Abstract
A major goal of phytoremediation is to transform fast-growing plants with genes from plant species that hyperaccumulate toxic trace elements. We overexpressed the gene encoding selenocysteine methyltransferase (SMT) from the selenium (Se) hyperaccumulator Astragalus bisulcatus in Arabidopsis and Indian mustard (Brassica juncea). SMT detoxifies selenocysteine by methylating it to methylselenocysteine, a nonprotein amino acid, thereby diminishing the toxic misincorporation of Se into protein. Our Indian mustard transgenic plants accumulated more Se in the form of methylselenocysteine than the wild type. SMT transgenic seedlings tolerated Se, particularly selenite, significantly better than the wild type, producing 3- to 7-fold greater biomass and 3-fold longer root lengths. Moreover, SMT plants had significantly increased Se accumulation and volatilization. This is the first study, to our knowledge, in which a fast-growing plant was genetically engineered to overexpress a gene from a hyperaccumulator in order to increase phytoremediation potential.
Although selenium (Se) is a necessary micronutrient for humans and animals at very low doses, it is extremely toxic at higher doses (Wilber, 1983). Excess Se has been implicated in birth defects, sterility, and disease in animals, fish, and wildlife and loss of hair, teeth, and nails, fatigue, and even death in humans (Moxon, 1937; Eisler, 1985; Lemly and Smith, 1987; Sorenson, 1991). Environmental Se pollution is a worldwide problem. Anthropogenic Se pollution arises from many sources, such as aqueous discharges from electric power plants, coal ash leachates, refinery effluents, and industrial wastewater (American Medical Association, 1989). Selenium also occurs naturally in soils formed from Se-bearing shales. This leads to Se-contaminated irrigation drainage water, one of the most serious agricultural problems in the western United States and other areas with similar environments and geological conditions (Presser and Ohlendorf, 1987).
Cleaning up Se-contaminated soil and water is a major concern. Phytoremediation, using plants to remove, stabilize, or detoxify pollutants, is a promising technology for remediating Se-contaminated soil and water (Terry et al., 2000). In a process called phytoextraction, plants extract Se from soils and water into their tissues, which can be harvested and removed. Selenium is unusual among trace elements because it can also be removed from the ground ecosystem by phytovolatilization, i.e. plants metabolize inorganic Se to relatively nontoxic, volatile forms (dimethyl selenide [DMSe] and dimethyl diselenide [DMDSe]), which escape to the atmosphere (Lewis et al., 1966; Terry et al., 2000). Selenium phytoremediation has been achieved under field conditions using fast-growing plant species, such as Indian mustard (Brassica juncea; Bañuelos et al., 1997), which accumulates Se to hundreds of parts per million (Bañuelos and Schrale, 1989). Hyperaccumulating plant species, such as Astragalus bisulcatus, have adapted to seleniferous soils and accumulate Se to thousands of parts per million (Brown and Shrift, 1981). Yet, their slow growth rate and small biomass limits their phytoremediation potential (Cunningham et al., 1997). Researchers hope to circumvent this problem by creating transgenic plants with superior phytoremediation potential by transforming fast-growing plants with genes from hyperaccumulator plants (Terry et al., 2000).
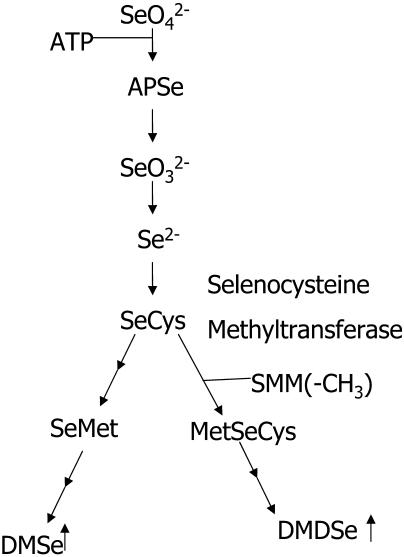
The key step in this strategy is to identify which hyperaccumulator genes to transfer to fast-growing plants. In this study, we selected the gene encoding selenocysteine methyltransferase (SMT) because it is thought to confer Se tolerance to A. bisulcatus. SMT specifically methylates selenocysteine (SeCys) to produce the nonprotein amino acid methylselenocysteine (MetSeCys; Fig. 1); this reduces the intracellular concentrations of SeCys and selenomethionine (SeMet; Neuhierl and Böck, 1996), thereby inhibiting the toxic misincorporation of these species into proteins. In support of this view, Escherichia coli recombinantly expressing SMT exhibited increased selenite tolerance and lowered concentrations of protein-bound Se (Neuhierl et al., 1999). In this study, we hypothesized that overexpressing SMT in the fast-growing phytoremediator Indian mustard would increase its ability to tolerate, accumulate, and volatilize high levels of Se. This hypothesis proved correct, and we report the first study, to our knowledge, to increase phytoremediation potential by overexpressing a hyperaccumulator gene in a fast-growing plant.
Figure 1.
A schematic representation of key metabolic intermediates of Se in nonaccumulators and hyperaccumulators. Reactions that may not be specific to hyperaccumulators convert MetSeCys, the end product of the hyperaccumulator pathway, to DMDSe, a volatile gas.
RESULTS
Molecular Characterization of SMT Transgenic Lines
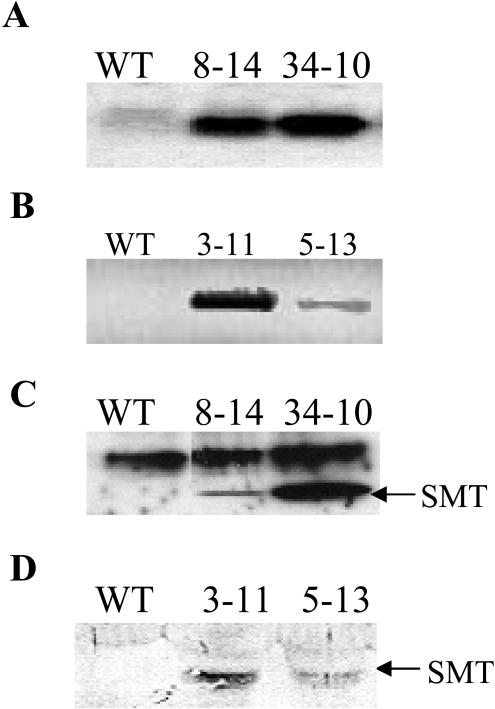
Homozygous SMT Arabidopsis and Indian mustard lines were identified by PCR using primers directed against SMT. No PCR product was amplified from wild-type DNA (data not shown). Arabidopsis DNA was analyzed by Southern blot to demonstrate that the SMT transgene is inserted into a different genomic site in each transgenic line, such that each line represents an independent transformation event. As expected, the SMT probe hybridized with different sized DNA fragments when genomic DNA from each transgenic line was digested with BamHI. No hybridization occurred when wild-type DNA was used as the template (data not shown). Similarly, transgenic Indian mustard plants were analyzed by inverse PCR. Different sized PCR products were amplified from each transgenic line using genomic DNA digested with BamHI and primers directed to the T-DNA border (data not shown). Gene copy number was estimated by Southern blotting (data not shown) Lines SMTAt34-10, SMTBj3-11, and SMTBj5-13 appear to have a single copy of the SMT gene, whereas SMTAt8-14 may have up to three copies.
SMT mRNA was detected in the Arabidopsis transgenic lines by northern blotting using the SMT cDNA as a probe and in the Indian mustard transgenic lines by reverse transcription (RT)-PCR using primers directed toward SMT (Fig. 2, A and B). No bands were detected from wild-type RNA. SMT protein was detected in Arabidopsis and Indian mustard transgenic lines using antiserum raised against A. bisulcatus SMT but not in the wild type (Fig. 2, C and D). Arabidopsis SMT lines used for all experiments discussed in this article are SMTAt8-14 and SMTAt34-10, and the Indian mustard SMT lines are SMTBj3-11 and SMTBj5-13. SMT lines At34-10 and Bj3-11 have greater SMT expression than lines At8-14 and Bj5-13 at both the RNA and protein levels.
Figure 2.
Molecular analysis of SMT transgenic plants. A, Northern-blot analysis of equimolar total RNA isolated from wild-type and SMT Arabidopsis probed with the SMT gene. B, RT-PCR analysis of cDNA derived from equimolar amounts of total RNA isolated from wild-type and SMT Indian mustard using PCR primers directed toward the SMT gene (shown in inverse). Immunoblot of equimolar amounts of protein isolated from wild-type and SMT Arabidopsis (C) and SMT Indian mustard (D) using an antibody specific for the A. bisulcatus SMT gene. The strong top band in the Arabidopsis immunoblot and the faint top band in the Indian mustard immunoblot correspond to a native protein, which has cross-reactivity with the SMT antibody. The SMT antibody did not bind to any protein At7-23. This line was excluded from further study.
SMT Plants Have Improved Se Tolerance
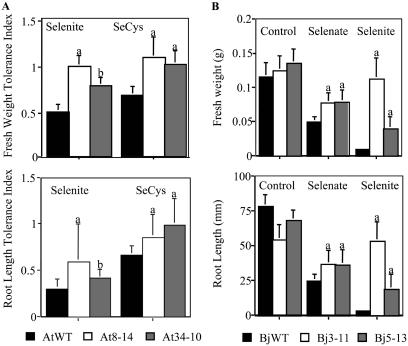
We assessed Se tolerance of SMT Arabidopsis and Indian mustard lines by measuring fresh weights and root lengths of seedlings grown in agar medium with selenocompounds (Murphy and Taiz, 1995). Fresh weights and root lengths of wild-type Arabidopsis were significantly reduced when grown on selenite (52% and 70%, respectively) and SeCys (34% and 30%; P < 0.01; Fig. 3A). Fresh weights but not root lengths were also reduced in the presence of selenate (52%; P < 0.05; data not shown). Both wild-type and SMT seedlings were most inhibited by SeMet, which reduced root lengths of all lines by 94% to 96% (data not shown). However, both SMT Arabidopsis lines tolerated selenite significantly better than the wild type. SMTAt8-14 seedling fresh weights were unaffected by selenite, and root lengths were reduced only 40% from control (P < 0.01; Fig. 3A). SMTAt34-10 seedling fresh weights were reduced by only 24% and root lengths by 59% when grown on selenite rather than control media (P < 0.05; Fig. 3A). SMT Arabidopsis also tolerated SeCys significantly better than the wild type (P < 0.01; Fig. 3A). SMTAt8-14 seedlings grown on SeCys had greater fresh weights than control and root lengths reduced only 10%, while SMTAt34-10 seedling fresh weights and root lengths were unchanged by SeCys (Fig. 3A).
Figure 3.
Seedling tolerance experiments with SMT transgenic plants. A, Fresh weight and root length Tolerance Index for wild-type and SMT (lines 8–14 and 34–10) Arabidopsis seedlings treated with 25 μm selenite or SeCys. The Tolerance Index is calculated by dividing treatment values with the average corresponding control values for that line. B, Fresh weights and root lengths of wild-type and SMT (lines 3–11 and 5–13) Indian mustard seedlings supplied with no selenium or 200 μm selenate or selenite. Experiments with other selenocompounds were conducted, but only statistically significant data are shown. Values for SMT transgenic lines that are statistically significantly different from wild-type values are denoted with either an a (P < 0.01) or b (P < 0.05).
SMT Indian mustard also tolerated certain selenocompounds better than the wild type. Wild-type fresh weights and root lengths were significantly reduced with all four treatments: selenate (58% and 68%, respectively), selenite (92% and 97%), SeCys (40% [P < 0.05] and 80%), and SeMet (73% and 94%; P < 0.01; Fig. 3B; data not shown). Both SMT Indian mustard lines tolerated selenite significantly better than the wild type. SMTBj3-11 seedling fresh weights were reduced only 10% and their root lengths not at all, whereas SMTBj5-13 seedling fresh weights and root lengths were reduced by 70% from their control values (P < 0.01; Fig. 3B). SMT Indian mustard tolerated selenate significantly better than the wild type (P < 0.01; Fig. 3B); fresh weights of both lines were reduced by 40% from control values, while the wild type was reduced by 58%. SMTBj3-11 root lengths were reduced by only 30% and SMTBj5-13 by only 50%, whereas wild-type root lengths were reduced by 68% from control values when treated with selenate (Fig. 3B).
SMT Plants Accumulate More Se
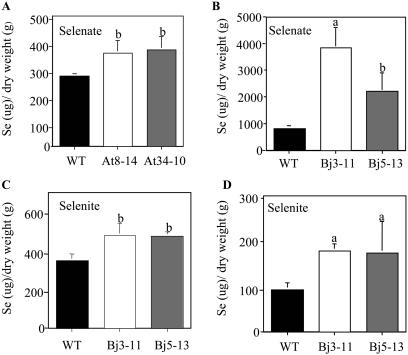
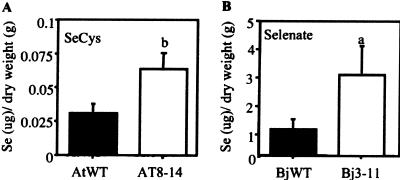
SMT plants accumulated more Se than the wild type (Fig. 4). Arabidopsis SMT seedlings supplied with selenate accumulated significantly more Se than the wild type (P < 0.05; Fig. 4A). Indian mustard SMT seedlings accumulated 2 to 4 times higher Se concentrations than the wild type when supplied with 200 μm SeO4 (P < 0.01 and 0.05; Fig. 4B) and significantly more Se when treated with 100 μm SeO3−2 (P < 0.05; Fig. 4C). SMT mature Indian mustard plants also accumulated 2-fold higher concentrations of Se than the wild type when treated with selenite (P < 0.01; Fig. 4D).
Figure 4.
Se accumulation in Arabidopsis seedlings treated with 25 μm selenate (A); Indian mustard seedlings treated with 200 μm selenate (B); Indian mustard seedlings treated with 100 μm selenite (C); and mature Indian mustard treated with 50 μm selenite (D). Experiments with other selenocompounds were conducted, but only statistically significant data are shown. Values for SMT transgenic lines that are statistically significantly different from wild-type values are denoted with either an a (P < 0.01) or b (P < 0.05).
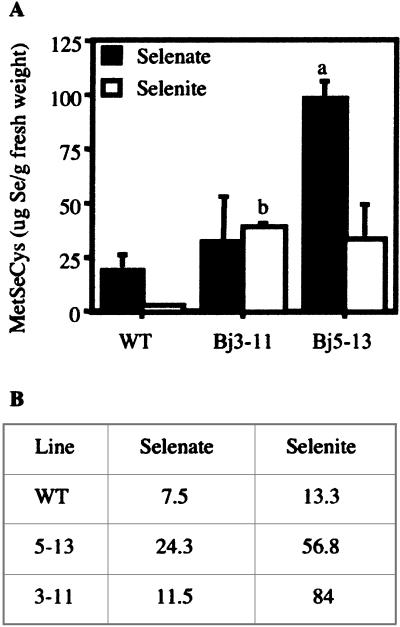
Indian Mustard SMT Plants Contain More MetSeCys
We determined how the proportion of Se accumulated as MetSeCys as well as the absolute amounts of MetSeCys accumulated depending on plant line and Se form supplied using HPLC-inductively coupled plasma (ICP)-mass spectrometry (MS; Fig. 5). Specific Se isotopes were monitored at mass-to-charge ratio (m/z) 77, 78, and 82. Although all selenate-treated Indian mustard plants accumulated predominantly inorganic Se, selenate-treated SMT plants had 1.5- to 3-fold higher proportions of MetSeCys than wild-type plants (Fig. 5B). Since the SMT lines also had greater overall accumulation of Se, they accumulated more Se in the form of MetSeCys than the wild type, more than 3 times as much in the case of SMTBj5-13 (P < 0.01; Fig. 5A). Although all selenite-treated plants accumulated primarily organic Se, the wild type accumulated more inorganic Se (data not shown). Whereas the selenite-treated SMT Indian mustard lines accumulated an average of 56.8% and 84% of their Se in the form of MetSeCys, MetSeCys Se accounted for only 10% of the Se accumulated in wild-type plants (Fig. 5B). Again, because of their greater total Se accumulation, the SMT lines accumulated more than 10 times higher concentrations of MetSeCys (Fig. 6A), a significant result in the case of SMTBj3-11 (P < 0.05).
Figure 5.
MetSeCys concentrations in leaves of mature Indian mustard plants treated with 50 μm selenate or 50 μm selenite (A). Values for SMT transgenic lines that are statistically significantly different from wild-type values are denoted with an a (P < 0.01) or b (P < 0.05). The values are calculated on a per fresh weight basis. B, The average percentage of HCl-extractable Se in the form of MetSeCys in mature Indian mustard leaves treated with selenate or selenite.
Figure 6.
Se volatilization in Arabidopsis mature plants treated with 25 μm SeCys (A) and mature Indian mustard treated with 20 μm selenate (B). Values for SMT transgenic lines that are statistically significantly different from wild-type values are denoted with either an a (P < 0.01) or b (P < 0.05).
SMT Plants Volatilize More Se
SMT plants volatilized significantly more Se than the wild type (Fig. 6). Arabidopsis SMTAt8-14 volatilized 1.5 times more Se than the wild type when treated with SeCys (P < 0.05; Fig. 6A). Indian mustard SMTBj3-11 produced 2.5 times more volatile Se than the wild type when supplied with selenate (P < 0.01; Fig. 6B).
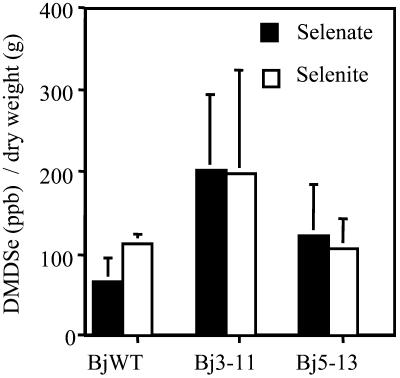
We have used gas chromatography (GC)-ICP-MS to detect the presence of DMDSe in the Se volatilized by wild-type and SMT Indian mustard seedlings treated with either selenate or selenite (Meija et al., 2002). Although both wild-type and SMT plants produce DMDSe, on average the SMT transgenic plants volatilize 2- to 3-fold more DMDSe than the wild type treated similarly, although differences are not statistically significant (Fig. 7).
Figure 7.
Amount of DMDSe present in the headspace of wild-type and SMT Indian mustard seedlings contained in airtight serum bottles and treated with 200 μm concentrations of selenate or selenite for 8 d. The gases were analyzed by GC-ICP-MS. The values are those calculated on a per gram dry weight basis.
DISCUSSION
Our results support the hypothesis that overexpressing the A. bisulcatus SMT gene in fast-growing plants increases their ability to tolerate, accumulate, and volatilize high levels of Se. SMT-overexpressing Arabidopsis and Indian mustard had increased Se tolerance and accumulation. Selenite concentrations that significantly reduced the fresh weights and root lengths of wild-type Arabidopsis and Indian mustard did not decrease fresh weights of Arabidopsis transgenic line SMTAt8-14 or root lengths of Indian mustard SMTBj3-11. Fresh weights and root lengths of Arabidopsis SMTAt34-10 and Indian mustard SMTBj5-13 were reduced by selenite treatment but less so than the wild type (Fig. 3, A and B). SMT overexpression increased Se accumulation. SMT Indian mustard seedlings accumulated significantly more Se from selenite than the wild type, and mature plants accumulated 2-fold more (Fig. 4). Overexpression of SMT increased the tolerance of SMT Indian mustard plants to toxic levels of Se supplied as selenate, although the differences were less dramatic than with selenite: selenate-treated SMT plants had fresh weights that were 60% and root lengths of 50% and 70% of their control values, whereas wild-type fresh weights were only 42% and root lengths 32% of their control values (Fig. 3B). Arabidopsis seedlings supplied with selenate also accumulated significantly more Se than the wild type, and selenate-treated Indian mustard seedlings accumulated 2 to 4 times higher Se concentrations than the wild type (Fig. 4). Although a precise explanation for the increase in Se accumulation is not clear, it is conceivable that the greater Se tolerance of the SMT plants allowed them to continue using metabolic energy for Se accumulation that might otherwise be diverted to stress responses. Alternatively, overexpressing the SMT enzyme may have reduced SeCys and Cys pools, which could lead to increased Se uptake by mass balance or up-regulation of the S/Se assimilation pathway, respectively (Terry et al., 2000).
Our results with Indian mustard clearly support the view that SMT confers Se tolerance to plants by synthesizing MetSeCys. Se-treated wild-type plants produced MetSeCys, most likely due to endogenous methyltransferases (Neuhierl et al., 1999), but overexpressing the A. bisulcatus SMT gene in these plants greatly increased their MetSeCys accumulation and tolerance to selenocompounds. We explain the differences between Indian mustard SMT plants' tolerance to selenite and selenate as follows. Selenate-supplied plants accumulated Se concentrations 10- to 20-fold higher than selenite-treated plants, mostly as selenate. Even though MetSeCys concentrations were higher in selenate- compared to selenite-treated plants (Fig. 6, A and B), the proportion of Se in the form of MetSeCys was much lower (Fig. 6C) and was accompanied by significantly higher concentrations of inorganic Se (data not shown). This is because reduction of selenate to selenite is rate limited by ATP sulfurylase (de Souza et al., 1998; Pilon-Smits et al., 1999), so a large selenate pool remains oxidized within plant tissues and is not converted to MetSeCys by SMT. Even though all selenate-treated plants accumulated primarily inorganic Se, SMT plants were still able to accumulate up to 3 times higher concentrations of MetSeCys than the wild type (Fig. 6A). Selenite-Se was much more readily detoxified by SMT transgenic plants than selenate-Se because selenite enters the pathway downstream of ATP sulfurylase and is therefore more rapidly reduced to form SeCys than is selenate. The rapid conversion of selenite to SeCys accounts for the increased production of MetSeCys by SMT transgenic plants treated with selenite. While all plants treated with selenite accumulated Se mainly in reduced organic Se forms (data not shown), SMT transgenic plants accumulated more than 10 times higher concentrations of MetSeCys than wild-type plants after selenite treatment (Fig. 6B).
Indian mustard and Arabidopsis SMT plants were not able to tolerate SeMet significantly better than the wild type (data not shown) and did not accumulate significantly more MetSeCys when supplied with SeMet (data not shown). This is expected since the overexpression of SMT, which diminishes the potentially toxic incorporation of SeCys into proteins, would be ineffective against SeMet, which enters the S/Se assimilation pathway downstream of SMT (Fig. 1). Therefore, SMT transgenic plants are expected to better tolerate SeCys but not SeMet.
Se volatilization is an important aspect of Se phytoremediation, as it removes Se completely from the ground ecosystem in significantly less toxic forms. Selenium is primarily volatilized as DMSe in most plants (Fig. 1). In Astragalus, Se is volatilized in the form of DMDSe using a different pathway (Fig. 1). We have used GC-ICP-MS to determine what form of Se is volatilized in the SMT plants. We found that the SMT seedlings produce slightly more DMDSe, on average, than the wild type (Fig. 7). However, this amount remains small compared with DMSe production. It was previously assumed that whereas SeMet was converted to DMSe, MetSeCys was converted to DMDSe. Recent evidence suggests that MetSeCys can also be used in the production of DMSe via enzymes such as thiopurine methyltransferase (Ranjard et al., 2002). Therefore, increased MetSeCys concentrations could lead to increases in both DMSe and DMDSe concentrations. Indeed, SMT-overexpressing Indian mustard volatilized significantly more Se from selenate, and Arabidopsis volatilized more from SeCys.
We have demonstrated that the transfer of a single gene from a Se hyperaccumulator to a fast-growing species can greatly enhance its phytoremediation potential. We expect that additional A. bisulcatus genes may also be necessary for Se hyperaccumulation, particularly with respect to its tolerance to selenate and almost complete conversion of selenate to organic forms, which we did not observe with our SMT plants (Orser et al., 1999). However, SMT plants do indeed have increased Se tolerance, accumulation, and volatilization ability, all the traits necessary for Se phytoremediation.
MATERIALS AND METHODS
Enzymes and Chemicals
DNA modifying enzymes were from New England Biolabs (Beverly, MA) and Promega (Madison, WI). Acrylamide and SDS-PAGE gel reagents were from Bio-Rad (Hercules, CA). SeCys was prepared as described (Terry et al., 2000). All other chemicals were from Sigma (St. Louis) and at least reagent grade.
Seeds, Bacterial, and Yeast Strains
Arabidopsis (Columbia) was from Lehle Seeds (Round Rock, TX). Indian mustard (Brassica juncea) seeds (PI no. 173874) were from the North Central Regional Plant Introduction Station (Ames, IA). Astragalus bisulcatus seeds were from the western Regional Plant Introduction Station (Pullman, WA; PI no. 372510). Escherichia coli strain XL-1 Blue MRF′ (Stratagene, La Jolla, CA) was used for cloning.
Plant Transformation and Characterization
The XbaI fragment containing the full-length A. bisulcatus SMT cDNA was isolated from p7AMT (Neuhierl et al., 1999) and cloned into the XbaI site of pBI121 containing the cauliflower mosaic virus 35S promoter (CLONTECH, Palo Alto, CA). Agrobacterium tumefaciens strain GV2260 was transformed with the resulting construct using the freeze-thaw method (An et al., 1988). Arabidopsis was transformed in planta by Agrobacterium infiltration (Clough and Bent, 1998) and Indian mustard as in Pilon-Smits et al. (1999). SMT transgenic plants were identified among the kanamycin-resistant lines by PCR using primers directed against the SMT gene.
Molecular Characterization
Total RNA was isolated from 8-d-old Arabidopsis seedlings using the RNeasy plant mini kit (Qiagen, Valencia, CA). For northern-blot analysis, 10 μg of total RNA per lane was fractionated on a 1.2% denaturating formaldehyde/agarose gel and transferred onto uncharged nylon membrane (Genescreen; Amersham, Buckinghamshire, UK). Hybridization, using Ultrahyb buffer, and radioactive detection were done following Ambion's instructions (Austin, TX). RNA blots were stained with methylene blue to confirm equal loading and transfer (Herrin and Schmidt, 1988). The SMT1 DNA probe was generated by PCR, purified from agarose, and labeled with 32P-dCTP by random priming (Ready-to-Go; Amersham). SMT transcript levels in Indian mustard plants were compared using RT-PCR. mRNA was converted to cDNA using the Superscript II RNaseH reverse transcriptase kit (Gibco-BRL, Cleveland). SMT cDNA was amplified using primers directed toward SMT.
Arabidopsis and Indian mustard genomic DNA was isolated using cetyl-trimethyl-ammonium bromide. For Southern-blot analysis, 10 to 15 μg of DNA/sample was digested with BamHI and fractionated on a 1% Tris-acetate EDTA agarose gel. Hybridization was done with 32P-dCTP-labeled probes using Ultrahyb (Ambion). SMT Indian mustard DNA was analyzed by inverse PCR. Genomic DNA (1 μg) was digested with BamHI, self-ligated, and amplified by PCR. PCR products were gel-purified using a GeneClean kit (Qbiogene, Montreal).
For immunoblot analysis, Arabidopsis and Indian mustard protein was isolated by grinding 1 g of seedling tissue in liquid nitrogen, homogenizing in 2 mL of extraction buffer (25 mm Tricine, pH 7.5, 1 mm EDTA, 10 mm β-mercaptoethanol, 1% polyvinylpolypyrrolidone, 1 μm leupeptin, 1 μm pepstatin, 200 μg phenylmethylsulfonyl fluoride), and precipitating with ammonium sulfate. Protein concentrations were estimated using Bradford reagent (Bio-Rad), with a bovine serum albumin standard. Equimolar amounts of protein (12.5 μg for Arabidopsis and 10 μg for Indian mustard) from each sample were separated on a 12% SDS-PAGE gel, transferred onto polyvinylidene difluoride membrane (Amersham Biosciences, Piscataway, NJ), and hybridized with anti-SMT polyclonal antibody (Wang et al., 1999). Blots were probed with anti-horseradish peroxidase or anti-alkaline phosphatase secondary antibodies and detected using an ECL chemiluminescence kit (Amersham) or colorometric method, respectively.
Selenium Tolerance, Accumulation, and Volatilization
To test Se tolerance and accumulation in Arabidopsis seedlings, seeds were sterilized by rinsing in isopropanol for 2 min, in 20% hypochlorite/Tween 20 for 15 min, and in sterile deionized water five times for 5 min, on a rocking platform. A total of 100 seeds were sown per 150-mm plate of Murashige and Skoog medium containing 10 g/L of Suc, 0.5 g/L of 2 N-morpholinoethanesulfonic acid monohydrate, 8 g/L of bactoagar (Difco, Detroit), and 25 to 100 μm selenate, 25 to 100 μm selenite, 25 μm SeCys, or 25 μm SeMet. Selenocysteine was synthesized by reducing one volume selenocystine with two volumes dithiothreitol in 1 m hydrochloric acid under nitrogen for 10 min. After 8 d at 25°C under continuous light, seedlings were harvested and washed with deionized water. Seedlings' fresh weights and root lengths were measured. Others were dried at 60°C, ground to fine powder, and digested for Se accumulation as described below.
To determine Se tolerance and accumulation in Indian mustard seedlings, seeds were sterilized by rinsing in 96% ethanol for 30 s, in 0.65% hypochlorite solution for 30 min, and in sterile deionized water five times for 10 min, on a rocking platform. A total of 25 sterilized seeds were sown in a grid pattern in Magenta boxes (Sigma) on half-strength Murashige and Skoog medium with 10 g L−1 Suc and 5 g L−1 phytagar (Sigma), with or without added selenocompounds (200 μm selenate, selenite, SeMet, or 100 μm SeCys [used because of its extreme toxicity to Indian mustard]). After 2 d at 4°C, the boxes were moved to a growth chamber kept at 25°C under continuous light. On day 7, individual seedlings were harvested, washed, weighed, and root lengths measured.
For Se accumulation and volatilization in mature plants, seedlings were instead transferred to 4-inch pots containing coarse sand and covered with a plastic dome. The pots were maintained in a greenhouse with controlled temperature (24°C) and a long-day (16-h) photoperiod. Plants were watered twice a day, once with tap water and once with half-strength Hoagland solution (Hoagland and Arnon, 1938). After 1 week, the dome was removed. After an additional week, plants were transferred to half-strength Hoagland solution in aerated plastic boxes. After 1 week, the nutrient solution was replaced with fresh solution containing 20 or 50 μm concentrations of selenocompounds. After 8 d of Se treatment, plants were harvested and weighed for Se accumulation or transferred to individual gastight glass volatilization chambers for volatile Se analysis. Harvested plants were washed in running deionized water to remove any Se externally bound to the roots, dried at 70°C, cut into small pieces, and acid digested (Martin, 1975). Volatile Se was collected from plants in 200 mL of Hoagland solution, through which a continuous air flow (1.5 L min−1) was passed and trapped in alkaline peroxide (Zayed and Terry, 1992). Ten-milliliter aliquots of trap solution were collected after 24 h and heated at 95°C to remove the peroxide. Selenate-Se was reduced to selenite by adding an equal volume of concentrated HCl and heating at 95°C for 30 min. Se concentration was measured by vapor-generation atomic absorption spectroscopy in combination with hydride generation (Zayed and Terry, 1992; Ward and Gray, 1996).
Biochemical Analysis of Transgenic Plants
For total Se analysis, powdered plant tissues (100 mg dry weight samples) were acid digested and analyzed using atomic absorption spectroscopy (Zayed and Terry, 1992; Ward and Gray, 1996). HPLC-ICP-MS was used to speciate and quantify organic Se (Montes-Bayón et al., 2002). GC-ICP-MS was used to speciate and quantify volatile Se forms (Meija et al., 2002). Statistical analyses were performed using the JMPIN statistical package (SAS Institute, Cary, NC).
Distribution of Materials
Upon request, all novel materials described in this publication will be made available in a timely manner for noncommercial research purposes, subject to the requisite permission from any third-party owners of all or parts of the material. Obtaining any permissions will be the responsibility of the requestor.
Sequence data from this article have been deposited with the EMBL/GenBank data libraries under accession number AJ131433.
This work was supported by the National Science Foundation SGER (grant no. 9900054), NSF Metabolic Biochemistry (grant no. 9904643), and the Novartis-Syngenta Research Foundation (grant to N.T.).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.103.026989.
References
- American Medical Association (1989) Home Medical Encyclopedia, Vols 1 and 2. Random House, New York
- An G, Ebert P, Mitra A, Ha S (1988) Binary vectors. In SB Gelvin, RA Schilperoort, eds, Plant Molecular Biology Manual. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp A3, 1-19
- Bañuelos GS, Ajwa HA, Mackey M, Wu L, Cook C, Akohoue S, Zambruzuski S (1997) Evaluation of different plant species used for phytoremediation of high soil selenium. J Environ Qual 26: 639–646 [Google Scholar]
- Bañuelos G, Schrale G (1989) Plants that remove selenium from soils. Calif Agric 43: 19–20 [Google Scholar]
- Brown T, Shrift A (1981) Exclusion of selenium from proteins in selenium-tolerant Astragalus species. Plant Physiol 67: 1951–1953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough S, Bent A (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Cunningham S, Shann J, Crowley D, Anderson T (1997) Phytoremediation of contaminated water and soil. In E Kruger, T Anderson, J Coats, eds, Phytoremediation of Soil and Water Contaminants, ACS Symposium Series No. 664. American Chemical Society, Washington, DC, pp 2–17
- de Souza MP, Pilon-Smits EAH, Lytle CM, Hwang S, Tai J, Honma TSU, Yeh L, Terry N (1998) Rate-limiting steps in selenium assimilation and volatilization by Indian mustard. Plant Physiol 117: 1487–1494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisler RS (1985) Selenium hazards to fish, wildlife and invertebrates: a synoptic review. US Fish Wildl Serv Biol Rep 85: 57 [Google Scholar]
- Herrin D, Schmidt G (1988) Rapid, reversible staining of northern blots prior to hybridization. Biotechniques 6: 196–200 [PubMed] [Google Scholar]
- Hoagland D, Arnon D (1938) The water culture method for growing plants without soil. Bull Calif Agric Stat 346
- Lemly AD, Smith GJ (1987) Aquatic cycling of selenium: implications for fish and wildlife. Fish and Wildlife Leaflet 12. U.S. Fish and Wildlife Service, Washington, DC
- Lewis B, Johnson C, Delwiche C (1966) Release of volatile selenium compounds by plants: collection procedures and preliminary observations. J Agric Food Chem 14: 638–664 [Google Scholar]
- Martin T (1975) Determining selenium in wastewater sediment and sludge by flameless atomic absorption. Atomic Abs Newslett 14: 109–116 [Google Scholar]
- Meija J, Montes-Bayon M, LeDuc DL, Terry N, Caruso JA (2002) Simultaneous monitoring of volatile selenium and sulfur species from Se accumulating plants (wild-type and genetically modified) by GC-MS and GC-ICP-MS using SPME for sample introduction. Anal Chem 74: 5837–5844 [DOI] [PubMed] [Google Scholar]
- Montes-Bayón M, LeDuc DL, Terry N, Caruso J (2002) Selenium speciation in wild-type and genetically modified Se accumulating plants with HPLC separation and ICP-MS/ES-MS detection. J Anal At Spectrom 17: 872–879 [Google Scholar]
- Moxon AL (1937) Alkali disease, or selenium poisoning. South Dakota State College Agriculture Experiment Station Bulletin 311. South Dakota State College, Brookings, SD
- Murphy A, Taiz L (1995) A new vertical mesh transfer technique for metal-tolerance studies in Arabidopsis. Ecotypic variation and copper-sensitive mutants. Plant Physiol 108: 29–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhierl B, Böck A (1996) On the mechanism of selenium tolerance in selenium-accumulating plants. Purification and characterization of a specific selenocysteine methyltransferase from cultured cells of Astragalus bisulcatus. Eur J Biochem 239: 235–238 [DOI] [PubMed] [Google Scholar]
- Neuhierl B, Thanbichler M, Lottspeich F, Böck A (1999) A family of S-methylmethionine-dependent thiol/selenol methyltransferases: role in selenium tolerance and evolutionary relation. J Biol Chem 274: 5407–5414 [DOI] [PubMed] [Google Scholar]
- Orser CS, Salt DE, Pickering IJ, Prince RC, Epstein A, Ensley BD (1999) Brassica plants to provide enhanced human mineral nutrition: selenium phytoenrichment and metabolic transformation. J Med Food 1: 253–261 [Google Scholar]
- Pilon-Smits E, Hwang S, Lytle CM, Zhu Y, Tai JC, Bravo RC, Chen Y, Leustek T, Terry N (1999) Over-expression of ATP sulfurylase in Brassica juncea leads to increased selenate uptake, reduction and tolerance. Plant Physiol 119: 123–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presser TS, Ohlendorf HM (1987) Biogeochemical cycling of selenium in the San Joaquin Valley California, USA. Environ Manage 11: 804–822 [Google Scholar]
- Ranjard L, Prigent-Combaret C, Nazaret S, Cournoyer B (2002) Methylation of inorganic and organic selenium by the bacterial thiopurine methyltransferase. J Bacteriol 184: 3146–3149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorenson EM (1991) Metal Poisoning in Fish. Lewis Publishers, Boca Raton, FL
- Terry N, Zayed A, de Souza M, Tarun A (2000) Selenium in higher plants. Ann Rev Plant Physiol Plant Mol Biol 51: 401–432 [DOI] [PubMed] [Google Scholar]
- Wang Y, Böck A, Neuhierl B (1999) Acquisition of selenium tolerance by a selenium non-accumulating Astragalus species via selection. Biofactors 9: 3–10 [DOI] [PubMed] [Google Scholar]
- Ward M, Gray A (1996) Vapor generation accessory VGA-77. Varian Australia Pty. Ltd., Publication No. 85-101047-00
- Wilber CG (1983) Selenium: A Potential Environmental Poison and a Necessary Food Constituent. Charles C. Thomas, Springfield, IL
- Zayed A, Terry N (1992) Selenium volatilization as influenced by sulfate supply. J Plant Physiol 140: 646–652 [Google Scholar]