Abstract
Proliferating cell nuclear antigen (PCNA) is a highly conserved protein necessary for proper component loading during the DNA replication and repair process. Proteins make a connection within the interdomain connector loop of PCNA, and much of the regulation is a result of the inherent competition for this docking site. If this target region of PCNA is modified, the DNA replication and repair process in cancer cells is potentially altered. Exploitation of this cancer-associated region has implications for targeted breast cancer therapy. In the present communication, we characterize a novel peptide (caPeptide) that has been synthesized to mimic the sequence identified as critical to the cancer-associated isoform of PCNA. This peptide is delivered into cells using a nine-arginine linking mechanism, and the resulting peptide (R9-cc-caPeptide) exhibits cytotoxicity in a triple-negative breast cancer cell line, MDA-MB-436, while having less of an effect on the normal counterparts (MCF10A and primary breast epithelial cells). The novel peptide was then evaluated for cytotoxicity using various in vivo techniques, including ATP activity assays, flow cytometry, and clonogenetic assays. This cytotoxicity has been observed in other breast cancer cell lines (MCF7 and HCC1937) and other forms of cancer (pancreatic and lymphoma). R9-cc-caPeptide has also been shown to block the association of PCNA with chromatin. Alanine scanning of the peptide sequence, combined with preliminary in silico modeling, gives insight to the disruptive ability and the molecular mechanism of action of the therapeutic peptide in vivo.
Introduction
Proliferating cell nuclear antigen (PCNA) is an evolutionarily conserved protein that is critically important to many cellular processes (Prosperi, 1997). During DNA replication, this 36-kDa protein forms a homotrimer encircling the DNA strand and acts as a scaffold to systematically load proteins and enzymes. Immunohistochemical (IHC) staining of breast cancer tissue samples exhibits a pattern of increased PCNA expression (Tahan et al., 1993), as compared with unaffected epithelial tissue adjacent to the tumor site. This increased PCNA expression in breast cancer is associated with axillary node status, p53 overexpression, shorter disease-free survival, and shorter overall survival (Chu et al., 1998).
Mutagenic analyses show that the DNA replication machinery derived from malignant breast cell lines and actual tumor tissue replicate DNA in a significantly more error-prone manner as compared with the replication machinery derived from nonmalignant counterparts (Sekowski et al., 1998). A structural comparison of the components from both normal and malignant cell lines using two-dimensional SDS-PAGE analysis revealed a unique form of PCNA present only in malignant breast cells (Bechtel et al., 1998). These malignant cells harbor an additional isoform of PCNA with an acidic pI, as opposed to the normal cells, which only contain PCNA with a basic pI. Similar PCNA profiles are present in other types of cancer, including neuroblastoma (Sandoval et al., 2005), hepatic carcinoma (Venturi et al., 2008), and high-grade prostatic intraepithelial neoplasia and prostate cancer (Wang et al., 2011). The newly identified cancer-associated acidic isoform of PCNA (caPCNA) results from a set of post-translational modifications (Hoelz et al., 2006). Previous studies have shown that PCNA can be post-translationally modified by phosphorylation (Wang et al., 2006), acetylation (Naryzhny and Lee, 2004), ubiquitination, and SUMOylation (Hoege et al., 2002; Stelter and Ulrich, 2003; Kannouche and Lehmann, 2004; Kannouche et al., 2004; Watanabe et al., 2004; Garg and Burgers, 2005; Sabbioneda et al., 2008; van der Kemp et al., 2009; Krijger et al., 2011). These modifications act as regulators of PCNA activity in normal cellular processes, whereas others have yet to be fully understood. These uncharacterized alterations could be key to cancer development and progression.
A PCNA monomer has two topologically similar domains linked head to tail. These domains are connected by a crossover loop, referred to as the interdomain connector loop (IDCL). X-ray crystallograms of PCNA have shown that PCNA exhibits increased mobility within the IDCL (Bruning and Shamoo, 2004), indicating that a number of conformations are possible in this region to accommodate a myriad of interactions. In fact, a majority of the proteins interacting with PCNA do so within the IDCL via a conserved motif known as the PCNA-interacting protein box (PIP-box). The PIP-box generally consists of an extended N-terminal region, a central conserved region containing hydrophobic residues, a 310-helix, and a C-terminal region that varies in length. The single-turn 310-helix displays a side chain residue that fits like a “plug” in the hydrophobic pocket of the PCNA IDCL (Bruning and Shamoo, 2004). The helical conformation brings the LXXFF region to the side of the structure, allowing for hydrogen bonding with the glutamine within the IDCL (Chapados et al., 2004).
The commonality of PCNA-binding motifs suggests that regulation depends on the competition of proteins within the interaction site, making the IDCL of PCNA a fascinating therapeutic target (Kontopidis et al., 2005). Much of the recent work to inhibit PCNA interactions focuses on blocking the IDCL region by synthesizing peptides and small molecules to mimic the binding of partner proteins, such as p21 (Zheleva et al., 2000; Kontopidis et al., 2005; Warbrick, 2006; Punchihewa et al., 2012). These p21-derived agents can specifically disrupt protein-PCNA interactions and have been shown to block replication and cell cycle progression, both in vitro and in vivo. This approach, however, most likely will not have the specificity required to block PCNA-protein interactions only in cells that harbor the caPCNA isoform, such as cancer cells.
We have successfully developed a rabbit polyclonal antibody that specifically recognizes the cancer-associated isoform of PCNA (caPCNAab). We then synthesized a peptide mimicking this region identified as specific to caPCNA, which is within the IDCL of PCNA and critical to protein binding. In the present communication, we show that the peptide exhibits cytotoxicity in a triple-negative breast cancer cell line (MDA-MB-436). The peptide inhibits binding of PCNA-interacting proteins required for DNA replication and repair, preventing normal cellular function and eventually resulting in cellular death. Because of the peptide’s specificity to caPCNA, it has the therapeutic capability to target DNA replication and repair in cells harboring the caPCNA isoform.
Materials and Methods
Antibody Production.
caPCNA antibodies were produced by Zymed Laboratories (South San Francisco, CA) and Yenzym Antibodies (South San Francisco, CA). All animal work was approved by the Institutional Animal Care and Use Committee. Peptides of varying sequence lengths within the PCNA IDCL were synthesized. To increase the length of the peptide and improve antigenicity, a spacer arm consisting of CGGG was added to the N-terminus of each antigenic peptide. The peptide was covalently conjugated to keyhole limpet hemocyanin, and 100 μg was injected into female rabbits (two per peptide) in complete Freund’s adjuvant. Four weeks after the initial injection, the rabbits were boosted with a second injection. The boost was repeated after another 4 weeks, and 12 days after the final boost, the rabbits were bled and 20 ml of serum was obtained. Affinity purification of the antisera was performed using the antigens’ peptide-coupled chromatography matrix. The affinity matrix was transferred to the antisera, diluted 1:1 with phosphate-buffered saline (PBS), and mixed thoroughly at 4°C for 2 hours. After collecting the flowthrough, the gel was washed extensively and the antibody was eluted with a low-pH elution buffer (0.1 M glycine, pH 2.5). The pH was neutralized with Tris buffer, and the eluted solution was dialyzed against PBS. A 0.1% sodium azide solution was added to the final purified antibody.
Peptide Production.
All peptides (excluding the all-d peptide) were synthesized and isolated to >95% purity by AnaSpec (San Jose, CA). The all-d peptide was synthesized on a PS3 Synthesizer (Protein Technologies, Tucson, AZ), purified by reversed-phase high-performance liquid chromatography, and characterized by matrix-assisted laser desorption ionization–time-of-flight mass spectrometry. The peptides were received in powder form and stored at −20°C. Prior to use, each peptide was solubilized in PBS (Mediatech, Manassas, VA) to a concentration of 10 mM, and aliquots were stored at −20°C.
Cell Culture.
All cancer cell lines were cultured according to procedures established by the American Type Culture Collection. MCF7 cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) (Mediatech, Manassas, VA) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin. PaCa-2 cells were cultured in DMEM supplemented with 10% FBS, 2.5% horse serum, and 1% penicillin-streptomycin. MDA-MB-436 cells were cultured in minimum Eagle’s medium (Gibco Life Technologies, Grand Island, NY) supplemented with 10% FBS, 1% penicillin-streptomycin, 2% minimum Eagle’s medium essential vitamins, 1% sodium pyruvate, and 1 mM HEPES. MCF10A cells were cultured in DMEM–F-12 (50:50) (Mediatech) supplemented with 5% donor horse serum, 20 ng/ml epidermal growth factor, 10 μg/ml insulin, 100 μg/ml hydrocortisone, and 100 ng/ml cholera toxin. Human mammary epithelial cells (HMECs) were obtained from Invitrogen Life Technologies (Grand Island, NY) and cultured according to the manufacturer’s specifications in the provided HuMEC Ready Medium. U937 and HCC1937 cells were cultured in RPMI 1640 medium (Mediatech) supplemented with 10% FBS. HCC1937 breast cancer susceptibility gene (BRCA)–positive cells were obtained from Dr. Stephen Elledge (Harvard Medical School, Boston, MA) and selectively cultured with an addition of 1 μg/ml puromycin. All cell cultures were maintained at 37°C supplemented with 5% CO2.
Cellular Fractionation.
Cultured MCF7 cells were homogenized with a homogenization buffer [200 mM sucrose, 50 mM HEPES-KOH (pH 7.5), 5 mM KCl, 5 mM MgCl2, 2 mM dithiothreitol, and 1 mM phenylmethanesulfonylfluoride (PMSF)] and pelleted. The resulting supernatant was supplemented with 5 mM EDTA and 5 mM EGTA to inhibit protein degradation. Differential centrifugation was used to pellet the mitochondria and microsomes, and the resulting supernatant was collected and stored at −80°C until used.
Two-Dimensional PAGE.
MCF7 fractions (150 μg) were desalted using Protein Desalting Spin Columns (Pierce, Rockford, IL) and lyophilized in a speed vac (ATR, Laurel, MD). The lyophilized samples were rehydrated in equal parts diH2O and first-dimension sample buffer (9 M urea, 2% Triton X-100, 5% β-mercaptoethanol, 1.6% Bio-lyte 5/7 ampholyte, and 0.4% Bio-lyte 3/10 ampholyte), loaded in a first-dimension tube gel (9.2 M urea, 4% acrylamide, 1.6% pH 5–7 ampholytes, 0.4% pH 3–10 ampholytes, and 2% Triton X-100), then overlaid with 30 μl of first-dimension sample overlay buffer (9 M urea, 0.8% Bio-lyte 5/7 ampholyte, 0.2% Bio-lyte 3/10 ampholyte, and 0.0025% bromophenol blue). Isoelectric focusing of the polypeptides was achieved using 10 mM NaOH and 10 mM H3PO4 to create a pH gradient. Focused tube gels were transferred to a 12% polyacrylamide-SDS gel to resolve the second dimension by molecular weight.
Western Blot Analysis.
The resolved proteins were electrophoretically transferred from the slab gel to polyvinylidene difluoride membranes. Membranes were blocked for 30 minutes in buffer containing 10 mM Tris-HCl (pH 7.5), 100 mM NaCl, 2.5 mM EDTA (pH 8.0), 0.1% Tween 20, and 5% nonfat dry milk, then exposed to either PCNA PC10 (1:2000 dilution) (Santa Cruz Biotechnology, Dallas, TX) or the epitope-mapping anti-rabbit caPCNA antibodies (1:500 dilution) for 90 minutes. Blots were washed with TNE [10 mM Tris-HCl (pH 7.5), 100 mM NaCl, and 2.5 mM EDTA (pH 8.0)] to remove background. Membranes were then incubated with horseradish peroxidase secondary antibodies for 1 hour. Final TNE washes were performed, and immunodetection was performed using the Visualizer Spray & Glow chemiluminescent system (Millipore, Billerica, MA).
Immunohistochemical Staining.
Formalin-fixed, paraffin-embedded sections of breast tissue [normal and ductal carcinoma in situ (DCIS) cases] were stained with the panel of purified caPCNA antibodies and the commercially available PC10 using the OptiView DAB IHC Detection Kit (Ventana Medical Systems, Tucson, AZ). All antibody staining was conducted and antibody dilutions were optimized (1:2500 to 1:7500) on the Ventana BenchMark XT IHC/ISH staining module (Ventana Medical Systems) to eliminate variability.
In Vitro Cytotoxicity of R9-cc-caPeptide.
Exponentially growing (3 × 103 cells/well) MDA-MB-436 cells were seeded in 96-well plates. In octuplicate, increasing concentrations of peptide were added to each well and incubated for 48 hours at 37°C with 5% CO2. Cell viability was determined using the CellTiter-Glo Luminescent Cell Viability Assay (Promega, Madison, WI), according to the manufacturer’s instructions. Activity was calculated as the percentage of cells killed/concentration versus control cells with no R9-cc-caPeptide treatment, where 0% indicates no cell death (high ATP levels) and 100% indicates complete cell death (low or no ATP levels). Data were analyzed, and EC50 values were determined following the guidelines described in Sebaugh (Gilmore et al., 2003), and using the sigmoidal dose-response equation in GraphPad Prism 5 software (GraphPad Software, La Jolla, CA).
MTT Cell Viability Assay.
MDA-MB-436 cells (5 × 103 cells/well) were seeded in 96-well plates and incubated overnight at 37°C with 5% CO2. In octuplicate, cells were then treated for 24 hours with increasing concentrations of caPeptide and its variants. After 24 hours, the medium was removed; cells were then washed with PBS and incubated with 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) solution (500 μg/ml) for 4 hours. The MTT solution was then removed, and cells were resuspended in dimethylsulfoxide. The absorbance at λ = 570 nm was then measured for each well. Cell proliferation was calculated as the percentage of surviving cells/concentration versus control cells with no R9-cc-caPeptide treatment.
Apoptotic Evaluation of Cells.
Exponentially growing cells (1 × 106 cells/well) were seeded in a 12-well plate. In triplicate, the appropriate concentration of peptide was then added to each well. Cells were treated for 48 hours, collected, washed twice with ice-cold PBS (Mediatech), and resuspended in PBS with 1 μg/ml propidium iodide (PI). The cellular PI intensity was measured using the CyAn ADP 9 Color (Beckman Coulter, Indianapolis, IN) flow cytometer. Gates were set using untreated MDA-MB-436 cells (control), with 50,000 events counted per sample. The cell death percentage for each sample was calculated relative to control.
For annexin V staining, cells were treated with 50 or 100 μM of either R9-cc-caPeptide or a scrambled version of the caPeptide (R9-cc-scrambled) (see below) for 4, 8, and 24 hours. After trypsinization, cells were washed twice with cold PBS and collected by centrifugation at 1000 rpm. Cells were then resuspended in 1× Binding Buffer (BD Biosciences, San Jose, CA) at a concentration of 1 × 106 cells/ml and 100 μl of the suspension (1 × 105 cells) was transferred to a polystyrene round-bottom tube. Cells were subsequently stained with fluorescein isothiocyanate–conjugated annexin V (4 μl) and PI (50 μg/ml, 5 μl). The mixture was gently vortexed and incubated for 15 minutes at room temperature, and 1× Binding Buffer (400 μl) was added to each tube prior to analysis using flow cytometry.
Colony Formation Assay.
MDA-MB-436 cells were seeded in 25-cm3 flasks (3 × 105 cells/flask) and incubated overnight at 37°C with 5% CO2. Each flask was then treated for 1 hour with increasing concentrations of R9-cc-caPeptide and R9-cc-scrambled peptide. After 1 hour, the medium was removed from the treated flasks and replaced by 1 ml of 0.25% trypsin (Invitrogen) to resuspend cells. Each treatment set of cells was then plated into 10-cm dishes at a density of 750 cells/dish, with three dishes for each concentration. Dishes were incubated for 14 days, and colonies counted.
Nuclear Fractionation and Chromatin Isolation.
PCNA association with chromatin was evaluated, as previously published (Tan et al., 2012). MDA-MB-436 cells were plated, treated with 30 μM R9-cc-caPeptide, and lysed in buffer A [10 mM Tris-HCl (pH 7.4), 2.5 mM MgCl2, 0.5% NP40, 1 mM dithiothreitol, 1 mM PMSF, and a protease inhibitor cocktail]. Samples were then pelleted by centrifugation (1500g, 2 minutes, 4°C), and the supernatant was collected as NP40-extractable (NP-E) fraction. The pellet was washed in buffer B [10 mM Tris-HCl (pH 7.4), 150 mM NaCl, 1 mM PMSF, and a protease inhibitor cocktail], resuspended, and digested in buffer C [10 mM Tris-HCl (pH 7.4), 10 mM NaCl, 5 mM MgCl2, 0.2 mM PMSF, and protease inhibitors] with 200 units/107 cells of DNase I for 30 minutes at 37°C. After centrifugation at 13,000g for 5 minutes at 4°C, the supernatant was collected as NP40-resistant (NP-R) fraction. NP-E and NP-R fractions of the protein were analyzed by Western blot.
Animal Model.
NOD/SCID/IL2Rγ-null mice 4–6 weeks of age were produced from an in-house breeding colony. MDA-MB-436 cells were harvested, washed twice in PBS, and suspended in Matrigel (BD Biosciences, San Jose, CA) at 5 × 107/ml. Suspended cells (0.1 ml) were injected into the mammary fat pads in the right flank of 20 mice. Seven days after tumor inoculation, mice were randomly grouped into three groups with six mice in each group. The mice were treated with PBS, R9-cc-srambled, or R9-cc-caPeptide three times a week via intratumoral injection. Tumor growth was measured weekly as well as at the end of the experiment by a dial caliper. Tumor volumes (V) were estimated based on the length (L) and width (W) of the tumors (V = L × W2 × 0.5). At the end of the experiment, tumors were isolated from sacrificed mice and their masses were measured. All experiments involving live animals were carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. This protocol (#11034) was reviewed and approved by the City of Hope Institutional Animal Care and Use Committee.
Results
Amino Acids Critical to caPCNA Specificity Are Identified by Sequence Mapping of the caPCNAab.
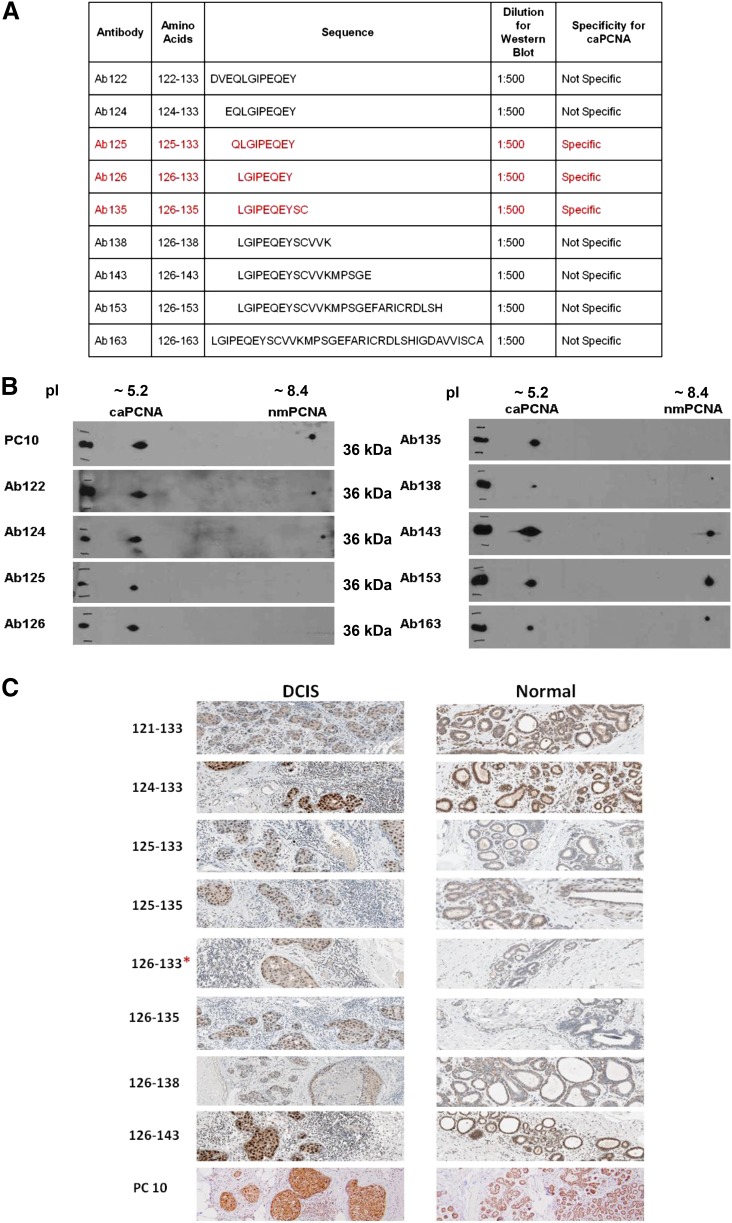
Commercially available antibodies used to identify PCNA are unable to distinguish between the normal PCNA and caPCNA isoforms (Malkas et al., 2006). The epitope these commercial antibodies (PC10 and C20) recognize spans amino acids 68–230 of PCNA, which are identical in both normal PCNA and caPCNA. In previous studies, we successfully developed a rabbit polyclonal antibody that specifically detects the caPCNA isoform through standard IHC staining procedures. To identify the amino acid sequence that establishes caPCNA specificity, a series of rabbit polyclonal antibodies were produced by sequentially adding amino acids around the N and C termini of the core region spanning amino acids 122–135 (listed in Fig. 1A). The specificity of each antibody to caPCNA in MCF7 protein extract was then evaluated using two-dimensional PAGE, followed by Western blot analysis. As illustrated in Fig. 1B, antibodies Ab125 (amino acids 125–133), Ab126 (amino acids 126–133), and Ab135 (amino acids 126–135) specifically recognize the caPCNA isoform. Antibodies composed of amino acids outside the 125–135 range resulted in a loss of specificity.
Fig. 1.
The epitope specific to caPCNA is identified by an overlapping peptide scan. (A) Rabbit polyclonal antibodies listed were generated from various overlapping PCNA amino acid sequences within the IDCL of PCNA. (B) Two-dimensional SDS-PAGE of an MCF7 S3 protein fraction followed by Western blotting (see Materials and Methods) was performed using each affinity-purified antibody. PC10 was diluted 1:2000; Ab122 through Ab163 were diluted 1:500. Specificity of the antibody for caPCNA expressed by cancer cells was previously described in Malkas et al. (2006). (C) Paraffin-embedded breast tissue samples were stained with the antibody series using IHC staining techniques, as detailed in Materials and Methods.
The results of the Western blot were then validated by IHC staining of paraffin-embedded breast tissue samples. Both DCIS and normal breast tissue samples were stained with the antibody panel. As shown in Fig. 1C, PCNA is identified in each slide with a brown stain, and a darker brown indicates increased binding to the cell nuclei. Typically, IHC staining for breast cancer using the commercially available antibody to PCNA (PC10) will stain all proliferating cells expressing PCNA. The expression level correlates with the degree of proliferation, as shown in the lowest panel of Fig. 1C. In practice, this will not necessarily aid the pathologist in distinguishing between a normal proliferating cell and a cancer cell. When the samples shown in Fig. 1C were stained with Ab126 (126–133), the cancer cells in the DCIS sample exhibited nuclear staining of caPCNA, while the normal tissue samples did not stain with the antibody. Similar results were obtained with Ab125 (125–135) and Ab135 (126–135). Antibodies raised against amino acid sequences outside the 125–135 amino acid region were unable to differentiate between PCNA expressed in normal cells and caPCNA expressed in cancer cells. Amino acids 125–135, identified from both Western blot analysis and IHC staining, represent the PCNA region unique to the cancer-associated isoform within the IDCL of PCNA. This is the region where numerous proteins required for proper DNA replication and repair interact with the PCNA molecule.
A Peptide Mimicking a Portion of the Interaction Region Is Developed for Efficient Cellular Uptake and Is Cytotoxic in a Triple-Negative Breast Cancer Cell Line.
In a separate study conducted by Roos et al. (1996), monoclonal antibodies created from shorter sequences of PCNA exhibited the ability to inhibit in vitro DNA replication. The epitopal region used in those experiments spanned residues 121–135, which is located within the IDCL of PCNA. From our antibody scanning study, Ab126 (recognizing amino acids 126–133) was selective for the caPCNA isoform expressed in cancer cells. Using this information, a peptide corresponding to PCNA amino acids 126–133 was synthesized (caPeptide) and tested for its therapeutic potential. One of the drawbacks in using peptides as therapeutics, however, is that most lack the polarity necessary to passively diffuse through the cell membrane. To aid in the uptake process, positively charged chains, such as a series of arginines, lysines, or histidines, have been used previously. Polyarginines display the ability to promote cellular uptake more efficiently than polylysines and polyhistidines (Wender et al., 2000; Deshayes et al., 2005). Detailed analyses of the polyarginine length indicate that a nine-arginine sequence is most effective for cellular penetration and uptake, with the sequence in “all-d” configuration to minimize proteolysis (Wender et al., 2000). Thus, for testing purposes, the caPeptide was linked to nine “d” arginine residues (R9-cc-caPeptide).
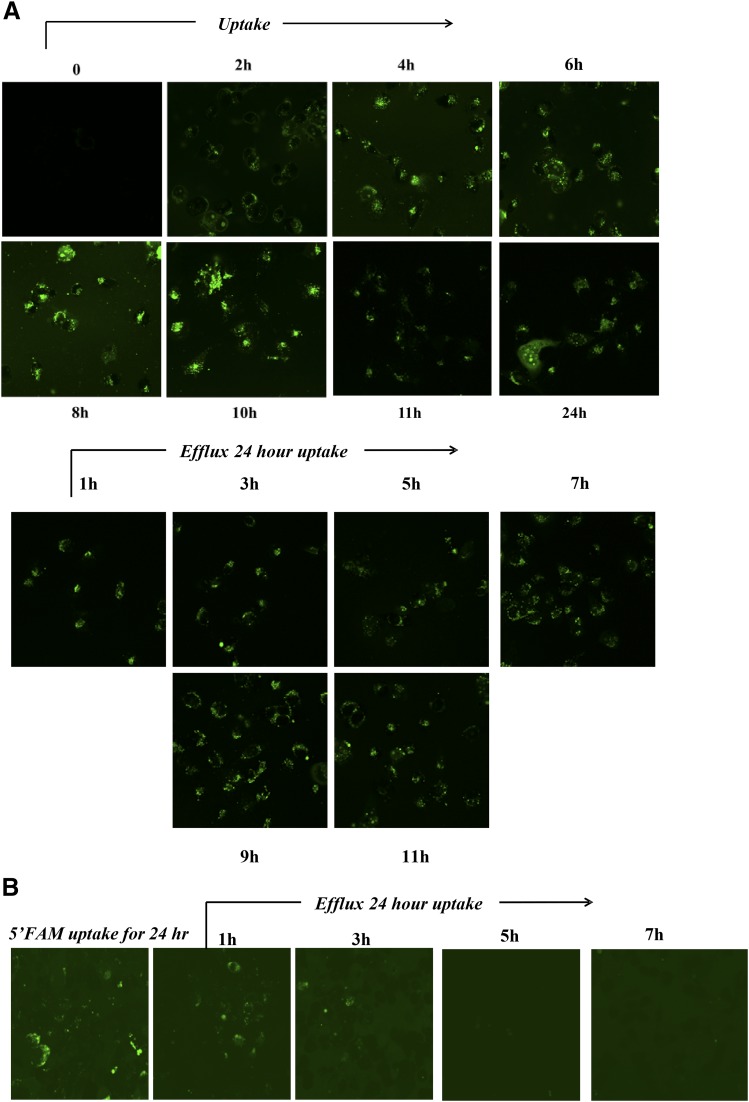
To establish that the R9 sequence is necessary for efficient cellular uptake, 5-carboxyfluorescein (FAM) was attached to the N-terminus of the R9-cc-caPeptide. MDA-MB-436 cells were treated with 10 μM FAM-tagged R9-cc-caPeptide, and the uptake was monitored and imaged using fluorescence microscopy at 2-hour intervals for 12 hours, followed by imaging at 24 hours. MDA-MB-436 cells were derived from the pleural fluid of a 43-year-old woman diagnosed with breast cancer (Brinkley et al., 1980). This particular cell line exhibits a triple-negative, highly invasive breast cancer phenotype (Gordon et al., 2003). As illustrated in Fig. 2A, FAM-labeled R9-cc-caPeptide entered MDA-MB-436 cells within 2 hours of treatment and accumulated in these cells up to 24 hours. After 24 hours, the cells were washed with PBS and monitored for efflux of the peptide over the next 12 hours. FAM-labeled R9-cc-caPeptide remained in the cell up to 11 hours after the wash step (Fig. 2A). To verify that the accumulation observed was not due to a background effect of FAM tag alone, the MDA-MB-436 cells were treated with FAM tag only. As shown in Fig. 2B, the FAM tag did not remain in the cells following the wash step as long as the FAM-tagged R9-cc-caPeptide, verifying that the fluorescence observed was not due to FAM tag alone.
Fig. 2.
The addition of an R9 sequence to caPeptide aids in the uptake and efflux in breast cancer cells. (A) MDA-MB-436 cells were treated with fluorescein-labeled R9-cc-caPeptide for 24 hours, and the uptake was monitored. Cells were subsequently washed, and the efflux of the fluorescein-labeled R9-cc-caPeptide was monitored for 11 hours. (B) As a control, MDA-MB-436 cells were treated with fluorescein alone for 24 hours, and the efflux was monitored for 7 hours.
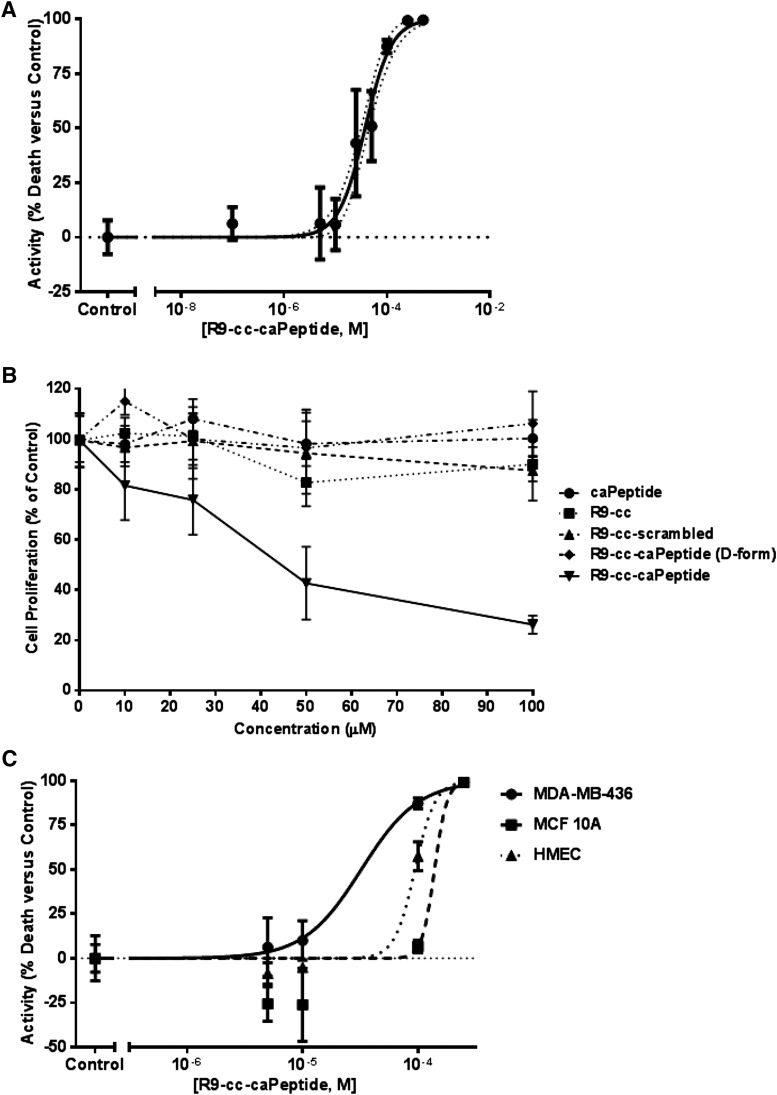
We then sought to verify cytotoxicity in MDA-MB-436 cells after R9-cc-caPeptide treatment. Increasing concentrations of the peptide were added to plated cells and analyzed using the CellTiter-Glo Luminescent Cell Viability Assay (Promega). In this approach used to measure cell viability, the luminescence measured is directly proportional to the amount of ATP produced by metabolically active cells. This measurement technique is reportedly the most sensitive cell-based assay for cytotoxicity (Petty et al., 1995). As shown in Fig. 3A, R9-cc-caPeptide exhibited a dose-dependent response in these particular breast cancer cells (MDA-MB-436). The dose-response data were then analyzed using GraphPad Prism 5 to fit a sigmoidal dose-response curve and determine the EC50 of R9-cc-caPeptide. The EC50 determined from fit was ∼28.8 μM (95% confidence interval, 23.5 to 35.3 μM).
Fig. 3.
The caPeptide sequence is required for cytotoxicity in MDA-MB-436 cells and targets caPCNA in cancer cells. (A) Exponentially growing (3 × 103) MDA-MB-436 cells were treated with increasing concentrations of R9-cc-caPeptide for 48 hours. Cell viability (CellTiter-Glo assay) was evaluated as detailed in Materials and Methods. Activity was calculated as percentage of cells killed at each concentration, normalized to untreated MDA-MB-436 cells (control). Activity of 100% refers to 100% cell death. EC50 with 95% confidence was evaluated using nonlinear regression in GraphPad Prism 5. (B) Exponentially growing MDA-MB-436 (5 × 103) cells were treated with increasing concentrations of caPeptide, R9-cc, R9-cc-scrambled, R9-cc-caPeptide, and R9-cc-caPeptide (d-form) for 48 hours. Cell proliferation (from MTT analysis) was evaluated as percentage of control cells, as detailed in Materials and Methods. (C) Exponentially growing MDA-MB-436 cells, MCF10A cells, and HMECs were treated with increasing concentrations of R9-cc-caPeptide for 48 hours. Cell viability (CellTiter-Glo assay) was evaluated as detailed in Materials and Methods. Activity was calculated as percentage of cells killed at each concentration, normalized to untreated MDA-MB-436 cells (control). EC50 was evaluated using nonlinear regression in GraphPad Prism 5. Data points are means (± S.D.) of octuplicate determinations in a representative experiment; similar results were obtained in at least three additional experiments.
To confirm that the specific sequence of the peptide is required for cytotoxic activity, a scrambled version of the caPeptide (R9-cc-scrambled) was constructed using the same eight amino acids randomly placed in a scrambled sequence. MDA-MB-436 cells were treated with up to 100 μM of both constructs for 48 hours and evaluated for viability using the MTT assay, as described in the Materials and Methods section. As shown in Fig. 3B, R9-cc-caPeptide treatment dose-dependently decreased cell viability. When MDA-MB-436 cells were treated with the R9-cc-scrambled construct, limited cellular toxicity was observed (Fig. 3B). In addition, treatment of the peptide in d-configuration with an R9-cc-linkage (all-d) elicited a response similar to scrambled peptide treatment (Fig. 3B). Both variations of the caPeptide (scrambled and all-d) confirmed that the specific caPeptide sequence is critical to the observed cytotoxic action.
To further demonstrate that the R9 sequence is an effective mechanism for delivery into the breast cancer cell line, treatment with the R9-cc-caPeptide construct was compared with the unlinked version (caPeptide). MDA-MB-436 cells were treated with up to 100 μM caPeptide for 48 hours and then evaluated for viability using the MTT assay. Figure 3B confirms that the R9 sequence is key to peptide delivery and cellular toxicity, as treatment with caPeptide alone had no effect on the MDA-MB-436 cells. These cells were also treated with increasing concentrations of the R9-cc alone to establish that the nine arginines in d-configuration did not contribute to the observed cytotoxicity, and MTT analysis (Fig. 3B) confirms this.
R9-cc-caPeptide Targets caPCNA Identified in Cancer Cells.
After demonstrating the effectiveness of the R9-cc-caPeptide in treating the selected triple-negative breast cancer cell line, we then sought to confirm that the caPeptide targets the caPCNA that is prevalent in cancer cells. Therefore, we used a conventional normal immortalized breast cancer cell line (MCF10A) and primary HMECs. Both cell types were treated with increasing concentrations of R9-cc-caPeptide, and the EC50 was evaluated. As shown in Fig. 3C, the R9-cc-caPeptide was well tolerated by HMEC and MCF10A cells, with EC50s of 94 and >100 μM, respectively, as compared with 28.8 μM in MDA-MB-436 cells. These results demonstrate that R9-cc-caPeptide treatment is more effective in cancer cells, and further confirms that the peptide preferentially targets PCNA-dependent interactions within cancer cells relative to those in noncancer cells.
R9-cc-caPeptide Induces Apoptosis in Triple-Negative Breast Cancer Cells.
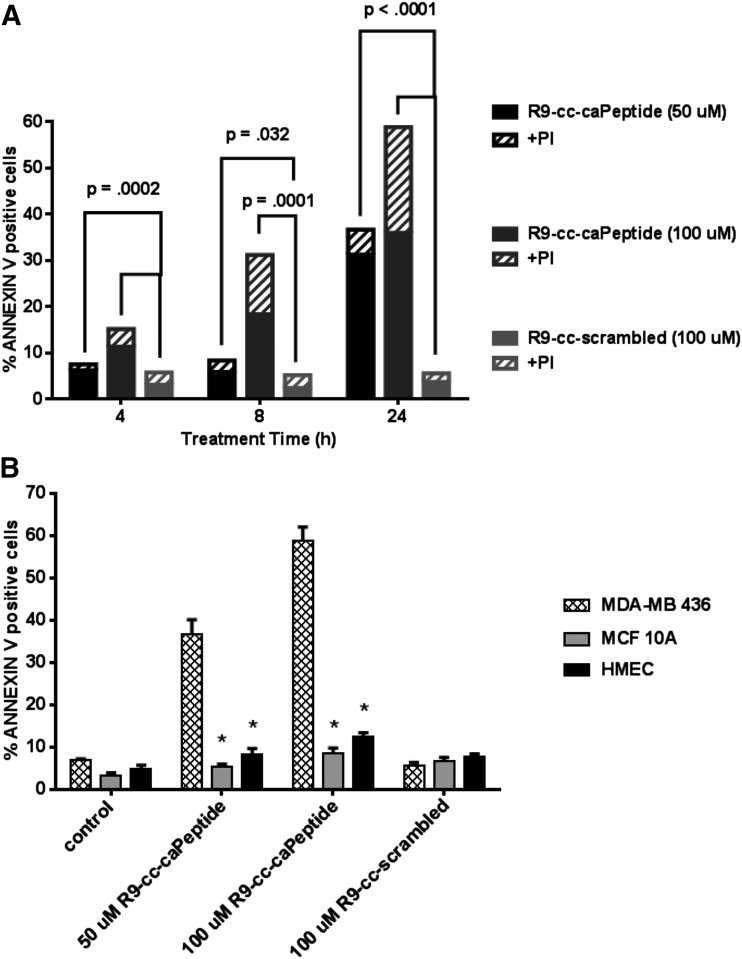
MDA-MB-436 cells (1 × 106 cells/sample) were treated with increasing concentrations of R9-cc-caPeptide for 24 hours, stained with annexin V and PI, and then analyzed using flow cytometry. After 24 hours of treatment, ∼30% of the cells exhibited apoptosis (annexin V positive, PI negative) when treated with 50 μM R9-cc-caPeptide (Fig. 4A; Supplemental Fig. 1). R9-cc-caPeptide treatment (100 μM) resulted in ∼20% of the cells becoming necrotic (annexin V positive, PI positive) over the same time period. When treated with 100 μM R9-cc-scrambled, <5% of cells underwent apoptosis. These results correlate with previous studies showing the induction of apoptosis in neuroblastoma cells following R9-cc-caPeptide treatment (Zhang and Powell, 2005).
Fig. 4.
Treatment of MDA-MB-436 cells with R9-cc-caPeptide results in cytotoxicity via apoptosis. Exponentially growing (1 × 106) MDA-MB-436 cells (A and B), MCF10A cells (B), and HMECs (B) were treated with increasing concentrations of R9-cc-caPeptide or 100 μM R9-cc-scrambled for up to 24 hours. Annexin V staining was then evaluated using flow cytometry, as detailed in Materials and Methods. The percentages of annexin V–positive cells (B) and of annexin V– and annexin V/PI–positive cells (A) were plotted as means of triplicate determinations in a representative experiment; similar results were obtained in at least two additional experiments. (A) Plotted data are the mean of each treatment set. Additional details of the data set (mean, S.D., number of observations) are provided as a table in Supplemental Fig. 1. P values are reported, as shown. (B) Mean ± S.D. are plotted for each measurement. *P < 0.001.
Staining was also performed in the nonmalignant cell lines (MCF10A and HMEC). As shown in Fig. 4B, treatment of these cells with R9-cc-caPeptide resulted in little apoptotic staining above that of the control cells receiving either no treatment or treatment with R9-cc-scrambled. These results further confirm the specificity of R9-cc-caPeptide for inducing apoptosis in triple-negative breast cancer cells.
R9-cc-caPeptide Treatment Inhibits the Clonogenicity of Triple-Negative Breast Cancer Cells.
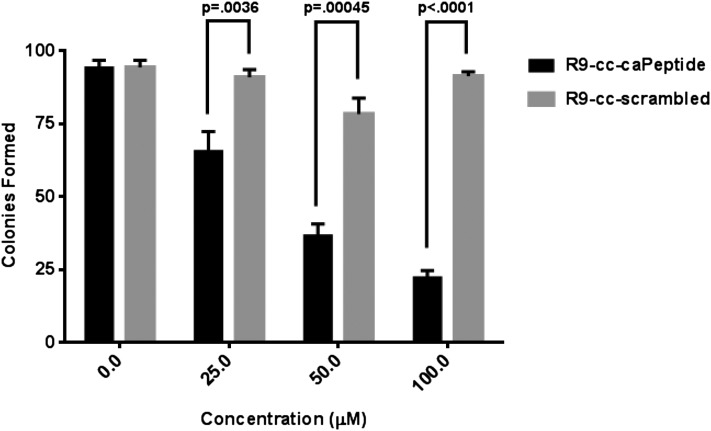
MDA-MB-436 cells were treated with increasing concentrations of R9-cc-caPeptide or R9-scrambled peptide for 1 hour, and 750 treated cells were seeded in 10-cm culture dishes. As shown in Fig. 5 (with images of representative plates in Supplemental Fig. 2), the R9-cc-caPeptide treatment decreased the ability of the triple-negative breast cancer cells to form colonies in a dose-dependent manner. Treatment with ∼40 μM R9-cc-caPeptide resulted in a 50% decrease of clonogenicity. Treatment with increasing concentrations of R9-cc-scrambled had little or no effect on the ability of cells to form colonies. This result is consistent with the previous cytotoxicity experiments (Figs. 3 and 4).
Fig. 5.
Treatment of MDA-MB-436 cells with R9-cc-caPeptide inhibits colony formation. (A) MDA-MB-436 (3 × 105) cells were treated with increasing concentrations of either R9-cc-caPeptide or R9-cc-scrambled for 1 hour, removed from the flask, plated at 750 cells per 10-cm dish, and incubated for 14 days. Colonies were then counted, as detailed in Materials and Methods. Colonies formed are the means (± S.D.) of triplicate determinations in a representative experiment; similar results were obtained in at least two additional experiments. Representative images of treated plates are provided in Supplemental Fig. 2.
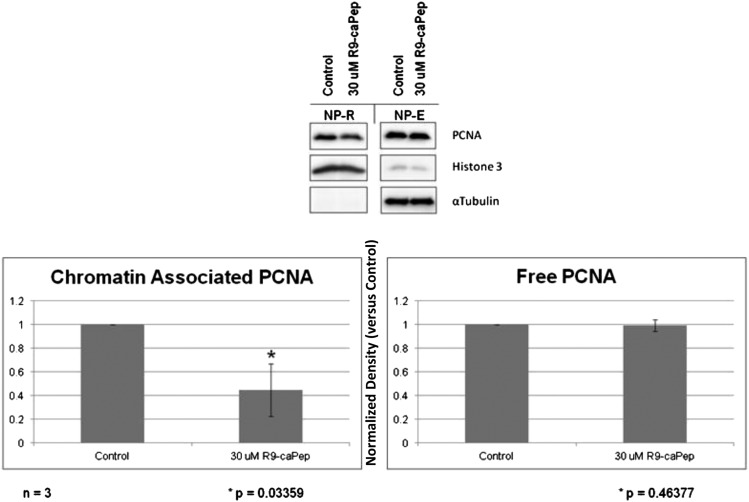
R9-cc-caPeptide Inhibits PCNA Association to Chromatin.
In addition to interacting with DNA replication and repair molecules, PCNA is essential in chromatin association. Previous studies have demonstrated that PCNA interacts with both chromatin assembly factor-1 (CAF-1) and replication factor C during chromatin assembly (Shibahara and Stillman, 1999; Moggs et al., 2000; Tan et al., 2012). CAF-1 has been shown to interact with PCNA within the region of interest (IDCL) (Moggs et al., 2000). Previous studies have shown that the inhibition of PCNA accumulation in chromatin correlates with the level of cytotoxicity observed in cells (Tan et al., 2012). We therefore wanted to assess whether treatment of cells with R9-cc-caPeptide alters the association of PCNA with chromatin. MDA-MB-436 cells were treated with 30 μM R9-cc-caPeptide for 8 hours, then separated into NP-E and NP-R fractions. As shown in Fig. 6, treatment of cells with R9-cc-caPeptide decreased the association of PCNA with chromatin in cells (NP-R). This establishes R9-cc-caPeptide’s ability to block the interaction of PCNA with chromatin-associated proteins, such as CAF-1.
Fig. 6.
R9-cc-caPeptide treatment inhibits the association of PCNA with chromatin. MDA-MB-436 cells were treated with 30 μM R9-cc-caPeptide for 8 hours, followed by separation of the NP-E fraction (free form of PCNA) and NP-R (chromatin-associated PCNA). Fractions were then analyzed, as detailed in Materials and Methods. Plotted values represent the mean (± S.D.) of the normalized density (versus the control) of a total of three different experiments.
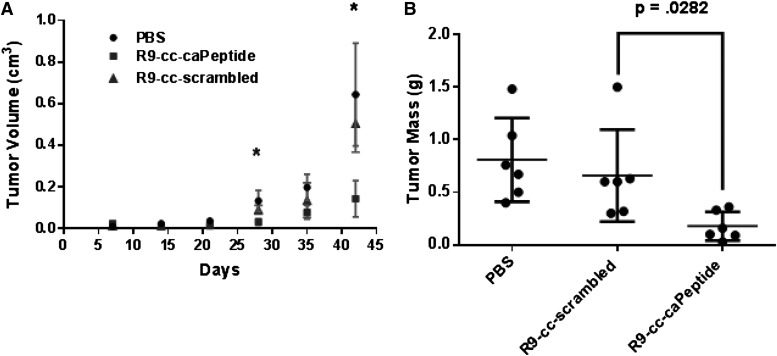
R9-cc-caPeptide Inhibits Tumor Growth in a Mouse Model.
Because R9-cc-caPeptide exhibits therapeutic potential in a number of in vitro assays using triple-negative breast cancer cells, we then sought to recapitulate this anticancer activity using an in vivo breast cancer model. We tested R9-cc-caPeptide in NOD/SCID/IL2Rg-null mice bearing xenograft tumors derived from MDA-MB-436 cells and found that R9-cc-caPeptide significantly and almost completely inhibited tumor growth, as assessed by measuring the tumor volume (Fig. 7A) and final mass (Fig. 7B) in comparison with the control groups that were treated with either PBS or R9-cc-scrambled. These in vivo results correlate with our in vitro results and further validate the therapeutic potential of targeting the PCNA region (L126-Y133) as a novel approach in treating triple-negative breast cancer.
Fig. 7.
R9-cc-caPeptide treatment effectively reduces tumor volume in a mouse model. (A) NOD/SCID/IL2Rg-null mice were randomly divided into three groups of six mice after each being injected with 5 × 106 MDA-MB-436 cells in Matrigel. Each group was treated with PBS, R9-cc-caPeptide, or R9-cc-scrambled three times a week via intratumoral injection. Tumor sizes were measured at indicated time points, and tumor volumes (V) were estimated. The mean tumor volume for each tumor group was graphed (± S.D.). *P < 0.05. (B) Tumor masses were measured at the end of the experiment and graphed in a scatter plot with mean (± S.D.).
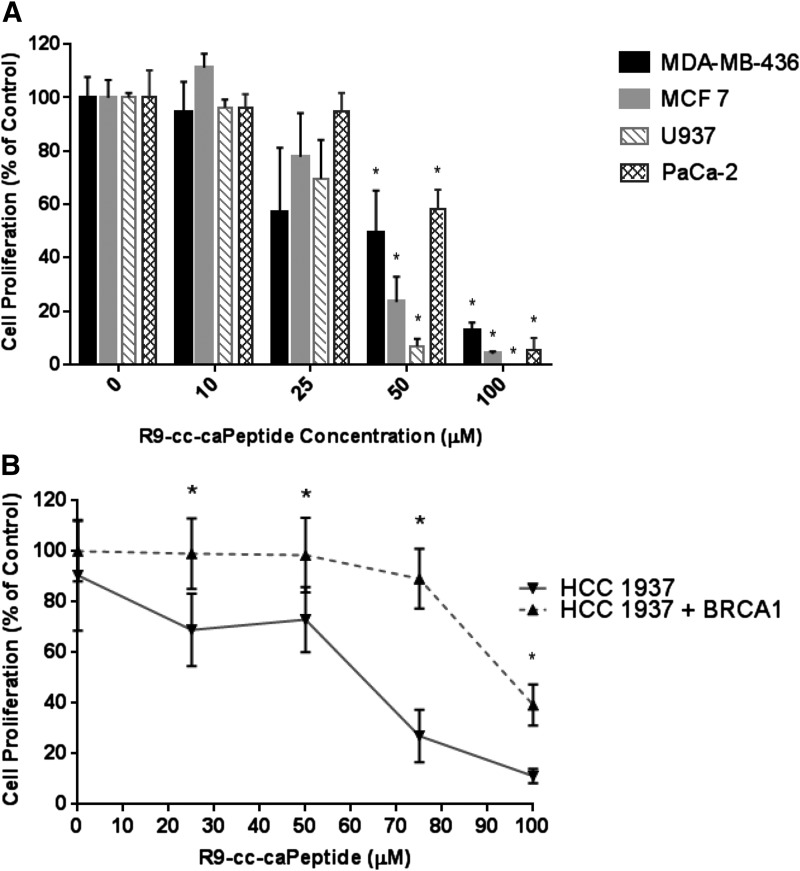
Various Cancer Cell Types Exhibit a Range of Sensitivity to R9-cc-caPeptide Treatment.
The cytotoxicity of R9-cc-caPeptide was tested in an estrogen receptor–positive breast cancer cell line (MCF7), along with two other cancer cell types, lymphoma (U937) and pancreatic cancer (PaCa-2). MTT analysis of each cell line treated with R9-cc-caPeptide is illustrated in Fig. 8A. The pancreatic cancer cell line exhibited increased sensitivity to R9-cc-caPeptide treatment (EC50 of 16 μM), as compared with lymphoma (30 μM) and the two breast cancer cell lines (28 μM for MDA-MB-436 and 60 μM for MCF7). The varying cytotoxic efficacy of the R9-cc-caPeptide on the various cancer cell lines tested may be related to alterations within the DNA replication and repair machinery that are inherent in different types of cancers or the increased uptake or reduced efflux of the peptide in different cell types. Cell lines exhibiting greater resistance to the peptide treatment may be more dependent on other repair pathways that do not require PCNA interaction. Further examination of the specificity of the peptide sequence for different PCNA-interacting proteins and enzymes within the DNA replication and repair process are currently under way.
Fig. 8.
Various cancer cell types exhibit a range of sensitivity to R9-cc-caPeptide treatment. (A) Exponentially growing (3 × 103) MDA-MB-436, MCF7, PaCa-2, and U937 cells were treated with increasing concentrations of R9-cc-caPeptide for 48 hours. (B) HCC1937 cells (both with and without BRCA1) were treated with increasing concentrations of R9-cc-caPeptide for 48 hours. All cells were evaluated for cell survival, as compared with untreated control cells for each cell type using the MTT assay (as detailed in the Materials and Methods section). Data points are means (± S.D.) of octuplicate determinations in a representative experiment; similar results were obtained in at least three additional experiments. *P < 0.001.
R9-cc-caPeptide Contributes to Synthetic Lethality in BRCA-Deficient Breast Cancer Cells.
BRCA1 has been identified as a critical tumor suppressor gene in breast cancer, and loss-of-function mutations confer up to an 82% risk of developing breast cancer by the age of 80 (King et al., 2003). BRCA1 is a 220-kDa nuclear phosphoprotein containing several functional domains that are able to interact with various proteins involved in DNA damage repair pathways. In response to DNA damage, BRCA1 participates in homologous recombination by forming a complex with BRCA2, RAD51, and PCNA (Karran, 2000; Gilmore et al., 2003). BRCA1 may also be involved in nonhomologous end joining, a less accurate repair pathway, by aligning short areas of base homology on either side of the DNA break before ligation (Zhong et al., 2002). BRCA1-deficient cells exhibit increased genomic instability, and this is most likely due to an inability to respond appropriately to DNA damage. This ultimately results in the accumulation of DNA damage (Zhang and Powell, 2005). In the absence of BRCA1, these repair pathways are rendered ineffective, increasing the toxicity of DNA-damaging agents (Kennedy et al., 2004).
As shown in Fig. 8B, HCC1937 cells expressing a functional BRCA1 transgene (HCC1937 BRCA+) are more resistant to increased R9-cc-caPeptide treatment; an IC50 of 90 μM was determined in BRCA+ cells, as compared with an IC50 of 60 μM in the BRCA-deficient HCC1937 cells. Previous investigations have shown that these BRCA-deficient HCC1937 cells are increasingly more sensitive to conventional DNA-damaging agents, such as cisplatin (Tassone et al., 2003; Bayraktar and Gluck, 2012). The results shown in Fig. 8B correlate with these observations, and this helps to confirm that the R9-cc-caPeptide has the ability to disrupt protein functions critical to DNA repair in cancer cells, especially those that require specific PCNA-dependent repair pathways for survival.
Specific Amino Acids within the caPeptide Sequence Are Critical to the Observed Cytotoxicity.
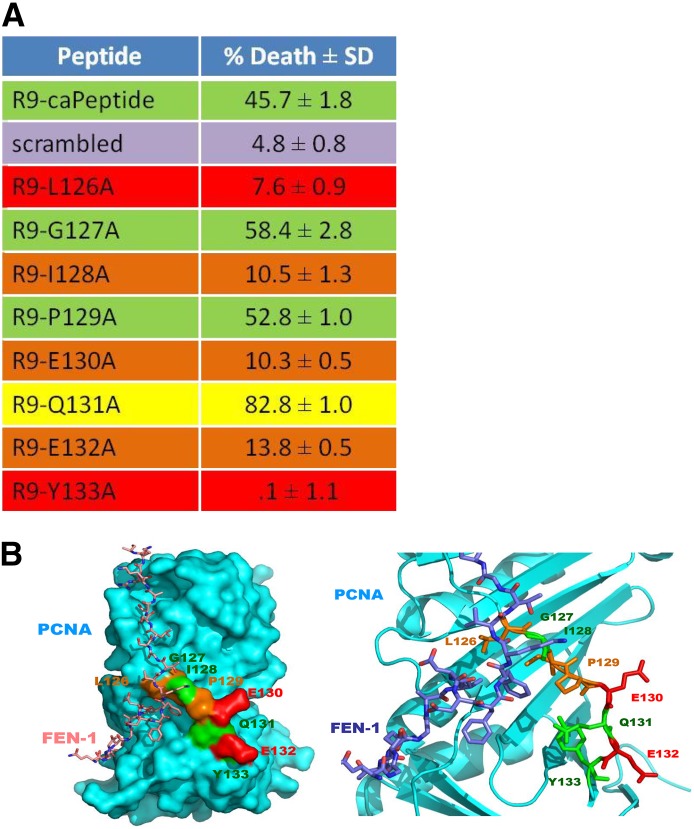
To further characterize the specific mechanism of interaction of the caPeptide contributing to cytotoxicity, peptide constructs, each containing a single alanine substitution within the peptide sequence, were synthesized (Fig. 9A). MDA-MB-436 cells (1 × 106) were individually treated with 75 μM of each of the R9-cc-alanine–substituted caPeptides for 24 hours and then analyzed using flow cytometry. The calculated cell death percentage of each R9-linked peptide is shown in Fig. 9A.
Fig. 9.
Specific amino acids within the caPeptide sequence are critical to the observed cytotoxicity and correlate with in silico molecular modeling of region of interest. (A) Exponentially growing (1 × 106) MDA-MB-436 cells were incubated with 75 μM R9-caPeptide or R9-cc-alanine–substituted caPeptide for 24 hours. The cells were then analyzed by flow cytometry. Cell death percentage was calculated in reference to control cells (no peptide treatment), with standard deviation displayed from an average of five separate experiments. Representative fluorescence-activated cell sorter analyses are shown in Supplemental Fig. 3. (B) Crystal structure of FEN-1 binding to PCNA, with caPeptide amino acids highlighted (PDB ID: 1UL1; Sakurai et al., 2005).
If a critical amino acid is substituted with an alanine, treatment with that particular peptide would presumably be less cytotoxic in cells, as compared with the original (R9-cc-caPeptide). Alanine substitution of an amino acid not critical to the protein-protein interactions, however, should not affect the observed cytotoxicity. Representative fluorescence-activated cell sorter data for each type of response are shown in Supplemental Fig. 3. As illustrated in Fig. 9A, alanine substitution at amino acids 126, 128, 130, 132, and 133 virtually eliminated the cytotoxicity of the peptide, and thus the native amino acids (leucine, isoleucine, glutamic acids, and tyrosine, respectively) are likely critical to the activity of the peptide. Alanine substitution at amino acids 127 and 129 exhibited little or no change in the observed cytotoxicity of the peptide, and thus the native amino acids (glycine and proline, respectively) most likely do not contribute to cytotoxicity. Interestingly, an alanine substitution at amino acid 131 increased the cytotoxicity of the peptide. This indicates that the R9-cc-Q131A is potentially a better therapeutic. Further studies are currently under way to investigate this phenomenon.
Discussion
As previously described, PCNA plays an integral role in DNA replication and repair. Therefore, if the PCNA function is altered in cancer cells, there is a greater potential for error propagation within the DNA structure itself. A characteristic common to cancer is accumulation of these mutations during the replication process, generating genomic instability within the cell. According to the “mutator phenotype” hypothesis, as DNA begins to replicate with less fidelity in cells, errors accumulate and the cells take on a “mutator phenotype,” eventually leading to the incidence of cancer (Loeb, 1998, 2001, 2010). Although the actual driving force behind this loss of DNA replication fidelity has yet to be determined, it is very possible that a malfunction within the replication machinery itself is the main cause of error propagation.
In the previous communication (Malkas et al., 2006), we had successfully developed a rabbit polyclonal antibody that specifically detects the cancer-associated isoform of PCNA (caPCNAab). This antibody has now been validated through both Western blot analysis of breast cancer cell extracts and IHC staining of breast tissue samples. Figure 1 demonstrates its specificity to the caPCNA isoform expressed in cancer cells and defines the cancer-selective epitope of the PCNA sequence (amino acids 125–135). From these findings, we then developed a peptide mimicking this caPCNA-specific region. This peptide exhibits the ability to compete with the binding of accessory proteins to caPCNA, thereby targeting cells containing caPCNA, inhibiting DNA replication and eventually leading to cellular apoptosis.
The caPeptide sequence alone was incubated in MDA-MB-436 cells for 48 hours in concentrations up to 100 μM, but no cytotoxicity was observed (Fig. 3B). Because most peptides cannot passively diffuse into cells, a linker of nine arginines in the d- configuration was attached to the N-terminus of the peptide to create R9-cc-caPeptide. Treatment of MDA-MB-436 cells with fluorescently tagged R9-cc-caPeptide demonstrated sufficient cellular uptake and accumulation of the peptide (Fig. 2). Subsequent rinsing of the cells (efflux) showed that the R9-cc-caPeptide remained in the cells beyond 12 hours. Treatment of MDA-MB-436 cells with increasing concentrations of R9-cc-caPeptide up to 100 μM resulted in dose-dependent cell killing, and the EC50 determined for MDA-MB-436 cells was ∼30 μM (Fig. 3A). As a control, cells treated with a scrambled peptide attached to the nona-arginine (R9-cc-scrambled) or the all-d peptide exhibited little cytotoxicity. The study also confirmed the importance of R9 for cellular uptake and the specificity of the caPeptide sequence for cytotoxic action. The specificity of the peptide for cells containing caPCNA was further demonstrated, as R9-cc-caPeptide exhibited limited cytotoxicity in the nonmalignant cell lines (MCF10A and HMECs) (Fig. 3C; Fig. 4B).
The cell line chosen for preliminary experiments with the caPeptide, MDA-MB-436, is a triple-negative breast cancer cell line. Treatment with R9-cc-caPeptide was shown to decrease cell viability through the measurement of ATP, flow cytometry, and clonogenic assays. It can also inhibit PCNA accumulation in chromatin. Peptide cytotoxicity was subsequently tested in two other cancer cell lines [lymphoma (U937) and pancreatic cancer (PaCa-2)] and breast cancers of different genetic makeup (HCC1937 and MCF7). The results demonstrate that the R9-cc-caPeptide displays cytotoxicity in various forms of cancer. Furthermore, the preliminary data from the in vivo mouse model show that the R9-cc-caPeptide may have potential as a therapeutic agent.
Existing X-ray crystallography data on the interaction site within PCNA were used to aid in visualizing the relationship between the observed cytotoxicity and specific amino acids within the peptide sequence that affect interactions with PCNA binding partners. The binding model shown in Fig. 9B is a reproduction of PCNA interacting with flap structure–specific endonuclease 1 (FEN-1) using previously published X-ray crystallography (PDB ID: 1UL1; Sakurai et al., 2005). The C terminus (amino acids 330–380) of the FEN-1 molecule forms the “plug” that fits into the IDCL pocket of the PCNA molecule. Closer examination shows that the PCNA peptide region encompassing amino acids 126–133 does, in fact, form a cavity for the FEN-1 molecule to bind. Amino acids 126 and 133 are positioned above the cavity in such a way as to presumably provide complementary van der Waals interactions between FEN-1 and PCNA. As demonstrated by alanine mutation analysis (Fig. 9A), substitution of these amino acids with an alanine resulted in a decrease of the cytotoxic activity, confirming their importance to the interaction. The model also reveals that amino acids 127 and 132 face away from the pocket and do not appear to be involved in the PCNA–FEN-1 interaction, correlating with the alanine mutation analysis. The nonpolar valine at residue 346 makes contact with the hydrophobic leucine at residue 126 on PCNA. Our mutational studies confirm that the replacement of either the leucine (126) or the tyrosine (133) with an alanine results in the loss of key protein interactions within the IDCL, thereby reducing the mimicking capability of the peptide. Previous work in the laboratory confirms that the caPeptide sequence interferes with FEN1 binding to PCNA, as demonstrated by surface plasmon resonance and immunofluorescence (Zhang and Powell, 2005).
Although only the interaction between FEN-1 and PCNA was applied to the current study, the cavity formed by PCNA amino acids 126–133 is a binding site for a variety of other binding partners (Gulbis et al., 1996; Bowman et al., 2004; Bruning and Shamoo, 2004; Chapados et al., 2004; Kontopidis et al., 2005; Sakurai et al., 2005; Vijayakumar et al., 2007). PCNA affinity column elution studies have confirmed the role of PCNA in linking DNA replication and cell cycle control of DNA replication via protein-protein interactions (Loor et al., 1997). Peptides identified as interacting with PCNA in this format include Pol δ (125-kDa subunit), Pol ε (145-kDa subunit), replication factor C (subunits 37 and 40), replication protein A (subunits 70, 34, and 11), DNA helicase II, and topoisomerase I. Each of these proteins contains a PIP-box that interacts with the pocket region formed in the IDCL of the PCNA molecule. Although there are many other amino acid interacting points between PCNA and its binding partners, the cavity formed in part by the caPeptide region is necessary for proper binding and the initiation of the cellular processes produced by the interaction.
Other studies involving a combination of the crystal structure and experimental techniques describe PCNA interaction with other proteins. X-ray crystallography of p21-PCNA interactions correlate with the flow cytometry results in this study (Fig. 9A). The crystal structure of p21 complexed with PCNA revealed major interactions with residues leucine-126, isoleucine-128, and tyrosine-133 of the PCNA IDCL (Gibbs et al., 1997). The hydroxyl group of tyrosine-151 of the p21 peptide forms a hydrogen bond with glutamine-131 and forms an ordered water pair with tyrosine-133 of PCNA (Pascal et al., 2006). When PCNA is mutated with an alanine at the isoleucine residue (position 128), Pol δ is unable to bind, resulting in a loss of processivity (Zhang et al., 1998). This correlates with our flow cytometry data of the I128A mutant, where cytotoxicity is virtually eliminated by this alanine substitution. The affinity for Pol δ presumably decreases, rendering it ineffective. These results further confirm that the caPeptide has the ability to disrupt the proper functioning of Pol δ. This type of disruption is important, as PCNA has been shown to stimulate Pol δ processivity by decreasing the dissociation of Pol δ from the template-primer complex (McConnell et al., 1996), while moderately increasing the rate of incorporation of a single nucleotide (Ng et al., 1991). PCNA can enhance Pol δ activity across DNA damage sites that include a model abasic site (modified tetrahydrofuran), 8-oxo-2′-deoxyguanosine, acetylaminofluorene-dG (Mozzherin et al., 1997), and thymine dimers (a common DNA photoproduct) (O'Day et al., 1992).
As mentioned previously, the caPeptide designed for these studies is derived from a portion of the flexible IDCL region of PCNA. This particular region of PCNA is considered to have a disordered structure (Bruning and Shamoo, 2004). Disordered regions in proteins indicate the locations for protein-protein interaction. The cytotoxicity observed with R9-cc-caPeptide treatment suggests that the peptide has the ability to compete with PCNA for its binding partners, thereby inhibiting their binding ability and proper functioning in normal DNA replication and repair processes.
Supplementary Material
Acknowledgments
The authors thank Drs. Hongzhi Li and Yate-Ching Yuan (Director) in Bioinformatics for their assistance with the illustration of the X-ray crystallography data and Lucy Brown (Manager) and her staff in the Analytical Cytometry Core for their assistance with the alanine scanning data. The authors also thank Dr. Fei Shen at Indiana University for conducting the peptide uptake and retention studies. Finally, the authors thank Angelica Ruiz for her assistance with the preparation of this manuscript.
Abbreviations
- BRCA
breast cancer susceptibility gene
- caPCNA
cancer-associated PCNA
- caPCNAab
cancer-associated PCNA–targeted antibody
- DCIS
ductal carcinoma in situ
- DMEM
Dulbecco’s modified Eagle’s medium
- FAM
5-carboxyfluorescein
- FBS
fetal bovine serum
- HMEC
human mammary epithelial cell
- IDCL
interdomain connector loop
- IHC
immunohistochemical
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- NP-E
NP40-extractable
- NP-R
NP40-resistant
- PBS
phosphate-buffered saline
- PCNA
proliferating cell nuclear antigen
- PI
propidium iodide
- PIP-box
PCNA-interacting protein box
- PMSF
phenylmethanesulfonylfluoride
Authorship Contributions
Participated in research design: Smith, Phipps, Dobrolecki, Mabrey, Chen, Gu, Ann, Hickey, Malkas.
Conducted experiments: Smith, Phipps, Dobrolecki, Mabrey, Gulley, Dillehay, Chen, Gu, Hickey.
Contributed new reagents or analytic tools: Dobrolecki, Mabrey, Dillehay, Dong, Fields, Ann.
Performed data analysis: Smith, Dobrolecki, Mabrey, Gulley, Dillehay, Dong, Chen, Gu, Hickey.
Wrote or contributed to the writing of the manuscript: Smith, Chen, Fields, Gu, Hickey, Malkas.
Footnotes
This work was supported by the Department of Defense [Grant W81XWH-11-1-0786 to L.H.M.] and by the National Institutes of Health National Cancer Institute [Grants R01-CA121289 and P30-CA033572]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
 This article has supplemental material available at molpharm.aspetjournals.org.
This article has supplemental material available at molpharm.aspetjournals.org.
References
- Bayraktar S, Glück S. (2012) Systemic therapy options in BRCA mutation-associated breast cancer. Breast Cancer Res Treat 135:355–366. [DOI] [PubMed] [Google Scholar]
- Bechtel PE, Hickey RJ, Schnaper L, Sekowski JW, Long BJ, Freund R, Liu N, Rodriguez-Valenzuela C, Malkas LH. (1998) A unique form of proliferating cell nuclear antigen is present in malignant breast cells. Cancer Res 58:3264–3269. [PubMed] [Google Scholar]
- Bowman GD, O’Donnell M, Kuriyan J. (2004) Structural analysis of a eukaryotic sliding DNA clamp-clamp loader complex. Nature 429:724–730. [DOI] [PubMed] [Google Scholar]
- Brinkley BR, Beall PT, Wible LJ, Mace ML, Turner DS, Cailleau RM. (1980) Variations in cell form and cytoskeleton in human breast carcinoma cells in vitro. Cancer Res 40:3118–3129. [PubMed] [Google Scholar]
- Bruning JB, Shamoo Y. (2004) Structural and thermodynamic analysis of human PCNA with peptides derived from DNA polymerase-delta p66 subunit and flap endonuclease-1. Structure 12:2209–2219. [DOI] [PubMed] [Google Scholar]
- Chapados BR, Hosfield DJ, Han S, Qiu J, Yelent B, Shen B, Tainer JA. (2004) Structural basis for FEN-1 substrate specificity and PCNA-mediated activation in DNA replication and repair. Cell 116:39–50. [DOI] [PubMed] [Google Scholar]
- Chu JS, Huang CS, Chang KJ. (1998) Proliferating cell nuclear antigen (PCNA) immunolabeling as a prognostic factor in invasive ductal carcinoma of the breast in Taiwan. Cancer Lett 131:145–152. [DOI] [PubMed] [Google Scholar]
- Deshayes S, Morris MC, Divita G, Heitz F. (2005) Cell-penetrating peptides: tools for intracellular delivery of therapeutics. Cell Mol Life Sci 62:1839–1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg P, Burgers PM. (2005) Ubiquitinated proliferating cell nuclear antigen activates translesion DNA polymerases eta and REV1. Proc Natl Acad Sci USA 102:18361–18366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs E, Kelman Z, Gulbis JM, O’Donnell M, Kuriyan J, Burgers PM, Hurwitz J. (1997) The influence of the proliferating cell nuclear antigen-interacting domain of p21(CIP1) on DNA synthesis catalyzed by the human and Saccharomyces cerevisiae polymerase delta holoenzymes. J Biol Chem 272:2373–2381. [DOI] [PubMed] [Google Scholar]
- Gilmore PM, Quinn JE, Mullan PB, Andrews HN, McCabe N, Carty M, Kennedy RD, Harkin DP. (2003) Role played by BRCA1 in regulating the cellular response to stress. Biochem Soc Trans 31:257–262. [DOI] [PubMed] [Google Scholar]
- Gordon LA, Mulligan KT, Maxwell-Jones H, Adams M, Walker RA, Jones JL. (2003) Breast cell invasive potential relates to the myoepithelial phenotype. Int J Cancer 106:8–16. [DOI] [PubMed] [Google Scholar]
- Gulbis JM, Kelman Z, Hurwitz J, O’Donnell M, Kuriyan J. (1996) Structure of the C-terminal region of p21(WAF1/CIP1) complexed with human PCNA. Cell 87:297–306. [DOI] [PubMed] [Google Scholar]
- Hoege C, Pfander B, Moldovan GL, Pyrowolakis G, Jentsch S. (2002) RAD6-dependent DNA repair is linked to modification of PCNA by ubiquitin and SUMO. Nature 419:135–141. [DOI] [PubMed] [Google Scholar]
- Hoelz DJ, Arnold RJ, Dobrolecki LE, Abdel-Aziz W, Loehrer AP, Novotny MV, Schnaper L, Hickey RJ, Malkas LH. (2006) The discovery of labile methyl esters on proliferating cell nuclear antigen by MS/MS. Proteomics 6:4808–4816. [DOI] [PubMed] [Google Scholar]
- Kannouche PL, Lehmann AR. (2004) Ubiquitination of PCNA and the polymerase switch in human cells. Cell Cycle 3:1011–1013. [PubMed] [Google Scholar]
- Kannouche PL, Wing J, Lehmann AR. (2004) Interaction of human DNA polymerase eta with monoubiquitinated PCNA: a possible mechanism for the polymerase switch in response to DNA damage. Mol Cell 14:491–500. [DOI] [PubMed] [Google Scholar]
- Karran P. (2000) DNA double strand break repair in mammalian cells. Curr Opin Genet Dev 10:144–150. [DOI] [PubMed] [Google Scholar]
- Kennedy RD, Quinn JE, Mullan PB, Johnston PG, Harkin DP. (2004) The role of BRCA1 in the cellular response to chemotherapy. J Natl Cancer Inst 96:1659–1668. [DOI] [PubMed] [Google Scholar]
- King MC, Marks JH, Mandell JB, New York Breast Cancer Study Group (2003) Breast and ovarian cancer risks due to inherited mutations in BRCA1 and BRCA2. Science 302:643–646. [DOI] [PubMed] [Google Scholar]
- Kontopidis G, Wu SY, Zheleva DI, Taylor P, McInnes C, Lane DP, Fischer PM, Walkinshaw MD. (2005) Structural and biochemical studies of human proliferating cell nuclear antigen complexes provide a rationale for cyclin association and inhibitor design. Proc Natl Acad Sci USA 102:1871–1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krijger PH, van den Berk PC, Wit N, Langerak P, Jansen JG, Reynaud CA, de Wind N, Jacobs H. (2011) PCNA ubiquitination-independent activation of polymerase η during somatic hypermutation and DNA damage tolerance. DNA Repair (Amst) 10:1051–1059. [DOI] [PubMed] [Google Scholar]
- Loeb LA. (1998) Cancer cells exhibit a mutator phenotype. Adv Cancer Res 72:25–56. [DOI] [PubMed] [Google Scholar]
- Loeb LA. (2001) A mutator phenotype in cancer. Cancer Res 61:3230–3239. [PubMed] [Google Scholar]
- Loeb LA. (2010) Mutator phenotype in cancer: origin and consequences. Semin Cancer Biol 20:279–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loor G, Zhang SJ, Zhang P, Toomey NL, Lee MY. (1997) Identification of DNA replication and cell cycle proteins that interact with PCNA. Nucleic Acids Res 25:5041–5046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malkas LH, Herbert BS, Abdel-Aziz W, Dobrolecki LE, Liu Y, Agarwal B, Hoelz D, Badve S, Schnaper L, Arnold RJ, et al. (2006) A cancer-associated PCNA expressed in breast cancer has implications as a potential biomarker. Proc Natl Acad Sci USA 103:19472–19477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell M, Miller H, Mozzherin DJ, Quamina A, Tan CK, Downey KM, Fisher PA. (1996) The mammalian DNA polymerase delta—proliferating cell nuclear antigen—template-primer complex: molecular characterization by direct binding. Biochemistry 35:8268–8274. [DOI] [PubMed] [Google Scholar]
- Moggs JG, Grandi P, Quivy JP, Jónsson ZO, Hübscher U, Becker PB, Almouzni G. (2000) A CAF-1-PCNA-mediated chromatin assembly pathway triggered by sensing DNA damage. Mol Cell Biol 20:1206–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozzherin DJ, Shibutani S, Tan CK, Downey KM, Fisher PA. (1997) Proliferating cell nuclear antigen promotes DNA synthesis past template lesions by mammalian DNA polymerase delta. Proc Natl Acad Sci USA 94:6126–6131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naryzhny SN, Lee H. (2004) The post-translational modifications of proliferating cell nuclear antigen: acetylation, not phosphorylation, plays an important role in the regulation of its function. J Biol Chem 279:20194–20199. [DOI] [PubMed] [Google Scholar]
- Ng L, Tan CK, Downey KM, Fisher PA. (1991) Enzymologic mechanism of calf thymus DNA polymerase delta. J Biol Chem 266:11699–11704. [PubMed] [Google Scholar]
- O’Day CL, Burgers PM, Taylor JS. (1992) PCNA-induced DNA synthesis past cis-syn and trans-syn-I thymine dimers by calf thymus DNA polymerase delta in vitro. Nucleic Acids Res 20:5403–5406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascal JM, Tsodikov OV, Hura GL, Song W, Cotner EA, Classen S, Tomkinson AE, Tainer JA, Ellenberger T. (2006) A flexible interface between DNA ligase and PCNA supports conformational switching and efficient ligation of DNA. Mol Cell 24:279–291. [DOI] [PubMed] [Google Scholar]
- Petty RD, Sutherland LA, Hunter EM, Cree IA. (1995) Comparison of MTT and ATP-based assays for the measurement of viable cell number. J Biolumin Chemilumin 10:29–34. [DOI] [PubMed] [Google Scholar]
- Prosperi E. (1997) Multiple roles of the proliferating cell nuclear antigen: DNA replication, repair and cell cycle control. Prog Cell Cycle Res 3:193–210. [DOI] [PubMed] [Google Scholar]
- Punchihewa C, Inoue A, Hishiki A, Fujikawa Y, Connelly M, Evison B, Shao Y, Heath R, Kuraoka I, Rodrigues P, et al. (2012) Identification of small molecule proliferating cell nuclear antigen (PCNA) inhibitor that disrupts interactions with PIP-box proteins and inhibits DNA replication. J Biol Chem 287:14289–14300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos G, Jiang Y, Landberg G, Nielsen NH, Zhang P, Lee MY. (1996) Determination of the epitope of an inhibitory antibody to proliferating cell nuclear antigen. Exp Cell Res 226:208–213. [DOI] [PubMed] [Google Scholar]
- Sabbioneda S, Gourdin AM, Green CM, Zotter A, Giglia-Mari G, Houtsmuller A, Vermeulen W, Lehmann AR. (2008) Effect of proliferating cell nuclear antigen ubiquitination and chromatin structure on the dynamic properties of the Y-family DNA polymerases. Mol Biol Cell 19:5193–5202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai S, Kitano K, Yamaguchi H, Hamada K, Okada K, Fukuda K, Uchida M, Ohtsuka E, Morioka H, Hakoshima T. (2005) Structural basis for recruitment of human flap endonuclease 1 to PCNA. EMBO J 24:683–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandoval JA, Hickey RJ, Malkas LH. (2005) Isolation and characterization of a DNA synthesome from a neuroblastoma cell line. J Pediatr Surg 40:1070–1077. [DOI] [PubMed] [Google Scholar]
- Sekowski JW, Malkas LH, Schnaper L, Bechtel PE, Long BJ, Hickey RJ. (1998) Human breast cancer cells contain an error-prone DNA replication apparatus. Cancer Res 58:3259–3263. [PubMed] [Google Scholar]
- Shibahara K, Stillman B. (1999) Replication-dependent marking of DNA by PCNA facilitates CAF-1-coupled inheritance of chromatin. Cell 96:575–585. [DOI] [PubMed] [Google Scholar]
- Stelter P, Ulrich HD. (2003) Control of spontaneous and damage-induced mutagenesis by SUMO and ubiquitin conjugation. Nature 425:188–191. [DOI] [PubMed] [Google Scholar]
- Tahan SR, Neuberg DS, Dieffenbach A, Yacoub L. (1993) Prediction of early relapse and shortened survival in patients with breast cancer by proliferating cell nuclear antigen score. Cancer 71:3552–3559. [DOI] [PubMed] [Google Scholar]
- Tan Z, Wortman M, Dillehay KL, Seibel WL, Evelyn CR, Smith SJ, Malkas LH, Zheng Y, Lu S, Dong Z. (2012) Small-molecule targeting of proliferating cell nuclear antigen chromatin association inhibits tumor cell growth. Mol Pharmacol 81:811–819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tassone P, Tagliaferri P, Perricelli A, Blotta S, Quaresima B, Martelli ML, Goel A, Barbieri V, Costanzo F, Boland CR, et al. (2003) BRCA1 expression modulates chemosensitivity of BRCA1-defective HCC1937 human breast cancer cells. Br J Cancer 88:1285–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Kemp PA, de Padula M, Burguiere-Slezak G, Ulrich HD, and Boiteux S (2009). PCNA monoubiquitylation and DNA polymerase eta ubiquitin-binding domain are required to prevent 8-oxoguanine-induced mutagenesis in Saccharomyces cerevisiae. Nucleic Acids Res 37:2549–2559. [DOI] [PMC free article] [PubMed]
- Venturi A, Piaz FD, Giovannini C, Gramantieri L, Chieco P, Bolondi L. (2008) Human hepatocellular carcinoma expresses specific PCNA isoforms: an in vivo and in vitro evaluation. Lab Invest 88:995–1007. [DOI] [PubMed] [Google Scholar]
- Vijayakumar S, Chapados BR, Schmidt KH, Kolodner RD, Tainer JA, Tomkinson AE. (2007) The C-terminal domain of yeast PCNA is required for physical and functional interactions with Cdc9 DNA ligase. Nucleic Acids Res 35:1624–1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SC, Nakajima Y, Yu YL, Xia W, Chen CT, Yang CC, McIntush EW, Li LY, Hawke DH, Kobayashi R, et al. (2006) Tyrosine phosphorylation controls PCNA function through protein stability. Nat Cell Biol 8:1359–1368. [DOI] [PubMed] [Google Scholar]
- Wang X, Hickey RJ, Malkas LH, Koch MO, Li L, Zhang S, Sandusky GE, Grignon DJ, Eble JN, Cheng L. (2011) Elevated expression of cancer-associated proliferating cell nuclear antigen in high-grade prostatic intraepithelial neoplasia and prostate cancer. Prostate 71:748–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warbrick E. (2006) A functional analysis of PCNA-binding peptides derived from protein sequence, interaction screening and rational design. Oncogene 25:2850–2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe K, Tateishi S, Kawasuji M, Tsurimoto T, Inoue H, Yamaizumi M. (2004) Rad18 guides poleta to replication stalling sites through physical interaction and PCNA monoubiquitination. EMBO J 23:3886–3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wender PA, Mitchell DJ, Pattabiraman K, Pelkey ET, Steinman L, Rothbard JB. (2000) The design, synthesis, and evaluation of molecules that enable or enhance cellular uptake: peptoid molecular transporters. Proc Natl Acad Sci USA 97:13003–13008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Powell SN. (2005) The role of the BRCA1 tumor suppressor in DNA double-strand break repair. Mol Cancer Res 3:531–539. [DOI] [PubMed] [Google Scholar]
- Zhang P, Sun Y, Hsu H, Zhang L, Zhang Y, Lee MY. (1998) The interdomain connector loop of human PCNA is involved in a direct interaction with human polymerase delta. J Biol Chem 273:713–719. [DOI] [PubMed] [Google Scholar]
- Zheleva DI, Zhelev NZ, Fischer PM, Duff SV, Warbrick E, Blake DG, Lane DP. (2000) A quantitative study of the in vitro binding of the C-terminal domain of p21 to PCNA: affinity, stoichiometry, and thermodynamics. Biochemistry 39:7388–7397. [DOI] [PubMed] [Google Scholar]
- Zhong Q, Chen CF, Chen PL, Lee WH. (2002) BRCA1 facilitates microhomology-mediated end joining of DNA double strand breaks. J Biol Chem 277:28641–28647. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.