Abstract
Temporomandibular joint (TMJ) discs frequently undergo degenerative changes in arthritis. However, the biomechanical properties of pathogenic discs remain to be explored. In this study, we evaluated the effects of chronic inflammation on the biomechanical properties of TMJ discs in rats. Chronic inflammation of TMJs was induced by double intra-articular injections of complete Freund’s adjuvant for 5 weeks, and biomechanical properties and ultrastructure of the discs were examined by mechanical testing, scanning electron microscopy, and transmission electron microscopy. The instantaneous compressive moduli of the anterior and posterior bands of discs in inflamed TMJs were decreased significantly compared with those in the control group. The instantaneous tensile moduli of the discs of inflamed TMJs also showed significant decreases in both the anterior-posterior and mesial-lateral directions. The relaxation moduli of the discs of inflamed TMJs showed nearly the same tendency as the instantaneous moduli. The surfaces of the discs of inflamed TMJs became rough and porous due to the loss of the superficial gel-like stratum, with many collagen fibers exposed and degradation of the sub-superficial collagen fibrils. Our results suggested that chronic inflammation of TMJ could lead to deterioration of mechanical properties and alteration of disc ultrastructure, which might contribute to TMJ disc displacement.
Keywords: temporomandibular joint, temporomandibular disorders, disc displacement, arthritis, biomechanics, collagen
Introduction
Internal derangement or articular disc displacement in the temporomandibular joint (TMJ) is characterized by different displacements of the discs in the joint, such as disc anterior displacement with or without reduction, and occupies a large sub-group in the classification of temporomandibular disorders (TMD) (Emshoff and Rudisch, 2001; Stegenga, 2001). The TMJ disc has a biconcave shape and functions in absorbing and redistributing stress, which plays an important role in protecting the joint (Tanaka and van Eijden, 2003).
Patients with severe anterior disc displacement in the TMJ show strong correlations with disc deformation, including thickening, lengthening, and folding (Westesson and Rohlin, 1984; Westesson et al., 1985; Orsini et al., 1996; Taskaya-Yilmaz and Ogutcen-Toller, 2001). Moreover, inflammation and degeneration were found in nearly one-third of TMJs with advanced internal derangement (Dimitroulis, 2005). However, the causes of disc displacement in the TMJ remain unknown. It has been noted that some TMJs had inflammation and disc degeneration but had no disc displacement (de Bont et al., 1986; Kondoh et al., 1998), although inflammation of the TMJ and deformation of discs are generally believed to be the consequence of disc displacement. However, in our recent study, deformity and thickening and changes in the disc contents of collagen and proteoglycan were observed in chronically inflamed TMJs in rats (Wang et al., 2012b). Therefore, we hypothesized that inflammation might cause deterioration of the biomechanical properties of discs.
The mechanical properties of the disc are indispensable to TMJ function. Several mechanical properties of normal TMJ discs from various species have been evaluated (Tanaka and van Eijden, 2003; Allen and Athanasiou, 2006; Koolstra and Tanaka, 2009; Kuo et al., 2011; Juran et al., 2013). Anisotropic and heterogeneous properties among diverse region of discs have been highlighted (Kuo et al., 2010; Yuya et al., 2010). Although the physiological functions and mechanical properties of TMJ discs have been documented to some extent, there is a lack of understanding regarding the mechanical properties of pathogenic discs. Moreover, little information is available on the relationship between biomechanical properties and ultrastructure changes of discs in inflamed TMJs.
In this study, we examined the biomechanical properties (tensile and compressive) and the ultrastructure of discs from chronically inflamed TMJs in rats.
Materials & Methods
Induction of Inflammation
Female Sprague–Dawley rats (7 wk old, 180-200 g) were divided randomly into inflammation and control groups. TMJ inflammation was induced by intra-articular injections of complete Freund’s adjuvant (CFA; Sigma, St. Louis, MO, USA) twice, as described previously (Wang et al., 2012b). Briefly, a 25-μL quantity of CFA was emulsified in 25 μL saline and then injected directly into the upper compartments of the TMJs on days 0 and 14. The control group received similar intra-articular injections of saline. All rats were sacrificed by pentobarbital overdose on day 35. Rats were housed under controlled temperatures with a 12-/12-hour light/dark cycle and free access to food and water.
Animal procedures were approved by the Peking University Animal Ethics Committee prior to the initiation of the study (approval number: LA2012-59). This investigation conformed to the ARRIVE guidelines for preclinical studies.
Macroscopic Mechanical Testing
We carefully dissected 16 discs from the TMJs of eight rats by cutting the disc-condylar ligaments and stored them in ice-cold 0.15 M phosphate-buffered saline (PBS, pH 7.4) until use (Juran et al., 2013). All samples were tested within 4 hr of dissection. Right and left discs were examined for compressive and tensile properties, respectively. Discs were kept moist by being soaked in PBS buffer at room temperature throughout the entire testing process (Tanaka et al., 2003).
Compressive and tensile properties, including instantaneous reaction and stress relaxation of the discs, were tested with the ElectroForce 5200 biodynamic test instrument (Bose Corporation, Bloomington, MN, USA). We modified the indenter for both compression and tension tests. The indenter in compression tests was designed to have a rounded cross-section (r = 0.37 mm), which was small enough for the rugged surface of the disc to be regarded as a regular sample (Appendix Fig., C). Analogously, the clamping apparatus for tensile testing was modified to have a square contact section (a = 0.3 mm), which is sufficiently small to allow us to ignore the deviation in internal thickness, and to consider the sample as a long bar-shape between the clamping apparatus (Appendix Fig., D). The cross-sectional areas of the compression and tensile apparatus were 0.43 mm2 and 0.3 mm2, respectively. Graphs of compressive and tensile tests are shown in Figs. 1A and 1B.
Figure 1.
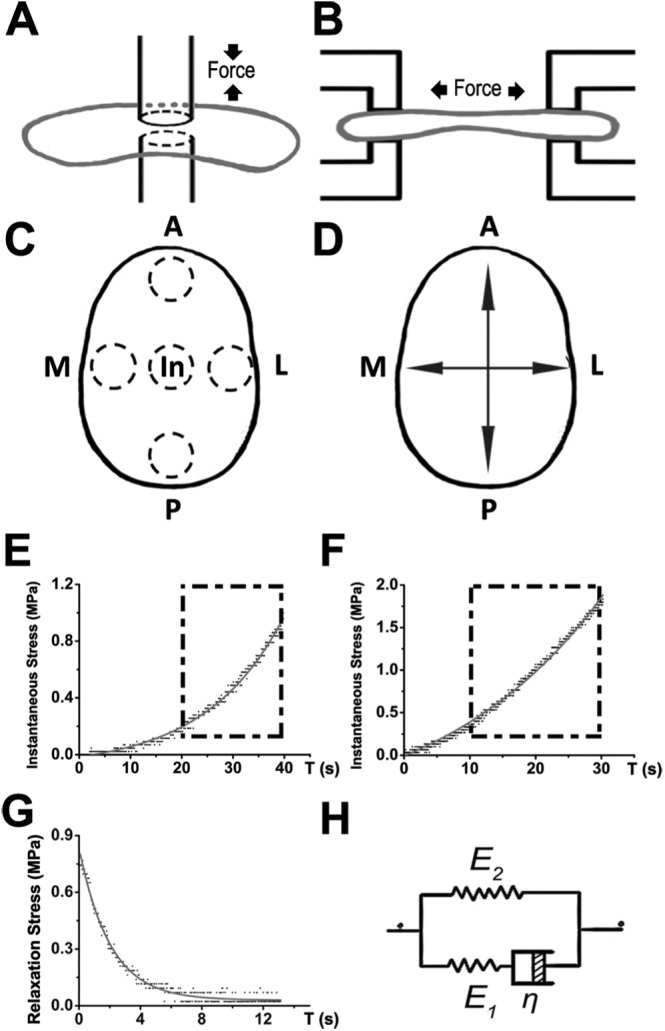
Description and representative stress-strain curves of biomechanical tests of TMJ disc. (A) Graph of the process of compressive tests. (B) Graph of the process of tensile tests. (C) Region division of compressive mechanical tests of TMJ disc. Dotted circle represents relevant compressive regions, including anterior, intermediate, posterior, mesial, and lateral regions. (D) Direction division of tensile mechanical tests of TMJ disc. The arrow represents the tensile direction, including anterior-posterior and mesial-lateral directions. (E) Linear fit of instantaneous compressive modulus. (F) Linear fit of instantaneous tensile modulus. (G) Nonlinear fit of relaxation modulus. (H) Kelvin model used to build viscoelastic model in relaxed tests. A, anterior; In, intermediate; P, posterior; M, mesial; L, lateral.
To maintain the integrity of discs, the tested regions were not isolated from the disc. For compressive testing, five equal regions (anterior, posterior, intermediate, medial, and lateral) were marked on each disc (Fig. 1C). For tensile testing, only anterior-posterior and medial-lateral directions were marked on the discs (Fig. 1D).
Following the application of a 0.05-N tare load, the initial thickness and length were calculated according to the exact location. During testing, the largest strain was defined (compressive strain, 40%; tensile strain, 30%). Stress and strain magnitudes were recorded at 0.02-second intervals, and stress-strain and strain-time curves were generated. Disc viscoelastic properties were evaluated in terms of the instantaneous and relaxation moduli. For the instantaneous modulus, stress-strain curves were intercepted at the transition zone (Tanaka and van Eijden, 2003), and fitted to a linear model. For the test, 20% to 40% compressive strain (Fig. 1E) and 10% to 30% tensile strain were used (Fig. 1F). In the stress-relaxation test, the Kelvin model was used to fit stress-time curves (Allen and Athanasiou, 2006; Kalpakci et al., 2011) (Figs. 1G, 1H). Using these original data, we calculated stress, strain, and modulus. The expression of the stress-time curve was as follows:
The relaxation modulus was determined according to this formula. All experimental data showed high accuracy ( = 0.94).
Scanning Electron Microscopy (SEM) and Transmission Electron Microscopy (TEM)
In total, six TMJ discs of three rats were fixed with 2.5% glutaraldehyde solution (Sigma) at 4°C for 12 hr, with half (right discs) for SEM and the other half (left discs) for TEM. SEM and TEM were performed as described previously (Wang et al., 2012a). For SEM, the superior surface of the anterior band, intermediate zone, and posterior band of the disc was scanned. For TEM, the superior surface of the anterior band, intermediate zone, and posterior band of the disc was examined. Samples were double-fixed with 1% osmium tetroxide (Sigma), stained with lead citrate and uranyl acetate, embedded in epoxy resin, and sectioned at 100 nm.
Statistical Analysis
Statistical analyses were performed with SPSS software (ver. 11.0 for Windows). Data for macroscopic mechanical testing are presented as means ± standard deviations (SDs). Two of the samples were damaged during tensile properties testing, and the corresponding data were excluded in statistical analysis. Thus, the sample sizes for compressive and tensile tests were eight and six, respectively. Statistical comparisons of the two groups were performed an independent t test. P values < .05 were considered to indicate statistical significance.
Results
Decreased Instantaneous and Relaxation Moduli of Discs in Chronically Inflamed TMJ
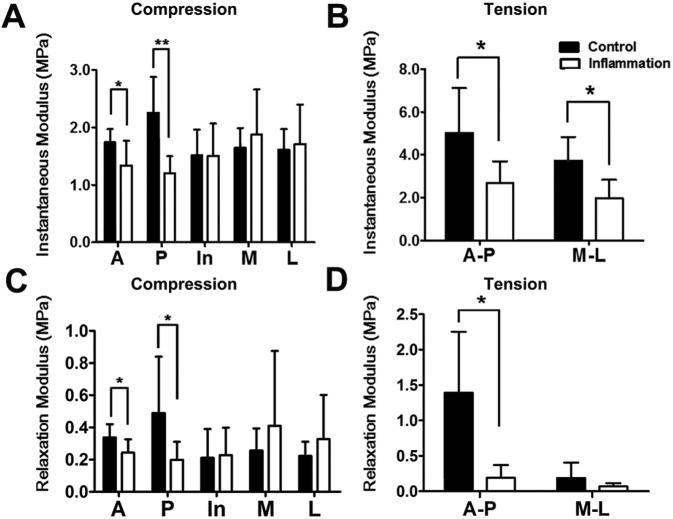
Biomechanical properties of discs from TMJs with and without chronic inflammation were evaluated by macroscopic mechanical testing. The instantaneous and relaxation moduli of discs in compression and tension tests are shown in the Table. The instantaneous compressive moduli of the anterior and posterior bands of the discs of inflamed TMJs were decreased significantly vs. those of control discs (1.34 ± 0.43 MPa and 1.20 ± 0.30 MPa vs. 1.74 ± 0.23 MPa and 2.26 ± 0.62 MPa, respectively; n = 8; p < .05; Fig. 2A). However, the compressive moduli of the intermediate, medial, and lateral regions of discs from inflamed TMJs did not differ from those of control discs. The instantaneous tensile moduli in the anterior-posterior and mesial-lateral directions of discs from inflamed TMJs showed significant decreases compared with those of the control group (2.69 ± 1.01 MPa and 1.98 ± 0.85 MPa vs. 5.03 ± 2.08 MPa and 3.74 ± 1.09 MPa, respectively, n = 6; p < .05; Fig. 2B). Two samples were broken during tension testing, and their data were excluded from the analyses. The relaxation compressive moduli of the anterior and posterior bands of discs from inflamed TMJs were also decreased compared with those of the control group (0.24 ± 0.08 MPa and 0.20 ± 0.11 MPa vs. 0.34 ± 0.08 MPa and 0.49 ± 0.35 MPa, respectively; n = 8; p < .05; Fig. 2C). The relaxation tensile modulus in the anterior-posterior direction of discs from inflamed TMJs showed a significant decrease compared with that of the control group (0.19 ± 0.18 MPa vs. 1.39 ± 0.88 MPa), whereas the relaxation tensile modulus in the mesial-lateral direction showed no difference between the groups (Fig. 2D).
Table.
Values of Compressive and Tensile Moduli of TMJ Discs Represented by the Mean ± Standard Deviation
| (A) | ||||
|---|---|---|---|---|
| Instantaneous Modulus (MPa) |
Relaxation Modulus (MPa) |
|||
| Location | Control | Inflammation | Control | Inflammation |
| A | 1.74 ± 0.23 | 1.34 ± 0.43 | 0.34 ± 0.08 | 0.24 ± 0.08 |
| P | 2.26 ± 0.62 | 1.20 ± 0.30 | 0.49 ± 0.35 | 0.20 ± 0.11 |
| In | 1.51 ± 0.45 | 1.51 ± 0.56 | 0.21 ± 0.18 | 0.23 ± 0.17 |
| M | 1.65 ± 0.35 | 1.88 ± 0.78 | 0.26 ± 0.14 | 0.41 ± 0.46 |
| L | 1.62 ± 0.36 | 1.71 ± 0.69 | 0.22 ± 0.09 | 0.33 ± 0.27 |
|
| ||||
| (B) | ||||
| Instantaneous Modulus (MPa) |
Relaxation Modulus (MPa) |
|||
| Direction | Control | Inflammation | Control | Inflammation |
| A-P | 2.69 ± 1.01 | 5.03 ± 2.08 | 1.39 ± 0.88 | 0.19 ± 0.18 |
| M-L | 1.98 ± 0.85 | 3.74 ± 1.09 | 0.19 ± 0.21 | 0.07 ± 0.05 |
(A) Compressive instantaneous and relaxation moduli of control and inflamed discs (n = 8).
(B) Tensile instantaneous and relaxation moduli of control and inflamed discs (n = 6).
A, anterior; In, intermediate; P, posterior; M, mesial; L, lateral.
Figure 2.
Biomechanical properties of TMJ discs with or without inflammation. (A) Instantaneous moduli under compression from 20% to 40% strain. Instantaneous compressive moduli of the anterior and posterior bands of discs of inflamed TMJs were significantly lower than those of the control group, whereas there was no statistically significant difference among the other three regions (n = 8; *p < .05, **p < .01). (B) Instantaneous moduli under tension from 10% to 30% strain. Instantaneous tensile moduli of anterior-posterior and mesial-lateral directions of discs of the inflamed TMJ were significantly lower than those of the control group (n = 6; *p < .05). (C) Relaxation moduli under compression. (D) Relaxation moduli under tension. Relaxation moduli of discs of the inflamed TMJs showed nearly the same trend as the instantaneous moduli. A, anterior zone; In, intermediate zone; P, posterior zone; M, mesial zone; L, lateral zone.
Loss of Gel-like Structure of Superior Surfaces of Discs from Inflamed TMJ
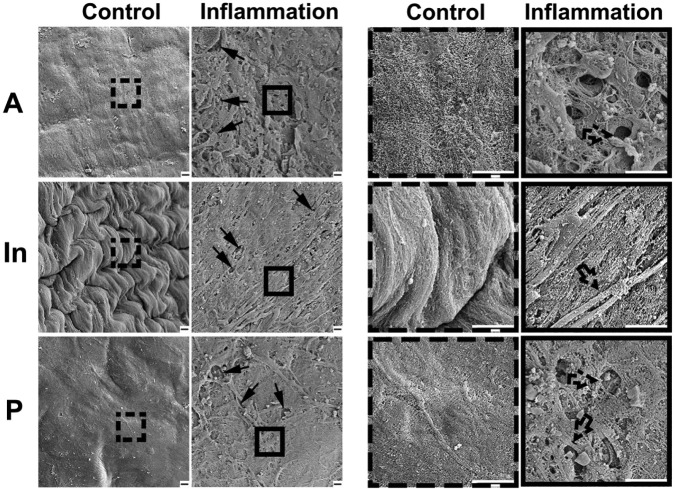
To evaluate the effects of inflammation on ultrastructural morphology in TMJ discs, we examined ultra-morphological changes of the superior surfaces of discs by SEM. At lower magnification (2,000×), the superficial strata of the anterior and posterior bands of the control disc appeared even and intact, covered with a gel-like structure; the superficial stratum of the intermediate zone of the control disc showed obvious folded features or a corrugated surface. Despite variation among bands, the surfaces of the control discs showed regularity at higher magnification (10,000×). All three control discs showed similar ultrastructural surfaces. However, at lower magnification, the superficial strata of discs from inflamed TMJs for all three discs examined were observed to lack the gel-like structure of the anterior and posterior bands and the corrugated surface of the intermediate zone; at higher magnification, superficial fractional damage and exposure of sub-superficial collagen fiber-like tissue were evident for all three discs examined (Fig. 3).
Figure 3.
SEM analysis of surface topography of discs of TMJs with or without inflammation. Left panels were lower magnification (magnified 2,000×), and right panels were higher magnification (magnified 10,000×) from the dashed and solid boxes on the left panels. Control group showed relative flat and smooth surfaces in anterior and posterior bands and folded integrated surface in the intermediate zone; the superficial gel-like layer was distinct at the lower magnification. Inflammation group showed rough and porous surfaces in anterior and posterior bands of discs of inflamed TMJs; the intermediate zone lost the folded structure and became flattened, and the gel-like layer was discontinued (arrow), with collagen fibers exposed (dotted arrow). Bar = 2 µm. Representative images of three samples.
Disordered Arrangement of Collagen Fibrils in Discs from Inflamed TMJ
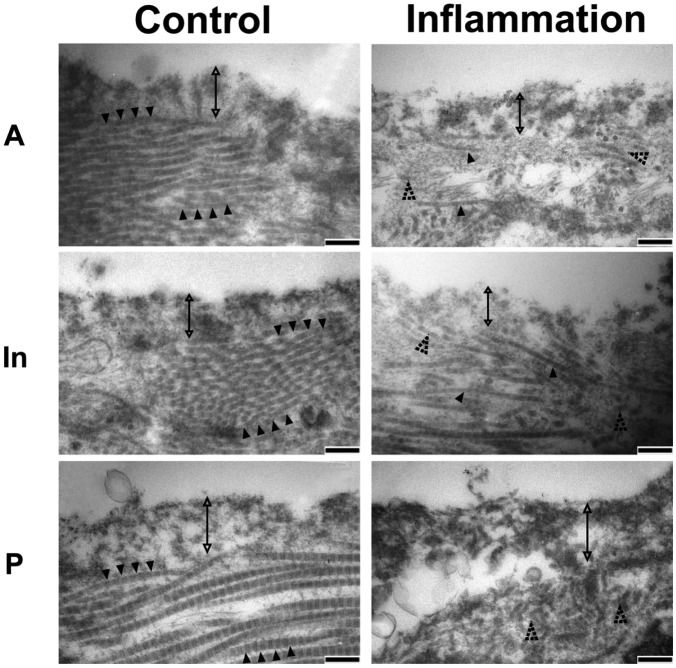
For further examination of the ultra-morphological changes in discs from inflamed TMJs, TEM was performed. The superior surfaces of the anterior band, intermediate zone, and posterior band of the control disc were covered by a 150- to 400-nm-thick superficial stratum, which had a collagen-free, electron-dense, and amorphous structure. A deeper stratum of less-electron-dense proteoglycan lamina with well-arranged collagen fibrils was also evident. However, in the inflamed group, the collagen-free superficial stratum was partially diminished; the collagen fibrils of the deeper stratum were disorganized and degraded, resulting in diffused and scattered individual collagen fibrils (Fig. 4).
Figure 4.
TEM analysis of superficial sections of discs of TMJs with or without inflammation. The surface of the control disc consisted of a collagen-free lamina (double arrow) covering the underlying collagen fibrils (black arrowhead), whereas the superficial structure became disorganized in the inflamed discs, with collagen-free lamina becoming irregular and the underlying collagen fibrils degraded and irregularly arranged (dotted arrowhead). Bar = 200 nm. Representative images of three samples.
Discussion
In this study, we demonstrated the deterioration of biomechanical properties and ultrastructural changes of discs from chronically inflamed TMJs. Both the instantaneous and relaxation moduli of discs from the inflamed TMJs were significantly lower than those of control discs. SEM and TEM showed disturbance of the superficial stratum, and exposure and degradation of the underlying collagen fibers in the discs of inflamed TMJs, suggesting a relationship between the biomechanical alterations and ultrastructural changes in the discs. To the best of our knowledge, this is the first report of a decrease in mechanical properties in discs from chronically inflamed TMJ discs.
The compressive and tensile moduli of discs were significantly decreased in chronically inflamed TMJs. This decrease in instantaneous moduli in different locations and directions of the discs from the inflamed TMJs indicated deterioration in the capacity to absorb and distribute stress from the inflamed TMJ discs. This deterioration of biomechanical properties of discs could be an indication of early degeneration, and is consistent with changes in articular cartilage due to inflammation (Silver et al., 2002). During mandibular movements, discs often endure and absorb peak loads by deformation in a certain area (Tanaka et al., 2004). In the present study, to observe biomechanical properties under significant deformation, we set 40% strain of compression and 30% strain of tension as loading limits. A diminished instantaneous modulus of the disc indicated that the disc was relatively softer or underwent greater strain under the same stress, which may subsequently cause a decrease in adaptive capacity of the disc and result in overloading of the TMJ, ultimately giving rise to tissue breakdown.
The decreasing instantaneous moduli of discs from the chronically inflamed TMJs were the opposite of the changes in aging discs, which showed an increase in instantaneous modulus (Tanaka et al., 2006; Ahn et al., 2007). These differing changes in mechanical properties may result from the different characteristics of the degeneration process. Age-related biomechanical changes of TMJ discs imply stiffening of discs (Tanaka et al., 2006) and may be related to an increase in calcium content in the discs (Takano et al., 1999). In contrast, the decreasing instantaneous moduli of the discs of chronically inflamed TMJs in the present model imply softening of the discs, which are more similar to the early degenerative changes of condylar cartilage, including swelling and softening (de Bont and Stegenga, 1993). Moreover, glycosaminoglycan (GAG) and collagen contents are considered to be responsible for the elastic properties of discs (Scapino et al., 1996; Tanaka et al., 2003; Tanaka and van Eijden, 2003). It is reported that the concentration of GAG showed a decrease with age in rats (Okazaki et al., 1996). However, in our previous study, both GAG and collagen, the composition of the disc matrix, were increased in the inflamed discs (Wang et al., 2012b). We evaluated the change of matrix of the disc as a whole, but did not examine the regional content of GAG and collagen in each band of the disc. Therefore, we could not exclude the possibility that changes of regional matrix components might also contribute to the regional alteration in biomechanical properties.
The relaxation modulus in discs from inflamed TMJs were also decreased. The stress relaxation of discs reflects their capacity to dissipate stress (Tanaka and van Eijden, 2003). Changes in the relaxation modulus are related to the moisture level of the disc and involve their viscoelastic properties, which play important roles in TMJ disc function (Kuboki et al., 1997; Yamashita et al., 2001). Previously, we showed that moisture content is increased in discs from inflamed TMJs (Wang et al., 2012b); thus, the decrease in viscoelasticity in the anterior and posterior bands may also be due to the increased moisture content of the disc following chronic inflammation of the TMJ.
The changes in morphological features may also be associated with changes in the mechanical and functional properties of discs from inflamed TMJs. The proteoglycans and arrangements of collagen fibers are considered to influence the compressive and tensile properties of discs (Stankovic et al., 2013). We observed by SEM and TEM that the superficial stratum was disordered, with a rough and porous structure in all three bands of discs from inflamed TMJs, and the underlying collagen fibers exhibited marked degradation. The superficial gel-like stratum of the three bands likely was comprised of chondroitin-sulfate-rich proteoglycans, similar to the surface layers of articular cartilage, providing lubrication and compression resistance to the articular surface (Orford and Gardner, 1985). Thus, the ultrastructural alterations observed may also account, in part, for the decrease in the mechanical properties of discs from inflamed TMJs.
Regional variations in compressive modulus were found in discs from inflamed TMJs. Studies have reported various magnitudes of tensile modulus or Young’s modulus and relaxation modulus. Analysis of data from animals showed marked differences in tension compared with humans, and regional variations are seen consistently among species (Kalpakci et al., 2011). We observed that the compressive modulus was higher in the anterior and posterior bands than in the intermediate zone, and the apparent Young’s modulus and ultimate tensile strength of the disc were higher for anterior-posterior loading than for mesial-lateral loading in both the control and inflammation groups. These results are consistent with those of other mechanical tests (Beatty et al., 2001; Kalpakci et al., 2011). Although the morphological changes were clear in all three zones of discs from inflamed TMJs, the intermediate zone showed no significant difference in mechanical tests compared with the control discs. This might be due to the thickness of the intermediate zone, which was the thinnest part of the TMJ disc. Thus, obtaining accurate data for the intermediate zone may not be feasible, resulting in the large variation. However, the compressive modulus of the mesial and lateral bands of the discs also showed no significant difference between the groups; this could be due to the fact that rats do not have lateral translator movement of the mandible (Hiiemäe and Ardran, 1968; Weijs, 1975), resulting in significant changes in compressive modulus only in the anterior and posterior bands. Moreover, various contents and types of proteoglycans have been found in different regions of discs in several mammals (Tanaka and van Eijden, 2003); this may also explain the differences in mechanical properties among regions. Additionally, regional variations were identified only in the viscoelasticity test under instantaneous loading; mechanical tests with a range of frequencies and cyclic loading, as well as shear stress testing, are necessary for a full assessment.
In this study, we demonstrated deterioration in the superficial ultrastructural morphology and mechanical properties of TMJ discs following the induction of chronic inflammation. Based on our previous and the current results, we suggest that TMJ inflammation-induced deformities and alterations in the contents and mechanical properties of discs might be a predisposing factor for disc displacement in the TMJ. Although there was a lack of direct evidence to support this suggestion, whether the distribution of stress of inflamed TMJ discs and surrounding tissues was also altered and how changes in disc mechanical properties contribute to functional changes in TMJ discs require further investigation.
There is a close relationship between arthritis and disc displacement, but the cause for disc displacement is still unclear. Guler et al. reported that 10 out of 16 discs with disc displacement without reduction presented joint effusion (Guler et al., 2005); Emshoff et al. reported that 11 out of 23 patients with arthritis had disc displacement without reduction (Emshoff et al., 2000). These clinical observations imply that there would be a correlation between the inflammatory changes and disc displacement. Nevertheless, the deterioration of disc biomechanical properties of chronic inflammation of TMJ in rats in the present study would suggest, or provide at least one possibility, that the discs of TMD patients, who usually have chronic inflammation in the TMJs, might also have decreased instantaneous and relaxation moduli, which would lead to the decrease in adaptive capacity of the disc and gradual breakdown of the harmony of the disc-condyle complex, ultimately leading to disc displacement. Therefore, our findings might be simply a new view to explain the cause of disc displacement.
In conclusion, chronic TMJ inflammation resulted in disruption of the surface morphology and deterioration of the viscoelasticity of the disc.
Supplementary Material
Footnotes
This project is supported by International Science and Technology Cooperation Program of China (Grant No. 2013DF B30360), and the National Natural Science Foundation of China (Grant No. 81300850).
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
A supplemental appendix to this article is published electronically only at http://jdr.sagepub.com/supplemental.
References
- Ahn HJ, Paik SK, Choi JK, Kim HJ, Ahn DK, Cho YS, et al. (2007). Age-related changes in the microarchitecture of collagen fibrils in the articular disc of the rat temporomandibular joint. Arch Histol Cytol 70:175-181. [DOI] [PubMed] [Google Scholar]
- Allen KD, Athanasiou KA. (2006). Viscoelastic characterization of the porcine temporomandibular joint disc under unconfined compression. J Biomech 39:312-322. [DOI] [PubMed] [Google Scholar]
- Beatty MW, Bruno MJ, Iwasaki LR, Nickel JC. (2001). Strain rate dependent orthotropic properties of pristine and impulsively loaded porcine temporomandibular joint disk. J Biomed Mater Res 57:25-34. [DOI] [PubMed] [Google Scholar]
- de Bont LG, Stegenga B. (1993). Pathology of temporomandibular joint internal derangement and osteoarthrosis. Int J Oral Maxillofac Surg 22:71-74. [DOI] [PubMed] [Google Scholar]
- de Bont LG, Boering G, Liem RS, Eulderink F, Westesson PL. (1986). Osteoarthritis and internal derangement of the temporomandibular joint: a light microscopic study. J Oral Maxillofac Surg 44:634-643. [DOI] [PubMed] [Google Scholar]
- Dimitroulis G. (2005). The prevalence of osteoarthrosis in cases of advanced internal derangement of the temporomandibular joint: a clinical, surgical and histological study. Int J Oral Maxillofac Surg 34:345-349. [DOI] [PubMed] [Google Scholar]
- Emshoff R, Rudisch A. (2001). Validity of clinical diagnostic criteria for temporomandibular disorders: clinical versus magnetic resonance imaging diagnosis of temporomandibular joint internal derangement and osteoarthrosis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 91:50-55. [DOI] [PubMed] [Google Scholar]
- Emshoff R, Puffer P, Rudisch A, Gassner R. (2000). Temporomandibular joint pain: relationship to internal derangement type, osteoarthrosis, and synovial fluid mediator level of tumor necrosis factor-alpha. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 90:442-449. [DOI] [PubMed] [Google Scholar]
- Guler N, Uckan S, Imirzalioglu P, Acikgozoglu S. (2005). Temporomandibular joint internal derangement: relationship between joint pain and MR grading of effusion and total protein concentration in the joint fluid. Dentomaxillofac Radiol 34:175-181. [DOI] [PubMed] [Google Scholar]
- Hiiemäe KM, Ardran GM. (1968). A cinefluorographic study of mandibular movement during feeding in the rat (Rattus norvegicus). J Zool 154:139-154. [Google Scholar]
- Juran CM, Dolwick MF, McFetridge PS. (2013). Shear mechanics of the TMJ disc: relationship to common clinical observations. J Dent Res 92:193-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalpakci KN, Willard VP, Wong ME, Athanasiou KA. (2011). An interspecies comparison of the temporomandibular joint disc. J Dent Res 90:193-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondoh T, Westesson PL, Takahashi T, Seto K. (1998). Prevalence of morphological changes in the surfaces of the temporomandibular joint disc associated with internal derangement. J Oral Maxillofac Surg 56:339-343. [DOI] [PubMed] [Google Scholar]
- Koolstra JH, Tanaka E. (2009). Tensile stress patterns predicted in the articular disc of the human temporomandibular joint. J Anat 215:411-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuboki T, Shinoda M, Orsini MG, Yamashita A. (1997). Viscoelastic properties of the pig temporomandibular joint articular soft tissues of the condyle and disc. J Dent Res 76:1760-1769. [DOI] [PubMed] [Google Scholar]
- Kuo J, Zhang L, Bacro T, Yao H. (2010). The region-dependent biphasic viscoelastic properties of human temporomandibular joint discs under confined compression. J Biomech 43:1316-1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo J, Wright GJ, Bach DE, Slate EH, Yao H. (2011). Effect of mechanical loading on electrical conductivity in porcine TMJ discs. J Dent Res 90:1216-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okazaki J, Kamada A, Higuchi Y, Kanabayashi T, Sakaki T, Gonda Y. (1996). Age changes in the rat temporomandibular joint articular disc: a biochemical study on glycosaminoglycan content. J Oral Rehabil 23:536-540. [DOI] [PubMed] [Google Scholar]
- Orford CR, Gardner DL. (1985). Ultrastructural histochemistry of the surface lamina of normal articular cartilage. Histochem J 17:223-233. [DOI] [PubMed] [Google Scholar]
- Orsini MG, Yatani H, Kuboki T, Yamashita A. (1996). Relationship between temporomandibular joint disc position and configuration on magnetic resonance imaging. Oral Radiol 12:39-47. [Google Scholar]
- Scapino RP, Canham PB, Finlay HM, Mills DK. (1996). The behaviour of collagen fibres in stress relaxation and stress distribution in the jaw-joint disc of rabbits. Arch Oral Biol 41:1039-1052. [DOI] [PubMed] [Google Scholar]
- Silver FH, Bradica G, Tria A. (2002). Elastic energy storage in human articular cartilage: estimation of the elastic modulus for type II collagen and changes associated with osteoarthritis. Matrix Biol 21:129-137. [DOI] [PubMed] [Google Scholar]
- Stankovic S, Vlajkovic S, Boskovic M, Radenkovic G, Antic V, Jevremovic D. (2013). Morphological and biomechanical features of the temporomandibular joint disc: an overview of recent findings. Arch Oral Biol 58:1475-1482. [DOI] [PubMed] [Google Scholar]
- Stegenga B. (2001). Osteoarthritis of the temporomandibular joint organ and its relationship to disc displacement. J Orofac Pain 15:193-205. [PubMed] [Google Scholar]
- Takano Y, Moriwake Y, Tohno Y, Minami T, Tohno S, Utsumi M, et al. (1999). Age-related changes of elements in the human articular disk of the temporomandibular joint. Biol Trace Elem Res 67:269-276. [DOI] [PubMed] [Google Scholar]
- Tanaka E, van Eijden T. (2003). Biomechanical behavior of the temporomandibular joint disc. Crit Rev Oral Biol Med 14:138-150. [DOI] [PubMed] [Google Scholar]
- Tanaka E, Aoyama J, Tanaka M, Van Eijden T, Sugiyama M, Hanaoka K, et al. (2003). The proteoglycan contents of the temporomandibular joint disc influence its dynamic viscoelastic properties. J Biomed Mater Res A 65:386-392. [DOI] [PubMed] [Google Scholar]
- Tanaka E, del Pozo R, Tanaka M, Asai D, Hirose M, Iwabe T, et al. (2004). Three-dimensional finite element analysis of human temporomandibular joint with and without disc displacement during jaw opening. Med Eng Phys 26:503-511. [DOI] [PubMed] [Google Scholar]
- Tanaka E, Hirose M, Yamano E, Dalla-Bona DA, Fujita R, Tanaka M, et al. (2006). Age-associated changes in viscoelastic properties of the bovine temporomandibular joint disc. Eur J Oral Sci 114:70-73. [DOI] [PubMed] [Google Scholar]
- Taskaya-Yilmaz N, Ogutcen-Toller M. (2001). Magnetic resonance imaging evaluation of temporomandibular joint disc deformities in relation to type of disc displacement. J Oral Maxillofac Surg 59:860-865. [DOI] [PubMed] [Google Scholar]
- Wang XD, Kou XX, He DQ, Zeng MM, Meng Z, Bi RY, et al. (2012a). Progression of cartilage degradation, bone resorption and pain in rat temporomandibular joint osteoarthritis induced by injection of iodoacetate. PLoS One 7:e45036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XD, Kou XX, Mao JJ, Gan YH, Zhou YH. (2012b). Sustained inflammation induces degeneration of the temporomandibular joint. J Dent Res 91:499-505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weijs WA. (1975). Mandibular movements of the albino rat during feeding. J Morphol 145:107-124. [DOI] [PubMed] [Google Scholar]
- Westesson PL, Rohlin M. (1984). Internal derangement related to osteoarthrosis in temporomandibular joint autopsy specimens. Oral Surg Oral Med Oral Pathol 57:17-22. [DOI] [PubMed] [Google Scholar]
- Westesson PL, Bronstein SL, Liedberg J. (1985). Internal derangement of the temporomandibular joint: morphologic description with correlation to joint function. Oral Surg Oral Med Oral Pathol 59:323-331. [DOI] [PubMed] [Google Scholar]
- Yamashita J, Furman BR, Rawls HR, Wang X, Agrawal CM. (2001). The use of dynamic mechanical analysis to assess the viscoelastic properties of human cortical bone. J Biomed Mater Res 58:47-53. [DOI] [PubMed] [Google Scholar]
- Yuya PA, Amborn EK, Beatty MW, Turner JA. (2010). Evaluating anisotropic properties in the porcine temporomandibular joint disc using nanoindentation. Ann Biomed Eng 38:2428-2437. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.