Abstract
In order to identify nuclear genes required for early chloroplast development, a collection of photosynthetic pigment mutants of Arabidopsis was assembled and screened for lines with extremely low levels of chlorophyll. Nine chloroplast biogenesis (clb) mutants that affect proplastid growth and thylakoid membrane formation and result in an albino seedling phenotype were identified. These mutations identify six new genes as well as a novel allele of cla1. clb mutants have less than 2% of wild-type chlorophyll levels, and little or no expression of nuclear and plastid-encoded genes required for chloroplast development and function. In all but one mutant, proplastids do not differentiate enough to form elongated stroma thylakoid membranes. Analysis of mutants during embryogenesis allows differentiation between CLB genes that act noncell autonomously, where partial maternal complementation of chloroplast development is observed in embryos, and those that act cell autonomously, where complementation during embryogenesis is not observed. Molecular characterization of the noncell autonomous clb4 mutant established that the CLB4 gene encodes for hydroxy-2-methyl-2-(E)-butenyl 4-diphosphate synthase (HDS), the next to the last enzyme of the methylerythritol 4-phosphate (MEP) pathway for the synthesis of plastidic isoprenoids. The noncell autonomous nature of the clb4 mutant suggests that products of the MEP pathway can travel between tissues, and provides in vivo evidence that some movement of MEP intermediates exists from the cytoplasm to the plastid. The isolation and characterization of clb mutants represents the first systematic study of genes required for early chloroplast development in Arabidopsis.
Chloroplasts are responsible for essential plant functions such as the fixation of CO2, manufacture of carbon skeletons, fatty acids and pigments, and the synthesis of amino acids from inorganic nitrogen, among others (Staehelin and Newcomb, 2000). In higher plants, chloroplasts develop from proplastids, small organelles (0.2–0.5 μm diameter) that lack thylakoid membranes and that are present primarily in meristematic cells and in young postmitotic cells (Vothknecht and Westhoff, 2001). As meristematic cells begin to differentiate into mesophyll and palisade cells, proplastids coordinately differentiate into chloroplasts. This differentiation process is also modulated by environmental cues such as light, and thus certain Arabidopsis mutants with an altered light signal transduction pathway are able to develop leaves even in the absence of chloroplast development (Li et al., 1994).
During development, the increase in plastid volume can be greater than 100-fold. When the proplastid reaches an average size of 1.0 μm the inner layer of the membrane begins to invaginate into the stroma (Mühlethaler and Frey-Wyssling, 1959). This process continues until there are many flat sacs or thylakoids lying in the stroma. The number of thylakoids in each stack increases until eventually the typical grana of the mature chloroplast are produced. It has been suggested that the progressive extension of the thylakoid system results from the fusion of vesicles derived from the inner plastid envelope (Kirk and Tilney-Bassett, 1978; Vothknecht and Westhoff, 2001).
The conversion of proplastids into chloroplasts is accompanied by high transcription levels of plastid- and nuclear-encoded genes involved in the transcription/translation apparatus (Baumgartner et al., 1989). The expression of these genes decreases once the mature chloroplast is established, suggesting that one central regulatory point during chloroplast differentiation is the activation of chloroplast transcription (Mache et al., 1997). Early plastid differentiation also correlates with an increase in chloroplast division (Pyke, 1999). In contrast, genes required for photosynthesis are highly expressed only later in development (Bisanz-Seyer et al., 1989). In spinach it has been shown that nuclear-encoded genes for plastid ribosomal proteins increase their expression soon after seed imbibition, 2 d in advance of the expression of the plastid-encoded and nuclear-encoded photosynthetic genes (Harrak et al., 1995). These results suggest that early signals for chloroplast development originate from the nucleus (Mullet, 1993; Harrak et al., 1995; Mache et al., 1997). Evidence also exists for retrograde regulation of the chloroplast developmental process from the developing plastid to the nucleus (Larkin et al., 2003; Strand et al., 2003). Despite the importance of plastids, an understanding of their differentiation process is far from complete.
Mutations that interfere with or block different stages of chloroplast development have been isolated from a variety of plant species, including maize, barley, tobacco, tomato, Antirrhinum, and Arabidopsis (Mascia and Robertson, 1978; Han et al., 1993; Meurer et al., 1996; Roy and Barkan, 1998; Rapp and Mullet, 1991; Wang et al., 2000; reviewed in León et al., 1998). Among these mutants, those that affect the onset of this developmental process give rise to plastids that are very small and lack internal membranes (i.e. resemble proplastids). Examples of this class of mutants include cla1, pac, edd1, slp, and apg2 in Arabidopsis (Reiter et al., 1994; Mandel et al., 1996; Uwer et al., 1998; Apuya et al., 2001; Motohashi et al., 2001) as well as dcl, dag, iojap, and vdl in other plants (Shumway and Weier, 1967; Chatterjee et al., 1996; Keddie et al., 1996; Wang et al., 2000). Plastids from pac, dcl, and cla1 display some single thylakoid membranes and accumulate chlorophyll and carotenoids at low levels. In dag, iojap, and vdl, no thylakoid differentiation occurs and plastids show the typical small vesicles and invaginations of the inner membrane characteristic of proplastids. Consistent with the multiple functions carried out in the chloroplast, these mutants affect proteins involved in processes such as RNA processing (PAC and VDL), protein translation and folding (Ij, EDD1, and SLP), and plastidic isoprenoid biosynthesis (DXS/CLA1; Han and Martienssen, 1994; Meurer et al., 1998; Uwer et al., 1998; Estévez et al., 2000; Wang et al., 2000; Apuya et al., 2001; Budziszewski et al., 2001).
Plant isoprenoids serve essential roles in photosynthesis, respiration, growth and development. These compounds include an enormous variety of natural products synthesized through the consecutive condensation and modification of two basic 5-carbon units, the isopentenyl diphosphate (IPP) and its isomer dimethylallyl diphosphate (DMAPP). In plants the biosynthesis of these two structural blocks takes place by two independent pathways localized in different cellular compartments. The well-characterized mevalonic pathway operates in the cytoplasm, while in plastids IPP and DMAPP are synthesized by the recently discovered methylerythritol 4-phosphate (MEP) pathway (Lichtenthaler, 1999; Rodríguez-Concepción and Boronat, 2002). Although clear evidence exists of cross talk between the two routes, each pathway seems primarily involved in the biosynthesis of specific isoprenoids (Lichtenthaler, 1999; Laule et al., 2003). Photosynthetic pigments such as chlorophyll and carotenoids are derived mainly from the MEP pathway, and thus it is not surprising that mutations in this pathway render albino phenotypes such as the cla1 mutant (Araki et al., 2000; Estévez et al., 2000; Budziszewski et al., 2001). Since the recent discovery of the MEP route, all the biosynthetic steps and their corresponding enzymes have been established, mainly in bacteria. Genes with sequence similarity to the Escherichia coli MEP pathway genes are found in Arabidopsis and other plants, but in many cases there is no functional evidence for the role of these genes in isoprenoid synthesis or chloroplast development (Rodríguez-Concepción and Boronat, 2002).
To identify new genes necessary for early steps of chloroplast biogenesis, we assembled a collection of pigmentation lines, and focused our subsequent analysis on the mutants with extremely low levels of chlorophyll. These albino lines define six novel genes, which we have named CHLOROPLAST BIOGENESIS (CLB) 1–6. Growth of clb seedlings under high and low light conditions demonstrated that the albino phenotype of clb seedlings is not a secondary effect of photooxidative damage. Our analysis demonstrates that CLB genes are required for plastid growth and the formation of thylakoid membranes, as well as for the expression of plastid- and nuclear-encoded genes required for early chloroplast biogenesis. By comparing the phenotype of clb mutants during embryogenesis and seedling growth, we show that CLB genes encode factors required for early chloroplast biogenesis that act both cell and noncell autonomously. Further, we found that CLB4 corresponds to the ISPG gene, which encodes the enzyme that participates in the next to last step in the plastidic isoprenoid biosynthesis pathway. clb4 corresponds to the first loss-of-function mutant for this enzyme in plants, and underscores the importance of isoprenoids in chloroplast development.
RESULTS
Survey of Photosynthetic Pigment Lines from Arabidopsis Stock Center
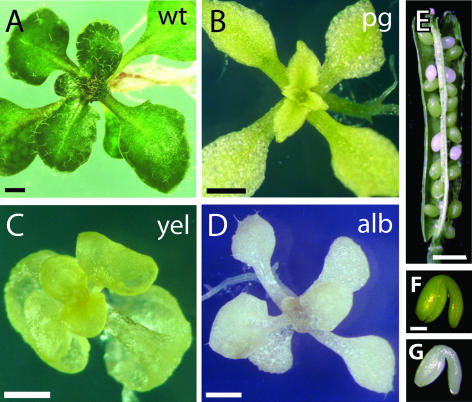
To identify new mutants that affect early stages of chloroplast development, 22 lines segregating seedling pigment mutations (classified as albino) were obtained from the ABRC. Lines were grown in tissue culture media supplemented with Suc and examined visually. Seedlings representative of the observed phenotypic spectrum are shown in Figure 1. Surprisingly, the majority of the ABRC mutants classified as albino had a considerable amount of chlorophyll (Fig. 1B) or carotenoid (Fig. 1C) pigments, and therefore are referred to here as pale green or yellow phenotypes, respectively. Only 2 of these lines, CS27 and CS213 (Fig. 1D), were visually severely lacking in photosynthetic pigments and thus fit our phenotypic criteria for albino mutants. The fact that only 2 out of 22 ABRC pigment lines displayed a true albino seedling phenotype suggested that the number of genes that when mutated render an albino phenotype might be relatively small. In view of this, a genetic screen to isolate more albino mutants was performed.
Figure 1.
Phenotypic spectrum of pigment mutants at seedling and late embryogenesis stages. Representative lines for phenotypic classes are shown. Seedlings were grown on GM medium for 21 d. A, wild type (wt); B, pale green (pg); C, yellow (yel); and D, albino (alb). E, Segregation of a pigment mutant in a silique from a heterozygous plant: approximately one-quarter of albino embryos segregate. F, wild-type embryo; G, albino embryo. Scale bars: A–D, 1 mm; E, 5 mm; F and G, 100 μm.
Identification of chloroplast biogenesis Mutants
Since wild-type Arabidopsis embryos develop chloroplasts during embryogenesis (Mansfield and Briarty, 1991), a plant heterozygous for a monogenic photosynthetic pigment mutation segregates approximately one-quarter pale or white embryos in developing fruits (Fig. 1E). Thus, mutagenized (M3) seed can be effectively screened for pigment phenotypes while developing in siliques of the mother (M2) plant, saving the step of harvesting individual families for screening by germinating seedlings on plates. Using this screening criteria, 1,000 ethyl methanesulfonate (EMS) M2 and 5,000 T-DNA mutagenized lines were examined for siliques containing approximately one-quarter pale or white embryos. A total of 77 lines segregating morphologically normal pale or white embryos were recovered and used for further analysis (Fig. 1G).
Based on subsequent visual inspection of seedlings of the 99 pigment lines in our collection (22 from the ABRC and 77 from our screen), 61 mutant lines were classified as pale green, 29 as yellow, and only 9 as albino (Table I). As our specific interest is in early chloroplast biogenesis, subsequent analysis was focused on the albino mutant lines (Table II). We chose the name chloroplast biogenesis (clb) for these mutants, to reflect the requirement of these genes for early chloroplast development. To corroborate the visual observation that our clb mutants were severely lacking in chlorophylls and carotenoids, both pigment levels were quantified. It was found that all clb mutants contained 2% or less chlorophyll levels per wet weight compared with wild-type seedlings (Table III). The carotenoid content in these mutants is also severely reduced, although not as much as the chlorophylls (Table III).
Table I.
Classification of pigment lines in this study
| Phenotype | Number |
|---|---|
| % of total | |
| Albino | 9 (9%) |
| Pale green | 61 (62%) |
| Yellow | 29 (29%) |
| Total | 99 (100%) |
Table II.
Mutant lines analyzed
| Allele | Line | Mutagen | Ecotype | Source |
|---|---|---|---|---|
| cla1-2 | CS213 | Ethylenimine | Dijon | ABRC |
| clb1-1 | CS27 | Nitrosomethyl urea | Ler/Limoges/Dijon | ABRC |
| clb2-1 | 7-2 | EMS | Ler | T.S. |
| clb3-1 | 12-3 | EMS | Ler | T.S. |
| clb4-1 | 14-6 | EMS | Ler | T.S. |
| clb4-2 | SALK | |||
| 017595 | T-DNA | Col | ABRC | |
| clb5-1 | 15-8 | EMS | Ler | T.S. |
| clb5-2 | FN110 | Fast Neutron | Ler | T.S. |
| clb5-3 | 7815 | T-DNA | C24 | T.S. |
| clb6-1 | 16-1 | EMS | Ler | T.S. |
Abbreviations used: EMS, Ethylmethanesulfonate; Ler, Landsberg erecta; ABRC, Arabidopsis Biological Resource Center; T.S., this study.
Table III.
Chlorophyll and carotenoid content of wild type and clb seedlingsa
| Strain | Chlorophyll A | Chlorophyll B | Total Chlorophyll | Total Carotenoids |
|---|---|---|---|---|
| Ler wt | 41 (1) | 32 (1.5) | 73 | 9 (0.3) |
| clb1-1 | 0.16 (0.02) | 0.31 (0.05) | 0.47 | 0.32 (0.01) |
| clb2-1 | 0.22 (0.06) | 0.30 (0.2) | 0.52 | 0.32 (0.01) |
| clb3-1 | 0.41 (0.08) | 0.70 (0.3) | 1.01 | 0.37 (0.08) |
| clb4-1 | 0.63 (0.02) | 0.42 (0.06) | 1.05 | 1.1 (0.05) |
| clb5-1 | 0.05 (0.05) | 0.07 (0.02) | 0.12 | 0.31 (0.01) |
| clb6-1 | 0.60 (0.07) | 0.80 (0.20) | 1.4 | 0.82 (0.13) |
| cla1-1 | 0.83 (0.2) | 0.56 (0.09) | 1.39 | 1.7 (0.4) |
Pigments were extracted from 18-d-old seedlings and quantified by the method of Lichtenthaler and Wellburn (1983). Values are expressed in micrograms pigment per grams fresh weight of seedling tissue. The average of three replicates is shown, with the sd in parentheses.
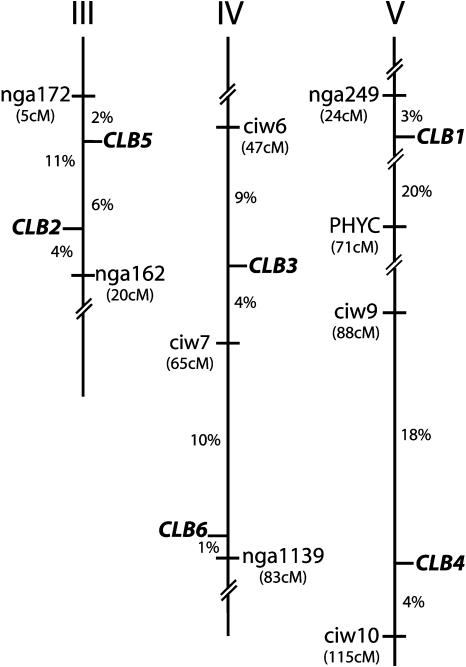
Complementation analysis of the nine clb mutants demonstrated that these mutations identified seven different genetic loci (see Table II). The mutation in ABRC stock center line CS213, was found to be allelic to the previously described cla1-1 (Mandel et al., 1996), and was designated cla1-2. Three additional mutants were found to be allelic and were designated clb5-1, clb5-2, and clb5-3. The remaining five nonallelic mutant lines were designated as clb1-1, clb2-1, clb3-1, clb4-1, and clb6-1. Plants heterozygous for all nine clb mutations segregated albino embryos as monogenic, nuclear recessive alleles with normal transmission (data not shown). clb mutants were mapped using cosegregation analysis of PCR-based molecular markers, and the map positions for CLB genes are shown in Figure 2.
Figure 2.
Map positions of CLB genes. The position of each CLB gene (boldface italics) relative to two flanking SSLP markers (normal font) is shown on the corresponding chromosome. Recombination frequencies between markers and genes are shown as percentages. The approximate position of each SSLP marker on the Recombinant Inbred map is shown in parentheses (Lukowitz et al., 2000). Recombination frequencies are based on the following numbers of recombinant chromosomes: CLB1 (138), CLB2 (140), CLB3 (138), CLB4 (136), CLB5 (102), and CLB6 (136).
Seedling Morphology of clb Mutants
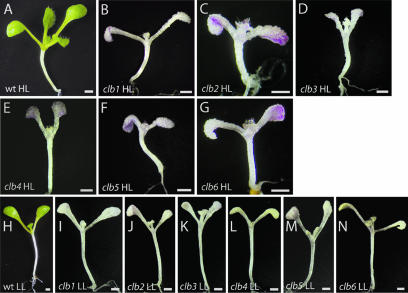
It is well known that the absence of carotenoid pigments can result in photo-oxidative stress under high/normal light conditions, resulting in pleiotropic effects on chloroplast development (Oelmüller, 1989). Because the initial selection of clb mutants was done under normal light conditions, it was possible that the albino phenotypes observed in these mutants were a consequence of chloroplast photooxidative damage due to a defect in carotenoid pigment biosynthesis, and not to a more specific block in chloroplast development. To differentiate between these two possibilities, all clb mutants were grown under both low (5 μE) and high light (120 μE) conditions. As shown in Figure 3, the albino phenotype of all clb mutants does not revert under low light conditions. This result supports the idea that the albino phenotype and the arrest of chloroplast development in clb mutants can be attributed to a direct block in plastid development, and not to a secondary effect of photooxidative stress.
Figure 3.
Morphology of wild-type and clb mutants grown under high (HL) and low light (LL) conditions. Wild-type (A), clb1-1 (B), clb2-1 (C), clb3-1 (D), clb4-1 (E), clb5-1 (F) and clb6-1 (G) seedlings grown under high light conditions. Wild-type (H), clb1-1 (I), clb2-1 (J), clb3-1 (K), clb4-1 (L), clb5-1 (M), and clb6-1 (N) seedlings grown under low light conditions. Plants were grown in 16 h light on GM medium for 10 d at 120 μm m−2 s−1 (high light) and 5 μm m−2 s−1 (low light). Scale bar: 1 mm.
After 10 d of growth on solidified germination media (GM) under high light conditions, all six clb mutants present a similar morphological phenotype. Seedling growth of clb mutants is retarded compared to wild-type. A typical 10-d-old wild-type seedling has four leaves and two cotyledons, while clb mutants have two cotyledons and only two small leaves (Fig. 3, B–G). Cotyledons of clb mutants have many swollen epidermal cells, and tend to curl under at the ends. With the exception of clb5, all clb mutants produce trichomes on the adaxial side of their first two leaves, similar to wild-type seedlings. clb seedlings accumulate considerable amounts of anthocyanin under high light conditions, but not when grown under low light conditions, presumably due to the decrease in photooxidative stress. With the exception of the difference in the accumulation of anthocyanin pigments, the effect of growing clb mutants under low light parallels that of wild-type seedlings grown under low light: seedlings show increased expansion of hypocotyls and petioles, and more oval shaped cotyledons (compare Fig. 3H with 3, I–N). Under low light conditions, clb4 (Fig. 3L) and clb6 (Fig. 3N) develop a subtle yellow color.
CLB Genes Are Required for Proplastid Growth and Thylakoid Membrane Formation
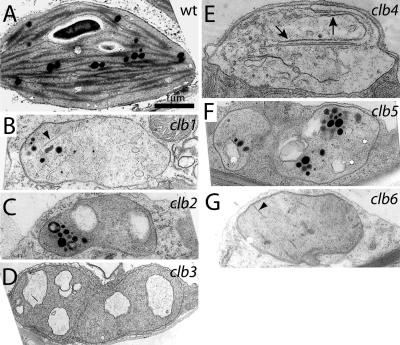
To assess the effect of clb mutations on chloroplast development, plastids from the first leaf of 3-week-old seedlings were examined by electron microscopy (EM). Compared with the wild-type chloroplast (Fig. 4A), the plastids of clb mutants are all arrested at an early stage of differentiation. Plastids of clb2 (Fig. 4C), clb3 (Fig. 4D), and clb5 (Fig. 4F) lack appressed internal membranes and have large vesicle-like structures with unknown contents, similar to those found in proplastids. By contrast, the chloropasts of clb1 (Fig. 4B) and clb6 (Fig. 4G) mutants contain short, linear appressed membranes, while clb4 (Fig. 4E) contains long appressed membranes. Based on plastid morphology, chloroplast development seems to be arrested earliest in clb5, clb2, and clb3, and slightly later in clb1, clb6, and clb4.
Figure 4.
Transmission electron microscopic examination of plastids of wild-type (A) and clb1 (B), clb2 (C), clb3 (D), clb4 (E), clb5 (F), and clb6 (G) mutant seedlings. Plants were grown on GM medium for 21 d and the second leaf of a representative plant for each phenotype was fixed for transmission electron microscopic analysis. Arrowheads, short appressed membranes; arrow, long appressed membranes. All images are shown at the same magnification. Scale bar: 1 μm.
CLB Genes Are Required for Expression of Nuclear- and Chloroplast-Encoded Genes Involved in Plastid Transcription, Translation, and Photosynthesis
EM analysis of plastids in clb mutants suggested that chloroplast differentiation is arrested at an early stage. To further characterize the plastid differentiation stage in clb mutants, the expression of several nuclear- and chloroplast-encoded genes known to be expressed at different moments of chloroplast development was determined.
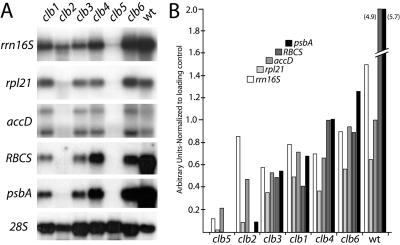
The rrn16S (16S rRNA) gene is the most highly expressed plastid-encoded gene in proplastids (Bisanz-Seyer et al., 1989; Krupinska and Falk, 1994), and is therefore a good molecular marker to measure plastid translational activities during the early phases of chloroplast development. As shown in Figure 5, the rrn16S transcript level is at approximately one-half of wild-type levels in clb1, clb2, clb3, clb4 and clb6. In clb5 however, the rrn16S transcript is barely detectable (around 10% of wild type), suggesting that in this mutant chloroplast biogenesis is affected earliest. This idea is also supported by the low or undetectable expression levels of other chloroplast developmental markers in the clb5 mutant. The nuclear gene rpl21 encodes a plastid ribosomal protein that is expressed very early after seed imbibition, preceding the expression of photosynthetic and nonphotosynthetic chloroplast-encoded genes (Lagrange et al., 1993; Harrak et al., 1995). Thus, rpl21 also constitutes a good marker for early plastid differentiation. As shown in Figure 5, rpl21 transcript was found at near wild-type levels in clb6, at 30% to 50% of wild type in clb1, clb3, and clb4, and was almost undetectable in clb2 and clb5.
Figure 5.
Northern analysis of plastid- and nuclear-encoded genes. A, Total RNA from clb and wild-type plants grown for 18 d in 16 h light was fractionated on agarose gels, transferred to nylon membrane, and hybridized with labeled probes for rrn16S, rpl21, accD, RBCS, psbA, and 28S (loading control). B, Band intensities in A were quantified and normalized to the 28S loading control. Relative band intensities are shown on the y axis in arbitrary units.
We also quantified transcript levels for accD, the chloroplast-encoded subunit of acetyl-CoA carboxylase, involved in lipid biosynthesis in the plastid. accD is exclusively transcribed by a nuclear-encoded plastid-localized RNA polymerase, required for proplastid maintenance, and is thus a marker of plastid transcription (Hajdukiewicz et al., 1997). accD transcript levels are detected at near wild-type levels in clb6, at about 70% of wild type in clb1 and clb4, at about 50% of wild type in clb2 and clb3, and at about 20% of wild type in clb5. The low accumulation of nuclear- and plastid-encoded ribosomal genes detected in clb5 suggests that plastids in this mutant are severely affected in translational competence.
Nuclear- and chloroplast-encoded genes required for photosynthetic reactions are late molecular markers for chloroplast biosynthesis and function. Thus, some of these genes were also chosen as markers for the molecular analysis of the clb mutants. The transcript level of nuclear-encoded RBCS, the essential enzyme of the Calvin cycle, and chloroplast-encoded psbA, which encodes the D1 protein of the reaction center of photosystem II (Bruick and Mayfield, 1999) was determined (Fig. 5). As expected, clb5 and clb2 accumulate undetectable or very little RBCS and psbA transcripts, while clb1, clb3, clb4, and clb6 show 10% to 20% of wild-type levels. In summary, these gene expression studies suggest that clb5 and clb2 are the earliest affected mutants, that clb3 and clb1 are less severe since they have a low level of expression of photosynthetic genes, and that clb4 and clb6 are the least severe mutants.
CLB Genes Act Cell and Noncell Autonomously
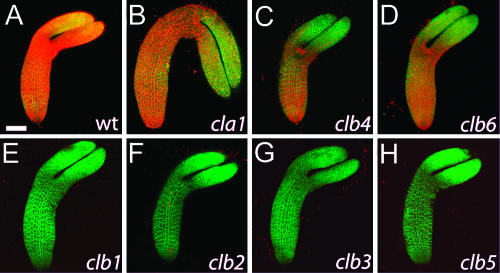
During Arabidopsis embryo development, differentiated chloroplasts are observed beginning at the heart stage, and their number continues to increase until the end of embryo growth (Mansfield and Briarty, 1991). As shown in Figure 6A, red chlorophyll autofluorescence from wild-type chloroplasts is clearly detected throughout the embryo. Surprisingly, even though clb mutant seedlings have almost undetectable levels of chlorophyll (Table III), significant levels of red chlorophyll autofluorescence are detected in cla1, clb4, and clb6 embryos, especially in the hypocotyl region (Fig. 6, B–D). This finding suggests the existence of mature chloroplasts that accumulate this pigment.
Figure 6.
Confocal reconstruction of wild-type (A) and cla1-1 (B), clb4 (C), clb6 (D), clb1 (E), clb2 (F), clb3 (G), and clb5 (H) embryos at the early bent cotyledon stage of development. Chlorophyll autofluorescence is shown in red, cell outlines are shown in green with plasma membrane-localized GFP fusion protein 29-1. Scale bar: 50 μm.
CLA1 is an essential gene in Arabidopsis that encodes for the DXS enzyme required in the first biosynthetic step of the MEP pathway (Estévez et al., 2000). It has previously been demonstrated that the albino phenotype of cla1-1 mutant seedlings can be complemented noncell autonomously by the addition of a synthetic version of the product of the DXS enzyme, 1-deoxyxylulose 5-P (DXP), to seedling growth medium (Estévez et al., 2000). Thus, diffusion of DXP or a downstream compound from the mother plant to cla1 embryos is probably responsible for the partial complementation that results in the observed chlorophyll accumulation during embryogenesis. Since clb4 and clb6 embryos also accumulate significant levels of chlorophyll, while clb4 and clb6 seedlings have almost no chlorophyll, the CLB4 and CLB6 genes can also be defined as acting noncell autonomously. By contrast, as shown in Figure 6, E–H, chlorophyll fluorescence was never detected in clb1, clb2, clb3, and clb5 embryos. These mutations can be defined as cell autonomous, as they are not complemented by maternal factors during embryogenesis.
Identification of the CLB4 Gene
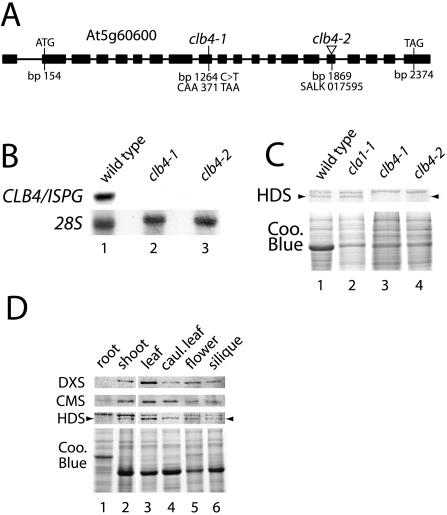
The observation that the CLA1, CLB4, and CLB6 genes behave noncell autonomously suggested that (similar to CLA1) CLB4 and CLB6 might also encode enzymes required for the MEP isoprenoid biosynthesis. To test this hypothesis, a candidate gene approach was used to identify the CLB4 gene. The mapping experiments described in Figure 2 placed the CLB4 gene approximately 4 cM north of the SSLP marker ciw10 on chromosome 5. Localization of genes involved in the MEP pathway showed the Arabidopsis homolog of the ISPG/GCPE gene (At5g60600) to be located in this region. In bacteria GcpE encodes for the 1-hydroxy-2-methyl-2-(E)-butenyl 4-diphosphate (HMBPP) synthase (HDS) enzyme, involved in the conversion of methylerythritol 2,4- cyclodiphosphate (ME-cPP) into HMBPP, which is the next to the last step of the MEP pathway (Hecht et al., 2001; Rohdich et al., 2003). At5g60600 is the only gene product in the Arabidopsis genome with sequence similarity to the HDS protein. The functionality of this HDS homolog has been analyzed by heterologous complementation of a GcpE E. coli mutant (Querol et al., 2002). The Arabidopsis HDS protein is predicted to consist of 741 amino acids, with an N-terminal chloroplast targeting sequence which has been shown to function in vivo (Querol et al., 2002).
Sequencing of the ISPG gene from genomic DNA of clb4-1 showed that this gene contained a cytosine to thymine change compared to wild type at bp 1264 of the ISPG cDNA, resulting in TAA stop at codon 371 (Fig. 7A). We subsequently identified a T-DNA insertion (SALK 017595) into the 16th exon of the ISPG gene (At5g60600). Plants heterozygous for this T-DNA insertion segregated albino seedlings with a phenotype identical to clb4-1, and crosses between clb4-1 and SALK 017595 heterozygous plants showed that this line failed to complement the clb4-1 mutation. Thus, the mutation in SALK 017595 was named clb4-2. To provide further evidence that clb4-1 and clb4-2 alleles affect the expression of the ISPG gene, the transcript levels of this gene were analyzed in both mutants. As shown in Figure 7B, while a transcript of the predicted molecular size for the ISPG gene was observed in wild-type seedlings, no transcript was detected in either clb4-1 or clb4-2 alleles. These results together demonstrate that both mutants behave as null alleles for the Arabidopsis ISPG gene, and strongly suggest that the disruption of this gene is responsible for the albino phenotype in both mutant lines.
Figure 7.
CLB4 is the Arabidopsis homolog of ISPG. A, Schematic representation of the CLB4/ISPG gene from Arabidopsis. The mutations found in the ISPG gene in clb4-1 and clb4-2 alleles are shown. Filled boxes correspond to exons of the transcript. Indicated features are the translation initiation codon (ATG) and the translation termination codon (TAG). Base pairs are numbered according to the ISPG cDNA clone, GenBank accession AF434673 (Querol et al., 2002). B, Northern-blot analysis of CLB4/ISPG transcript in wild-type, clb4-1, and clb4-2 seedlings. Each lane contains 5 μg of total RNA extracted from 5-d-old seedlings. A fragment of 768 bp of the 3′ region of the ISPG cDNA was used as a probe. The membrane was rehybridized with the 28S rRNA (28S) as a loading control. C, Western-blot analysis of the HDS protein. Total protein extracts were isolated from wild-type (1), cla1-1 (2), clb4-1 (3), and clb4-2 (4) mutants. Immunoblots were perfomed using antisera made against the HDS protein. Each lane contains 10 μg of total protein extract. A Coomassie blue stained gel is shown as a loading control. D, Expression level of the HDS protein in different tissues. Total protein extracts from roots (1), shoots (2), young leaves (3), cauline leaves (4), flowers (5), and siliques (6) were used to perform an immunoblot analysis with antibodies against DXS, CMS, and HDS proteins. Ten μg of protein were loaded from each extract and the stained gel is shown as a control.
To examine whether the effect observed at the transcript level was reflected at the protein level, we probed extracts of wild-type, cla1-1, clb4-1, and clb4-2 seedlings with polyclonal antibodies raised against a synthetic peptide of the HDS protein, the product of the ISPG gene from Arabidopsis. In wild-type and cla1-1 seedlings, this antibody recognized two bands of similar size, in the expected size range for the HDS protein. In protein extracts from clb4-1 and clb4-2 seedlings, the lower of these two bands was undetectable (Fig. 7C). While the identity of the upper band is unclear, these results indicate that the lower band corresponds to the HDS protein and that both mutant alleles have undetectable levels of the HDS protein.
Although there has been important progress in the elucidation of the MEP pathway genes in recent years, little is known about the expression profile and regulation of most of the genes of the pathway in plants. It has been reported that the first enzymes of the pathway (DXS and DXR) are expressed in most plant tissues but with highest levels in young photosynthetic tissues (Estévez et al., 2000; Carretero-Paulet et al., 2002). At the transcript level a similar pattern has been reported for ISPG, but little is known about the corresponding levels of HDS protein (Rodríguez-Concepción et al., 2003). We decided to analyze the level of the HDS protein in different plant tissues. This analysis also included two additional enzymes of the pathway; DXS as a comparative control, as well as 4-diphosphocytidyl methylerythriol synthase (CMS), the enzyme that catalyses the third step of this pathway (Rodríguez-Concepción and Boronat, 2002). As seen in Figure 7D, all three proteins are detected in all the tissues analyzed, but much higher levels are present in the young leaves, as compared to roots. This expression profile is consistent with the fact that photosynthetic pigments such as chlorophyll and carotenoids, synthesized from this pathway, are required in higher abundance in green tissues. A substantial protein level is also observed in flowers, especially for the DXS and HDS enzymes. These results are in agreement with the published data (Carretero-Paulet et al., 2002), suggesting that flowers are a site for high expression of several of the MEP genes.
DISCUSSION
In this work we report the isolation and characterization of mutations in six independent CHLOROPLAST BIOGENESIS genes, identified in a systematic visual screen for pigment mutants of Arabidopsis. Analysis of photosynthetic pigment levels, chloroplast ultrastructure, and expression of genes required for chloroplast transcription, translation, and photosynthesis demonstrated that chloroplasts in clb mutants are arrested at early stages of development with phenotypes similar to proplastids. It was further demonstrated that the albino phenotype of clb mutants is not due to photooxidation. The CLB4 gene was identified based on its map position, and demonstrated to correspond to the ISPG gene, which encodes for the HDS, the second-to-last acting enzyme in the MEP isoprenoid biosynthesis pathway. The noncell autonomous nature of the clb4 mutation provides evidence that products of the MEP pathway can move between tissue types, and suggests that there is at least some movement of MEP products from the cytoplasm to the chloroplast.
Noncell Autonomous CLB Genes Encode Enzymes of the Plastidic (MEP) Isoprenoid Biosynthesis Pathway
Isoprenoids play a fundamental role in plant development as they include molecules such as chlorophyll and carotenoids, as well as the plant hormones GA3 and abscisic acid (Rodríguez-Concepción and Boronat, 2002). Though plants have both cytosolic (MVA) and plastidic (MEP) pathways for the synthesis of the universal precursors (IPP and DMAPP), the above isoprenoids have been shown to be mainly synthesized by the MEP pathway (Lichtenthaler, 1999; Okada et al., 2002). Loss of the MEP pathway due to mutation leads to an albino seedling phenotype, supporting that this pathway is required for the synthesis of chlorophyll and carotenoid pigments (Estévez et al., 2000; Budziszewski et al., 2001). It has previously been suggested that chlorophyll is required for the formation of stacked (grana) thylakoids, although conflicting evidence for this exists (for review, see von Wettstein et al., 1995). However, mutations in the MEP pathway lead to defects in chloroplast biogenesis that are more severe than those in mutants that simply lack chlorophyll. cla1 mutant organelles are mostly lacking any kind of thylakoid membranes (Mandel et al., 1996) and we have shown that clb4 and clb6 mutants have very rudimentary internal membranes. Thus, it seems likely that the early arrest of chloroplast differentiation in mutants lacking the MEP pathway is due to lack of chlorophyll, plus some additional factor(s). One possibility is the hormone abscisic acid, whose synthesis comes from the MEP pathway. It has been shown that high concentrations of this hormone result in albino seedlings (Lopez-Molina et al., 2001). Also, abi3 mutations or the constitutive expression of the ABI3 gene affect plastid development (Rohde et al., 2000). However, a role for other isoprenoids such as gibberellins cannot be excluded.
The work described here demonstrated that CLB4 corresponds to the ISPG gene, which encodes the HDS enzyme, and is the second-to-last acting enzyme in the MEP plastidic isoprenoid biosynthesis pathway. Although a previous report has shown that the ISPG gene homolog from Arabidopsis complements a bacterial mutant affected in the HDS enzyme (Querol et al., 2002), clb4-1 and clb4-2 alleles represent the first characterized mutants in the ISPG gene from Arabidopsis. Based on the phenotype of these mutants we can conclude that no other gene in Arabidopsis is able to perform this function. In addition, we have recently discovered that clb6-1 is a null mutation in the ISPH gene, which encodes isopentenyl diphosphate synthase (IDS), the last-acting enzyme in the MEP pathway (A. Guevara, C. San Roman, M.E. Cortés, A. Arroyo, M. Gutiérrez-Nava, and P. León, unpublished data). Thus, of the CLB genes described in this paper, both noncell autonomous mutants affect the MEP isoprenoid biosynthesis pathway.
CLB4 and CLB6 genes were proposed to act noncell autonomously based on the observation that chlorophyll fluorescence accumulates in embryos of clb4 and clb6. A similar pattern of chlorophyll accumulation was also seen in cla1 embryos, and it was previously shown that cla1 seedlings can be rescued by growth on medium containing the MEP intermediate produced by the CLA1 gene product, demonstrating that CLA1 can act noncell autonomously (Estévez et al., 2000). As both CLB4 and CLB6 are single copy genes, and clb4-1 and clb6-1 are null mutations, the activity of a CLB4 or CLB6 homolog expressed during embryogenesis is excluded. Thus, it can be concluded that the chlorophyll accumulation (and presumably functional chloroplasts) during embryogenesis in both mutant embryos is likely due to an active or passive transport of product(s) of the MEP pathway from maternal tissues. Chlorophyll fluorescence was seen to accumulate primarily in the hypocotyl region of cla1-1, clb4, and clb6 embryos, the tissue closest to the suspensor that is the putative entry point for maternally supplied molecules (Yeung and Meinke, 1993). Interestingly, wild-type embryos show the opposite pattern, where higher chlorophyll fluorescence is seen in the cotyledons than in the hypocotyl. Because of the growing evidence suggesting that either IPP or DMAPP can move between the chloroplast and the cytoplasm (Rodríguez-Concepción and Boronat, 2002; Bick and Lange, 2003; Laule et al., 2003), these molecules are good candidates to also be transported from the mother to the embryonic tissue. Recent evidence suggests a preferential movement of IPP and/or DMAPP between chloroplast to the cytoplasm (Bick and Lange, 2003; Laule et al., 2003). However, based on our results it appears that under certain circumstances or developmental conditions, active movement of these molecules might also exist from the cytoplasm to the chloroplast.
The protein expression found for CMS and HDS is similar to the one observed for DXS and DXR (Estévez et al., 2000; Carretero-Paulet et al., 2002), with higher protein levels in young photosynthetic tissues and flowers. This expression is consistent with the high demand for chlorophyll and carotenoid pigments during the onset of photosynthesis. It is also notable that high protein levels exist in flowers and siliques, which has been suggested to be involved in the synthesis of specialized isoprenoids (Carretero-Paulet et al., 2002). This is of particular interest in view of the maternal flow to the developing embryo observed in the MEP affected mutants.
The phenotype of clb4 and clb6 seedlings may be alleviated by the presence of some functional chloroplasts during embryogenesis, allowing mutant embryos to produce a low level of essential compounds required during embryogenesis. These molecules, including MEP-derived isoprenoid such as hormones, could persist during the first stages of seedling growth, lessening the consequences of lack of CLB4 or CLB6 activity. To our knowledge, this is the first time that partial complementation of a mutation during embryogenesis by diffusion of maternal factors has been observed in plants. Conceivably, this could occur with any embryo defective mutant lacking a molecule that can diffuse or is actively transported from the mother plant to the embryo. If this is a general phenomenon, mutations affecting gene products that act noncell autonomously and that are required both during embryogenesis and seedling growth may only express a fully penetrant mutant phenotype beginning at the seedling stage.
It is intriguing that the three noncell autonomous mutants identified in this analysis affect the same biosynthetic pathway. Although the size of our mutant screen could be far from saturation, it is difficult to believe that the isolation of three different noncell autonomous mutants in the MEP pathway might result from a random chance. One explanation is that few noncell autonomous biosynthetic pathways render true albino phenotypes in Arabidopsis. Interestingly, similar results have been also obtained in a large-scale genetic screen to identify seedling lethal mutants (Budziszewski et al., 2001). It is well known that the extensive gene duplication in Arabidopsis has caused important functional redundancies in this plant (Vision et al., 2000). With the exception of CLA1, the enzymes of the Arabidopsis MEP pathway are encoded by unique genes, and thus a mutation in a single gene would be expected to result in an albino phenotype. This may not be true for other biochemical pathways that are more genetically redundant. It is also possible that mutants in other noncell autonomous pathways have been excluded by our selection due to complementation of the mutant phenotype either by the maternal tissue or by the growing media. An example of this could be vitamin biosynthetic pathways, as our selection media is supplemented with vitamins (Koornneef and Hanhart, 1981).
CLB Mutants Define a Set of Genes Required at Different Stages of Early Chloroplast Biogenesis
Although the seedling phenotype of all six clb mutants is similar, the mutants can be grouped by severity when plastid morphology and marker gene expression are taken into consideration. clb5 and clb2 show the most severe phenotypes, as their plastids lack appressed internal membranes and resemble proplastids. Both mutants had undetectable or nearly undetectable levels of the photosynthetic genes RBCS and psbA. While clb5 also had low or undetectable expression of early-expressed plastid genes related to transcription and translation, clb2 was less severe in showing significant levels of rrn16S and accD transcripts. clb3 plastids are similar to those of clb5 and clb2, in that they lack appressed internal membranes, and have large electron translucent cavities. However, clb3 differs in that photosynthetic genes show detectable expression, and early plastid genes are expressed at about 50% of wild-type levels. clb1 mutants have a similar gene expression profile to clb3, but clb1 plastids show a less severe phenotype in that some partially appressed internal membranes are visible. Therefore, with respect to chloroplast development, the phenotype of clb1 and clb3 can be considered intermediate among the clb mutants. Finally, clb4 and clb6 mutants have the highest marker gene expression, with early genes expressed slightly less than in wild type, and photosynthetic genes expressed at about 20% of wild-type levels. Plastids of clb6 show short, appressed internal membranes, while clb4 plastids have long single membranes.
Therefore, the mutants clb1, clb2, clb3, and clb5 show more severe albino phenotypes than clb4 and clb6. Based on these phenotypic observations and on the fact that clb4 and clb6 carry null mutations in their respective genes, we can conclude that the cell autonomous genes CLB1, CLB2, CLB3, and CLB5 are required earlier in chloroplast biogenesis than the noncell autonomous genes CLB4 and CLB6. Due to their cell autonomous nature, these genes are more likely to be required for processes such as plastid transcription or translation, or the biogenesis of thylakoid membranes.
The systematic isolation and analysis of mutants of Arabidopsis that affect early chloroplast differentiation represents an important step forward in studies of chloroplast biogenesis. The extreme phenotype and early arrest of chloroplast differentiation in cell autonomous clb mutants suggests that one or more of these CLB genes may be involved in the early signaling that initiates the chloroplast differentiation process in plastids. Cloning and characterization of these cell autonomous CLB genes, a current focus of research in our laboratory, will begin to allow the determination of their precise molecular role in chloroplast biogenesis, and should contribute to the overall understanding of this fascinating area of biology.
MATERIALS AND METHODS
Plant Material and Growth Conditions
Arabidopsis L. Heyhn. ecotypes Landsberg erecta (Ler), Columbia (Col), Wassilewskija (WS), C24, and Dijon were used in this study. For experiments involving plants grown under sterile conditions, seeds were surface-sterilized and plated on GM containing 1× Murashige and Skoog basal salts (Gibco BRL, Grand Island, NY), 2% (w/v) Suc, 1× B5 vitamin solution (Gamborg's, Sigma, St. Louis ), 0.05% (w/v) MES [2-(N-morpholino) ethanesulfonic acid], solidified with 0.8% (w/v) phytoagar. Adult plants were grown in Metro-Mix 200 (Grace Sierra, Milpitas, CA) under 16 h light:8 h dark at 24°C. Seedlings were grown under 16 h light:8 h dark cycle at 120 μE for high light conditions or at 5μE for low light conditions, at 22°C in growth chambers. Seeds were incubated at 4°C for 5 d to break dormancy prior to germination. Pigmentation mutant lines CS27, CS213, CS214, CS215, CS2751, CS2771, CS2790, CS2791, CS2793, CS2794, CS2795, CS2797, CS2798, CS2800, CS2801, CS2802, CS2804, CS2805, CS2806, CS2807, CS2808, and CS2809, T-DNA pools, and SALK insertion line 017595 were obtained from the Arabidopsis Biological Resource Center (ABRC, http://www.biosci.ohio-state.edu/plantbio/Facilities/abrc/abrchome.htm). EMS mutagenized seed, fast neutron mutagenized lines, and some T-DNA pools were generously provided by Chris Somerville (Carnegie Institute of Washington, Stanford, CA).
For the analysis of mutant embryos during development, each clb mutant line was crossed to the homozygous green fluorescent protein marker line 29-1 (Ler ecotype). Plants heterozygous for the corresponding clb mutation and homozygous for the 29-1 transgene were selected in the F2 generation, and used for confocal analysis. Seed expressing plasma membrane-localized GFP (line 29-1) were obtained from Sean Cutler, Joel Griffits, and David Ehrhardt (Carnegie Institution of Washington, Stanford, CA; Cutler et al., 2000; http://deepgreen.stanford.edu).
Complementation analysis was done by crossing heterozygous mutant plants in all possible combinations and scoring resulting F1 embryos for the mutant phenotype. Genetic mapping was performed according to Lukowitz et al. (2000). Primer sequences for all SSLP markers used in this study are available at www.Arabidopsis.org. Genomic DNA for PCR was prepared using the simplified 2× CTAB protocol (carnegiedpb.stanford.edu/publications/methods/ppsuppl.html).
Pigment Determination
Total carotenoids and chlorophylls were determined according to Lichtenthaler and Wellburn (1983). Extracts were obtained from 80 mg of fresh tissue from 18-d-old Arabidopsis seedlings and homogenized in 100 μL 100% acetone. Spectrophotometric quantification was carried out in a Beckman DUR650 spectrophotometer. Pigment measurements were repeated in three independent experiments.
Northern-Blot Analysis and DNA Sequencing
Total RNA was prepared from 18-d-old seedlings grown on GM medium plates by extraction with Trizol Reagent (Sigma). 15 μg of total RNA was fractionated by electrophoresis in 1.2% (w/v) agarose gel and transferred onto Gene Screen nylon membrane (New Life Science Products, Boston). Hybridization was done with PSE buffer (1 m sodium phosphate buffer, pH 7.2, plus 10% sodium dodecyl sulfate), and washes were done under high stringency conditions according to standard procedures (Sambrook et al., 1989). DNA probes for hybridization were labeled with 32P using an Amersham All-In-One labeling kit (Amersham, Piscataway, NJ). DNA probes for labeling reactions were obtained by PCR, or from specific DNA fragments, as follows: rrn16S probe (640 bp) was amplified from Arabidopsis nuclear and plastid genomic DNA using the following primers: rrn16S F: aaa tgc gta gag atc gga aag; rrn16S R: ttc atg cag gcg agt tg. accD probe (658 bp) using the primers accD F: tat ggt tgg gat gag cgt tct; accD R: ccg gat caa tcg aaa gc. rpl21 probe was obtained by amplifying the entire 750-bp insert from the Arabidopsis rpl21 EST 146E8 (obtained from the ABRC stock center), using standard T7 and SP6 primers. Specific fragments were obtained from the Arabidopsis CAB1 gene (GenBank accession no. At1g29930) and the spinach chloroplast psbA gene (GenBank accession no. NC 002202). The fragment used as a probe for CLB4 northern analysis includes 768 bp of the 3′ region of the CLB4/ISPG/GCPE cDNA (AF434673). Primers used were ISPG F: 5′-CCA TGG TTC TTG TCA ACC TC-3′ and ISPG R: 5′-CCA TGG TTC TTG TCA ACC TC-3′. All probes were gel purified prior labeling. Band signal intensities were quantified using Kodak 1D image analysis software (Kodak, Rochester, NY).
For sequencing of the ISPG gene (At5g60600) from the clb4-1 allele, the entire At5g60600 gene was amplified from genomic DNA isolated from homozygous clb4-1 seedlings, and directly sequenced using the ABI Big Dye Terminator 2.0 cycle sequencing kit. Samples were analyzed on an ABI 377 sequencer. The position of the T-DNA insertion site into the At5g60600 gene in the clb4-2 allele was verified by amplifying and sequencing the genomic T-DNA border.
Western-Blot Analysis
Total protein samples were obtained from frozen tissue ground in liquid nitrogen and thawed in SDS sample buffer (0.125 m Tris-Cl pH 6.8, 20% v/v glycerol, 4% w/v SDS, 2% v/v 2-mercaptoethanol). The protein concentration was determined with Bradford reagent (Bio-Rad, Hercules, CA) using BSA as a standard, and then separated by SDS-PAGE (SDS-PAGE). To verify equal protein loading, a parallel gel was run and stained with Coomassie Brilliant Blue R-250. The proteins were transferred onto nitrocellulose (Hybond C, Amersham) by electroblotting for 1 h at 200 mA in 25 mm Tris, 0.2 m Gly, and 20% (w/v) methanol. Immunodetection was performed using a 1:1000 dilution of a polyclonal antibody against DXS1 (Estévez et al., 2000), 1:2500 for CMS, and 1:1500 for HDS. The HDS antibody was raised against a synthetic peptide NH2-C*QEISAEIREKTSH-CONH that was conjugated with KLH and used as an antigen to immunize rabbits. The amino-terminal Cys residue (indicated as an asterisk) was used to conjugate to KLH. This antibody was kindly provided by Drs. Shinjiro Yamaguchi, Hiroyuki Kasahara, and Yuji Kamiya, from the Plant Science Research Center RIKEN. The antibody used against CMS protein corresponds to a polyclonal antibody generated against a fusion of the GST-CMS protein. For antibody generation, 50 μg of purified fusion protein in 1 mL of PBS (140 mm NaCl, 2.8 mm NaH2PO4, 7.2 mm Na2HPO4 pH 7.4) and complete Freund's adjuvant (Sigma) were injected subcutaneously as 1:1 (v:v) emulsion into 8-week-old female New Zealand rabbits. Six additional injections (50 μg each) with incomplete Freund's adjuvant (Sigma) were administrated at 10 d intervals starting 15 d after the initial injection. The serum was collected 3 d after the last injection and the titer determined for each protein. An anti-rabbit inmunoglobulin alkaline phosphatase-conjugate was used as a secondary antibody and was detected using the BCIP/NBT substrate kit (Zymed Laboratories, San Francisco).
Confocal, Light, and Electron Microscopy
For confocal microscopy, late stage embryos were dissected from heterozygous plants, mounted in a solution of 300 mm mannitol on large coverslips, and imaged on a Nikon inverted fluorescence microscope equipped with a 20× Nikon objective (Tokyo) and a Bio-Rad MRC 1024 confocal head. Confocal reconstructions were made from approximately 1 μm optical sections using the public domain NIH Image program (developed at the United States National Institutes of Health and available on the Internet at http://rsb.info.nih.gov/nih-image). Digital images of seedlings were obtained with a Sony DSC-F707 digital camera using a Wild M3Z dissecting microscope. Transmission electron micrographs were obtained exactly as described in Mandel et al. (1996). All images were processed using Adobe Photoshop (Mountain View, CA).
Distribution of Materials
Upon request, all novel materials described in this publication will be made available in a timely manner for noncommercial research purposes.
Sequence data from this article have been deposited with the EMBL/GenBank data libraries under accession numbers AF434673, At5g60600, At1g29930, and NC 002202.
Acknowledgments
Many thanks to Chris Somerville for providing laboratory and greenhouse space, reagents, and support during the course of this work. Thanks to Drs. Shinjiro Yamaguchi, Hiroyuki Kasahara, and Yuji Kamiya, from the Plant Science Research Center RIKEN, for providing the antibodies against the HDS protein. Thanks to Rogene Gillmor for expert assistance with mapping, Carolina San Roman for her assistance in protein analysis, Araceli Cantero for plant maintenance, and Daniel Grimanelli and Olivier Leblanc for support during the preparation of this manuscript. We gratefully acknowledge the Salk T-DNA insertion project for the production of sequenced T-DNA insertion lines and the Arabidopsis Biological Resource Center for providing seeds.
This work was supported by CONACYT (31791–N), DGAPA IN210200, BASF, and Howard Hughes grants. M.G.-N. was supported by fellowships from CONACYT, SNI, the Wood-Whelan Research Foundation, and by a UNESCO Short-Term Biotechnology Fellowship. C.S.G. was partially supported by a U.S. Department of Energy/National Science Foundation/U.S. Department of Agriculture tri-agency training grant, and by a grant from the U.S. Department of Energy (DE–FG02–97ER20133) to C.R. Somerville (Carnegie Institution of Washington, Department of Plant Biology).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.103.036996.
References
- Apuya NR, Yadegari R, Fischer RL, Harada JJ, Zimmerman JL, Goldberg RB (2001) The Arabidopsis embryo mutant schlepperless has a defect in the Chaperonin-60alpha gene. Plant Physiol 126: 717–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki N, Kusumi K, Masamoto K, Niwa Y, Iba K (2000) Temperature-sensitive Arabidopsis mutant defective in 1-deoxy-d-xylulose 5-phosphate synthase within the plastid non-mevalonate pathway of isoprenoid biosynthesis. Physiol Plant 108: 19–24 [Google Scholar]
- Baumgartner BJ, Rapp JC, Mullet JE (1989) Plastid transcription activity and DNA copy number increase early in Barley chloroplast development. Plant Physiol 89: 1011–1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bick JA, Lange M (2003) Metabolic cross talk between cytosolic and plastidial pathways of isoprenoid biosynthesis: unidirectional transport of intermediates across the chloroplast envelope membrane. Arch Biochem Biophys 415: 146–154 [DOI] [PubMed] [Google Scholar]
- Bisanz-Seyer C, Li Y-F, Seyer P, Mache R (1989) The components of the plastid ribosome are not accumulated synchronously during the early development of spinach plants. Plant Mol Biol 12: 201–211 [DOI] [PubMed] [Google Scholar]
- Bruick RK, Mayfield SP (1999) Light-activated translation of chloroplast mRNAs. Trends Plant Sci 4: 190–195 [DOI] [PubMed] [Google Scholar]
- Budziszewski GJ, Lewis SP, Glover LW, Reineke J, Jones G, Schlater Ziemnik L, Lonowski J, Nyfeler B, Aux G, Zhou Q, et al. (2001) Arabidopsis genes essential for seedling viability: isolation of insertional mutants and molecular cloning. Genetics 159: 1765–1778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carretero-Paulet L, Ahumada I, Cunillera N, Rodríguez-Concepción M, Ferrer A, Boronat A, Campos N (2002) Expression and molecular analysis of the Arabidopsis thaliana DXR gene encoding 1-deoxy-d-xylulose 5-phosphate reductoisomerase, the first committed enzyme of the 2-C-methyl-d-erythritol 4-phosphate pathway. Plant Physiol 129: 1581–1591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee M, Sparvoli S, Edmunds C, Garosi P, Findlay K, Martin C (1996) DAG, a gene required for chloroplast differentiation and palisade development in Antirrhinum majus. EMBO J 15: 4194–4207 [PMC free article] [PubMed] [Google Scholar]
- Cutler SR, Ehrhardt DW, Griffits JS, Somerville CR (2000) Random GFP::cDNA fusions enable visualization of subcellular structures in cells of Arabidopsis at a high frequency. Proc Natl Acad Sci USA 97: 3718–3723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estévez JM, Cantero A, Romero C, Kawaide H, Jiménez LF, Kuzuyama T, Seto H, Kamiya Y, León P (2000) Analysis of the expression of CLA1, a gene that encodes the 1-deoxyxylulose 5-phosphate synthase of the 2-C-methyl-D-erythritol-4-phosphate pathway in Arabidopsis. Plant Physiol 124: 95–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajdukiewicz PTJ, Allison LA, Maliga P (1997) The two RNA polymerases encoded by the nuclear and the plastid compartments transcribe distinct groups of genes in tobacco plastids. EMBO J 16: 4041–4048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han C-D, Martienssen RA (1994) Molecular characterization of Iojap in maize. Cold Spring Harbor Laboratory Annual Report. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY
- Han C-D, Patrie W, Polacco M, Coe EH (1993) Aberrations in plastid transcripts and deficiency of plastid DNA in striped and albino mutants in maize. Planta 191: 552–563 [Google Scholar]
- Harrak H, Lagrange T, Bisanz-Seyer C, Lerbs-Mache S, Mache R (1995) The expression of nuclear genes encoding plastid ribosomal proteins precedes the expression of chloroplast genes during early phases of chloroplast development. Plant Physiol 108: 685–692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht S, Eisenreich W, Adam P, Amslinger S, Kis K, Bacher A, Arigoni D, Rohdich F (2001) Studies on the nonmevalonate pathway to terpenes: the role of the GcpE (IspG) protein. Proc Natl Acad Sci USA 26: 14837–14842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keddie JS, Carroll B, Jones JDG, Gruissem W (1996) The DCL gene of tomato is required for chloroplast development and palisade cell morphogenesis in leaves. EMBO J 15: 4208–4217 [PMC free article] [PubMed] [Google Scholar]
- Kirk JTO, Tilney-Bassett RAE (1978) The Plastids. Elsevier/North Holland Biomedical Press, Amsterdam
- Koornneef M, Hanhart CJ (1981) A new thiamine locus in Arabidopsis. Arabidopsis Inf Serv 18: 52–58 [Google Scholar]
- Krupinska K, Falk J (1994) Changes in RNA-polymerase activity during biogenesis, maturation and senescence of barley chloroplasts: comparative analysis of transcripts synthesized either in run-on assays or by transcriptionally active chromosomes. Plant Physiol 143: 298–305 [Google Scholar]
- Lagrange T, Franzetti B, Axelos M, Mache R, Lerbs-Mache S (1993) Structure and expression of the nuclear gene coding for the chloroplast ribosomal protein L21: developmental regulation of a housekeeping gene by alternative promoters. Mol Cell Biol 13: 2614–2622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin RM, Alonso JM, Ecker JR, Chory J (2003) GUN4, a regulator of chlorophyll synthesis and intracellular signaling. Science 299: 902–906 [DOI] [PubMed] [Google Scholar]
- Laule O, Führholz A, Chang H-S, Zhu T, Wang X, Heifetz PB, Gruissem W, Lange BM (2003) Crosstalk between cytosolic and plastidial pathways of isoprenoid biosynthesis in Arabidopsis thaliana. Proc Natl Acad Sci USA 100: 6866–6871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- León P, Arroyo A, Mackenzie S (1998) Nuclear control of plastid and mitochondrial development in higher plants. Annu Rev Plant Physiol Plant Mol Biol 49: 453–480 [DOI] [PubMed] [Google Scholar]
- Li HM, Altschmied L, Chory J (1994) Arabidopsis mutants define downstream branches in the phototransduction pathway. Genes Dev 8: 339–349 [DOI] [PubMed] [Google Scholar]
- Lichtenthaler HK (1999) The 1-deoxy-D-xylulose-5-phosphate pathway of isoprenoid biosynthesis in plants. Annu Rev Plant Phys Plant Mol Biol 50: 47–65 [DOI] [PubMed] [Google Scholar]
- Lichtenthaler HK, Wellburn AR (1983) Determination of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem Soc Trans 11: 591–592 [Google Scholar]
- Lopez-Molina L, Mongrand S, Chua NH (2001) A postgermination developmental arrest checkpoint is mediated by abscisic acid and requires the ABI5 transcription factor in Arabidopsis. Proc Natl Acad Sci USA 98: 4782–4787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukowitz W, Gillmor CS, Scheible WR (2000) Positional cloning in Arabidopsis. Why it feels good to have a genome initiative working for you. Plant Physiol 123: 795–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mache R, Zhou D-X, Lerbs-Mache S, Harrak H, Villain P, Gauvin S (1997) Nuclear control of early plastid differentiation. Plant Physiol Biochem 35: 199–203 [Google Scholar]
- Mandel MA, Feldmann KA, Herrera-Estrella L, Rocha-Sosa M, León P (1996) CLA1, a novel gene required for chloroplast development, is highly conserved in evolution. Plant J 9: 649–658 [DOI] [PubMed] [Google Scholar]
- Mansfield SG, Briarty LG (1991) Early embryogenesis in Arabidopsis thaliana. II. The developing embryo. Can J Bot 69: 461–476 [Google Scholar]
- Mascia PN, Robertson DR (1978) Studies of chloroplast development in four maize mutants defective in chlorophyll biosynthesis. Planta 143: 207–211 [DOI] [PubMed] [Google Scholar]
- Meurer J, Grevelding C, Westhoff P, Reiss B (1998) The PAC protein affects the maturation of specific chloroplast mRNAs in Arabidopsis thaliana. Mol Gen Genet 258: 342–351 [DOI] [PubMed] [Google Scholar]
- Meurer J, Meierhoff K, Westhoff P (1996) Isolation of high-chlorophyll-fluorescence mutants of Arabidopsis thaliana and their characterization by spectroscopy, immunoblotting and Northern hybridization. Planta 198: 385–396 [DOI] [PubMed] [Google Scholar]
- Motohashi R, Nagata N, Ito T, Takahashi S, Hobo T, Yoshida S, Shinozaki K (2001) An essential role of a TatC homologue of a DpH-dependent protein transporter in thylakoid membrane formation during chloroplast development in Arabidopsis thaliana. Proc Natl Acad Sci USA 98: 10499–10504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mühlethaler K, Frey-Wyssling A (1959) Entwicklung und struktur der proplastiden. J Biophys Biochem Cytol 6: 507–512 [PMC free article] [PubMed] [Google Scholar]
- Mullet JE (1993) Dynamic regulation of chloroplast transcription. Plant Physiol 103: 309–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oelmüller R (1989) Photooxidative destruction of chloroplasts and its effect on nuclear gene expression and extraplastidic enzyme levels. Photochem Photobiol 49: 229–239 [Google Scholar]
- Okada K, Kawaide H, Kuzuyama T, Seto H, Curtis IS, Kamiya Y (2002) Antisense and chemical suppression of the nonmevalonate pathway affects ent-kaurene biosynthesis in Arabidopsis. Planta 215: 339–344 [DOI] [PubMed] [Google Scholar]
- Pyke KA (1999) Plastid division and development. Plant Cell 11: 549–556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Querol J, Campos N, Imperial S, Boronat A, Rodríguez-Concepción M (2002) Functional analysis of the Arabidopsis thaliana GCPE protein involved in plastid isoprenoid biosynthesis. FEBS Lett 514: 343–346 [DOI] [PubMed] [Google Scholar]
- Rapp JC, Mullet JE (1991) Chloroplast transcription is required to express the nuclear genes rbcS and cab. Plastid DNA copy number is regulated independently. Plant Mol Biol 17: 813–823 [DOI] [PubMed] [Google Scholar]
- Reiter RS, Coomber SA, Bourett TM, Bartley GE, Scolnik PA (1994) Control of leaf and chloroplast development by the Arabidopsis gene pale cress. Plant Cell 6: 1253–1264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Concepción M, Boronat A (2002) Elucidation of the methylerythritol phosphate pathway for isoprenoid biosynthesis in bacteria and plastids. A metabolic milestone achieved through genomics. Plant Physiol 103: 1079–1089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Concepción M, Querol J, Lois LM, Imperial S, Boronat A (2003) Bioinformatic and molecular analysis of hydroxymethylbutenyl diphosphate synthase (GCPE) gene expression during carotenoid accumulation in ripening tomato fruit. Planta 217: 476–482 [DOI] [PubMed] [Google Scholar]
- Rohde A, De Rycke R, Beekman T, Engler G, Van Montagu M, Boerjan W (2000) ABI3 affects plastid differentiation in dark-grown Arabidopsis seedlings. Plant Cell 12: 35–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohdich F, Zepeck F, Adam P, Hecht S, Kaiser J, Laupitz R, Grawert T, Amslinger S, Eisenreich W, Bacher A, et al. (2003) The deoxyxylulose phosphate pathway of isoprenoid biosynthesis: studies on the mechanisms of the reactions catalyzed by IspG and IspH protein. Proc Natl Acad Sci USA 100: 1586–1591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy LM, Barkan A (1998) A SecY homolog is required for the elaboration of the chloroplast thylakoid membrane and for normal chloroplast gene expression. J Cell Biol 141: 385–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T (1989) Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY
- Shumway LK, Weier TE (1967) The chloroplast structure of iojap maize. Am J Bot 54: 773–780 [Google Scholar]
- Staehelin LA, Newcomb EH (2000) Membrane structure and membranous organelles. In RB Buchanan, W Gruissem, RL Jones, eds, Biochemistry and Molecular Biology of Plants. American Society of Plant Biology, Rockville, MD, pp 2–50
- Strand A, Asami T, Alonso J, Ecker JR, Chory J (2003) Chloroplast to nucleus communication triggered by accumulation of Mg-protoporphyrin IX. Nature 421: 79–83 [DOI] [PubMed] [Google Scholar]
- Uwer U, Willmitzer L, Altmann T (1998) Inactivation of a Glycyl-tRNA synthetase leads to an arrest in plant embryo development. Plant Cell 10: 1277–1294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vision TJ, Brown DG, Tanksley SD (2000) The origins of genomic duplications in Arabidopsis. Science 290: 2114–2117 [DOI] [PubMed] [Google Scholar]
- von Wettstein D, Gough S, Kannangara CG (1995) Chlorophyll biosynthesis. Plant Cell 7: 1039–1057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vothknecht UC, Westhoff P (2001) Biogenesis and origin of thylakoid membranes. Biochim Biophys Acta 1541: 91–101 [DOI] [PubMed] [Google Scholar]
- Wang Y, Duby G, Purnelle B, Boutry M (2000) Tobacco VDL gene encodes a plastid DEAD box RNA helicase and is involved in chloroplast differentiation and plant morphogenesis. Plant Cell 12: 2129–2142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung EC, Meinke DW (1993) Embryogenesis in angiosperms: development of the suspensor. Plant Cell 5: 1371–1381 [DOI] [PMC free article] [PubMed] [Google Scholar]