Abstract
The dimorphic fungus Histoplasma capsulatum exists in two phases: a unicellular yeast form at 37 C and a mycelium at 25 C. We have found that these two phases have selective drug susceptibilities. The mycelial form of H. capsulatum was much more susceptible to the polyene antibiotic amphotericin B than the yeast form; in contrast, the yeast form was more susceptible to the antibiotic actinomycin D. The changes in susceptibility occurred early in the transition between the two phases and permitted the transitions to be blocked by sublethal concentrations of the appropriate drugs.
Full text
PDF
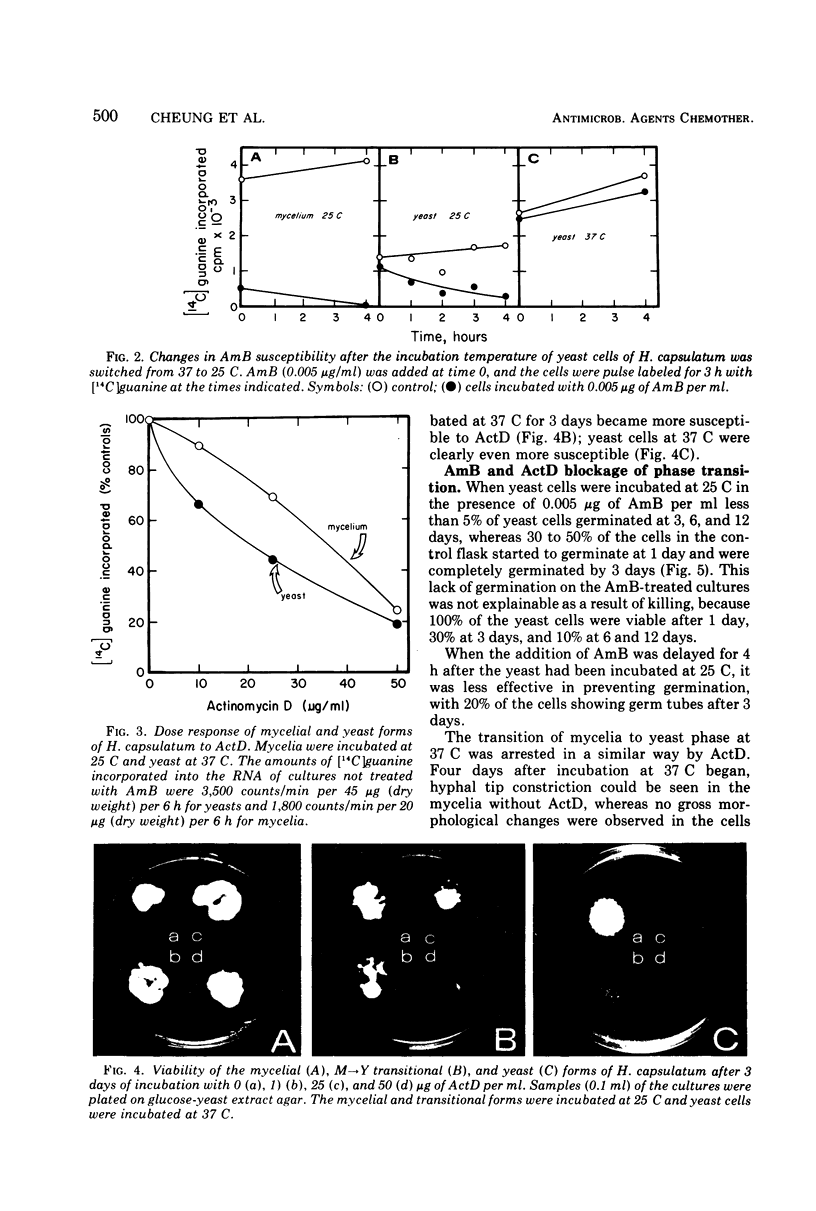
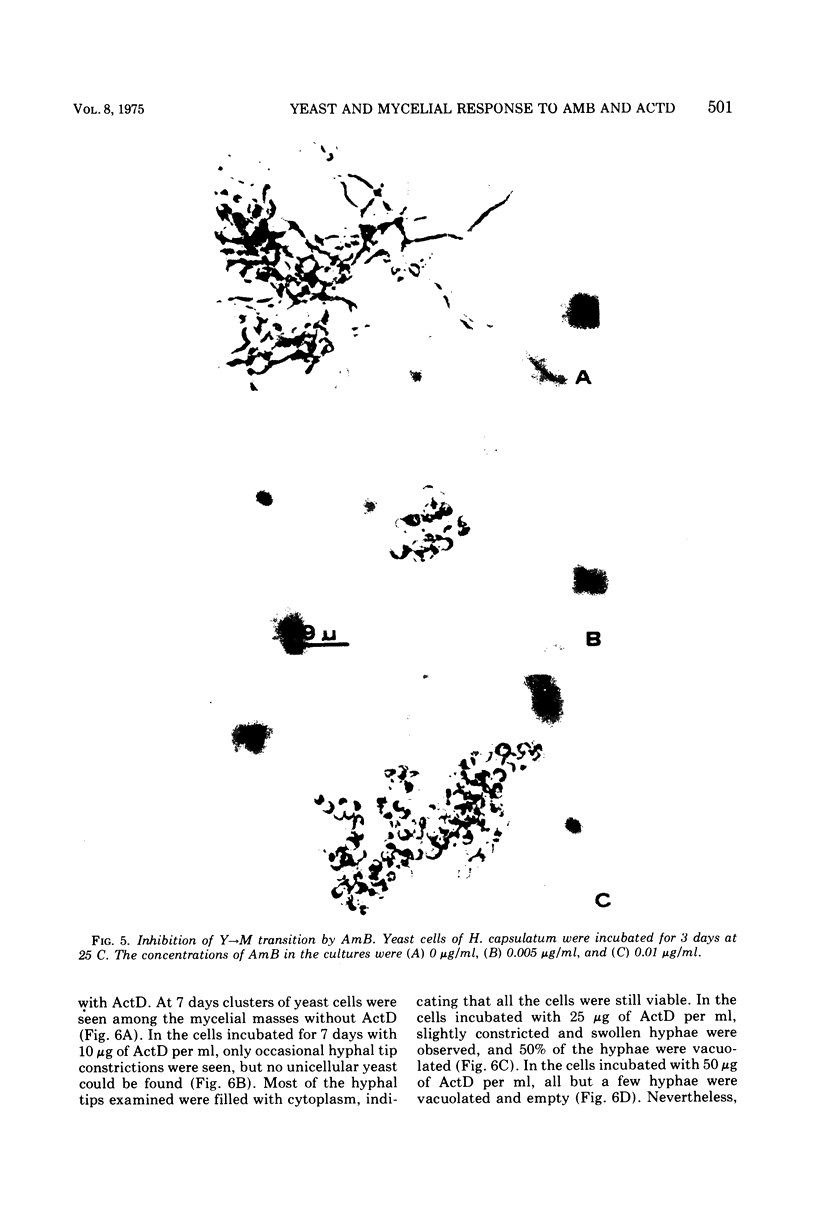
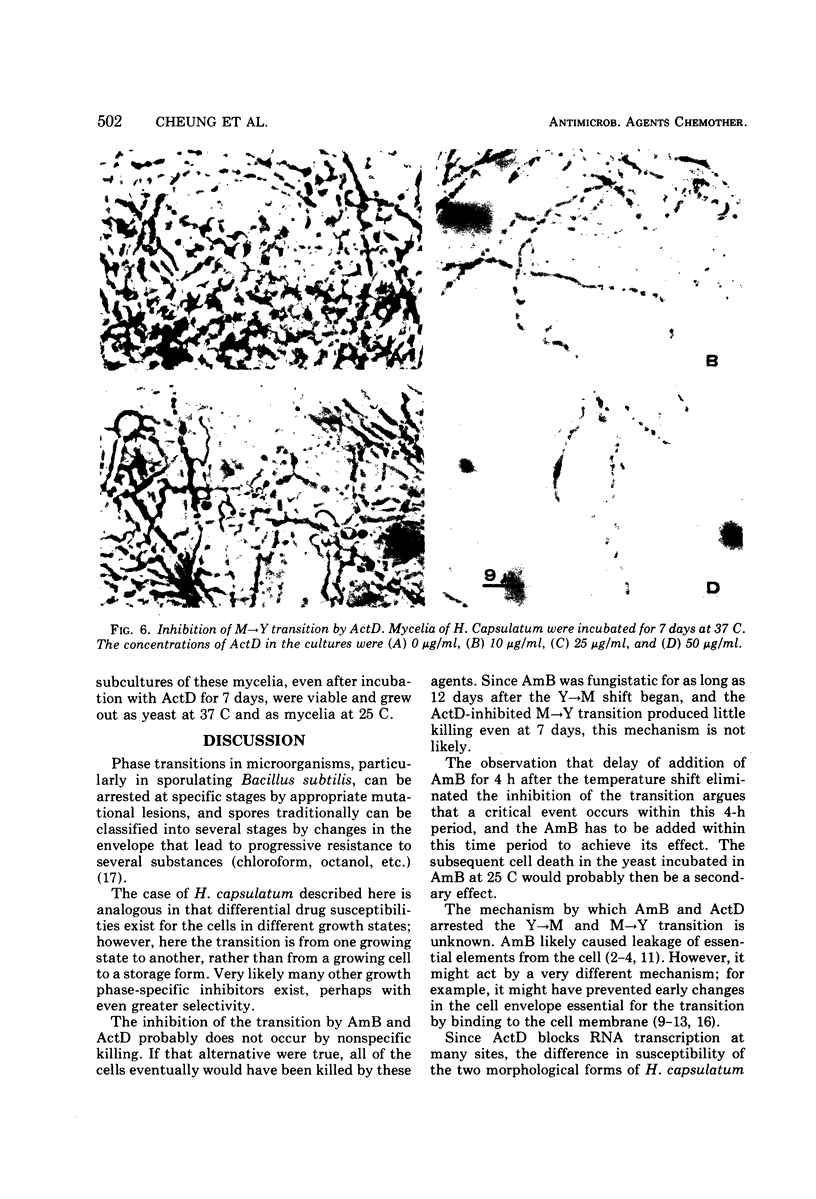

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cheung S. S., Kobayashi G. S., Schlessinger D., Medoff G. RNA metabolism during morphogenesis in Histoplasma capsulatum. J Gen Microbiol. 1974 Jun;82(2):301–307. doi: 10.1099/00221287-82-2-301. [DOI] [PubMed] [Google Scholar]
- FLECK A., MUNRO H. N. The precision of ultraviolet absorption measurements in the Schmidt-Thannhauser procedure for nucleic acid estimation. Biochim Biophys Acta. 1962 May 14;55:571–583. doi: 10.1016/0006-3002(62)90836-3. [DOI] [PubMed] [Google Scholar]
- Garrison R. G., Lane J. W., Field M. F. Ultrastructural changes during the yeastlike to mycelial-phase conversion of Blastomyces dermatitidis and Histoplasma capsulatum. J Bacteriol. 1970 Feb;101(2):628–635. doi: 10.1128/jb.101.2.628-635.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein M. N., Hamm K., Amrod E. Incorporation of triated actinomycin D into drug-sensitive and drug-resistant HeLa cells. Science. 1966 Mar 25;151(3717):1555–1556. doi: 10.1126/science.151.3717.1555. [DOI] [PubMed] [Google Scholar]
- Hamilton-Miller J. M. Chemistry and biology of the polyene macrolide antibiotics. Bacteriol Rev. 1973 Jun;37(2):166–196. [PMC free article] [PubMed] [Google Scholar]
- HsuChen C. C., Feingold D. S. Two types of resistance to polyene antibiotics in Candida albicans. Nature. 1974 Oct 18;251(5476):656–659. doi: 10.1038/251656a0. [DOI] [PubMed] [Google Scholar]
- Kotler-Brajtburg J., Medoff G., Schlessinger D., Kobayashi G. S. Characterization of the binding of amphotericin B to Saccharomyces cerevisiae and relationship to the antifungal effects. Antimicrob Agents Chemother. 1974 Dec;6(6):770–776. doi: 10.1128/aac.6.6.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nozawa Y., Kitajima Y., Sekiya T., Ito Y. Ultrastructural alterations induced by amphotericin B in the plasma membrane of Epidermophyton floccosum as revealed by freeze-etch electron microscopy. Biochim Biophys Acta. 1974 Oct 10;367(1):32–38. doi: 10.1016/0005-2736(74)90132-1. [DOI] [PubMed] [Google Scholar]
- Russell N. J., Kerridge D., Gale E. F. Polyene sensitivity during germination of conidia of Aspergillus fumigatus. J Gen Microbiol. 1975 Apr;87(2):351–358. doi: 10.1099/00221287-87-2-351. [DOI] [PubMed] [Google Scholar]
- SALVIN S. B. Growth of the yeastlike phase of Histoplasma capsulatum in a fluid medium. J Bacteriol. 1950 Feb;59(2):312–313. doi: 10.1128/jb.59.2.312-313.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherrer R., Louden L., Gerhardt P. Porosity of the yeast cell wall and membrane. J Bacteriol. 1974 May;118(2):534–540. doi: 10.1128/jb.118.2.534-540.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kruijff B., Demel R. A. Polyene antibiotic-sterol interactions in membranes of Acholeplasma laidlawii cells and lecithin liposomes. 3. Molecular structure of the polyene antibiotic-cholesterol complexes. Biochim Biophys Acta. 1974 Feb 26;339(1):57–70. doi: 10.1016/0005-2736(74)90332-0. [DOI] [PubMed] [Google Scholar]
- de Kruijff B., Gerritsen W. J., Oerlemans A., Demel R. A., van Deenen L. L. Polyene antibiotic-sterol interactions in membranes of Acholeplasma laidlawii cells and lecithin liposomes. I. Specificity of the membrane permeability changes induced by the polyene antibiotics. Biochim Biophys Acta. 1974 Feb 26;339(1):30–43. doi: 10.1016/0005-2736(74)90330-7. [DOI] [PubMed] [Google Scholar]
- de Kruijff B., Gerritsen W. J., Oerlemans A., van Dijck P. W., Demel R. A., van Deenen L. L. Polyene antibiotic-sterol interactions in membranes of Acholesplasma laidlawii cells and lecithin liposomes. II. Temperature dependence of the polyene antibiotic-sterol complex formation. Biochim Biophys Acta. 1974 Feb 26;339(1):44–56. doi: 10.1016/0005-2736(74)90331-9. [DOI] [PubMed] [Google Scholar]