Abstract
Capsicum annuum tobacco mosaic virus (TMV)-induced clone 1 (CaTin1) gene was expressed early during incompatible interaction of hot pepper (Caspsicum annuum) plants with TMV and Xanthomonas campestris. RNA-blot analysis showed that CaTin1 gene was expressed only in roots in untreated plants and induced mainly in leaf in response to ethylene, NaCl, and methyl viologen but not by salicylic acid and methyl jasmonate. The ethylene dependence of CaTin1 induction upon TMV inoculation was demonstrated by the decrease of CaTin1 expression in response to several inhibitors of ethylene biosynthesis or its action. Transgenic tobacco (Nicotiana tabacum) plants expressing CaTin1 gene in sense- or antisense-orientation showed interesting characteristics such as the accelerated growth and the enhanced resistance to biotic as well as abiotic stresses. Such characteristics appear to be caused by the elevated level of ethylene and H2O2. Moreover, in transgenic plants expressing antisense CaTin1 gene, the expression of some pathogenesis-related genes was enhanced constitutively, which may be mainly due to the increased ethylene level. The promoter of CaTin1 has four GCC-boxes, two AT-rich regions, and an elicitor-inducible W-box. The induction of the promoter activity by ethylene depends on GCC-boxes and by TMV on W-box. Taken together, we propose that the CaTin1 up-regulation or down-regulation interferes with the redox balance of plants leading to the altered response to ethylene and biotic as well as abiotic stresses.
Multiple preformed antimicrobial compounds contribute to the constitutive defense machinery of plants against pathogenic organisms. In addition, plants can trigger the inducible defense programs upon the perception of invaders. The early signaling molecules leading to R-gene-mediated resistance are ion fluxes, GTP-binding proteins, protein kinases, phosphatases, and phospholipases (Hammond-Kosack and Jones, 1996). Plants also respond to external stimuli such as microbial elicitors of cell death and/or defense responses by changing the calcium influx of the cell (Ebel and Scheel, 1997; Higgins et al., 1998). The increase of cytosolic calcium leads to cell death, which seems necessary for hypersensitive cell death triggered by rust fungi (Xu and Heath, 1998). The calcium channel blocker La3+, on the other hand, prevents the bacteria-induced hypersensitive response (HR) in soybean (Glycine max) leaves (Levine et al., 1996). The burst in the oxidative metabolism generated within minutes of infection leads to the accumulation of the reactive oxygen intermediates (ROI) such as H2O2 and superoxide anion (O2•−), involving NADPH-dependent oxidase. ROI serve as signaling molecules, for example in the recognition of the attack by fungal pathogens (Lamb and Dixon, 1997).
Ethylene is widely known to modulate the organ senescence induced by various stress factors such as plant pathogens (Moore et al., 2000), O3− (Pell et al., 1997), and hypoxia (He et al., 1996). O3− induces genes involved in the ethylene biosynthesis (Overmyer et al., 2000). The study of O3−sensitive Arabidopsis rcd1 mutant demonstrated that lesion-initiation processes in plants were independent of ethylene while lesion-propagation processes were ethylene-dependent (Overmyer et al., 2000). In addition, Arabidopsis ctr1 mutants exhibit the constitutive activation of the ethylene-response pathway. It is proposed that exposure to ethylene inactivates a CTR1 MAP kinase cascade, triggering plant ethylene responses (Bleecker and Kende, 2000). The study of the role of ethylene in the disease resistance revealed the interrelationship between ethylene and another stress hormone, methyl jasmonate (MeJA). MeJA appears to stimulate the ethylene biosynthesis and vice versa (Laudert and Weiler, 1998). In general, during the disease resistance signal transduction, at least two separate pathways are required for the resistance against different pathogens: salicylic acid (SA)-dependent pathway and MeJA/ethylene-dependent pathway. However, our study demonstrates that the newly isolated gene Caspsicum annuum TMV-induced clone 1 (CaTin1) expression is dependent only on ethylene but not MeJA or SA. Previous studies showed that ethylene exerted both the positive and the negative effect on its own biosynthesis (Johnson and Ecker, 1998).
The TMV-induced hot pepper (Caspsicum annuum) gene, CaTin1 gene, was isolated as an incompatible interaction-specific gene through the differential screening between TMV inoculated and mock inoculated hot pepper plants (Shin et al., 2001). CaTin1 was characterized further because CaTin1 was induced by ethylene but not by SA or MeJA. CaTin1 gene has the PKD1, lipoxygenase, alphatoxin, and triacylglycerol lipase (PLAT) domain that is known to exist in PKD gene whose function is unknown (Bateman and Sanford, 1998). The three-dimensional structure of the PLAT domain of human pancreatic lipase (van Tibeurgh et al., 1993), rabbit 15-lipoxygenase (Gillmor et al., 1997), and alpha toxin from Clostridim perfringens (Naylor et al., 1998) is characterized. In plants, the PLAT domain may interact directly with the membrane in a Ca2+ dependent manner. Or the PLAT domain may be involved in the protein-protein and/or protein-lipid interaction (Bateman and Sanford, 1998). An ethylene-induced cDNA encoding a lipase that contains the PLAT domain was expressed at the onset of senescence (Hong et al., 2000). In tobacco, ethylene induces the expression of basic pathogenesis-related (PR) genes by a calcium-dependent pathway that requires protein phosphorylation (Raz and Fluhr, 1992, 1993). Calcium could influence the ethylene biosynthesis by affecting ACC oxidase transcription (Kwak and Lee, 1997) and its activity (Gallardo et al., 1999). Calcium also regulates the ethylene-dependent pathway of the PR gene induction that is required for the ethylene-promoted morphogenic responses in seedling (Raz and Fluhr, 1992). The data suggest that CaTin1 may function on the ethylene-dependent incompatible interaction between plants and avirulent pathogens.
CaTin1 gene was induced by ethylene and methyl viologen (MV). The promoter of CaTin1 gene has four GCC-boxes and one W-box. The analysis of PR gene promoters has led to the identification of the11-bp ethylene-responsive element TAAGAGCCGCC, which has been referred as the GCC-box (Ohme-Takagi and Shinshi, 1995). This element was shown to be required for the regulation of ethylene in plants. Several ethylene-responsive element binding proteins function by binding to the ethylene-responsive GCC-box that is involved in the pathogen-activated transcription of PR genes (Leubner-Metzger et al., 1998; Park et al., 2001b). The elicitor responsive element, W-box (T)TGAC(C), was first identified within the parsley (Petroselinum crispum) PR1-1 and PR1-2 promoters and the WRKY transcription factor is known to bind to the W-box (Rushton et al., 1996). Another WRKY transcription factor, TDBA12, also recognizes a W-box. TDBA12 is suggested to serve as a component in pathogen-induced signal transduction pathways in plant cells and to regulate the expression of certain plant defense genes (Yang et al., 1999).
RESULTS
The CaTin1 Gene Has the PLAT Domain and Is Expressed Only in Roots
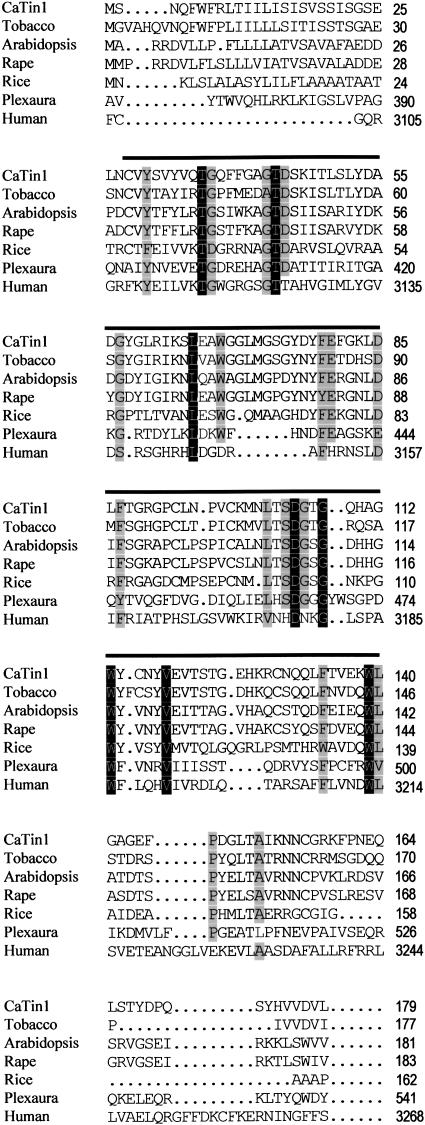
In the previous study, we isolated N6-11 gene that was one of the genes induced specifically during the incompatible interaction between hot pepper plants and TMV (Shin et al., 2001). The clone, renamed as CaTin1, contains the 775-bp insert encoding a protein of 180 amino acid residues (Fig. 1). The molecular mass of the predicted mature protein is 19.8 kD. The sequence of CaTin1 is not homologous to any known genes except the PLAT domain (lined in Fig. 1). The function of this domain in plants is not known. However, a few elicitor-induced clones having the PLAT domain were found (AF314810, AB040407, and AC083945).
Figure 1.
Comparison of the predicted amino acid sequence of CaTin1 (AF242731) with various PLAT domain-containing genes. Compared genes are tobacco (AB040407), Arabidopsis (NP_195683, chromosome 4), rape (AF314810), rice (AC083945), the PLAT domain part of Plexaura lipoxigenase gene (T30903), and the PLAT domain part of human PKD gene (2203412A). The black bold line indicates a PLAT domain and each black, dark gray, and light gray box indicates 100%, 75%, and 50% homology, respectively.
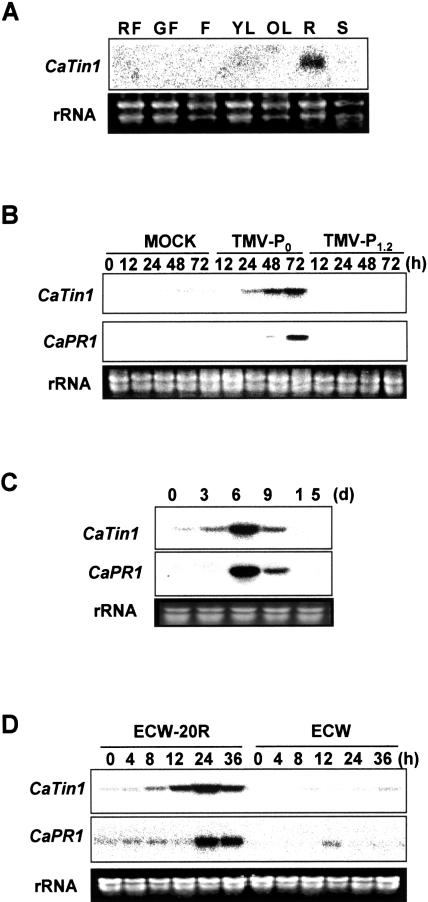
Based on the high stringent Southern-blot analysis, a few copies of CaTin1 gene were detected in pepper genome (data not shown). To examine the steady state transcription level of CaTin1 gene in various organs of pepper plants, the total RNA was extracted from the red ripe fruits, green unripe fruits, young leaves, old leaves, roots, stems, and flowers. As shown in Figure 2A, the transcription of CaTin1 was detected only in roots and not in any other organs.
Figure 2.
Expression patterns of the CaTin1 gene in various organs and in response to biotic stresses. A, Organ-specific expression of the CaTin1 gene. In each lane, 15 μg of total RNAs prepared from various organs were loaded. RF, red ripe fruit; GF, green unripe fruit; F, flower; YL, young leaf; OL, old leaf; R, root; S, stem. B, Expression pattern of CaTin1 gene upon TMV-P0 or TMV-P1.2 inoculation. As a control, leaves were mock-inoculated with phosphate buffer. C, Expression pattern of CaTin1 gene to TMV-P0 during SAR. To monitor expression of CaTin1 gene, 2 leaves of hot pepper plant were inoculated with TMV-P0 sap (approximately 20 μg/mL) and upper uninoculated leaves were detached at 0, 3, 6, 9, and 15 d postinoculation. D, Expression pattern of pepper CaTin1 gene upon Xcv inoculation. A hot pepper cultivar (ECW-20R) resistant to Xcv and a susceptible cultivar (ECW) were infiltrated with Xcv culture (1 × 108 cells/mL). Total RNA was extracted from the leaves harvested at time points indicated after inoculation. RNA was separated in formaldehyde-agarose gel and transferred onto nylon membrane. The membranes were hybridized with CaTin1 cDNA probe. CaPR1 was used as a control for HR. The rRNA bands in ethidium bromide-stained gels are shown as a loading control. Experiments were performed at least three or four times for each treatment and the representative result was shown here.
The CaTin1 Expression during the Incompatible Interaction with TMV and Xanthomonas campestris
The expression pattern of CaTin1 gene was monitored in hot pepper cv Bugang plants after TMV-P0 (avirulent strain) inoculation. A mock inoculation treatment was carried out as a control to rule out any CaTin1 gene expression that might arise from wounding due to rubbing with carborundum. As shown in Figure 2B, the accumulation of CaTin1 transcripts was barely detected in mock-inoculated or TMV-P1.2 (virulent strain) inoculated leaves. However, CaTin1 transcripts started to accumulate from 24 h after the inoculation with TMV-P0 and increased until 72 h after the inoculation. As a positive control, the expression pattern of the CaPR1 gene, one of the PR genes isolated from hot pepper, was also monitored (Shin et al., 2001). The induction of CaPR1 gene was observed from 48 h after the inoculation. This indicates that the expression of CaTin1 gene was induced earlier than CaPR1 gene.
We also examined whether CaTin1 could be induced during the systemic acquired resistance (SAR) after TMV inoculation. The transcripts corresponding to CaTin1 gene were accumulated in the distant uninoculated leaves 3 d after TMV inoculation (Fig. 2C). The systemic induction of the CaTin1 gene was earlier than CaPR1 gene that is known to be induced during SAR (Sticher et al., 1997).
It was investigated whether the TMV-P0-inducible CaTin1 gene in plants is also involved in the defense against other pathogens. Pepper leaves were challenged with a pathogenic bacterium Xanthomonas campestris pv vesicatoria (Xcv). When the leaves of the Xcv resistant cv ECW-20R were infiltrated with Xcv, the leaves became dark purple within 24 h of inoculation and subsequently became necrotic. In contrast, within 48 h after the infiltration, the susceptible cv ECW leaves were damaged severely and exhibited chlorosis (data not shown). Northern-blot analysis carried out with the CaTin1 probe revealed that the transcripts of CaTin1 gene accumulated only in ECW-20R. This suggested HR-specific induction of CaTin1 by viral and bacterial challenges (Fig. 2D). Thus, the CaTin1 expression upon biotic stresses had a tendency of earlier induction than that of well-known CaPR1 gene.
The CaTin1 Gene Expression Was Related to Ethylene but Not to SA or MeJA Signal
To examine whether CaTin1 gene can be induced by other stimuli, plants were treated with various abiotic inducers and the expression of CaTin1 gene was examined at the times indicated.
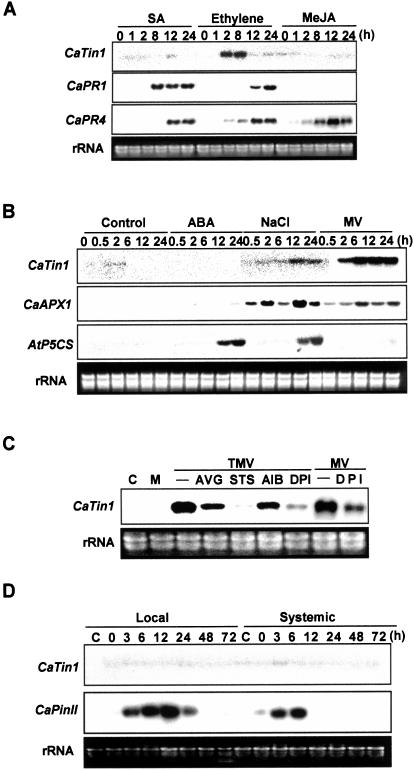
In pepper leaves sprayed with SA, the transcripts of CaTin1 gene were not detected. This is clearly different from the induction of CaPR1 and CaPR4 transcripts. Also CaTin1 gene was not induced by MeJA treatment but the CaPR4 transcripts were well induced. CaPR1 was known to be not induced by MeJA (Park et al., 2001a; Fig. 3A). On the other hand, CaTin1 gene expression started to increase 2 h after ethylene treatment, and then maintained until 8 h at similar level. Compared to the CaPR1 gene expression, the CaTin1 gene expression started very early and quickly disappeared (Fig. 3A).
Figure 3.
Expression of the CaTin1 gene in response to abiotic stresses. A, Expression patterns of CaTin1 gene upon SA, ethylene, or MeJA treatment. Hot pepper leaves were sprayed with 2 mm SA, 50 μL/L gaseous ethylene, or 10 μm MeJA and detached at time points designated. The CaPR1 and CaPR4 expression was compared as a control for treatment. B, Expression patterns of CaTin1 gene upon ABA, NaCl, and MV treatments. Hot pepper leaves were sprayed and the unrooted plants were soaked in each solution with 10 μm ABA, 250 mm NaCl, or 0.5 mm MV and detached at time points designated for total RNA extraction. CaAPX1 or AtP5CS gene was used as a control for MV, salt, or ABA treatment. C, CaTin1 gene expression in the presence of ethylene synthesis or action inhibitors and H2O2 production inhibitor. RNA was isolated from the 2 leaves that were inoculated with TMV-P0, incubated for 24 h, and then treated for an additional 24 h with water, 2 mm AIB, 1 mm AVG, or 0.5 mm STS. The unrooted plants were soaked in solution with 100 μm DPI for 4 h, were inoculated with TMV- P0 and treated with 0.5 mm MV for 24 h. C indicates the uninoculated leaves of a control plant at 0 h and M indicates the mock-inoculated leaves of control plant after 24 h. D, Expression patterns of CaTin1 gene by incision-wounding. Hot pepper leaves were incised with a pair of scissors and RNA was extracted from the incised leaves (Local) and the upper unincised leaves to detect systemic induction (Systemic). The CaPinII gene expression was compared as a control for wounding treatment. The membranes were hybridized with CaTin1-specific cDNA probe. The rRNA bands in ethidium bromide-stained gels were shown as a loading control. Experiments were performed at least three times for each treatment and the representative result was shown here.
To see whether other abiotic inducers induce the CaTin1 expression, ABA at a concentration of 10 μm, 250 mm NaCl, or 0.5 mm MV was applied to the unrooted pepper plants. MV induces H2O2 (Dodge, 1994). NaCl induced the expression of CaTin1 gene slightly. MV, on the other hand, induced the expression of CaTin1 gene significantly (Fig. 3B). In pepper plants, MV induced the expression of CaTin1 gene more efficiently than the expression of Capsicum annuum ascorbate peroxidase (CaAPX1) gene that was used as the positive control (Yoo et al., 2002).
In regard to the response to various stimuli, the expression of CaTin1 was different from previously characterized PR genes that are responsive to ethylene and MV only. We thus examined the role of ethylene and H2O2 in the induction of the CaTin1 gene expression in response to biotic stresses. The leaves of hot pepper inoculated with TMV were treated with the ethylene biosynthesis inhibitors aminoethoxyvinylglycine (AVG) and α-aminoisobutyric acid (AIB), the ethylene action inhibitor silver thiosulfate (STS), or the H2O2 production enzyme NADPH oxidase inhibitor (diphenylene iodonium, DPI) and the induction of the CaTin1 gene was examined. Treated with various ethylene synthesis inhibitors or ethylene action inhibitors, the CaTin1 gene expression in the TMV-inoculated plants was reduced. Particularly, the expression was markedly diminished in plants treated with STS (Fig. 3C). Treated with DPI, the CaTin1 gene expression induced by TMV or MV was reduced in hot pepper plants (Fig. 3C). The data showed that the induction of the CaTin1 gene expression by TMV is associated with the ethylene-dependent and/or the H2O2-dependent signal pathway.
To examine whether the expression of CaTin1 gene is induced by incision-wounding, leaves were cut randomly with a pair of scissors and their expression of CaTin1 gene was examined. Control was the untreated upper leaves of the same plant. The proteinase inhibitor II gene (CaPinII) that has been reported to be induced by local and systemic wounding was used as the positive control (Shin et al., 2001). Northern-blot analysis using the duplicate RNA samples showed that the transcripts of CaPinII were dramatically induced by local as well as by systemic incision-wounding, whereas the transcripts of CaTin1 gene was not detected (Fig. 3D).
The Altered Gene Expression and the Growth Rate in CaTin1 Transgenic Tobacco Plants
To understand the biological function of CaTin1 in planta, we generated transgenic tobacco plants harboring the sense CaTin1 gene or the anti-sense CaTin1 gene. Although CaTin1 gene is a hot pepper gene, we used the tobacco system because of the difficulty of obtaining a large number of transgenic pepper plants (Shin et al., 2002). Tobacco plants have the CaTin1 homolog whose expression pattern is similar to CaTin1 under normal conditions as well as in response to TMV or ethylene (data not shown). CaTin1 gene and its tobacco homolog showed 84% identity at the nucleotide level (data not shown). Tobacco plants were transformed with a binary vector carrying the fusion of the double 35S promoter and CaTin1 cDNA in the sense or antisense orientation by the Agrobacterium-mediated transformation. Transgenic plants were regenerated from the verified transformants and used for the analysis. The expression of CaTin1 and several other PR genes in transgenic plants was compared with control plants transformed with the empty pMBP2 vector (Han et al., 1999). All experiments of the transgenic plants were performed in triplicates using the T1 descendents of independent T0 lines that had single copy of CaTin1.
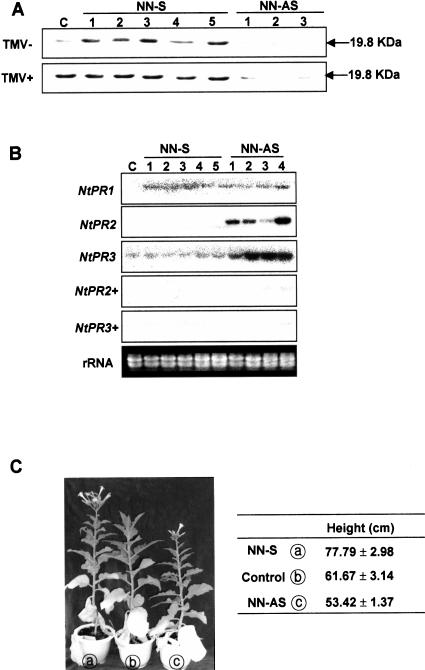
As we observed that ethylene was the key regulator of the CaTin1 gene expression, we examined specifically whether the expression of CaTin1 gene is induced by the ethylene-dependent but SA-independent signal transduction pathway. Since the expression of CaTin1 was quite fast, it was possible that this gene might be regulated earlier than other PR genes in defense signal transduction pathway. At first, we compared the level of protein expression in transgenic plants and control plants. The expression level in transgenic plants harboring the sense CaTin1 gene (sense transgenic plants) was higher than control plants. The expression level in control plants, in turn, was higher than in transgenic plants harboring the antisense gene (antisense transgenic plants). In fact, CaTin1 protein in antisense transgenic plants was barely detectable (Fig. 4A). And after TMV inoculation, CaTin1 protein level was high in sense transgenic plants but very low in antisense transgenic plants even though the plants were infected by TMV. We also investigated the expression of PR genes in these transgenic plants. NtPR1, NtPR2, and NtPR3 show 100% homology to X06361, M59442, and S44869 at NCBI database and the full-length NtPR1, the 3′ 1 kb region of NtPR2, or NtPR3 were used as probes. As shown in Figure 4B, NtPR2 and NtPR3 but not NtPR1 gene were expressed in the untreated antisense transgenic plants. Acidic NtPR1 was known to show SA-dependent gene expression pattern but basic NtPR2 and NtPR3 ethylene-dependent expression pattern. The above results suggest the expression of NtPR2 and NtPR3 might have been related to ethylene dependent CaTin1 expression change. To investigate the possible relationship of their expressions with ethylene, transgenic plants were treated with ethylene blocker (STS) and the expression of CaTin1 was examined (+ signs in Fig. 4B). The expression of NtPR2 and NtPR3 gene in the antisense transgenic plants disappeared or decreased. From this result, we speculated that the expression of NtPR2 and NtPR3 gene in the antisense transgenic plants is regulated mainly by ethylene. Interestingly, both the sense and antisense CaTin1 transgenic plants flowered earlier than control plants. Furthermore, the sense transgenic plants were taller than controls, whereas the antisense transgenic plants were shorter than control plants (Fig. 4C). The data suggest that the expression of CaTin1 may be an important factor of the ethylene signal transduction pathway.
Figure 4.
Expression pattern of genes and growth difference in sense- and antisense-oriented CaTin1 transgenic tobacco plants and control plants. A, Western-blot analysis of CaTin1 transgenic plants. Western-blot analysis using anti-rat-CaTin1 antibody (2 μg/mL) was carried out for detection of CaTin1 in transgenic plants. TMV+ means western-blot analysis performed with TMV-P0-inoculated plants and TMV-means western-blot analysis performed with no TMV- P0-inoculated plants. B, RNA gel blotting was conducted to measure the amount of NtPR1, NtPR2, or NtPR3 mRNA in empty-vector transformed control and sense- and antisense-oriented independent transgenic tobacco plants (T1 generation) when treated with ethylene blocker, STS (+), or no treatment. C, Comparison of the height of transgenic plants and control plants. a, sense transgenic plant; b, control plant; c, antisense transgenic plant. Their heights were measured from 100-d-old plants. The heights are the average of experiments performed three times, each time with 10 T1 descendents of typical transgenic line. NN-S designates the CaTin1 sense transgenic tobacco cv Samsun NN T1 plants and NN-AS designates the CaTin1 antisense transgenic tobacco cv Samsun NN T1 plants. The numbers indicate independent lines of transgenic T1 plants. C indicates control plants. The rRNA bands in ethidium bromide-stained gels were shown as a loading control. Experiments were performed at least three times for each treatment and the representative result was shown for blot analyses.
The Alteration of the HR Lesion Formation and Ion Conductivity in CaTin1 Transgenic Plants after TMV Inoculation
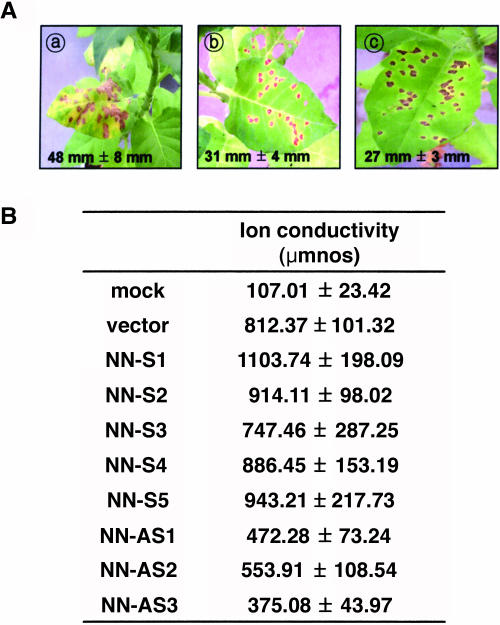
The HR response elicited by avirulent pathogens is rapid cell death. We noticed that the sense transgenic plants showed the irregular and bigger HR lesion formation after TMV inoculation. As shown in Figure 5A, 10 d after TMV inoculation, the HR lesions of the sense transgenic plants were over 40% bigger than control plants (a versus b). The lesion in the antisense plants was smaller than control (b versus c). The correlation between the cell death severity and the ion leakage level has been reported (Greenberg and Ausubel, 1993). We thus compared the ion leakage level of the transgenic plants with control plants. Two days after TMV inoculation, the ion conductivity level of the sense transgenic plants was lower than control plants (data not shown). The level in the transgenic plants was increased more rapidly than control plants. Ten days after inoculation, the ion leakage of the sense transgenic plants was slightly but consistently higher than control plants (Fig. 5B). However, the antisense transgenic plants showed lower ion conductivity than control plants, which correlates to the smaller size of the HR lesions.
Figure 5.
Comparison of HR lesion formation and ion conductivity after TMV inoculation between control plants and CaTin1 transgenic T1 plants. A, Comparison of HR lesion formation. a, Sense transgenic plant; b, control plant; c, antisense transgenic plant. Each leaf here shows typical HR lesion formation 10 d after TMV inoculation. Each number in the figures indicates the average size of 100 HR lesions' diameter in each case. B, Comparison of ion conductivity during HR lesion formation between control plants and CaTin1 transgenic T1 plants 10 d after TMV inoculation. The mean number of five experiments was shown on the table. ± indicates sd. NN-S designates the CaTin1 sense transgenic tobacco cv Samsun NN T1 plants and NN-AS designates the CaTin1 antisense transgenic tobacco cv Samsun NN T1 plants. The numbers indicate independent lines of transgenic T1 plants.
The Elevated Level of Ethylene and H2O2 in CaTin1 Transgenic Plants
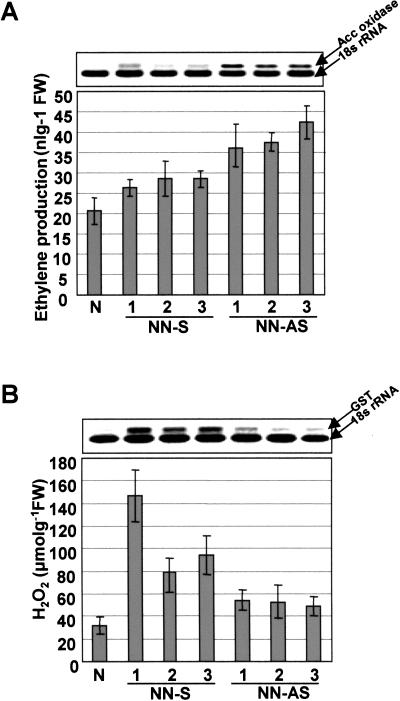
When ethylene blockers were applied to the transgenic plants, the expression of CaTin1 as well as PR genes was decreased or disappeared (Figs. 3C and 4B). In addition, the sense transgenic plants showed the significant progression of the HR lesion and increased ion conductivity in response to TMV inoculation (Fig. 5). Based on these results, we postulated that the ethylene and/or H2O2 level may be elevated in the transgenic plants. Hence, in addition to measuring the in vivo ethylene and H2O2 level directly, we evaluated the expression of 1-aminocyclopropane-1-carboxylic acid (ACC) oxidase gene and glutathione-S-transferase (GST) gene in the reverse transcription (RT)-PCR analysis. The former gene is the marker for the ethylene level and the latter gene is the marker for the H2O2 level (Fig. 6). In the antisense transgenic plants, the expression of the ACC oxidase gene and the ethylene level were higher than control plants. In the sense transgenic plants, the levels were slightly elevated (Fig. 6A). In contrast, the H2O2 level and the GST expression level were higher in the sense transgenic plants than control plants. The levels in the antisense plants were slightly higher than control plants not undergoing HR (Fig. 6B). However, the H2O2 level of TMV inoculated control plants undergoing HR was about 5 times higher than that of sense transgenic plants.
Figure 6.
Measurements of ethylene and H2O2 levels in planta in transgenic plants and control plants. A, Analysis of ACC oxidase transcripts using RT-PCR and measurement of ethylene level in planta through gas chromatography. B, Analysis of GST transcripts using RT-PCR and measurement of H2O2 level in planta. The mean number of five experiments was shown on the graph. Error bars indicate sds. 18S indicates 18S rRNA products.
CaTin1 Transgenic Plants Are Enhanced in Tolerance against Virus Infection, Drought, and Salt Treatments
Our data show that the sense as well as the antisense CaTin1 transgenic plants have the higher ethylene and H2O2 levels than control plants (Fig. 6). We thus examined whether CaTin1 sense and antisense transgenic plants showed altered tolerance against biotic and/or abiotic stresses. To determine whether CaTin1 transgenic plants showed enhanced resistance to pathogens, we inoculated control and transgenic plants with the virulent viral pathogen cucumber mosaic virus Y (CMV-Y) that infects hot pepper systemically. Uninoculated upper leaves were taken for infection analysis after 21 d of virus inoculation. As shown in Table I, resistance to CMV-Y of the sense as well as the antisense transgenic plants was increased by 19% to 73%.
Table I.
Analyses of various stress tolerance in transgenic CaTin1 tobacco plants
| Absorbance of A405a | Chlorophyll | Chlorophyll | |
|---|---|---|---|
| μg/mgb | μg/mgc | ||
| mockd | 0.42 ± 0.17 | 20.42 ± 1.42 | 20.64 ± 0.42 |
| vectoe | 1.51 ± 0.21 | 7.01 ± 1.22 | 1.58 ± 0.32 |
| NN-S1 | 0.68 ± 0.31 | 10.88 ± 1.49 | 6.49 ± 0.99 |
| NN-S2 | 1.19 ± 0.47 | 16.42 ± 2.49 | 3.88 ± 0.49 |
| NN-S3 | 0.72 ± 0.20 | 11.26 ± 2.03 | 2.87 ± 0.25 |
| NN-S4 | 0.41 ± 0.09 | 20.02 ± 1.99 | 5.71 ± 1.09 |
| NN-S5 | 0.62 ± 0.14 | 16.23 ± 2.17 | 12.03 ± 0.73 |
| NN-AS1 | 0.98 ± 0.11 | 15.84 ± 2.37 | 13.21 ± 1.24 |
| NN-AS2 | 1.27 ± 0.32 | 9.26 ± 0.94 | 5.44 ± 0.54 |
| NN-AS3 | 1.12 ± 0.13 | 16.43 ± 1.86 | 11.62 ± 0.97 |
NN-S designates the CaTin1 sense transgenic tobacco cv Samsun NN T1 plants and NN-AS the CaTin1 antisense transgenic tobacco cv Samsun NN T1 plants. The control plants indicated empty vector transgenic plants. Each number represents an independent T0 transgenic plant. Each mean number is derived from 10 combined T1 descendents of each T0 line and experiments were performed 5 times. ± indicates sd.
The absorbance of transgenic T1 plants compared to control plants was measured 21 d after inoculation with CMV-Y. Virus was detected by indirect ELISA using anti-CMV IgG (3 μg/mL).
Leaf discs from the transgenic plants carrying the CaTin1 gene in the sense and antisense orientation and the empty vector transgenic tobacco plants were floated in 6% mannitol solution for 1 week under continuous white light at 25°C and chlorophyll content (milligrams per gram fresh weight) was measured.
Leaf discs were floated in 400 mm NaCl solution for 1 week under continuous white light at 25°C and chlorophyll content (milligrams per gram fresh weight) was measured.
Nontreated control plants.
CMV, mannitol, or NaCl treated control plants.
We also examined whether the higher levels of ethylene and H2O2 in transgenic plants alter their tolerance to drought or salt. The leaf discs of transgenic tobacco plants were immersed in 6% mannitol or 400 mm NaCl for 7 d. Subsequently the chlorophyll content in transgenic tobacco and control plants was compared. As shown in Table I, the tolerance in the sense and the antisense transgenic plants to drought and salt was increased by 2.8 times and from 1.5 times to the maximum 11 times, respectively.
The data demonstrated that the resistance to various stresses in the sense transgenic plants might be caused mainly by the elevated H2O2 level. The increased resistance in the antisense transgenic plants may be due to the higher ethylene level resulting in the PR gene expression.
The GCC-Boxes and W-Box Were Important Elements for the CaTin1 Gene Expression
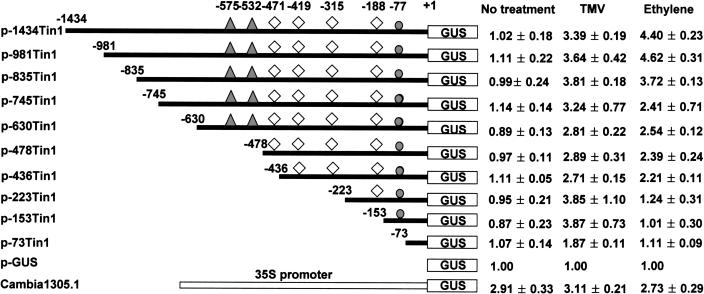
To characterize the sequences involved in the CaTin1 transcriptional regulation, we generated the deletions of the full-length promoter region of CaTin1, connected them to GUS coding gene, and produced transgenic tobacco plants. The CaTin1 promoter has four ethylene responsive GCC-boxes, two AT-rich regions known to be responsible for the root-specific expression, and an elicitor-induced WRKY transcription factor-binding W-box. The activity of each deletion construct was measured by using the leaf samples, which showed almost no expression of CaTin1 without any treatment. The activity was not detected by the histochemical GUS staining in the leaf even when the full-length promoter construct (p-1434Tin1) was used. However, constructed with the AT-rich regions, the GUS activity could be detected specifically in the apical meristem of roots without any treatment (data not shown).
When the transgenic tobacco plants of the deletion constructs of CaTin1 promoter were inoculated with TMV, they had higher GUS activity compared to the promoterless GUS construct as long as they had a W-box. In contrast, the GUS activity of the deletion promoters treated with ethylene was dependent on the GCC-boxes as expected. The construct with one GCC-box showed weak activity. The constructs with three or four GCC-boxes showed strong activity (Fig. 7).
Figure 7.
The deletion constructs of CaTin1 promoter and their GUS activities analysis upon TMV and ethylene treatments. Deletion analysis of the CaTin1 promoter fused to the promoterless GUS in Cambia 1305.1 vector (p-Gus) was carried out. The numbers of each construct indicate the distance from the CaTin1 transcription start site. Diamonds indicate the GCC-box, a circle indicates W-box, and triangles indicate AT-rich region. For each construct 10 independent transgenic lines were investigated. Average value of GUS activities (pmol methylumbelliferone/min/mg protein) resulted from five independent experiments were shown. We normalized the activities of deletion constructs results by comparing those to the activity of p-GUS construct of which activity adjusted to 1.00.
DISCUSSION
The expression of CaTin1 gene containing the PLAT domain is induced by avirulent pathogens, ethylene, NaCl, and MV. SA or MeJA is ineffective. Many genes containing the PLAT domain, such as lipoxygenase and lipase, have other functional domains in addition to the PLAT domain. CaTin1 and its homologs of other plants have only PLAT domain (Fig. 1). Although PKD1 and PKD2 appear to form homo- and heterodimer with each other (Tsiokas et al., 1997), we found that CaTin1 does not form homodimers based on the yeast-two-hybrid interaction experiment (data not shown). CaTin1 gene may be different from other known genes containing the PLAT domain in terms of not having other functional domain such as the lipoxygenase domain of lipoxygenase gene. However, our results together with previous reports suggest that the function of CaTin1 gene may be to help the communication between the ethylene signal pathway and the abiotic/biotic stress-related pathway through the protein-protein or protein-lipid interaction in a Ca2+ dependent fashion (Gillmor et al., 1997; Tsiokas et al., 1997; Bateman and Sanford, 1998).
Several studies confirmed the importance of SA for the establishment of disease resistance (Dempsey et al., 1999). Plants with the defect in the SA-dependent response show the enhanced susceptibility to fungus. SA, however, is not required for the defense against all pathogens. The plant hormones MeJA and ethylene are implicated as mediators in many physiological processes. MeJA and ethylene coregulate a subset of PR genes PR2, PR3, and PR12 in Arabidopsis that encode antimicrobial proteins (Penninckx et al., 1998; Thomma et al., 1998). For the expression of PR12 (PDF1.2), the concomitant activation of both the MeJA and ethylene response pathway is required. Although the mechanism of the regulation of PR2, PR3, and PR12 by MeJA and ethylene is still obscure, the regulation is clearly distinct from that of SA-dependent PR genes such as PR1 and PR5 (Thomma et al., 1998). CaTin1 is induced only by ethylene and not by SA and MeJA (Fig. 3A). In many cases, the SA-dependent and the SA-independent signals of disease response were divided. The SA-independent signal of disease response was not divided between MeJA-specific signal and ethylene-specific signal, however (Thomma et al., 2001). The ethylene signal pathway of fruit development or biosynthesis is relatively well characterized (Bleecker, 1999). In contrast, the ethylene dependent signaling of disease response is not well characterized. The expression of CaTin1 was specific to ethylene signal judging from ethylene synthesis or the action inhibitor treatment experiments (Figs. 3C and 4B), in addition to the CaTin1 induction result upon ethylene treatment, but not SA or MeJA treatment. The CaTin1 expression was also related to the incompatible plant-pathogen interaction (Fig. 2, B and D). Thus, CaTin1 gene seemed to be related to the SA/MeJA independent and the ethylene dependent defense signal transduction.
The expression of CaTin1 was induced during SAR (Fig. 2C). SAR induction was known to require the signal molecule SA. The removal of SA in transgenic plants expressing salicylate hydroxylase (nahG) prevents the establishment of SAR (Gaffney et al., 1993). Lawton et al. (1994) reported that the pathogen-induced SAR is ethylene independent. Similarly, many reports have shown that the low expression level of genes and proteins are regulated by ethylene during SAR (Brederode et al., 1991; Ward et al., 1991; Cordelier et al., 2003). However, SA-independent but ethylene-dependent CaTin1 was induced during SAR (Fig. 2C). Furthermore, CaTin1 transgenic plants showed tolerance at systemic leaves after CMV inoculation (Table I). A recent report has shown convincingly that ROI generated during HR may serve as signals mediating the SAR (Alvarez et al., 1998). The systemic expression of CaTin1 showed the possibility that the SA-independent SAR mechanism exists in plants. As shown in Figure 6, the sense transgenic plants showed the higher H2O2 level. The antisense transgenic plants showed the higher ethylene level than control plants. Plants may have to maintain the optimal CaTin1 level. Once its homeostasis is broken as in the case of transgenic plants, many changes including the elevated ethylene or H2O2 levels may occur.
The promoter of CaTin1 has bidirectional activity. In addition, another homologous gene (CaTin1-2) is located in the front of CaTin1 in the head-to-head fashion. The expression of CaTin1 and CaTin1-2 in response to several elicitors was very similar except for the slightly different induction time upon TMV challenge (Shin et al., 2003). Even though the CaTin1 and its homolog CaTin1-2 are closely located in hot pepper, the homologs of CaTin1 in Arabidopsis are located at different chromosomes (2 and 4). Furthermore, so far only one CaTin1 homolog of tobacco has been reported at the National Center for Biotechnology Information. It is not known how the homologs of CaTin1 are regulated in other plants. The promoter of CaTin1 may finely tune up the expression of CaTin1 and CaTin1-2 for keeping their homeostasis in hot pepper.
CaTin1 antisense transgenic tobacco plants expressed some basic PR genes constitutively, especially NtPR2 and NtPR3, without any treatment (Fig. 4B). As mentioned earlier, NtPR2 and NtPR3 are SA-independent PR genes. On the other hand, NtPR1 gene belonging to SA-dependent PR genes was not expressed in these transgenic plants under the same conditions. The CaTin1 protein expression level in the sense transgenic plants was higher than the antisense transgenic plants (Fig. 4A). The CaTin1 protein level homeogenesis in planta may be important. If CaTin1 is more underexpressed than the normal biological level, plants should have to make CaTin1 and the ethylene level may be increased for producing CaTin1. Moreover, because the CaTin1 level seems to be important in planta, the promoter of CaTin1 should regulate its homolog in hot pepper at the same time (Shin et al., 2003). Thus, to supply proper amount of CaTin1, the CaTin1 antisense transgenic plants may continuously make the CaTin1 transcripts. Because of the antisense transcripts, however, they could be unable to make CaTin1 protein resulting in the higher ethylene level continuously in vivo (Figs. 4A and 6A). The antisense transgenic plants express the ethylene-related PR genes due to the higher ethylene level. The ethylene blockers suppress the PR gene expression. This confirms that the PR gene expression was mainly due to the increased ethylene level in the antisense transgenic plants (Fig. 4B).
The CaTin1 sense transgenic plants were taller than control plants, whereas the antisense plants were shorter than control plants (Fig. 4C). Both the sense and the antisense transgenic plants flowered earlier than control plants. Although the CaTin1 sense transgenic plants grew more rapidly than control plants, the number of internodes was not changed (data not shown). After TMV inoculation, the sense transgenic plants showed bigger, and the antisense plants smaller, HR lesion than control plants (Fig. 5A). Furthermore, 10 d after the inoculation, ion conductivity was higher in the sense transgenic plants than control plants (Fig. 5B). Bent et al. (1992) reported that ethylene controls the amplitude and development of disease symptom after inoculation with virulent pathogen. Overmyer et al. (2000) reported that ethylene acted as a promoting factor during the propagation phase of oxygen radical-dependent lesion development, whereas MeJA plays a role in the lesion containments. In addition, when HR is generated, the activation of ion fluxes and the production of H2O2 are the initial response detected in plant cells. Biochemical evidences suggest that the processes that occur prior to the transcriptional activation of defense-related genes appear to be mediated through the regulation of plasma-membrane-bound enzymes. These include changes in Ca2+-ATPase and H+-ATPase activation of plasma membrane-bound ion channels (Blumwald et al., 1998). As shown in Figure 5, the changes of the size of the HR lesion and ion conductivity may have been due to ethylene as well as the ROI changes. The data demonstrated that CaTin1 may be affected by the level of ethylene and H2O2. Hence, the CaTin1 transgenic plants may mimic the state of being affected in the signal pathway involving ethylene and H2O2 action. Transgenic tobacco plants of reduced catalase showed that although PR-1 protein was not accumulated in these plants, their ion leakage was increased during the HR (Mittler et al., 1999). Transgenic tobacco deficient in the H2O2-removing enzyme catalase showed the enhanced tolerance to pathogens and the rapid activation of the defense system (Chamnongpol et al., 1998). Pellinen et al. (2002) reported that during the progression of HR lesion formation, H2O2 was detected not only in the area with the undetectable damage but also the necrotic area, so an internal signal that was positively regulated by H2O2 induced systemic oxidative burst. Levine et al. (1994) showed that H2O2 could play a dual role in the plant defense response depending on its concentration. At high concentrations, it triggers HR, whereas at low concentrations it functions as a diffusible signal that induces cellular protecting genes involved in the blocking of the oxidant-mediated cell death. CaTin1 sense transgenic plants also seem to show the rapid strong activation of some defense signals because of the elevated H2O2 level even though this activation could not induce the expression of PR genes resulting in CaTin1 sense transgenic plant showing the enhanced stress tolerance (Table I). Figures 4B and 6A show that the increase of ethylene by at least 2-fold may be required to induce PR genes. The slight ethylene increase may not induce the PR gene expression in the sense transgenic plants. The transgenic plants may have experienced some cellular and biochemical changes especially in the ethylene signal pathway(s). Several PR genes may be expressed probably due to the increased ethylene level in the antisense transgenic plants. Due to the elevated ethylene level and/or H2O2 level and the expression of PR genes, both the sense and the antisense plants may show the biotic and abiotic stress tolerance (Table I).
Previous studies show that WRKY transcription factors and their downstream genes such as NPR1 are associated with the pathogen- and SA-induced pathway (Chen and Chen, 2000; Du and Chen, 2000; Kirsch et al., 2001; Yu et al., 2001). Although the promoter of CaTin1 has a W-box, CaTin1 was not related to the SA-dependent signal pathway. Further studies of finding any WRKY transcription factor that binds to this W-box may elucidate the function of W-box more conclusively. The GCC-box has been found in the promoters of various elicitor-responsive genes, such as genes encoding the basic-type pathogenesis proteins PR2, PR3, and the osmotin PR5 (Eval et al., 1993; Ohme-Takagi and Shinshi, 1995). Two GCC-boxes in the osmotin promoter are required, but not sufficient, for the maximal ethylene responsiveness (Xu and Heath, 1998). The GCC-boxes in CaTin1 promoter are important key elements for the response to ethylene, similarly to other PR genes. Other PR genes have two GCC-boxes on the average (Xu et al., 1998), whereas the promoter of CaTin1 has four GCC-boxes. This suggests that the CaTin1 expression is more dependent on ethylene than other PR genes. We have observed that CaTin1 gene is expressed only in roots under normal condition and the promoter seems to be responsible for root specific expression under normal condition. The reports describing the regulation of the expression of genes in roots are scarce. The cis-acting sequences regulating the root-specific expression have not been identified. Furthermore, only a few root specific promoters were described and whose activity was rather restricted to some parts of the root (Nitz et al., 2001). The known cis-acting sequences specific to roots are ocs-elements (Ellis et al., 1987), as-1-elements (Lam et al., 1989), and AT-rich region (Reisdorf-Cren et al., 2002). CaTin1 promoter has two AT-rich regions, which may be related to the root specific expression of CaTin1.
The CaTin1 gene expression was regulated by ethylene and the ethylene level seemed to be controlled by the CaTin1 expression level. We propose that the perturbation of the CaTin1 expression alters the response of plants to ethylene and interferes with the redox homeostasis.
MATERIALS AND METHODS
Plant Cultivation and Pathogen Inoculation
Hot pepper (Capsicum annuum) L. cv Bugang that is susceptible to the P1.2 pathotype of TMV but resistant to the P0 pathotype was used. Plants were grown in a growth chamber or greenhouse at 25°C with the photoperiod cycle of 16 h light and 8 h dark. Healthy and well-expanded leaves of 2-month-old plants were used. TMV-P0 and TMV-P1.2 strains were maintained in the leaves of tobacco (Nicotiana tabacum cv Burley 21) in petri dishes containing CaCl2. The leaf sap of TMV-P0 or TMV-P1.2 was prepared by grinding infected leaves in 0.25 m phosphate buffer containing 5 mm EDTA (pH 7.4). To inoculate plants, virus-containing sap was applied to the surface of 4 to 5 fully expanded leaves of hot pepper plants and rubbed with carborundum (Hayashi Chemical, Osaka). Mock-inoculated plants were rubbed with phosphate buffer and carborundum only. To assess systemic responses, 2 lower leaves were inoculated with TMV-P0 sap and the upper leaves were harvested at 0, 3, 6, 9, and 15 d after inoculation. Two cultivars of hot pepper, Early Calwonder (ECW; bs1/bs1, bs2/bs2, and bs3/bs3) and Early Calwonder-20R (ECW-20R; bs1/bs1, Bs2/Bs2, and bs3/bs3) were used for infiltration with Xcv (avrBs2). Xcv was cultured in the medium containing yeast extract, dextrose, and calcium carbonate. Cell suspensions (1 × 108 cells/mL) were infiltrated into leaves, and RNA was extracted from the infiltrated leaves.
Treatments with the Inhibitors of Ethylene Synthesis or Its Action, the Inhibitors of H2O2 Synthesis, and Other Chemicals
Pepper leaves were sprayed with 5 mm SA or 10 μm MeJA solution. Control plants were sprayed with distilled water. Leaves thus treated were harvested at the times indicated, quickly frozen in liquid nitrogen, and stored at −80°C. For the treatments of abscisic acid (ABA), NaCl, or MV, unrooted pepper plants were placed in Falcon tubes filled with the chemicals or water for various durations and frozen in liquid nitrogen. The ethylene treatment was performed by keeping pepper plants in a chamber equilibrated with 50 μL/L gaseous ethylene for the durations indicated. Ethylene concentration was determined by gas chromatography. Control plants were kept in a chamber without ethylene. For the wound treatment, leaves were cut randomly with a pair of scissors. RNA was extracted from the wound-treated leaves and the nontreated upper leaves of the same plant at various times after wounding. CaPR1 was used as a control for HR, SA, or ethylene treatment. CaPR4 was used as a control for MeJA treatment. CaAPX1 was used as a control for MV treatment. Arabidopsis pyrroline-5-carboxylate synthase (AtP5CS) was used as a control for salt or ABA treatment. CaPinII was used as a control for wound treatment (Kasuga et al., 1999; Shin et al., 2001).
Two leaves from hot pepper plants were inoculated with 1 μg/mL TMV. After 24 h incubation at 22°C, the infected leaves from 2 plants were injected with either water, 2 mm AIB, 1 mm AVG, or 0.5 mm STS. Immediately following the injection, the treated leaves were detached and immersed in the corresponding solution. The unrooted plants were soaked in 100 μm DPI solution for 4 h and were inoculated with TMV- P0 or was treated with 0.5 mm MV for 24 h (Orozco-Cárdenas et al., 2001).
After 24 h incubation in various solutions, the leaves were harvested and used for RNA analysis (Guo et al., 2000).
DNA, RNA, RT-PCR, and Protein Analyses
Genomic DNA and RNA were isolated from the pepper leaves by the method of Ausubel et al. (1995). Southern- and RNA-blot hybridizations were carried out as described by Shin et al. (2001). For RT-PCR, the total RNA (5 μg) was reverse-transcribed in the total volume of 20 μL according to Sambrook et al. (1989). One microliter of the 1:10 diluted RT reaction product was used as a template in 40 μL PCR premix (Bioneer, Taejun, Korea) with the sense primer (GST; 5′-ttggtcaagtaccagcatttg, ACC oxidase; 5′-agtggagaaaatgacaaagg), the antisense primer (GST; 5′-ttgtactcatcaagtaatatat, ACC oxidase; 5′-ccaaggttaaccacaatagaga) or QuantumRNA Universal 18S (Ambion, Austin, TX). The condition of RT-PCR was 94°C for 2 min, 25 cycles of 94°C for 30 s, 55°C for 30 s, 72°C for 30 s, and 72°C for 5 min. After RT-PCR, the products were cloned into pGEM Easy Vector (Promega, Madison, WI) and sequenced for their confirmation.
Indirect ELISA was used for the detection of CMV-Y using anti-rabbit-CMV-Y antibody (3 μg/mL). For the detection of CaTin1 in transgenic plants, western-blot analysis using anti-rat-CaTin1 antibody (2 μg/mL) was performed.
Analysis of CaTin1 Transgenic Plants
Plasmids used in the transformation of tobacco were prepared with the sense and antisense orientation of the full-length CaTin1 cDNA cloned into the polylinker site of the binary vector pMBP2 (Han et al., 1999). The constructs were introduced into tobacco cv Samsun NN using Agrobacterium-mediated transformation as described (Hoekema et al., 1983).
To investigate the tolerance to the salt and drought stress, healthy and fully expanded leaves of wild-type and transgenic plants were detached and washed briefly in distilled water. The 1-cm diameter leaf disc was cut and immersed in Murashige and Skoog medium containing 400 mm NaCl or 6% mannitol for 7 d (Veena and Sopory, 1999). The treatment was performed in the continuous white light at 25°C. The chlorophyll content was measured as described by Aono et al. (1993).
Ethylene, H2O2, and Ion Conductivity Measurements
For the measurement of ethylene evaporation, 3 to 5 plants per transgenic T1 line were analyzed. Plants were kept in a sealed test bottle for 12 h. Each 1-mL gas sample was withdrawn from the bottle with a hypodermic syringe and ethylene was assayed on a gas chromatography equipped with an aluminum column and flame ionization detector (Yi et al., 1999). For H2O2 measurements, frozen leaves (1 g) were ground to powder in liquid nitrogen, homogenized, and H2O2 was measured as described by Rao et al. (2000).
The ion leakage was measured with the Orion ion conductivity meter (model 150; Beverly, MA). Leaves of 2-month-old plants were inoculated with TMV. 10 d later, leaf discs obtained by punching with a number 4 cork borer (diameter 7.5 mm) were immersed in 2 mL of distilled water with the abaxial side down. After 2 h immersion at room temperature, the conductivity of the bathing solution was measured (Greenberg and Ausubel, 1993).
The Generation and the Analysis of the Promoter Region of CaTin1
By using the CaTin1 gene specific primer 1 (5′-AGCCTGAAATAGAAGAAACGGAGATGGAGATGAGA-3′) and 2 (5′-GGAACCAGAATTGGTTACTCATGGCTACCTGAAC-3′), CaTin1 promoter region was obtained from the adaptor-ligated genomic DNA fragments in GenomeWalker libraries according to the manufacturer's instructions (CLONTECH, Palo Alto, CA). The deletion fragments of CaTin1 promoter were generated by PCR using the primers corresponding to serial 100-bp deletions and containing the EcoRI restriction site (forward primers) and the NcoI site (reverse primer). Amplified products were cloned into pGEM Easy Vector (Promega, Madison, WI), digested with EcoRI and NcoI, and cloned into pCambia 1305.1 (CAMBIA, Canberra, Australia) that contains a gus reporter gene and the hygromycin selectable marker flanked by T-DNA border sequence. The constructs were introduced into tobacco cv Samsun NN using Agrobacterium-mediated transformation.
For the GUS activity assay, 112.5 μL 4-methylunbelliferyl β-d-glucuronide solution in a microtube was preincubated in a water bath for 5 min at 37°C. Then, 75 μL of cell extract in GUS/ luciferase buffer (0.1 m KPO4, pH 7.8, 2 mm Na2-EDTA, 2 mm dithiothreitol, and 5% glycerol) was added and incubated at 37°C. After 30 min or 60 min, 50 μL was removed from each assay tube and transferred to a test tube containing 1 mL stop solution (0.2 m Na2CO3). The progress of the reactions was quantitatively assessed under the emission wavelength of 455 nm. The standard curve was generated by determining the fluorescence of various concentrations of 4-methylumbelliferone standards ranged from 0 to 10 μm (Maliga et al., 1995).
Sequence data from this article have been deposited with the EMBL/GenBank data libraries under accession numbers AF242731 for CaTin1 and AF480414 for CaTin1-2.
This work was supported by a grant (CG1223) from the Crop Functional Genomics Center of the 21st Century Frontier Research Program funded by the Korea Ministry of Science and Technology, a Biogreen grant from the Rural Development Administration, and by a grant from the Plant Signaling Network Research Center of Korea Science and Engineering Foundation.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.103.035436.
References
- Alvarez ME, Pennell RI, Meijer PJ, Ishikawa A, Dixon RA, Lamb C (1998) Reactive oxygen intermediates mediate a systemic signal network in the establishment of plant immunity. Cell 92: 773–784 [DOI] [PubMed] [Google Scholar]
- Aono M, Kubo A, Saji H, Tanaka K, Kondo N (1993) Enhanced tolerance to photooxidative stress of transgenic Nicotiana tabacum with high chloroplastic glutathione reductase activity. Plant Cell Physiol 34: 129–136 [Google Scholar]
- Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman G, Smith JA, Struhl K (1995) Short Protocols in Molecular Biology. John Wiley & Sons, NY
- Bateman A, Sanford R (1998) The PLAT domain: a new piece in the PKD1 puzzle. Curr Biol 9: 588–590 [DOI] [PubMed] [Google Scholar]
- Bent AF, Innes RW, Ecker JR, Staskawicz B (1992) Disease development in ethylene-insensitive Arabidopsis thaliana infected with virulence and avirulence Pseudomonas and Xanthomoas pathogens. Mol Plant Microbe Interact 5: 372–378 [DOI] [PubMed] [Google Scholar]
- Bleecker AB (1999) Ethylene perception and signaling: an evolutionary perspective. Trends Plant Sci 4: 269–274 [DOI] [PubMed] [Google Scholar]
- Bleecker AB, Kende H (2000) Ethylene: a gaseous signal molecule in plants. Annu Rev Cell Dev Biol 16: 1–18 [DOI] [PubMed] [Google Scholar]
- Blumwald E, Gilad S, Lam BCH (1998) Early signal transduction pathways in plant-pathogen interaction. Trends Plant Sci 3: 342–346 [Google Scholar]
- Brederode FT, Linthorst JM, Bol JF (1991) Differential induction of acquired resistance and PR gene expression in tobacco by virus infection, ethophon treatment, UV light and wounding. Plant Mol Biol 17: 1117–1125 [DOI] [PubMed] [Google Scholar]
- Chamnongpol S, Willekens H, Moeder W, Langebartels C, Sandermann H, Van Montagu M, Inzé D, Van Camp W (1998) Defense activation and enhanced pathogen tolerance induced by H2O2 in transgenic tobacco. Proc Natl Acad Sci USA 95: 5818–5823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Chen Z (2000) Isolation and characterization of two pathogen- and salicylic acid-induced genes encoding WRKY DNA-binding proteins from tobacco. Plant Mol Biol 42: 387–396 [DOI] [PubMed] [Google Scholar]
- Cordelier S, de Ruffray P, Fritig B, Kauffmann S (2003) Biological and molecular comparison between localized and systemic acquired resistance induced in tobacco by Phytophthora megasperma glycoprotein elicitin. Plant Mol Biol 51: 109–118 [DOI] [PubMed] [Google Scholar]
- Dempsey DA, Shah J, Klessig DF (1999) Salicylic acid and disease resistance in plants. Crit Rev Plant Sci 18: 547–575 [Google Scholar]
- Dodge AD (1994) Herbicide action and effects in detoxification processes. In CH Foyer and DM Mullineaux, eds, Causes of Photooxidative Stress and Amelioration of Defense Systems in Plants. CRC Press, Boca Raton, FL, pp 219–236
- Du L, Chen Z (2000) Identification of genes encoding receptor-like protein kinases as possible targets of pathogen- and salicylic acid-induced WRKY DNA-binding proteins in Arabidopsis. Plant J 24: 837–847 [DOI] [PubMed] [Google Scholar]
- Ebel J, Scheel D (1997) Signals in host-parasite interactions. In GC Carroll and P Tudzynski, eds, The Mycota, Vol. 5, Plant Relationships Part A. Springer-Verlag, Heidelberg, pp 85–105
- Ellis JG, Llewellyn DJ, Walker JC, Dennis ES, Peacock WJ (1987) The ocs-element: a 16 bp palindrome essential for activity of the octopine synthase enhancer. EMBO J 6: 3203–3208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eval Y, Meller Y, Lev-Yadun S, Fluhr R (1993) A basic-type PR-1 promoter directs ethylene responsiveness, vascular and abscission zone-specific expression. Plant J 4: 225–234 [DOI] [PubMed] [Google Scholar]
- Gaffney J, Friedrich L, Vemooij B, Negrotto D, Hye G, Uknes S, Ward E, Kessmann H, Ryals J (1993) Requirement of salicylic acid for the induction of systemic acquired resistance. Science 261: 754–756 [DOI] [PubMed] [Google Scholar]
- Gallardo M, Gomez-Jimenez MC, Matilla A (1999) Involvement of calcium in ACC-oxidase activity from Cicer arietinum seed embryonic axes. Phytochemistry 50: 373–376 [DOI] [PubMed] [Google Scholar]
- Gillmor SA, Villasenor A, Fletterick R, Sigal E, Browner MF (1997) The structure of mammalian 15-lipoxygenase reveals similarity to the lipases and the determinants of substrate specificity. Nat Struct Biol 4: 1003–1009 [DOI] [PubMed] [Google Scholar]
- Greenberg JT, Ausubel FM (1993) Arabidopsis mutants compromised for the control of cellular damage during pathogenesis and aging. Plant J 4: 327–341 [DOI] [PubMed] [Google Scholar]
- Guo A, Salih G, Klessig DF (2000) Activation of a diverse set of genes during the tobacco resistance to TMV is independent of salicylic acid; induction of a subset is also ethylene independent. Plant J 21: 409–418 [DOI] [PubMed] [Google Scholar]
- Hammond-Kosack KE, Jones JDG (1996) Resistance gene-dependent plant defense responses. Plant Cell 8: 1773–1791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S-J, Cho HY, You J-S, Nam Y-W, Park EK, Shin J-S, Park YI, Park WM, Paek K-H (1999) Gene silencing-mediated resistance in transgenic tobacco plants carrying potato virus Y coat protein gene. Mol Cells 9: 376–383 [PubMed] [Google Scholar]
- He C-J, Morgan PW, Drew MC (1996) Transduction of an ethylene signal is required for cell death and lysis in the root cortex of maize during aerenchyma formation induced by hypoxia. Plant Physiol 112: 463–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins VJ, Lu H, Xing T, Gellie A, Blumwald E (1998) The gene-for-gene concept and beyond: interactions and signals. Can J Plant Pathol 20: 150–157 [Google Scholar]
- Hoekema A, Hirsch P, Hooykaas PJJ, Schilperoort R (1983) A binary plant vector strategy based on separation of vir- and T-region of the Agrobacterium tumefaciens Ti-plasmid. Nature 303: 179–180 [Google Scholar]
- Hong Y, Wang T-W, Hudak KA, Schade F, Froese CD, Thompson JE (2000) An ethylene-induced cDNA encoding a lipase expressed at the onset of senescence. Proc Natl Acad Sci USA 97: 8712–8722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PR, Ecker JR (1998) The ethylene gas signal transduction pathway: a molecular perspective. Annu Rev Genet 32: 227–254 [DOI] [PubMed] [Google Scholar]
- Kasuga M, Liu Q, Miura S, Yamaguchi-Shinozaki K, Shinozaki K (1999) Improving plant drought, salt and freezing tolerance by gene transfer of a single stress-inducible transcription factor. Nat Biotechnol 17: 287–291 [DOI] [PubMed] [Google Scholar]
- Kirsch C, Logemann E, Lippok B, Schmelzer E, Hahlbrock KA (2001) A highly specific pathogen-responsive promoter element from the immediate-early activated CMPG1 gene in Petroselinum crispum. Plant J 26: 217–227 [DOI] [PubMed] [Google Scholar]
- Kwak SH, Lee SH (1997) The requirements for Ca2+, protein phosphorylation, and dephosphorylation for ethylene signal transduction in Pisum sativum L. Plant Cell Physiol 38: 1142–1149 [DOI] [PubMed] [Google Scholar]
- Lam E, Benfey PN, Gilmartin PM, Fang R-X, Chua NH (1989) Site specific mutations alter in vitro factor binding and change promoter expression pattern in transgenic plants. Proc Natl Acad Sci USA 86: 7890–7894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb CJ, Dixon RA (1997) The oxidative burst in plant disease resistance. Annu Rev Plant Physiol Plant Mol Biol 76: 419–422 [DOI] [PubMed] [Google Scholar]
- Laudert D, Weiler EW (1998) Allene oxide synthase: a major control point in Arabidopsis thaliana octadecanoid signaling. Plant J 15: 675–684 [DOI] [PubMed] [Google Scholar]
- Lawton K, Pottter SC, Friedrich L, Vernooij B, Uknes S, Ryals J (1994) Acquired resistance signal transduction in Arabidopsis is ethylene independent. Plant Cell 6: 581–588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leubner-Metzger G, Petruzzelli L, Waldvogel R, Vögeli-Lange R, Meins F (1998) Ethylene-responsive element binding protein (EREBP) expression and the transcriptional regulation of class I beta-1,3-glucanase during tobacco seed germination. Plant Mol Biol 38: 785–795 [DOI] [PubMed] [Google Scholar]
- Levine A, Tenhaken R, Dixon R, Lamb C (1994) H2O2 from the oxidative burst orchestrates the plant hypersensitive disease resistance response. Cell 79: 583–593 [DOI] [PubMed] [Google Scholar]
- Levine A, Pennell RI, Alvarex ME, Palmer R, Lamb C (1996) Calcium-mediated apoptosis in a plant hypersensitive disease of plants. Curr Biol 6: 427–437 [DOI] [PubMed] [Google Scholar]
- Maliga P, Klessig DF, Cashnore AR, Gruissem W, Varner JE (1995) Methods in Plant Molecular Biology: A Laboratory Course Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, pp 29–39
- Mittler R, Herr EH, Orvar BL, van Camp W, Willekens H, Inzé D, Ellis BE (1999) Transgenic tobacco plants with reduced capability to detoxify reactive oxygen intermediates are hyperresponsive to pathogen infection. Proc Natl Acad Sci USA 96: 14165–14170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore T, Martineau B, Bostock RM, Lincoln JE, Gilchrist DG (2000) Molecular and genetic characterization of ethylene involvement in mycotoxin-induced plant cell death. Physiol Mol Plant Pathol 54: 73–85 [Google Scholar]
- Naylor CE, Eaton JT, Howells A, Justin N, Moss DS, Titball RW, Basak AK (1998) Structure of the key toxin in gas gangrene. Nat Struct Biol 5: 738–746 [DOI] [PubMed] [Google Scholar]
- Nitz I, Berkefeld H, Puzio PS, Grundler FMW (2001) Pyk10, a seedling and root specific gene and promoter from Arabidopsis thaliana. Plant Sci 161: 337–346 [DOI] [PubMed] [Google Scholar]
- Ohme-Takagi M, Shinshi H (1995) Ethylene-inducible DNA binding proteins that interact with an ethylene-responsive element. Plant Cell 7: 173–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orozco-Cárdenas ML, Narváez-Vásquez J, Ryan CA (2001) Hydrogen peroxide acts as a second messenger for the induction of defense genes in tomato plants in response to wounding, systemin, and methyl jasmonate. Plant Cell 13: 179–191 [PMC free article] [PubMed] [Google Scholar]
- Overmyer K, Tuomainen H, Kettunen R, Betz C, Langebartels C, Sandermann H, Kangasjärvi J (2000) Ozone-sensitive Arabidopsis rcd1 mutant reveals opposite roles for ethylene and jasmonate signaling pathways in regulating superoxide-dependent cell death. Plant Cell 12: 1849–1862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park C-J, Shin R, Park JM, Lee G-J, Yoo TH, Paek K-H (2001. a) A hot pepper cDNA encoding a pathogenesis-related protein 4 is induced during the resistance response to tobacco mosaic virus. Mol Cells 11: 122–127 [PubMed] [Google Scholar]
- Park JM, Park C-J, Lee S-B, Ham B-K, Shin R, Paek K-H (2001. b) Overexpression of the tobacco Tsi1 gene encoding an EREBP/AP2-type transcription factor enhances resistance against pathogen attack and osmotic stress in tobacco. Plant Cell 13: 1035–1046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pell E, Schlagnhaufe CD, Arteca RN (1997) Ozone-induced oxidative stress: mechanisms of action and reaction. Physiol Plant 100: 264–273 [Google Scholar]
- Pellinen RI, Korhonen M-S, Tauriainen AA, Palva TP, Kangasjärvi J (2002) Hydrogen peroxide activates cell death and defense gene expression in birch. Plant Physiol 130: 549–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penninckx IAMA, Thomma BPHJ, Buchala A, Metraux J-P, Broekaert WF (1998) Concomitant activation of jasmonate and ethylene response pathways is required for induction of a plant defensin gene in Arabidopsis. Plant Cell 10: 2103–2113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao MV, Lee H-I, Creelman RA, Mullet JE, Davis KR (2000) Jasmonic acid signaling modulates ozone-induced hypersensitive cell death. Plant Cell 12: 1633–1646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz V, Fluhr R (1992) Calcium requirement for ethylene-dependent responses. Plant Cell 4: 1123–1130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz V, Fluhr R (1993) Ethylene signal is transduced via protein phosphorylation events in plants. Plant Cell 5: 523–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisdorf-Cren M, Carrayol E, Terce-Laforgue T, Hirel B (2002) A novel HMG A-like protein binds differentially to the AT-rich regions located in the far distal and proximal parts of a soybean glutamine synthetase gene (GS15) promoter. Plant Cell Physiol 43: 1006–1016 [DOI] [PubMed] [Google Scholar]
- Rushton PJ, Torees JT, Parniske M, Wernert P, Hahlbrock K, Somissch IE (1996) Interaction of elicitor-induced DNA-binding proteins with elicitor response elements in the promoters of parsley PR1 genes. EMBO J 20: 5690–5700 [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T (1989) Molecular Cloning. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY
- Shin R, Han J-H, Lee G-J, Paek K-H (2002) The potential use of a viral coat protein gene as a transgene screening marker and multiple virus resistance of pepper plants coexpressing coat proteins of cucumber mosaic virus and tomato mosaic virus. Transgenic Res 11: 215–219 [DOI] [PubMed] [Google Scholar]
- Shin R, Kim MJ, Paek K-H (2003) The CaTin1 (Capsicum annuum TMV-induced clone 1) and CaTin1-2 genes are linked head-to-head and share a bidirectional promoter. Plant Cell Physiol 44: 549–554 [DOI] [PubMed] [Google Scholar]
- Shin R, Lee G-J, Park C-J, Kim T-Y, You J-S, Nam Y-W, Paek K-H (2001) Isolation of pepper mRNAs differentially expressed during the hypersensitive response to tobacco mosaic virus and characterization of a proteinase inhibitor gene. Plant Sci 161: 727–737 [Google Scholar]
- Sticher L, Mauch-Mani B, Metraux JP (1997) Systemic acquired resistance. Annu Rev Phytopathol 35: 235–270 [DOI] [PubMed] [Google Scholar]
- Thomma BPHJ, Eddermont K, Pennickx IAMA, Mauch-Mani B, Broekaert WF, Cammue BPA (2001) The complexity of disease signaling in Arabidopsis. Curr Opin Plant Biol 13: 63–68 [DOI] [PubMed] [Google Scholar]
- Thomma BPHJ, Eddermont K, Pennickx IAMA, Mauch-Mani B, Vodelsang R, Cammue BPA, Broekaert WF (1998) Separate jasmonate-dependent and salicylate-dependent defense response pathways in Arabidopsis are essential for resistance to distinct microbial pathogens. Proc Natl Acad Sci USA 95: 15107–15111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsiokas L, Kim E, Arnould T, Sukhatme VP, Walz G (1997) Homo- and heterodimeric interactions between the gene products of PKD1 and PKD2. Proc Natl Acad Sci USA 94: 6965–6970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Tibeurgh H, Egloff MP, Martinez C, Rugani N, Verger R, Cambillau C (1993) Interfacial activation of the lipase-prolipase complex by mixed micelles revealed by X-ray crystallography. Nature 326: 814–820 [DOI] [PubMed] [Google Scholar]
- Veena RVS, Sopory SK (1999) Glyoxylase I from Brassica juncea: Molecular cloning, regulation and its over-expression confer tolerance in transgenic tobacco under stress. Plant J 17: 385–395 [DOI] [PubMed] [Google Scholar]
- Ward ER, Uknes SJ, Williams SC, Dincher SS, Wiederhold DL, Alexander DC, Ahl-Goy P, Métrauz J-P, Ryals JA (1991) Coordinate gene activity in response to agents that induce systemic acquired resistance. Plant Cell 3: 1085–1094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Heath MC (1998) Role of calcium in signal transduction during the hypersensitive response caused by basidiospore-derived infection of the cowpea rust fungus. Plant Cell 10: 585–597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu P, Narasimhan ML, Samson T, Coca MA, Huh G-H, Zhou J, Martin GB, Hasegawa PM, Bressan RA (1998) A nitrilase-like protein interacts with GCC box DNA binding proteins involved in ethylene and defense responses. Plant Physiol 118: 867–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang P, Chen C, Wang Z, Fan B, Chen Z (1999) A pathogen- and salicylic acid-induced WRKY DNA-binding activity recognizes the elicitor response element of the tobacco class I chitnase gene promoter. Plant J 18: 141–149 [Google Scholar]
- Yi HC, Joo S, Nam KH, Lee JS, Kang BG, Kim WT (1999) Auxin and brassinosteroid differentially regulate the expression of three members of the 1-aminocyclopropane-1-carboxylate synthase gene family in mung bean (Vigna radiate L.). Plant Mol Biol 41: 443–454 [DOI] [PubMed] [Google Scholar]
- Yoo TH, Park C-J, Lee G-J, Shin R, Yun J-H, Kim K-J, Rhee K-H, Paek K-H (2002) A hot pepper cDNA encoding ascorbate peroxidase is induced during the incompatible interaction with virus and bacteria. Mol Cells 14: 75–84 [PubMed] [Google Scholar]
- Yu D, Chen C, Chen Z (2001) Evidence for an important role of WRKY DNA binding proteins in the regulation of NPR1 gene expression. Plant Cell 13: 1527–1540 [DOI] [PMC free article] [PubMed] [Google Scholar]