1.5 × 106 cells). The spleen/pancreas were then returned into the abdominal cavity, and the incision (both muscle and skin layers) was reapproximated with surgical sutures. Sutures were removed 1 week post< >procedure. Treatment with ESI-09 (injection of 10 mg/kg i.p.) was initiated 2 days after injection of cells. For in vivo imaging, mice were injected with d-Luciferin (150 mg/kg in PBS; PerkinElmer, Waltham, MA), anesthetized with isoflurane, and then imaged with the IVIS Spectrum Pre-clinical In Vivo Imaging System (PerkinElmer).
Statistical Analysis.
Student t test was used for data analysis in this study, and results were considered as statistically significant if P values were <0.05.
Results
EPAC1 Facilitates Invasion and Metastasis of MIA PaCa-2 Cells.
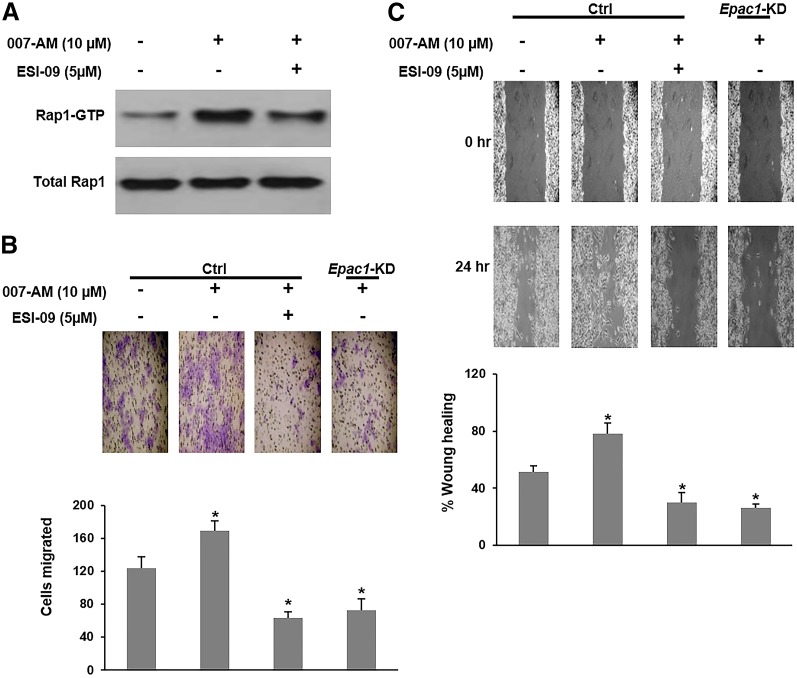
We have previously shown that EPAC1 is overexpressed in the PDA cells AsPC-1 and PANC-1 and facilitates their invasion/migration in vitro (Almahariq et al., 2013). To further determine whether EPAC1 plays an important role in PDA metastasis in vivo, we developed an orthotopic metastatic PDA mouse model using the PDA cells MIA PaCa-2. EPAC1 is highly expressed in MIA PaCa-2 cells, and its expression was successfully suppressed by shRNA (Supplemental Fig. 1A). In contrast, EPAC2 expression is undetectable (Supplemental Fig. 1B). To verify EPAC1’s activity in these cells, we examined the impact of its activation on the level of GTP-bound Rap1 (active form). Treatment with the EPAC-specific agonist 007-AM led to a significant increase in activation of the EPAC effector Rap1, and the EPAC inhibitor ESI-09 blunted its activation (Fig. 1A). Furthermore, similar to our findings in AsPC-1 and PANC-1 cells, activation of EPAC1 with 007-AM significantly increased invasion/migration of MIA PaCa-2 cells in wound-healing and Transwell invasion/migration assays, whereas pharmacologic inhibition with ESI-09 or shRNA silencing (clone 32) of EPAC1 expression completely abolished 007-AM’s stimulatory effect (Fig. 1B, 1C). To confirm the specificity of the antimigratory effect seen with EPAC1 suppression, we employed another shRNA sequence (clone 28) and obtained similar results (Supplemental Fig. 2). The pharmacologic treatment had no impact on cell viability in the time frame of the employed assays (Supplemental Fig. 3). These results confirm that EPAC1 plays an important role in facilitating PDA invasion and migration in vitro and MIA PaCa-2 cells are a viable candidate for testing EPAC1’s function in PDA metastasis.
Fig. 1.
EPAC1 inhibition or knockdown decreases invasion and migration of MIA PaCa-2. (A) Cells were treated with the EPAC agonist 007-AM in the presence or absence of the EPAC inhibitor ESI-09, and Rap1 activation (GTP-bound) was probed by Western blotting. (B) An invasion/migration assay showing an increase in invasion/migration of MIA PaCa-2 cells with 007-AM treatment and a decrease by Epac1-KD or ESI-09 treatment. (C) A wound-healing assay showing an increase in wound-closure rate of MIA PaCa-2 cells with 007-AM treatment and a decrease by Epac1-KD or ESI-09 treatment. Wound closure is presented as the distance traveled by the edge of the wound relative to the wound’s initial size. *Significantly higher or lower than vehicle-treated Ctrl MIA PaCa-2 cells (P < 0.03). Bars represent mean ± S.D. (n = 3).
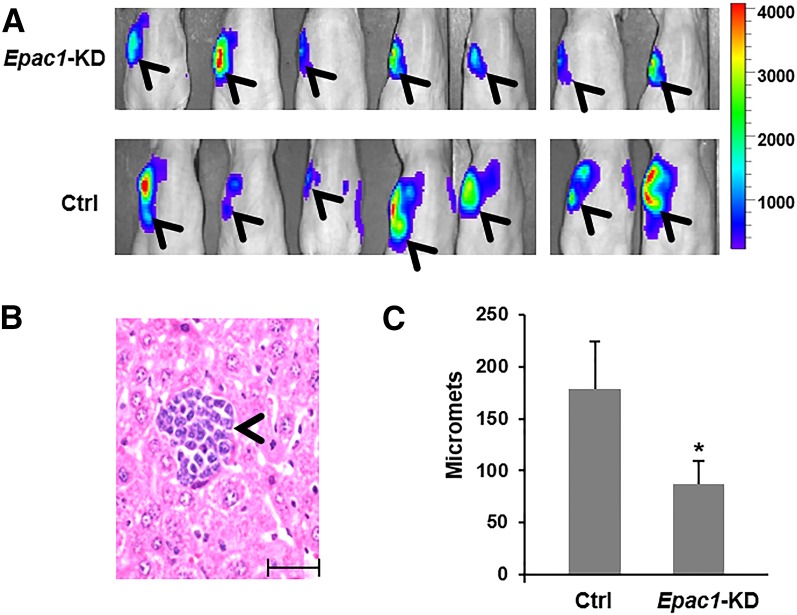
Subsequently, we transduced luciferase into Ctrl or Epac1-KD MIA PaCa-2 cells. Cells with comparable luciferase activity were then injected into the pancreas of athymic nude mice (Supplemental Fig. 4). Local invasion and metastasis were monitored in vivo using bioluminescence imaging, and, at the end of the experiment, metastasis was further quantified by the number of metastatic foci in the liver, which is one of the most common sites of PDA metastasis (Paik et al., 2012). Suppression of EPAC1 reduced local and distant spread of MIA PaCa-2 cells (Fig. 2A) and significantly decreased metastasis to the liver (Fig. 2B, 2C).
Fig. 2.
Suppression of EPAC1 reduces metastasis of MIA PaCa-2. (A) Cells were injected into the pancreas of athymic nude mice, and invasion/metastasis was monitored in vivo by bioluminescence imaging. The image shown was obtained 3 weeks post< >injection. Arrowheads show signal from the primary tumor and local invasion. (B) Representative image of H&E staining of the liver showing a metastatic focus (micromets) of MIA PaCa-2 cells (arrowhead); scale bar, 10 µm. (C) Quantification of liver micromets (number of micromets/H&E slide). For each mouse, the number of micromets is the average of two slides taken ∼20 µm apart. *Significantly lower than vehicle-treated group (P < 0.02). Bars represent mean ± S.D.
EPAC1 Promotes Trafficking of Itgβ1.
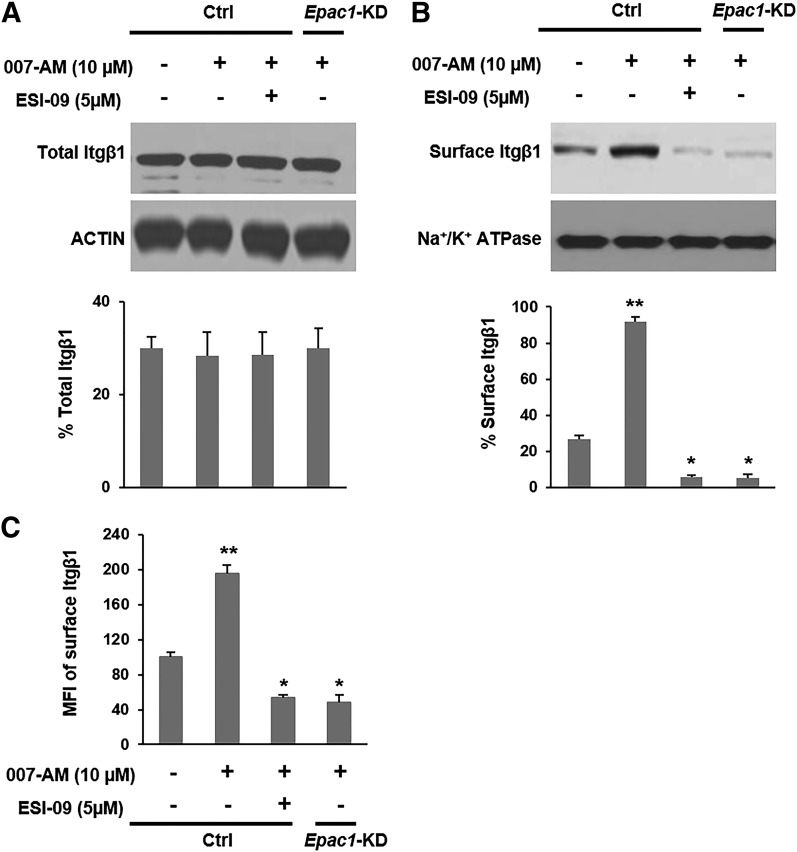
The expression, distribution, trafficking, and function of integrins are frequently altered in tumor cells in a manner that promotes cancer migration (Caswell and Norman, 2006). Itgβ1 is particularly important for invasion of PDA and plays an essential role in facilitating its metastasis (Vogelmann et al., 1999; Grzesiak et al., 2011). Several reports have implicated EPAC1 and its effector Rap1 in Itgβ1-mediated adhesion and migration of endothelial progenitor and immune cells (Lorenowicz et al., 2006; Carmona et al., 2008). Hence, we hypothesized that EPAC1 facilitates invasion/migration of PDA through an Itgβ1-related pathway. Neither activation nor pharmacologic inhibition or genetic knockdown of EPAC1 altered expression levels of Itgβ1 in MIA PaCa-2 cells (Fig. 3A). However, when cells were treated with the EPAC activator 007-AM (45 minutes), followed by biotinylation and isolation of surface proteins, the plasma membrane fraction of Itgβ1 was significantly increased, and this observed rise was completely negated by ESI-09 treatment or knockdown of EPAC1 (Fig. 3B). In fact, inhibition or suppression of EPAC1 reduced the membrane fraction of Itgβ1 below the basal level determined for vehicle-treated parental MIA PaCa-2 cells (Fig. 3B).
Fig. 3.
EPAC1 increases trafficking of integrin β1 to the plasma membrane. (A and B) MIA PaCa-2 cells were treated with 007-AM in the presence or absence of ESI-09, and total or plasma membrane proteins were isolated, respectively. Itgβ1 levels were probed by Western blotting, quantified by densitometry, and presented as a percentage of the indicated loading control. (C) Cells were trypsinized, and recovery of surface integrin β1 was probed by FACS. Data are presented as mean fluorescence intensity (MFI) and normalized to vehicle-treated Ctrl MIA PaCa-2 cells. **Significantly higher than vehicle-treated Ctrl cells (P < 0.01). *Significantly lower than vehicle-treated Ctrl cells (P < 0.02). Bars represent mean ± S.D. (n = 3).
Additionally, after cells were trypsinized, recovery of cell surface Itgβ1, even after only 15 minutes, was significantly enhanced by EPAC activation and attenuated by its inhibition or suppression (Fig. 3C). We also followed membrane expression levels of Itgβ1 by immunofluorescence in the PDA cell lines AsPC-1 and PANC-1 and found that inhibition/suppression of EPAC1 decreased the cell surface levels of Itgβ1 (Supplemental Fig. 5). Together, these results suggest that EPAC1 facilitates trafficking of Itgβ1 to the plasma membrane during invasion/migration.
EPAC1 Promotes Trafficking of Itgβ1 through PKC.
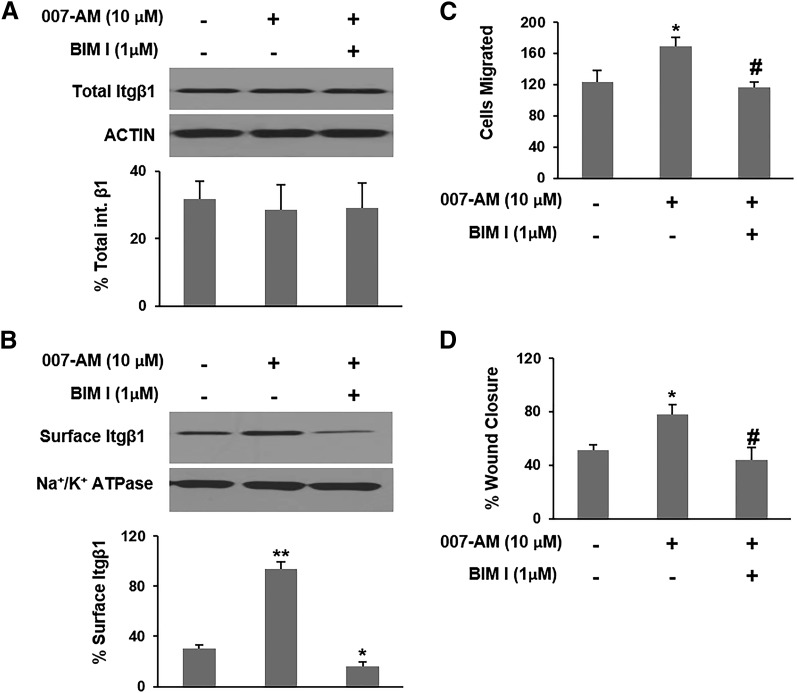
PKC regulates integrin trafficking and has been shown to promote migration of cancer cells (Wang et al., 2011; Al-Alem et al., 2013). Of note, during the integrin trafficking cycle, PKCε mediates the movement of Itgβ1 from the perinuclear recycling compartment (PNRC) to the plasma membrane (Caswell and Norman, 2006). Numerous studies have shown that EPAC activates PKC in various cell contexts, including PKCε (Hucho et al., 2005; Borland et al., 2009; Almahariq et al., 2014). Therefore, we reasoned that EPAC1 enhances trafficking of Itgβ1 to the plasma membrane in part through PKC activation. Similarly to the impact of inhibition and suppression of EPAC1, inhibition of PKC with the PKC-specific inhibitor BIM I had no impact on total Itgβ1 expression (Fig. 4A), but completely abrogated the increase in surface Itgβ1 seen with EPAC1 activation by 007-AM (Fig. 4B). Furthermore, inhibition of PKC negated the rise in invasion/migration observed with EPAC1 activation in Transwell invasion/migration and wound-healing assays (Fig. 4C, 4D).
Fig. 4.
EPAC1 increases trafficking of integrin β1 to the plasma membrane through PKC. MIA PaCa-2 cells were treated with 007-AM in the presence or absence of BIM I. (A and B) Total or plasma membrane proteins were isolated, respectively. Itgβ1 levels were probed by Western blotting, quantified by densitometry, and presented as a percentage of the indicated loading control. (C) Invasion/Migration was examined by a Transwell invasion/migration assay. (D) Wound-healing rate was examined in a wound-healing assay. Wound closure rate is presented as the distance traveled by the edge of the wound relative to the wound’s initial size. *Significantly higher or lower than vehicle-treated cells (P < 0.05). **Significantly higher than vehicle-treated cells (P < 0.02). #Significantly lower than 007-AM–treated cells (P < 0.03). Bars represent mean ± S.D. (n = 3).
To confirm the specificity of the observed response to BIM I treatment, we used two other PKC-specific inhibitors (NPC 15437 and Gö 6983). These inhibitors also blocked 007-AM’s stimulatory effect on invasion/migration of MIA PaCa-2 and Itgβ1 trafficking (Supplemental Fig. 6). The used PKC inhibitors had no impact on the viability of MIA PaCa-2 cells during the time frame of the essay (Supplemental Fig. 3). Together, these findings suggest that EPAC1 promotes Itgβ1 trafficking through the PKC pathway. Noticeably though, PKC inhibition (Fig. 4C, 4D) didn't reduce invasion/migration as effectively as did the inhibition of EPAC1 (Fig. 1B, 1C). This prompted us to search for potential additional mechanisms by which EPAC1 facilitates invasion/migration of PDA cells.
EPAC1 Promotes Activation of Itgβ1.
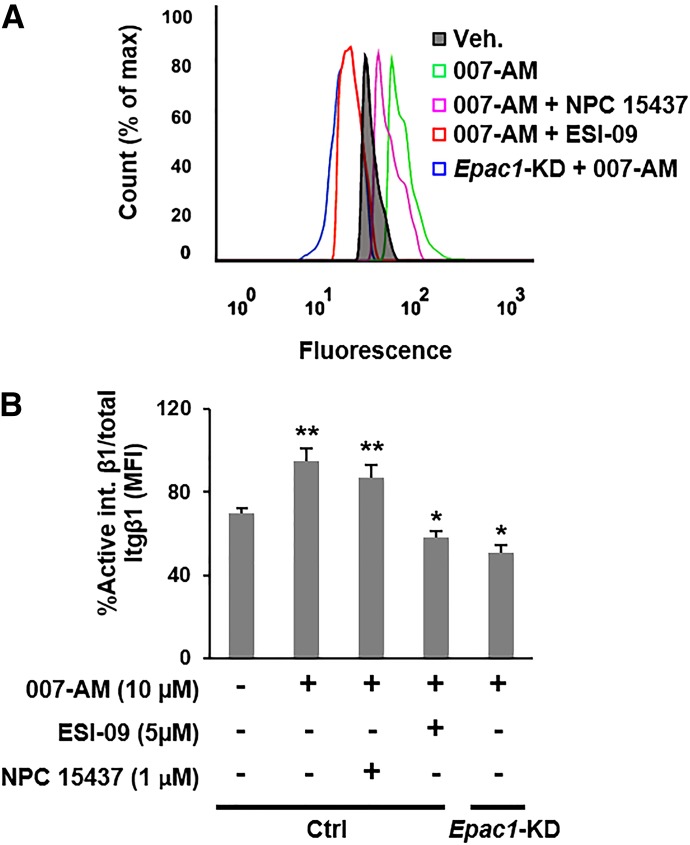
Integrins are usually present in an inactive conformation that has a low affinity for their ligand. A series of signaling events that involve the recruitment of various adaptor proteins to the cytosolic domain of the integrin are required for activation (inside-outside signaling) (Banno and Ginsberg, 2008). Several reports have shown that Rap1 plays a role in the integrin activation cascade (Bos, 2005; Han et al., 2006). To determine whether EPAC1 facilitates activation of Itgβ1 in PDA, we probed the activation status of Itgβ1 after EPAC1 activation or inhibition/suppression using an antibody that only recognizes the active conformation of Itgβ1 (12G10) (Byron et al., 2009). To account for the change in total surface Itgβ1 in response to altering EPAC1 activity, we normalized the fluorescence intensity of the integrin’s active conformation to the intensity of total surface Itgβ1 staining. Activation of EPAC1 by 007-AM significantly increased the fraction of active Itgβ1, and inhibition/suppression of EPAC1 lowered it below basal levels (Fig. 5). Moreover, inhibition of PKC with NPC 15437 did not affect Itgβ1 activation (Fig. 5). BIM I could not be used in FACS-based assays due to its strong and broad-spectrum autofluorescence. These results suggest that, in addition to regulating integrin trafficking, EPAC1 mediates activation of Itgβ1, and this effect is most likely PKC independent.
Fig. 5.
EPAC1 facilitates activation of integrin β1. Cells were treated with 007-AM in the presence or absence of ESI-09 or NPC 15437, and activation of integrin β1 was probed by FACS using the antibody 12G10, which only binds to the active form of integrin β1. Total integrin β1 was probed with the antibody K-20. (A) A representative histogram showing the binding of 12G10. (B) Quantification of active Itgβ1 relative to total Itgβ1 [mean fluorescence intensity (MFI)active/MFItotal]. **Significantly higher than vehicle-treated Ctrl cells (P < 0.03). *Significantly lower than vehicle-treated Ctrl cells (P < 0.04). Bars represent mean ± S.D. (n = 3).
Pharmacological Inhibition of EPAC Reduces PDA Metastasis.
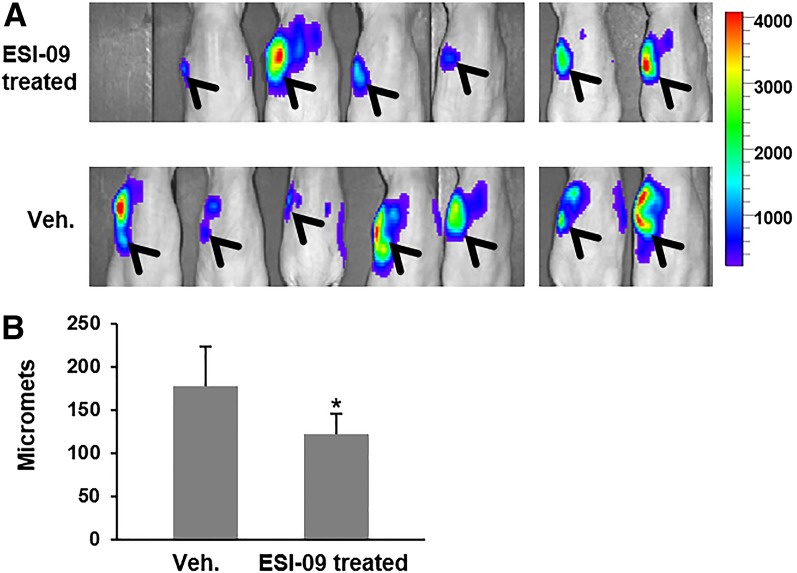
To determine whether inhibition of EPAC1 is a potentially viable therapeutic strategy for reducing metastasis of PDA, we employed the EPAC inhibitor recently discovered by our group, ESI-09 (Almahariq et al., 2013), in the orthotopic metastatic mouse model described earlier. We have previously shown that ESI-09 has excellent pharmacological activity in vivo and is capable of protecting mice from a lethal dose of rickettsial infection and recapitulating the EPAC1 knockout phenotype (Gong et al., 2013). Treatment with ESI-09 (10 mg/kg daily starting 2 days post-tumor injection) appeared to reduce local and distant spread of MIA PaCa-2 cells and significantly decreased metastasis to the liver (Fig. 6). These results suggest that EPAC1 is a potential target for developing antimetastasis agents for treatment of PDA.
Fig. 6.
Pharmacologic inhibition of EPAC1 reduces metastasis of MIA PaCa-2. Luciferase-transduced MIA PaCa-2 cells were injected into the pancreas of athymic nude mice, and animals were treated with ESI-09 (daily injection of 10 mg/kg i.p.) or vehicle. (A) In vivo bioluminescence image taken 3 weeks post injection of cells. Arrowheads show signal from the primary tumor and local invasion. (B) Quantification of liver micromets (number of micromets/H&E slide). For each mouse, the number of micromets is the average of two slides taken ∼20 µm apart. *Significantly lower than vehicle-treated group (P < 0.04). Bars represent mean ± S.D.
Discussion
Our study shows that EPAC1 promotes invasion and metastasis in PDA. Genetic suppression of EPAC1 in the PDA cell line MIA PaCa-2 inhibited their invasion/migration in vitro and local spread and metastasis to the liver in an orthotopic metastatic mouse model. Given previous reports by our group and others (Almahariq et al., 2013; Burdyga et al., 2013), showing that EPAC1 increases invasion/migration of the PDA cell lines AsPC-1 and PANC-1, it appears that, within the context of PDA, EPAC1’s promigratory role is a general one.
Mechanistically, our results show that EPAC1 promotes invasion/migration of PDA by enhancing Itgβ1 trafficking, which plays a crucial role in controlling cell mobility. During cell migration, integrins are internalized from the cell surface into endosomes and accumulate in the PNRC before being shuttled to the leading edge of the migrating cell (Caswell and Norman, 2006; Shin et al., 2012). This process is dynamic and occurs rapidly, with each step being tightly regulated by various interactions of a complex network of proteins (Shin et al., 2012). The PKC isoform PKCε is particularly important for mediating movement of Itgβ1 vesicles from the PNRC pool to the plasma membrane (Caswell and Norman, 2006). We found that the PKC-specific inhibitors BIM I and NPC 15437 negated the stimulatory impact of the EPAC agonist 007-AM on trafficking of Itgβ1 and invasion/migration of PDA. The inhibitor NPC 15437 preferentially targets novel isoenzymes of PKC, such as PKCε (Sullivan et al., 1992). Therefore, our results suggest that it is likely through the PKCε pathway that EPAC1 promotes Itgβ1 trafficking. This is in agreement with numerous reports showing EPAC activates PKC, including PKCε, through the phospholipase C pathway (Hucho et al., 2005; Borland et al., 2009; Almahariq et al., 2014). However, we cannot rule out the involvement of other PKC isoforms, and further studies are needed to determine the specific isoenzyme targeted by EPAC1 in the context of PDA.
Interestingly, although the impact of EPAC1 inhibition/suppression on Itgβ1 trafficking was mimicked by PKC inhibition, the latter did not suppress invasion/migration of MIA PaCa-2 to the same extent as the former did, prompting us to investigate additional mechanisms underlying EPAC1’s role in PDA migration. In addition to integrin trafficking, cells control their adhesion and migration by regulating the activation status of integrins (Banno and Ginsberg, 2008). Our results show that EPAC1 facilitates the activation of Itgβ1. This is in concordance with several reports showing the EPAC effector Rap1 activates integrins, including Itgβ1, through Rap1-GTP–interacting adaptor molecule, without affecting the overall surface expression levels of these proteins (Reedquist et al., 2000; Lafuente et al., 2004). In contrast, inhibition of PKC had no impact on the activation status of Itgβ1, suggesting EPAC1’s role in this mechanism is PKC independent.
To determine the druggability of EPAC1, we used ESI-09, an EPAC-specific inhibitor recently discovered in our laboratory (Almahariq et al., 2013). This compound was able to reduce invasion and metastasis of MIA PaCa-2, suggesting that EPAC1 is a potentially viable target for antimetastasis agents. However, the in vivo role of EPAC1 in metastasis of additional PDA cell lines and/or tumors extracted from patients must be examined to better assess and confirm the therapeutic potential of this protein in PDA treatment.
The findings of our study might carry significant clinical implications, as there is a dire need for mechanism-based therapeutic strategies for pancreatic cancer, especially ones that target the Itgβ1 activation and/or trafficking pathways. Numerous studies have shown that Itgβ1 mediates the malignant phenotype of PDA and facilitates loss of epithelial integrity and oncogenic transformation in epithelial tumors (Grzesiak and Bouvet, 2006; Lee et al., 2013; Onodera et al., 2014). In fact, constitutive activation of Itgβ1 is correlated with higher grade carcinomas (Lee et al., 2013). There are currently no available small molecules that target Itgβ1, but monoclonal antibodies and synthetic peptides against this integrin have shown significant clinical efficacy (Barkan and Chambers, 2011). Hence, EPAC inhibitors might provide a new approach to target Itgβ1 in cancer treatment.
Supplementary Material
Abbreviations
- 007-AM
8-(4-chlorophenylthio)-2′-O-methyladenosine-3′,5′-cyclic monophosphate, acetoxymethyl ester
- BIM I
bisindolylmaleimide I
- BSA
bovine serum albumin
- Ctrl
nontargeting shRNA clone control
- EPAC
exchange protein directly activated by cAMP
- ESI-09
3-(5-tert-butyl-isoxazol-3-yl)-2-[(3-chloro-phenyl)-hydrazono]-3-oxo-propionitrile
- FACS
fluorescence-activated cell sorter
- FBS
fetal bovine serum
- FITC
fluorescein isothiocyanate
- Gö 6983
3-[1-[3-(dimethylamino)propyl]-5-methoxy-1H-indol-3-yl]-4-(1H-indol-3-yl)-1H-pyrrole-2,5-dione
- NPC 15437
2,6-diamino-N-([1-(1-oxotridecyl)-2-piperidinyl] methyl) hexanamide
- PBS
phosphate-buffered saline
- PDA
pancreatic ductal adenocarcinoma
- PKC
protein kinase C
- PNRC
perinuclear recycling compartment
- shRNA
short hairpin RNA
Authorship Contributions
Participated in research design: Almahariq, Chao, Mei, Hellmich, Cheng.
Conducted experiments: Almahariq, Chao, Mei.
Contributed new reagents or analytic tools: Motamedi.
Performed data analysis: Almahariq, Patrikeev, Cheng.
Wrote or contributed to the writing of the manuscript: Almahariq, Cheng.
Footnotes
M.A. is a recipient of training fellowships from the Keck Center for Interdisciplinary Bioscience Training of the Gulf Coast Consortia supported by the National Institutes of Health National Institute of General Medical Sciences [Grant T32-GM89657-3], and the Biodefense Training Program at the University of Texas Medical Branch supported by the National Institutes of Health National Institute of Allergy and Infectious Diseases [Grant T32-AI60549-10]. X.C. is supported by National Institutes of Health National Institute of General Medical Sciences [Grants R01-GM066170 and R01-GM106218]. C.C. is a recipient of the National Cancer Institute Mentored Clinical Scientist Development Award [K08-CA125209]. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
 This article has supplemental material available at molpharm.aspetjournals.org.
This article has supplemental material available at molpharm.aspetjournals.org.
References
- Al-Alem LF, McCord LA, Southard RC, Kilgore MW, Curry TE., Jr (2013) Activation of the PKC pathway stimulates ovarian cancer cell proliferation, migration, and expression of MMP7 and MMP10. Biol Reprod 89:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almahariq M, Mei FC, Cheng X. (2014) Cyclic AMP sensor EPAC proteins and energy homeostasis. Trends Endocrinol Metab 25:60–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almahariq M, Tsalkova T, Mei FC, Chen H, Zhou J, Sastry SK, Schwede F, Cheng X. (2013) A novel EPAC-specific inhibitor suppresses pancreatic cancer cell migration and invasion. Mol Pharmacol 83:122–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey CL, Kelly P, Casey PJ. (2009) Activation of Rap1 promotes prostate cancer metastasis. Cancer Res 69:4962–4968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baljinnyam E, De Lorenzo MS, Xie LH, Iwatsubo M, Chen S, Goydos JS, Nowycky MC, Iwatsubo K. (2010) Exchange protein directly activated by cyclic AMP increases melanoma cell migration by a Ca2+-dependent mechanism. Cancer Res 70:5607–5617. [DOI] [PubMed] [Google Scholar]
- Baljinnyam E, Umemura M, Chuang C, De Lorenzo MS, Iwatsubo M, Chen S, Goydos JS, Ishikawa Y, Whitelock JM, Iwatsubo K. (2014) Epac1 increases migration of endothelial cells and melanoma cells via FGF2-mediated paracrine signaling. Pigment Cell Melanoma Res 27:611–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baljinnyam E, Umemura M, De Lorenzo MS, Iwatsubo M, Chen S, Goydos JS, Iwatsubo K. (2011) Epac1 promotes melanoma metastasis via modification of heparan sulfate. Pigment Cell Melanoma Res 24:680–687. [DOI] [PubMed] [Google Scholar]
- Banno A, Ginsberg MH. (2008) Integrin activation. Biochem Soc Trans 36:229–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkan D, Chambers AF. (2011) β1-integrin: a potential therapeutic target in the battle against cancer recurrence. Clin Cancer Res 17:7219–7223. [DOI] [PubMed] [Google Scholar]
- Bastian P, Balcarek A, Altanis C, Strell C, Niggemann B, Zaenker KS, Entschladen F. (2009) The inhibitory effect of norepinephrine on the migration of ES-2 ovarian carcinoma cells involves a Rap1-dependent pathway. Cancer Lett 274:218–224. [DOI] [PubMed] [Google Scholar]
- Borland G, Bird RJ, Palmer TM, Yarwood SJ. (2009) Activation of protein kinase Calpha by EPAC1 is required for the ERK- and CCAAT/enhancer-binding protein beta-dependent induction of the SOCS-3 gene by cyclic AMP in COS1 cells. J Biol Chem 284:17391–17403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos JL. (2005) Linking Rap to cell adhesion. Curr Opin Cell Biol 17:123–128. [DOI] [PubMed] [Google Scholar]
- Burdyga A, Conant A, Haynes L, Zhang J, Jalink K, Sutton R, Neoptolemos J, Costello E, Tepikin A. (2013) cAMP inhibits migration, ruffling and paxillin accumulation in focal adhesions of pancreatic ductal adenocarcinoma cells: effects of PKA and EPAC. Biochim Biophys Acta 1833:2664–2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byron A, Humphries JD, Askari JA, Craig SE, Mould AP, Humphries MJ. (2009) Anti-integrin monoclonal antibodies. J Cell Sci 122:4009–4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmona G, Chavakis E, Koehl U, Zeiher AM, Dimmeler S. (2008) Activation of Epac stimulates integrin-dependent homing of progenitor cells. Blood 111:2640–2646. [DOI] [PubMed] [Google Scholar]
- Caswell PT, Norman JC. (2006) Integrin trafficking and the control of cell migration. Traffic 7:14–21. [DOI] [PubMed] [Google Scholar]
- Chepurny OG, Leech CA, Kelley GG, Dzhura I, Dzhura E, Li X, Rindler MJ, Schwede F, Genieser HG, Holz GG. (2009) Enhanced Rap1 activation and insulin secretagogue properties of an acetoxymethyl ester of an Epac-selective cyclic AMP analog in rat INS-1 cells: studies with 8-pCPT-2′-O-Me-cAMP-AM. J Biol Chem 284:10728–10736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cress RD, Yin D, Clarke L, Bold R, Holly EA. (2006) Survival among patients with adenocarcinoma of the pancreas: a population-based study (United States). Cancer Causes Control 17:403–409. [DOI] [PubMed] [Google Scholar]
- de Rooij J, Zwartkruis FJ, Verheijen MH, Cool RH, Nijman SM, Wittinghofer A, Bos JL. (1998) Epac is a Rap1 guanine-nucleotide-exchange factor directly activated by cyclic AMP. Nature 396:474–477. [DOI] [PubMed] [Google Scholar]
- Enserink JM, Price LS, Methi T, Mahic M, Sonnenberg A, Bos JL, Taskén K. (2004) The cAMP-Epac-Rap1 pathway regulates cell spreading and cell adhesion to laminin-5 through the alpha3beta1 integrin but not the alpha6beta4 integrin. J Biol Chem 279:44889–44896. [DOI] [PubMed] [Google Scholar]
- Gong B, Shelite T, Mei FC, Ha T, Hu Y, Xu G, Chang Q, Wakamiya M, Ksiazek TG, Boor PJ, et al. (2013) Exchange protein directly activated by cAMP plays a critical role in bacterial invasion during fatal rickettsioses. Proc Natl Acad Sci USA 110:19615–19620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandoch M, Rose A, ter Braak M, Jendrossek V, Rübben H, Fischer JW, Schmidt M, Weber AA. (2009) Epac inhibits migration and proliferation of human prostate carcinoma cells. Br J Cancer 101:2038–2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grzesiak JJ, Bouvet M. (2006) The alpha2beta1 integrin mediates the malignant phenotype on type I collagen in pancreatic cancer cell lines. Br J Cancer 94:1311–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grzesiak JJ, Tran Cao HS, Burton DW, Kaushal S, Vargas F, Clopton P, Snyder CS, Deftos LJ, Hoffman RM, Bouvet M. (2011) Knockdown of the β(1) integrin subunit reduces primary tumor growth and inhibits pancreatic cancer metastasis. Int J Cancer 129:2905–2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gschwendt M, Dieterich S, Rennecke J, Kittstein W, Mueller HJ, Johannes FJ. (1996) Inhibition of protein kinase C mu by various inhibitors: differentiation from protein kinase C isoenzymes. FEBS Lett 392:77–80. [DOI] [PubMed] [Google Scholar]
- Han J, Lim CJ, Watanabe N, Soriani A, Ratnikov B, Calderwood DA, Puzon-McLaughlin W, Lafuente EM, Boussiotis VA, Shattil SJ, et al. (2006) Reconstructing and deconstructing agonist-induced activation of integrin alphaIIbbeta3. Curr Biol 16:1796–1806. [DOI] [PubMed] [Google Scholar]
- Hezel AF, Kimmelman AC, Stanger BZ, Bardeesy N, Depinho RA. (2006) Genetics and biology of pancreatic ductal adenocarcinoma. Genes Dev 20:1218–1249. [DOI] [PubMed] [Google Scholar]
- Hucho TB, Dina OA, Levine JD. (2005) Epac mediates a cAMP-to-PKC signaling in inflammatory pain: an isolectin B4(+) neuron-specific mechanism. J Neurosci 25:6119–6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamangar F, Dores GM, Anderson WF. (2006) Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol 24:2137–2150. [DOI] [PubMed] [Google Scholar]
- Kawasaki H, Springett GM, Mochizuki N, Toki S, Nakaya M, Matsuda M, Housman DE, Graybiel AM. (1998) A family of cAMP-binding proteins that directly activate Rap1. Science 282:2275–2279. [DOI] [PubMed] [Google Scholar]
- Keleg S, Büchler P, Ludwig R, Büchler MW, Friess H. (2003) Invasion and metastasis in pancreatic cancer. Mol Cancer 2:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafuente EM, van Puijenbroek AA, Krause M, Carman CV, Freeman GJ, Berezovskaya A, Constantine E, Springer TA, Gertler FB, Boussiotis VA. (2004) RIAM, an Ena/VASP and Profilin ligand, interacts with Rap1-GTP and mediates Rap1-induced adhesion. Dev Cell 7:585–595. [DOI] [PubMed] [Google Scholar]
- Lee YC, Jin JK, Cheng CJ, Huang CF, Song JH, Huang M, Brown WS, Zhang S, Yu-Lee LY, Yeh ET, et al. (2013) Targeting constitutively activated β1 integrins inhibits prostate cancer metastasis. Mol Cancer Res 11:405–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenowicz MJ, van Gils J, de Boer M, Hordijk PL, Fernandez-Borja M. (2006) Epac1-Rap1 signaling regulates monocyte adhesion and chemotaxis. J Leukoc Biol 80:1542–1552. [DOI] [PubMed] [Google Scholar]
- Lorenz R, Aleksic T, Wagner M, Adler G, Weber CK. (2008) The cAMP/Epac1/Rap1 pathway in pancreatic carcinoma. Pancreas 37:102–103. [DOI] [PubMed] [Google Scholar]
- Mei FC, Qiao J, Tsygankova OM, Meinkoth JL, Quilliam LA, Cheng X. (2002) Differential signaling of cyclic AMP: opposing effects of exchange protein directly activated by cyclic AMP and cAMP-dependent protein kinase on protein kinase B activation. J Biol Chem 277:11497–11504. [DOI] [PubMed] [Google Scholar]
- Menon J, Doebele RC, Gomes S, Bevilacqua E, Reindl KM, Rosner MR. (2012) A novel interplay between Rap1 and PKA regulates induction of angiogenesis in prostate cancer. PLoS One 7:e49893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra UK, Pizzo SV. (2009) Epac1-induced cellular proliferation in prostate cancer cells is mediated by B-Raf/ERK and mTOR signaling cascades. J Cell Biochem 108:998–1011. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Misra UK, Pizzo SV. (2012) Upregulation of mTORC2 activation by the selective agonist of EPAC, 8-CPT-2Me-cAMP, in prostate cancer cells: assembly of a multiprotein signaling complex. J Cell Biochem 113:1488–1500. [DOI] [PubMed] [Google Scholar]
- Onodera Y, Nam JM, Bissell MJ. (2014) Increased sugar uptake promotes oncogenesis via EPAC/RAP1 and O-GlcNAc pathways. J Clin Invest 124:367–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paik KY, Choi SH, Heo JS, Choi DW. (2012) Analysis of liver metastasis after resection for pancreatic ductal adenocarcinoma. World J Gastrointest Oncol 4:109–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raimondi S, Maisonneuve P, Lowenfels AB. (2009) Epidemiology of pancreatic cancer: an overview. Nat Rev Gastroenterol Hepatol 6:699–708. [DOI] [PubMed] [Google Scholar]
- Rangarajan S, Enserink JM, Kuiperij HB, de Rooij J, Price LS, Schwede F, Bos JL. (2003) Cyclic AMP induces integrin-mediated cell adhesion through Epac and Rap1 upon stimulation of the beta 2-adrenergic receptor. J Cell Biol 160:487–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reedquist KA, Ross E, Koop EA, Wolthuis RM, Zwartkruis FJ, van Kooyk Y, Salmon M, Buckley CD, Bos JL. (2000) The small GTPase, Rap1, mediates CD31-induced integrin adhesion. J Cell Biol 148:1151–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin S, Wolgamott L, Yoon SO. (2012) Integrin trafficking and tumor progression. Int J Cell Biol 2012:516789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan JP, Connor JR, Shearer BG, Burch RM. (1992) 2,6-Diamino-N-([1-(1-oxotridecyl)-2-piperidinyl] methyl)hexanamide (NPC 15437): a novel inhibitor of protein kinase C interacting at the regulatory domain. Mol Pharmacol 41:38–44. [PubMed] [Google Scholar]
- Toullec D, Pianetti P, Coste H, Bellevergue P, Grand-Perret T, Ajakane M, Baudet V, Boissin P, Boursier E, Loriolle F, et al. (1991) The bisindolylmaleimide GF 109203X is a potent and selective inhibitor of protein kinase C. J Biol Chem 266:15771–15781. [PubMed] [Google Scholar]
- Vliem MJ, Ponsioen B, Schwede F, Pannekoek WJ, Riedl J, Kooistra MR, Jalink K, Genieser HG, Bos JL, Rehmann H. (2008) 8-pCPT-2′-O-Me-cAMP-AM: an improved Epac-selective cAMP analogue. ChemBioChem 9:2052–2054. [DOI] [PubMed] [Google Scholar]
- Vogelmann R, Kreuser ED, Adler G, Lutz MP. (1999) Integrin alpha6beta1 role in metastatic behavior of human pancreatic carcinoma cells. Int J Cancer 80:791–795. [DOI] [PubMed] [Google Scholar]
- Wang J, Wu J, Hong J, Chen R, Xu K, Niu W, Peng C, Liu E, Wang J, Liu S, et al. (2011) PKC promotes the migration of colon cancer cells by regulating the internalization and recycling of integrin αvβ6. Cancer Lett 311:38–47. [DOI] [PubMed] [Google Scholar]
- Winter JM, Cameron JL, Campbell KA, Arnold MA, Chang DC, Coleman J, Hodgin MB, Sauter PK, Hruban RH, Riall TS, et al. (2006) 1423 pancreaticoduodenectomies for pancreatic cancer: a single-institution experience. J Gastrointest Surg 10:1199–1210. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.