Abstract
Aging may negatively affect gingival wound-healing. However, little is known about the mechanisms underlying this phenomenon. The present study examined the cellular responses associated with gingival wound-healing in aging. Primary cultures of human gingival fibroblasts were obtained from healthy young and aged donors for the analysis of cell proliferation, cell invasion, myofibroblastic differentiation, and collagen gel remodeling. Serum from young and old rats was used to stimulate cell migration. Gingival repair was evaluated in Sprague-Dawley rats of different ages. Data were analyzed by the Mann-Whitney and Kruskal-Wallis tests, with a p value of .05. Fibroblasts from aged donors showed a significant decrease in cell proliferation, migration, Rac activation, and collagen remodeling when compared with young fibroblasts. Serum from young rats induced higher cell migration when compared with serum from old rats. After TGF-beta1 stimulation, both young and old fibroblasts demonstrated increased levels of alpha-SMA. However, alpha-SMA was incorporated into actin stress fibers in young but not in old fibroblasts. After 7 days of repair, a significant delay in gingival wound-healing was observed in old rats. The present study suggests that cell migration, myofibroblastic differentiation, collagen gel remodeling, and proliferation are decreased in aged fibroblasts. In addition, altered cell migration in wound-healing may be attributable not only to cellular defects but also to changes in serum factors associated with the senescence process.
Keywords: gingiva, fibroblasts, myofibroblast, granulation tissue, regeneration, actin cytoskeleton
Introduction
Aging is a biological process characterized by a decrease in cell function which may result in a gradual impairment of the regenerative properties of most tissues (Conboy and Rando, 2005; Chung et al., 2011). During wound-healing, cells are committed to repopulate damaged tissues through cell proliferation, differentiation, and migration (Martin, 1997). Connective tissue cells surrounding the lesion receive signals from extracellular matrix molecules and soluble factors that stimulate cell migration to conform the granulation tissue (Greiling and Clark, 1997; Martin, 1997). At the intracellular level, cell migration is driven by changes in the polymerization and contraction of acto-myosin fibers (Martin, 1997). This process is controlled, at least in part, by the small GTPase Rac1 that commands cell migration during wound-healing in vivo (Liu et al., 2009). During granulation tissue maturation, fibroblasts differentiate into a specific phenotype known as myofibroblasts (Hinz et al., 2012). Myofibroblasts correspond to a population of mesenchymal cells exhibiting high contractile activity due to the expression of the actin isoform α-smooth-muscle actin (α-SMA). These cells are actively involved in collagen secretion and matrix remodeling (Hinz et al., 2012). Aging causes a delay in the regeneration of periodontal tissues (Benatti et al., 2006), probably due to modifications in cell phenotype (Lossdörfer et al., 2010), defects in cell proliferation, and changes in the regulation of inflammatory cytokines (Benatti et al., 2009). In addition, aging may decrease the synthesis of collagen types I and III (Benatti et al., 2008). All these studies have made important contributions to our understanding of the effects of aging on periodontal tissues. However, critically important aspects of wound-healing, including cell migration, myofibroblastic differentiation, and matrix remodeling, remain to be explored. The present study has evaluated the role of aging in cell migration, proliferation, matrix remodeling, myofibroblastic differentiation, and gingival wound-healing in primary cultures of gingival fibroblasts and rats challenged with gingival wounds.
Materials & Methods
Cell Culture
Explants were obtained from healthy gingiva surrounding third molars or during crown-lengthening surgery. Periodontal examination demonstrated sites with probing depth < 4 mm, no loss of attachment, and no bleeding on probing. Tissues were obtained from five young (15-25 yrs old) and five aged (50-70 yrs old) individuals with the informed consent of the patients. The Ethical Committee, Pontificia Universidad Católica de Chile approved the protocol for obtaining tissue. Patients reported no pre-existing medical or drug histories in the preceding 6 mos, and no smokers were included. Cells were cultured in α-Minimal Essential Medium (α-MEM) (Gibco BRL, Grand Island, NY, USA) containing 10% fetal bovine serum (FBS) (Hyclone Laboratories Inc., Logan, UT, USA), and Normocin (Invivogen, San Diego, CA, USA) at 37°C in a 5% CO2 atmosphere. All experiments were performed with cells expanded between passages 3 and 8.
Wound-healing Studies
Sixteen young (2 mos old; average weight, 336 ± 10 gr) and 16 old (18 mos old; average weight, 738 ± 7.8 gr) male Sprague-Dawley rats were used for wound-healing studies. Full-thickness excisional wounds were made in the palatal gingiva close to the first and second upper molars (1.5 mm x 3 mm) by means of a 15c blade with the aid of a periodontal probe (Hu-Friedy, Chicago, IL, USA). Animal procedures were conducted in accordance with regulations established by the Administrative Panel on Laboratory Animal Care at Pontificia Universidad Católica de Chile. During these studies, we followed the ARRIVE (Animal Research Reporting of in vivo Experiments) guidelines for animal experimentation (Kilkenny et al., 2010). Animals were sacrificed at 2, 7, 14, and 21 days post-wounding (n = 4 for each time-point for both age groups). Tissues were fixed in 10% buffered formalin, decalcified in 10% formic acid, and stained with Giemsa.
Quantification of Wound Closure
In total, 10 sections were obtained from the mesial aspect of the upper first molar. After this, 10 additional sections were obtained for morphological analysis. Quantification of results was performed in the tenth section. Digital images were obtained from each specimen at 20x by means of a light microscope (Leica DM 1000, Wetzlar, Germany) and a digital camera (Leica DFC290HD). Using ImageJ, we defined a rectangular area to quantify the newly generated tissue as follows. A first line was drawn from the alveolar crest to the cemento-enamel junction. Two perpendicular lines, 500 μm in length, were drawn, and a fourth line was created to enclose the area used for quantification. An example of this area is provided as the Appendix Fig. The amount (percentage) of epithelial and connective tissue present in this area was calculated by a blinded examiner who delineated both tissues using ImageJ and obtained the area of regenerated tissue.
Collagen Gel Contraction and Cell Invasion Assays
Restrained or stressed collagen gel experiments were performed over a 24-hour period, and cell invasion assays were performed as previously described (Cáceres et al., 2012). To stimulate cell invasion, we added 10% FBS, 10% rat young serum, or 10% rat old serum to the lower compartment of the transwell chamber. A 2-mL quantity of venous blood was obtained at the time of animal sacrifice. Blood was incubated for 4 hrs at 37°C and then centrifuged for 10 min at 1,800 rpm.
Spreading Assay
Two thousand cells were seeded over fibronectin-coated plates (10 μg/mL) in the presence of 1% FBS. After 45 min, cells were fixed-stained with 0.2% crystal violet. Cell area was quantified with ImageJ.
Rac Activation Assay
Rac activation was evaluated with a G-ELISA Rac Activation Assay Kit (Cytoskeleton, Denver, CO, USA). To assess Rac activation, HGFs from old and young donors were stimulated with 10% serum from young and old rats for 30 min.
MTT Assay
Cell viability was evaluated through the reduction of tetrazolium salt, MTT, to form a blue formazan product (Sigma, St. Louis, MO, USA). Formazan crystals were solubilized with dimethyl sulfoxide and read at 570 nm.
Immunofluorescence
Immunofluorescence was performed with cells grown on coverslips as previously described (Cáceres et al. 2012) using the following antibodies: Ki67 (Novocastra-Leica, Wetzlar, Germany), BrdU (Dako, Carpinteria, CA, USA), and α-smooth-muscle actin (Sigma). F-actin was stained with Alexa-Fluor-555-phalloidin (Invitrogen Molecular Probes, Carlsbad, CA, USA), and nuclei were detected with Hoechst 33342 (Invitrogen Molecular Probes).
Western Blotting
Cell lysis, protein transfer, and electrophoresis were performed as previously described (Cáceres et al., 2012). Membranes were exposed to primary antibodies against α-SMA (Sigma) and β-tubulin (Pierce, Rockford, IL, USA), and secondary antibodies were coupled to horseradish peroxidase and developed (ECL kit, Amersham Biosciences, Piscataway, NJ, USA).
Statistical Analysis
All experiments were performed at least four times on separate occasions using cells from different patients. Statistical analysis was performed by the Mann-Whitney and Kruskal-Wallis non-parametric tests. The statistical software Prism 5.0 from GraphPad was used (La Jolla, CA, USA). In all analyses, p < .05 was considered to indicate statistical significance.
Results
Cell Proliferation, Migration, and Collagen Gel Contraction
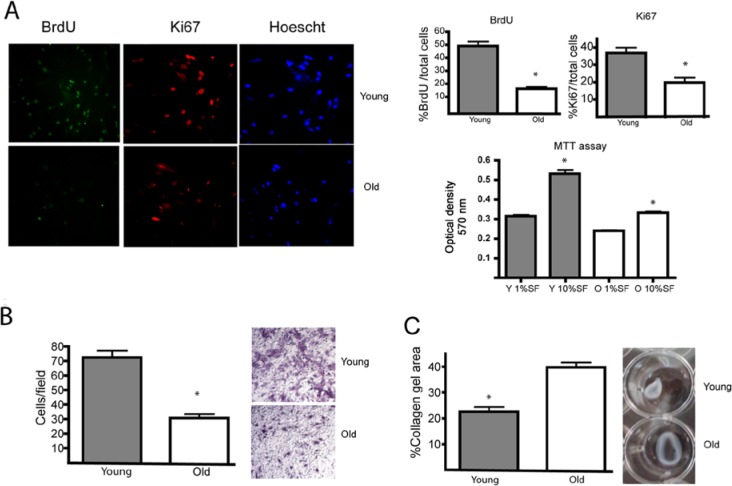
Young and aged HGFs were depleted of serum for 24 hrs and then incubated in the presence of 2 μg/mL BrdU and 10% FBS for 24 hrs. Cells were stained for Ki67 and BrdU through immunofluorescence. As shown in Fig. 1A, young fibroblasts displayed a higher proportion of BrdU (52% young vs. 18% aged) and Ki67 (38% young vs. 18% aged) staining. Using an MTT assay, we observed that young fibroblasts displayed increased cell viability when compared with old cells after 72 hrs (Fig. 1A). Cell migration assessed in a bicameral cell migration system demonstrated that young fibroblasts migrated 2.4 times faster than aged cells (Fig. 1B). Using a restrained or stressed collagen gel assay, we observed that aged fibroblasts displayed a reduced capacity to remodel collagen gels when compared with young cells. The gel area from young fibroblasts was 22% of the total area vs. 41% from aged cells (Fig. 1C).
Figure 1.
Cellular responses associated with gingival repair are decelerated in aged HGFs. (A) Young (Y) and old (O) HGFs were incubated with 2 μg/mL BrdU for 24 hrs in the presence of 10% FBS. To evaluate cell proliferation, we stained cells for BrdU and Ki67. Magnification bar equals 10 μm. Graph indicates average and standard error of positive nuclei for BrdU (+) and Ki67 (+) cells normalized against total cells stained with Hoechst. MTT assay: Graph indicates average and standard error of OD at 570 nm. Asterisks indicate statistically significant differences between young and old fibroblasts. (B) Young and old HGFs were placed in the upper compartment of a transwell chamber. In the lower chamber, 10% FBS was added. Migration was allowed to occur for 16 hrs. Quantification of migration assay is expressed as the average number of migrating cells detected in the lower side of the filter. Young fibroblasts were more migratory than old fibroblasts (p < .001). Magnification bar equals 50 μm. (C) Young and old HGFs were cultured within collagen gels (1 mg/mL) and exposed to 10% FBS. Collagen gel areas are represented as average ± standard error. Young HGFs were more contractile than old HGFs (p < .001). Bars indicate standard error. All assays were performed in quadruplicate. All assays were performed in cell lines derived from 4 different donors.
Myofibroblastic Differentiation
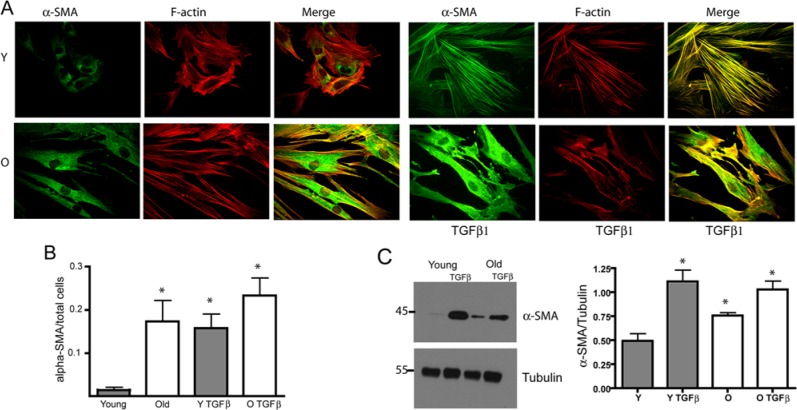
In non-stimulated fibroblasts, a higher proportion of aged fibroblasts demonstrated α-SMA staining when compared with young fibroblasts (Figs. 2A, 2B). When fibroblasts were stimulated with 10 ng/mL TGF-β1 for 72 hrs, both young and aged fibroblasts responded with an increase in the proportion of α-SMA stained cells. However, in aged fibroblasts, actin stress fibers were not well-developed and were not associated with α-SMA (Fig. 2A). Using Western blot of the cell lysates, we evaluated the protein levels for α-SMA. As shown in Fig. 2C, α-SMA protein levels were increased in non-stimulated aged fibroblasts when compared with young cells. However, α-SMA levels were increased in both young and aged fibroblasts after TGF-β1 stimulation.
Figure 2.
Myofibroblastic differentiation. (A) Serum-starved young and old HGFs were stimulated with 10 ng/mL TGF-β1 for 72 hrs. Actin cytoskeleton distribution and α-SMA staining were evaluated through immunofluorescence. Scale bar equals 10 μm. (B) Graph indicates average ± standard error of α-SMA (+) cells normalized against total cells. Asterisk indicates a statistically significant difference between young group compared with all other conditions (p < .0001, Kruskal-Wallis test). (C) Serum-starved young and aged HGFs were stimulated with 10 ng/mL TGF-β1 for 72 hrs, and α-SMA were analyzed through Western blot of the cell lysates. Graph represents the average ± standard error of 4 independent experiments from different cell lines. Asterisks indicate a statistically significant difference between young and old fibroblasts in both resting and stimulated (TGFβ1) conditions.
Cell Migration and Rac Activation
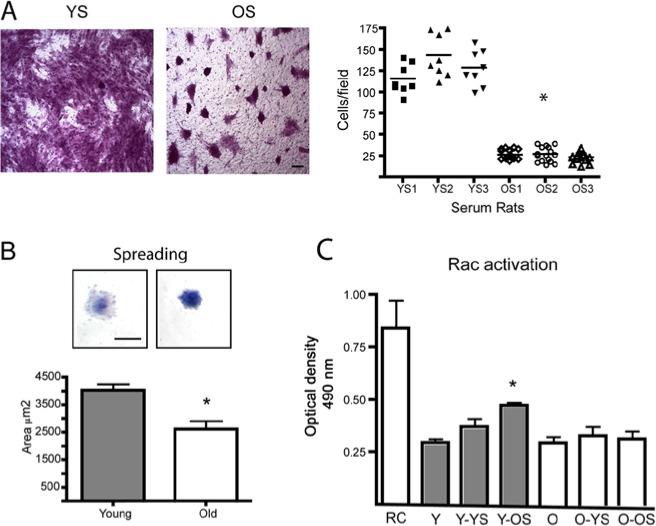
To determine whether defects in wound-healing were due to differences in the chemotactic properties of factors present in serum, we evaluated the migration capacities of fibroblasts derived from young donors challenged with serum obtained from young or old rats. As shown in Fig. 3A, young fibroblasts actively migrated, attracted by serum derived from young rats. However, young fibroblasts showed a reduced migration capacity when exposed to serum obtained from old rats. These responses were quantified using serum from 6 different rats (3 young and 3 old animals). Since cell spreading represents a key step in cell migration, we evaluated the capacity of cells to spread over a fibronectin matrix. Fig. 3B shows that young cells demonstrated increased cell spreading when compared with old fibroblasts. Due to the critical role of the GTPase Rac in cell migration and spreading (Liu et al., 2009; Steffen et al., 2013), we evaluated the activation of Rac in young and aged fibroblasts challenged with serum derived from young or aged rats. In young fibroblasts, Rac was activated by serum derived from young or aged rats. Surprisingly, a higher level of Rac activation was detected in young fibroblasts stimulated by old serum than with young serum (Fig. 3C). Finally, no Rac activation was detected in old fibroblasts stimulated with serum derived from young or old rats.
Figure 3.
Rac1 activation in young and old fibroblasts. (A) Young HGFs were placed in the upper compartment of a transwell chamber with an 8.0-µm-pore polycarbonate filter. In the lower chamber, 10% serum derived from 3 different young (YS) and old (OS) rats was used to stimulate cell migration. After 16 hrs, the migratory cells were fixed and stained, and the quantified graph represents the average ± standard error of migrating cells. Asterisk indicates a statistically significant difference between cells stimulated with 3 different samples of young serum (YS1, YS2, YS3) and cells stimulated with old serum (OS1, OS2, OS3) (Kruskal-Wallis test, p < .0001). Magnification bar equals 10 μm. (B) Cell-spreading assay was performed by plating cells over fibronectin-coated dishes. After 45 min, cells were stained with crystal violet, and the cell area was quantified with ImageJ. Magnification bar equals 15 μm. Asterisk indicates statistically significant differences between young and old fibroblasts (p < .005). (C) Young and old HGFs were stimulated with 10% serum obtained from young and old rats for 30 min. Rac activation was evaluated with a G-ELISA assay of commercial origin. Graph represents the average ± standard error of optical density (OD) measurements. Asterisk indicates a statistically significant difference between non-stimulated young cells (Y) and young cells stimulated with old serum (Y-OS) (Kruskal-Wallis test, p < .05). RC corresponds to a positive control of Rac activation. Y and O correspond to non-stimulated young and old fibroblasts. All assays were performed in cell lines derived from 4 different donors.
Retarded Gingival Wound-healing in Aged Rats
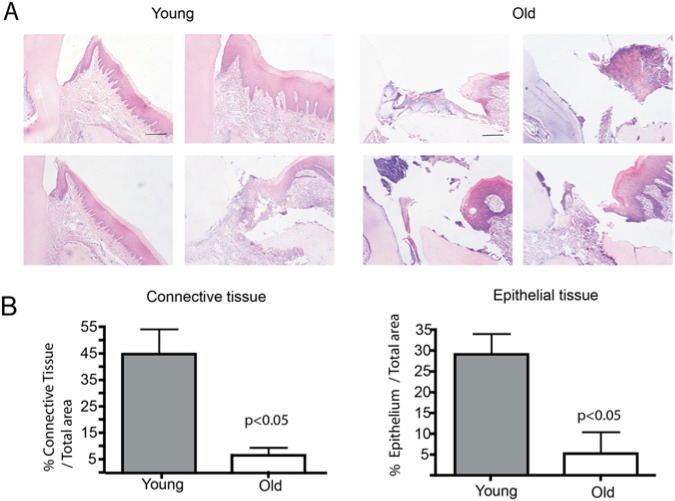
Gingival repair was evaluated in Sprague-Dawley rats 2 and 18 mos of age and analyzed histologically at 2, 7, 14, and 21 days. Wound closure was successfully achieved at 21 days in both young and old rats (Appendix Fig.). However, a significant delay in wound-healing was observed at 7 days in old rats. Fig. 4A shows representative images of 4 young and 4 old rats demonstrating important defects in the migration of both connective tissue and epithelium. As quantitated by the graphs in Fig. 4B, the amount of both gingival epithelium and connective tissue in old rats was significantly reduced when compared with that in young rats.
Figure 4.
Re-epithelialization and formation of connective tissue is decelerated in aged rats. (A) Full-thickness wounds were made in the palatal gingiva. Sections of wounded gingiva from 4 young and 4 aged rats were stained with Giemsa for examination of re-epithelialization and connective tissue at day 7 post-wounding. N = 4. Scale bar equals 100 μm. (B) Graph represents average ± standard error of tissue area (μm2) covered by connective tissue and epithelium of wounds in young and old rats. Asterisk indicates statistically significant differences between old and young rats (Mann-Whitney U test, p < .05 for epithelial tissue, and p < .05 for connective tissue).
Discussion
The present study found important deficiencies in gingival wound-healing associated with the aging process. We have identified that fibroblasts derived from aged donors display diminished cell proliferation, defective migration, altered myofibroblastic differentiation, and collagen remodeling. Using rats of different ages, we have recognized defects in gingival wound-healing characterized by delayed epithelial migration and deficient formation of connective tissue. Our results also show that serum derived from old rats induced a poor chemotactic response, even in fibroblasts derived from young donors. Gingival wound-healing may be compromised at the clinical level in elderly individuals (Lindhe et al., 1985). However, few studies have described the specific aspects of the tissue repair process that may be modified by aging. Benatti et al. (2006) reported that aging may alter the formation of new bone and periodontal ligament in rats. We believe that the present results contribute to understand how aging may affect wound-healing in gingival tissues.
Our study identified deficiencies in cell proliferation in gingival fibroblasts derived from aged donors. This result is consistent with those of previous studies in periodontal ligament cells (Benatti et al., 2009). Defects in cell proliferation in aged dermal fibroblasts have been explained by the loss of epidermal growth factor (EGF) receptors, leading to a reduced response to this mitogen (Shiraha et al., 2000). Although we did not evaluate apoptotic events in our study, previous studies have identified a high expression of pro-apoptotic genes in aged gingival tissues (González et al., 2011). In addition, aging affects telomeric length, pluripotency, and the cell proliferation potential of mesenchymal stem cells (Guillot et al., 2007). Future studies should explore the underlying cellular and molecular defects that may explain altered cell proliferation in aged gingival cells and tissues.
According to our study, gingival fibroblasts derived from aged donors exhibited a decreased capacity to contract collagen gels when compared with young fibroblasts. On the contrary, skin fibroblasts from old rats showed a significant increase in their collagen-remodeling activity (Ballas and Davidson, 2001). Although contradictory, these disparities may be explained by important differences in the behavior of fibroblasts derived from gingiva and skin during aging (Enoch et al., 2010). Importantly, collagen remodeling is also modulated by the differentiation of cells into myofibroblasts, since α-SMA increases the ability of cells to contract collagen matrices (Arora and McCulloch, 1994; Hinz et al., 2012). In our study, primary cultures derived from old donors showed a diffuse cytoplasmic staining for α-SMA in unstimulated conditions. Moreover, when challenged with TGF-β1, α-SMA levels were increased in old fibroblasts and young cells that responded with an increment in α-SMA levels. However, α-SMA was incorporated into actin stress fibers only in young cells. We believe that this defect in acto-myosin fiber remodeling and α-SMA incorporation into stress fibers may also contribute to explaining the defective collagen-remodeling ability identified in old fibroblasts in our study. Fibrotic tissues are characterized by the deposition of disorganized collagen fibers (Chan et al., 2010). Since actin stress fibers, focal adhesions, and integrins may play a critical role in collagen remodeling (Leask, 2013), we believe that aged animals might also carry defects in wound-healing and fibrosis that should be explored in future studies. A pronounced defect in myofibroblastic differentiation has been identified in mesenchymal cells from aged cardiac tissue (Cieslik et al., 2011) and in old skin fibroblasts (Simpson et al., 2010). These results suggest that old fibroblasts may display an interrupted differentiation process into the myofibroblastic phenotype. We propose that defects in myofibroblastic differentiation may have an impact in wound-healing in old gingival tissues.
Cell migration is a critical biological response necessary to provide cells for wound-healing. During tissue repair, soluble factors and plasma proteins are released from serum and activated platelets to initiate cell migration (Martin, 1997). Cells that receive these signals modify their phenotypes and migrate into the wounded tissue. Our study identified important defects in cell migration in fibroblasts derived from aged donors. Moreover, Rac1 was activated only in fibroblasts derived from young individuals. Fibroblasts deficient in Rac1 show important defects in cell migration and lamellipodia formation (Steffen et al., 2013) that derive from deficiencies in wound-healing in vivo (Liu et al., 2009). Importantly, Rac acts through WAVE and Arp2/3 proteins to promote actin polymerization at the front of migrating cells (Jaffe and Hall, 2005). Therefore, several signaling pathways regulating actin polymerization and cell locomotion may be affected or modified by the aging process. Another important finding in our study was that serum derived from old rats showed important deficiencies in the modulation of cell migration. Serum contains several growth factors and cytokines that modulate the responses of cells during wound-healing (Iyer et al., 1999). A paradoxical observation was the finding of increased Rac1 activation in rats exposed to serum from aged rats compared with young rats. EGF receptor (EGFR) potently activates Rac in fibroblasts (Wertheimer et al., 2012). Interestingly, increased levels of EGF and TGF-α (ligand for EGFR) have been found in the serum of aged individuals (Kim et al., 2011), giving a possible explanation for this result.
The present study provides a mechanistic explanation that may help to identify significant deficiencies in the wound-healing process of aging gingival tissues.
Supplementary Material
Acknowledgments
We appreciate the contribution of Claudio Lillo for the immunofluorescence staining of gingival fibroblasts.
Footnotes
A supplemental appendix to this article is published electronically only at http://jdr.sagepub.com/supplemental.
This study was financed by a post-doctoral grant to MC (3120041) and by a research grant to PS (1130618) from the National Fund for Science and Technology (FONDECYT) of Chile.
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Arora PD, McCulloch CA. (1994). Dependence of collagen remodeling on alpha-smooth muscle actin expression by fibroblasts. J Cell Physiol 159:161-175. [DOI] [PubMed] [Google Scholar]
- Ballas CB, Davidson JM. (2001). Delayed wound healing in aged rats is associated with increased collagen gel remodeling and contraction by skin fibroblasts, not with differences in apoptotic or myofibroblast cell populations. Wound Repair Regen 9:223-237. [DOI] [PubMed] [Google Scholar]
- Benatti BB, Neto JB, Casati MZ, Shallum EA, Shallum AW, Nociti FH., Jr (2006). Periodontal healing may be affected by aging: a histologic study in rats. J Periodontal Res 41:329-333. [DOI] [PubMed] [Google Scholar]
- Benatti BB, Silvério KG, Casati MZ, Salloum EA, Nociti FH., Jr (2008). Influence of aging on biological properties of periodontal ligament cells. Connect Tissue Res 49:401-408. [DOI] [PubMed] [Google Scholar]
- Benatti BB, Silvério KG, Casati MZ, Sallum EA, Nociti FH., Jr (2009). Inflammatory and bone-related genes are modulated by aging in human periodontal ligament cells. Cytokine 46:176-181. [DOI] [PubMed] [Google Scholar]
- Cáceres M, Martínez C, Martínez J, Smith PC. (2012). Effects of platelet rich and poor plasma on the reparative response of gingival fibroblasts. Clin Oral Implants Res 23:1104-1111. [DOI] [PubMed] [Google Scholar]
- Chan MW, Hinz B, McCulloch CA. (2010). Mechanical induction of gene expression in connective tissue cells. Methods Cell Biol 98:178-205. [DOI] [PubMed] [Google Scholar]
- Chung HY, Lee EK, Choi YJ, Kim JM, Kim DH, Zou Y, et al. (2011). Molecular inflammation as an underlying mechanism of the aging process and age-related diseases. J Dent Res 90:830-840. [DOI] [PubMed] [Google Scholar]
- Cieslik KA, Trial J, Entman ML. (2011). Defective myofibroblast formation from mesenchymal stem cells in the aging murine heart rescue by activation of the AMPK pathway. Am J Pathol 179:1792-1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conboy I, Rando T. ( 2005). Aging, stem cells and tissue regeneration: lessons from muscle. Cell Cycle 4:407-410. [DOI] [PubMed] [Google Scholar]
- Enoch S, Peake MA, Wall I, Davies L, Farrier J, Giles P, et al. (2010). ‘Young’ oral fibroblasts are geno/phenotypically distinct. J Dent Res 89:1407-1413. [DOI] [PubMed] [Google Scholar]
- González OA, Stromberg AJ, Huggins PM, Gonzalez-Martinez J, Novak MJ, Ebersole JL. (2011). Apoptotic genes are differentially expressed in aged gingival tissue. J Dent Res 90:880-886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greiling D, Clark RA. (1997). Fibronectin provides a conduit for fibroblast transmigration from collagenous stroma into fibrin clot provisional matrix. J Cell Sci 110(Pt 7):861-870. [DOI] [PubMed] [Google Scholar]
- Guillot PV, Gotherstrom C, Chan J, Kurata H, Fisk NM. (2007). Human first-trimester fetal MSC express pluripotency markers and grow faster and have longer telomeres than adult MSC. Stem Cells 25:646-654. [DOI] [PubMed] [Google Scholar]
- Hinz B, Phan SH, Thannickal VJ, Prunotto M, Desmoulière A, Varga J, et al. (2012). Recent developments in myofibroblast biology: paradigms for connective tissue remodeling. Am J Pathol 180:1340-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer VR, Eisen MB, Ross DT, Schuler G, Moore T, Lee JC, et al. (1999). The transcriptional program in the response of human fibroblasts to serum. Science 283:83-87. [DOI] [PubMed] [Google Scholar]
- Jaffe AB, Hall A. (2005). Rho GTPases: biochemistry and biology. Annu Rev Cell Dev Biol 21:247-269. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. (2010). Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol 8:e1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HO, Kim HS, Youn JC, Shin EC, Park S. (2011). Serum cytokine profiles in healthy young and elderly population assessed using multiplexed bead-based immunoassays. J Transl Med 9:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leask A. (2013). Integrin β1: a mechanosignaling sensor essential for connective tissue deposition by fibroblasts. Adv Wound Care 2:160-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindhe J, Socransky S, Nyman S, Westfelt E, Haffajee A. (1985). Effect of age on healing following periodontal therapy. J Clin Periodontol 12:774-787. [DOI] [PubMed] [Google Scholar]
- Liu S, Kapoor M, Leask A. (2009). Rac1 expression by fibroblasts is required for tissue repair in vivo. Am J Pathol 174:1847-1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lossdörfer S, Kraus D, Jäger A. (2010). Aging affects the phenotypic characteristics of human periodontal ligament cells and the cellular response to hormonal stimulation in vitro. J Periodontal Res 45:764-771. [DOI] [PubMed] [Google Scholar]
- Martin P. (1997). Wound healing—aiming for perfect skin regeneration. Science 276:75-81. [DOI] [PubMed] [Google Scholar]
- Shiraha H, Gupta K, Drabik K, Wells A. (2000). Aging fibroblasts present reduced epidermal growth factor (EGF) responsiveness due to preferential loss of EGF receptors. J Biol Chem 275:19343-19351. [DOI] [PubMed] [Google Scholar]
- Simpson RM, Wells A, Thomas D, Stephens P, Steadman R, Phillips A. (2010). Aging fibroblasts resist phenotypic maturation because of impaired hyaluronan-dependent CD44/epidermal growth factor receptor signaling. Am J Pathol 176:1215-1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffen A, Ladwein M, Dimchev GA, Hein A, Schwenkmezger L, Arens S, et al. (2013). Rac function is crucial for cell migration but is not required for spreading and focal adhesion formation. J Cell Sci 126(Pt 20):4572-4588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wertheimer E, Gutierrez-Uzquiza A, Rosemblit C, Lopez-Haber C, Sosa MS, Kazanietz MG. (2012). Rac signaling in breast cancer: a tale of GEFs and GAPs. Cell Signal 24:353-362. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.