Abstract
Objective
We have previously reported the combined effect of SNPs perturbing insulin signaling (ENPP1 K121Q, rs1044498; IRS1 G972R, rs1801278; TRIB3 Q84R, rs2295490) on insulin resistance (IR), type 2 diabetes (T2D) and cardiovascular events.
We here investigated whether such a combined effect affects also all-cause mortality in a sample of 1,851 Whites of European ancestry.
Methods
We investigated a first sample of 721 patients, 232 deaths, 3,389 person-years (py). Replication was assessed in two samples of patients with T2D: the Gargano Mortality Study (GMS) of 714 patients, 127 deaths, 5,426 py and the Joslin Kidney Study (JKS) comprising 416 patients, 214 deaths, 5,325 py.
Results
In the first sample, individuals carrying 1 or ≥ 2 risk alleles had 33% (p=0.06) and 51% (p=0.02) increased risk of mortality, as compared with individuals with no risk alleles. A similar, though not significant, trend was obtained in the two replication samples only for subject carrying ≥ 2 risk alleles. In a pooled analysis, individuals carrying ≥ 2 risk alleles had higher mortality rate as compared to those carrying 0 risk alleles (HR=1.34, 95%CI=1.08–1.67; p=0.008), and as compared to those carrying only one risk allele (HR=1.41, 95%CI=1.13–1.75; p=0.002). This association was independent from several possible confounders including sex, age, BMI, hypertension and diabetes status.
Conclusion
Our data suggest that variants affecting insulin signaling exert a joint effect on all-cause mortality and is consistent with a role of abnormal insulin signaling on mortality risk.
Keywords: ENPP1, IRS1, TRIB3, prospective study
Type 2 diabetes (T2D) and cardiovascular (CV) disease are major determinants of all-cause mortality [1] and are both characterized by insulin resistance (IR) [2, 3], which is itself a predictor of all-cause mortality [4, 5].
IR is, at least in part, genetically determined [6]. Thus, it is conceivable that genetic factors affecting IR may also affect all-cause mortality. Some clues on the genetic of IR have been provided by recent genome-wide association studies [7, 8]. In addition, non-synonymous, functional variants of genes affecting the insulin signaling pathway (rs1044498 - ENPP1 K121Q; rs1801278 - IRS1 G972R; and rs2295490 - TRIB3 Q84R; the only ones which have been thoroughly characterized in transfected cells as well as in human cells naturally carrying them [9–19]), have also been reported to exert a combined effect on IR, T2D and CV disease [20, 21].
Based upon this background, we investigated the combined effect of these insulin signaling single nucleotide polymorphism (SNPs) on all-cause mortality in a total of 1,851 white individuals of European ancestry.
Materials and Methods
Study design
Based on our previous observation of a combined SNPs effect on CV events in three cohorts analyzed together [21], we used these same pooled studies as a first sample to test the hypothesis of an association with all-cause mortality.
Subsequently, we tried to increase the robustness of our finding by investigating two additional replication cohorts.
First combined sample
This sample comprises the following cohorts:
Gargano Heart Study (GHS)-prospective design
Three-hundred-fifty-four Whites with T2D (ADA 2003 criteria) and coronary artery disease who were consecutively recruited at “Casa Sollievo della Sofferenza” Institute in San Giovanni Rotondo (Gargano, Center East Coast of Italy) from 2001 to 2008 [21, 22]. All patients had either a stenosis >50% in at least one coronary major vessel at coronary angiography or a previous myocardial infarction (MI). The only exclusion criterion was the presence of poor life expectancy for non diabetes-related diseases.
Tor Vergata Atherosclerosis Study (TVAS)
One-hundred-two Whites were consecutively recruited from 2005 to 2007 at “Tor Vergata” University Hospital (Rome); they all had been diagnosed with an acute MI. Exclusion criteria were the presence of malignancies and a previous medical record of diabetes, although 22 (15.7%) study participants turned out to have subclinical diabetes after an OGTT [21].
Cardiovascular Risk Extended Evaluation in Dialysis (CREED) database
Two-hundred-sixty-five Whites with end stage renal disease (ESRD) were recruited at the Reggio Calabria Hospital. Exclusion criteria were dialysis for less than 6 months, left ventricular ejection fraction <35%, history of circulatory congestion and hospitalization for inter-current illness including major infections. Out of these, 43 (16.2%) had diabetes [23].
Replication samples
Gargano Mortality Study (GMS)
Seven-hundred-fourteen Whites with T2D (ADA 2003 criteria) were consecutively recruited from November 1th 2000 to September 30th 2005 at “Casa Sollievo della Sofferenza” Institute, for a study having all-cause mortality as the end-point [22, 24]. The only exclusion criterion was the presence of poor life expectancy due to non diabetes-related disorders.
Joslin Kidney Study in type 2 diabetes (JKS)
This cohort consists of a random sample (n=516) of T2D patients from the Joslin Clinic enriched with individuals with proteinuria, who were recruited between 1993 and 1996 at the Joslin Diabetes Center, Boston, MA as previously described [25].
All subjects had diabetes diagnosed after age 25 according to WHO criteria and were treated with diet or oral agents for at least two years after the diagnosis. The present study was limited to 416 self-reported Whites for whom DNA samples were still available in 2013. Their survival status was updated as of December 31, 2011 by matching with the National Death Index.
Subjects from all studies underwent clinical examination and standardized interview at the time of recruitment, as previously reported [21–25]. Smoking habits and history of hypertension were recorded at time of examination. Hypertension was defined as systolic blood pressure > 130 mmHg or diastolic blood pressure > 85 mmHg or presence of antihypertensive therapy. For all studies the end-point was all-cause mortality. Confirmation of the event was obtained from death certificates or, in the case of GMS, by direct contact with patients and/or their relatives or by queries to the registry offices of the cities of residence.
Study protocol and the informed consent procedure were approved by each Institutional Ethic Committee. All participants gave written informed consent.
Genotyping
Genotyping was performed by TaqMan allele discrimination as previously described [20]. Genotyping quality was assessed by including in each 96 wells plate positive controls with known genotypes (i.e. homozygous for the major allele, heterozygous or homozygous for the minor allele, previously evaluated for each SNP of interest by direct Sanger sequencing, each in duplicates). The agreement rate was >99% for each SNP assay. Genotype distribution was in Hardy-Weinberg equilibrium (HWE) in all study samples (p value >0.1). Minor allele frequency of ENPP1, TRIB3 and IRS1 are shown in Supplemental Table 1.
Statistical Methods
Patients’ baseline characteristics were reported as mean±standard deviation (SD) and percentages for continuous and categorical variables, respectively.
In all prospective studies, time-to-death analyses were performed using multivariable Cox proportional hazards regression models, and risks were reported as hazards ratios (HR) along with their 95 % confidence intervals (95 % CI). The overall survival was defined as the time between enrollment and death. For subjects who did not experience the end point, survival time was censored at the time of the last available follow-up attempt. Incidence rates for overall mortality were expressed as the number of events per 100 person-years (py%). Since no evidence of a linear association was observed between the number of risk alleles carried by each individual and the rate of all-cause mortality, a Cox-based tree regression analysis (RECPAM) was performed. A deviance test confirmed that a non-linear, dichotomized model of risk alleles was to be preferred as compared to the linear (additive) one (p<0.01).
Pooled data analyses were performed in an individual patient data meta-analysis fashion [26] (i.e. adjusting for “study sample”) after having observed no genotype-by-sample interaction.
In the whole sample (n=1,851), we had 80% power, at alpha=0.05, to detect an HR=1.35 or 1.49, for either 1 or ≥ 2 risk alleles with respect to 0 risk allele, assuming an incidence rate in the 0 risk allele group of 4% py.
A p-value <0.05 was considered as significant. All analyses were performed using SAS Release 9.1.3 (SAS Institute, Cary, NC, USA).
Results
First sample
Clinical features of study participants from GHS-prospective design, TVAS and CREED cohorts are summarized in Table 1 (first three panels).
Table 1.
Baseline clinical characteristics and follow-up measures of participants from all study cohorts.
| GHS | TVAS | CREED | GMS | JKS | |
|---|---|---|---|---|---|
| Number | 354 | 102 | 265 | 714 | 416 |
| Male (%) | 67.8 | 86.3 | 55.8 | 46.1 | 56.7 |
| Age (yrs) | 64.5±8.1 | 61.9±9.3 | 61.0±15.4 | 61.8±9.8 | 43.7±9.0 |
| BMI (kg/m2) | 30.2±4.8 | 28.0±3.9 | 24.9±4.4 | 31.1±5.9 | 29.9±6.5 |
| Diabetes (%) | 100 | 15.7 | 16.2 | 100 | 100 |
| anti-hyperglycaemic Tx (%) | 89.6 | 0* | 100 | 86.8 | 91.5 |
| Hypertension (%) | 84.7 | 99.0 | 72.8 | 50.3 | 55 |
| anti-hypertensive Tx (%) | 84.3 | 95.4 | 41 | 49.2 | 46.5 |
| Follow-up (yrs) | 5.3±2.5 | 5.9±1.9 | 3.4±1.8 | 7.6±2.1 | 12.8±5.8 |
| Follow-up (py) | 1,876 | 602 | 901 | 5,426 | 5,325 |
| Events (n) | 79 | 12 | 141 | 127 | 214 |
Continuous variables were reported as mean±SD whereas categorical variables as percentages.
GHS: Gargano Heart Study-prospective design; TVAS: Tor Vergata Atherosclerosis Study; CREED: Cardiovascular Risk Extended Evaluation in Dialysis database; GMS: Gargano Mortality Study; JKS: Joslin Kidney Study in type 2 diabetes; BMI: body mass index; py: person-years. Tx: treatment.
TVAS diabetic patients were all neodiagnosed at study entry.
The association between each SNP, considered individually, and all-cause mortality is shown in Supplemental Table 2. Although formal statistical significance was reached only with IRS1 G972R, all three SNPs tended to be associated with all-cause mortality toward the direction one would have expected if risk alleles for metabolic and cardiovascular abnormalities (ENPP1 Q121, IRS1 R972 and TRIB3 R84) had to be associated with increased mortality rate (Supplemental Table 2).
Since no heterogeneity was observed in the HRs across studies in any SNP (p for SNP-by-cohorts interaction ranging 0.31–0.98), the data from the three cohorts were pooled, analyzed together as the first sample in which to test our hypothesis. In this pooled sample, a total of 721 individuals were followed up for 4.7±2.4 years with an annual incidence rate of all-cause mortality equal to 6.8 % (i.e. 232 events, 3,389 py). Overall, 306, 292, 99, 22 and 2 individuals carried 0, 1, 2, 3 and 4 risk alleles, respectively. Since the number of individuals carrying 3 and 4 risk alleles was small (n=24, 6 events), these subjects were pooled and analyzed together with those having 2 risk alleles. Individuals carrying 1 or ≥ 2 risk alleles had 33% (HR=1.33, 95%CI=0.99–1.78; p=0.06) and 51% (HR=1.51, 95%CI=1.06–2.15; p=0.02) increased risk of mortality respectively as compared with individuals with no risk alleles.
Replication samples
Clinical features of study participants from GMS and JKS are shown in Table 1 (last two panels). The HRs (95% CI) for all-cause mortality of ENPP1 K121Q, IRS1 G972R and TRIB3 Q84R, considered individually, are shown in Supplemental Table 2. Overall, 312, 272, 102, 24, 4 and 167, 171, 64, 12, 2 individuals carried 0, 1, 2, 3 and 4 risk alleles, respectively in GMS and in JKS. Individuals carrying 1 risk allele showed no association (HR=0.72, 95%CI=0.47–1.08; p=0.11 and HR=0.77, 95%CI=0.57–1.05; p=0.10 in GMS and JKS, respectively); contrariwise, individuals with ≥ 2 risk alleles displayed a directionally consistent but statistically insignificant association with all-cause mortality in both studies (HR=1.37, 95%CI=0.89–2.11; p=0.16 and HR=1.19, 95%CI=0.84–1.70; p=0.34 in GMS and JKS, respectively).
Pooled analysis
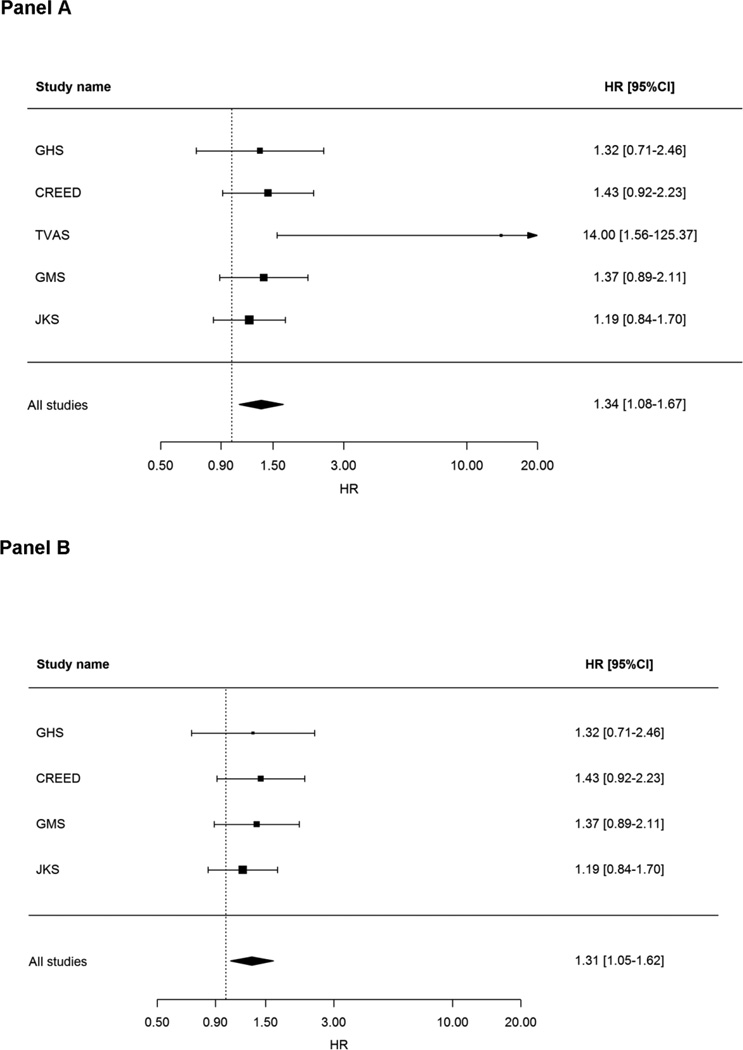
When all cohorts were analyzed together (n=1,851 individuals; 573 deaths), individuals carrying ≥ 2 risk alleles had higher mortality rate as compared to those carrying 0 risk alleles (HR=1.34, 95%CI=1.08–1.67; p=0.008; Figure 1, Panel A). This association was independent from several possible confounders including sex, age, BMI, hypertension and diabetes status (data not shown).
Figure 1.
Forest plots of random effects meta-analysis of HRs estimates according to individuals with ≥ 2 risk alleles vs individuals with 0 risk allele.
Figure 1A: meta-analysis was carried out on GHS, TVAS, CREED, GMS and JKS; Figure 1B: meta-analysis was carried out on GHS, CREED, GMS and JKS.
Similar results were obtained when TVAS, which showed an extremely strong association was not included in the analysis (Figure 1, Panel B). A similar increased risk was observed when comparing individuals with ≥ 2 vs. 1 risk allele (HR=1.41, 95%CI=1.13–1.75; p=0.002).
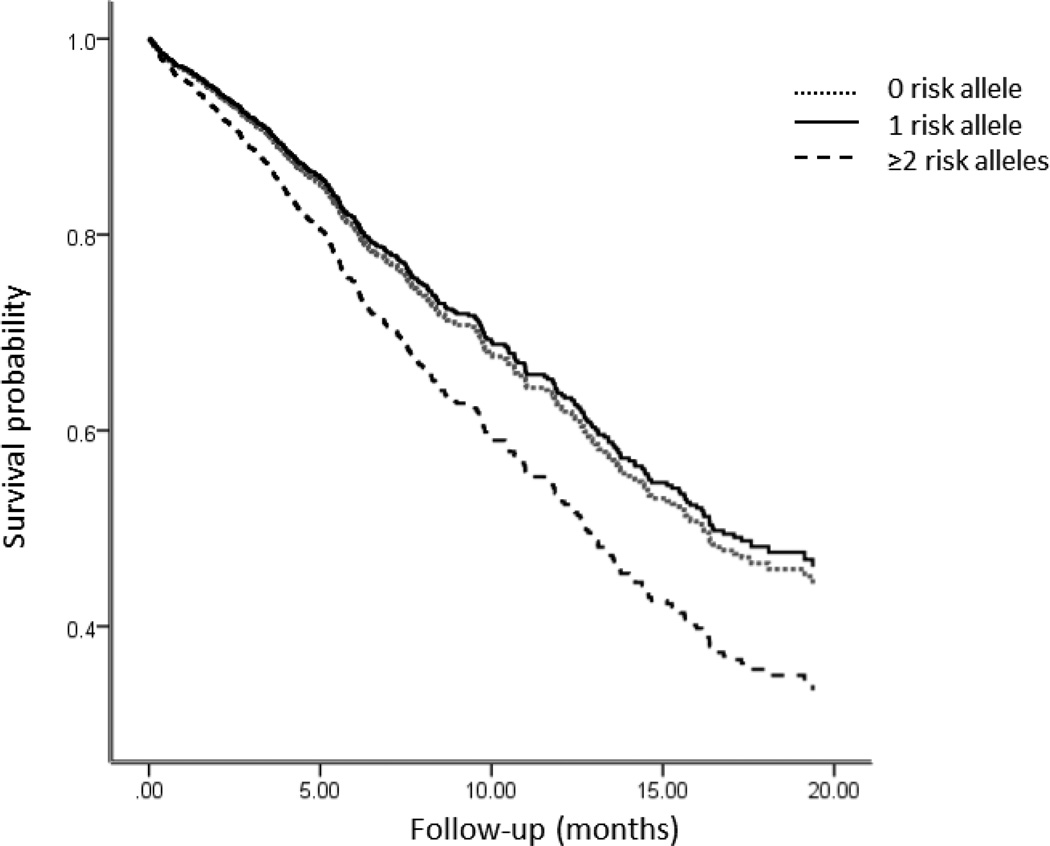
HRs for all-cause mortality in the pooled analysis of individuals carrying 0, 1 and ≥ 2 risk alleles are shown in Figure 2.
Figure 2.
Hazard ratios (95% CI) of all-cause mortality in all samples according to genotype risk alleles. Hazard ratios were estimated by Cox regression after adjusting for all five study cohorts.
When looking individually, only the IRS1 G972R SNP showed a nominal association; this association was especially strong in TVAS and virtually absent in GMS (Supplementary Table 2). The reason for such inconsistencies across different SNPs as well as across different samples may simply due to chance. Population stratification, though not excludable, is unlikely due to the fact that four out of the five study populations are from the same geographical region, with all individuals being Whites of Italian ancestry. Of note, no specific two-SNPs combination emerged as driving most of the observed association (data not shown). Among others, the combination of ENPP1 K121Q and TRIB3 Q84R (i.e. thus excluding IRS1 G972R SNP, the only significantly associated one) displayed a directionally consistent association with all-cause mortality (i.e. 20% increased risk). Since no evidence of a linear association between the number of risk alleles carried by each individual and the rate of all-cause mortality was observed, a RECPAM (i.e. a Cox-based tree regression) analysis was performed, which identified two patient subgroups at different risks, with subjects carrying ≥ 2 risk alleles showing a HR of all-cause mortality of 1.38 (95% CI=1.13–1.68; p=0.001) as compared to those carrying 0 – 1 risk alleles.
Post-hoc analysis on cause of mortality
In four of the five available samples (GHS, TVAS, CREED and JKS for a total of 1,137 individuals) also CV causes of mortality were recorded (i.e. 294 out of 446 total deaths). In this subgroup, the risk of all-cause mortality for individuals with ≥ 2 risk alleles compared to those carrying 0 risk allele was similar to that observed in the whole pooled sample (HR=1.33, 95%CI=1.03–1.70; p=0.03). In addition, CV and non-CV mortality risks of individuals carrying ≥ 2 risk alleles vs. those carrying 0 risk alleles were largely overlapping: HR=1.23, (95%CI=0.90–1.67; p=0.20) and 1.56, (95%CI=1.01–2.37; p=0.045), respectively.
Discussion
In this study, more than addressing the genetic of all-cause mortality (a task which would have needed a much more comprehensive approach) we used genetic data (i.e. variability at three functional SNPs affecting key components of the insulin signaling pathway) as a tool to address the role of abnormal insulin signaling on all-cause mortality. When considered in this perspective, our data suggest a deleterious role of increasingly defective insulin signaling on mortality risk. Unfortunately, in the most comprehensive analyses of the genetic contribution to IR, very few variants strictly related to the insulin signaling pathway have emerged [7, 8]. This does not allow to construct a strong multi-variant genetic risk score able to specifically address our hypothesis, which is focused on the role of insulin signaling, rather than IR as a whole.
The biology of the increased mortality we observed, might be related to the previously reported combined effect of the same three SNPs on IR [21], abnormal glucose homeostasis [20] and major cardiovascular events [21], all main causes of mortality of different origin and/or by other pathogenic effects of hyperinsulinemia [27]. This is also suggested by our post-hoc analysis, which showed no substantial difference in the association between study SNPs and either CV or non-CV mortality.
Of note, it appears that at least two risk alleles are needed for the deleterious effect to be detected, thus speaking in favor of a threshold effect, rather than a linear relationship, of the genetic load, and therefore on the burden of abnormal insulin signaling on mortality risk.
We acknowledge that the significance level of this association is still compatible with a false-positive result. Thus, further and larger samples have to be investigated before to consider our findings as established. Unfortunately, to the best of our knowledge, neither rs1801278 and rs2295490, nor other SNPs in linkage disequilibrium with them, were included in the recent genome wide association studies of IR [7, 8], T2D [28], CV disease [29] and lifespan [30, 31]. Therefore, it is not possible to draw any information from these datasets about a combined effect of the three study SNPs we are focused on.
It must also be considered that the five study cohorts, although all including individuals at high mortality risk, are quite heterogeneous in terms of presence/absence of diabetes, MI and end stage renal diseases as well as in terms of mortality rate. Although all these differences were taken into account by adjusting pooled analyses for “study sample”, this may be viewed as a limitation of our study. Conversely, the risk of all-cause mortality in individuals carrying ≥2 risk alleles were similar in all samples, suggesting that this finding is generalizable to high risk patients, regardless of their baseline clinical characteristics. Contrariwise, it remains to be investigated whether our finding applies also to individuals with low risk profile. It also remains to be addressed whether our finding can be extended to populations of non European ancestry.
A further limitation of our study is the lack of additional clinical information, including - for example - kidney dysfunction and MI in some samples, as well as specific treatment of both hyperglycemia and hypertension in others, thus making impossible more detailed analyses.
In conclusion, our data suggest that functional non-synonymous variants affecting insulin signaling exert a joint effect on all-cause mortality and are consistent with a role of abnormal insulin signaling on mortality risk.
Supplementary Material
Highlights.
-
➢
Non-synonymous, functional variants of genes affecting the insulin signaling pathway exert a joint effect on all-cause mortality.
-
➢
In details, high-risk individuals of European ancestry carrying > 2 risk variants show a 30–40% increased mortality rate.
-
➢
Such association was homogeneous across study samples from different clinical sets; this suggests that our finding is generalizable to high-risk patients, regardless of their baseline clinical characteristics.
-
➢
In all, our data suggest a deleterious role of increasingly defective insulin signaling on mortality risk.
Acknowledgments
We are indebted to the staffs and participants of the GHS, TVAS, CREED, GMS and JKS study for their dedication and contributions.
We thank Sabrina Prudente (Rome) who contributed in providing the intellectual background on the role of insulin signaling genes upon which our present investigation was based.
This study was supported by Accordo Programma Quadro in Materia di Ricerca Scientifica nella Regione Puglia-PST 2006 and PO Puglia FESR 2007–2013, Italian Ministry of Health grants RC2011, RC2012, RC2013, RC2014 (CM), and NIH grants R01 DK073168 and DK110400 (AD).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing interests
No potential conflicts of interest relevant to this article were reported.
Authors’ contributions
C.M. and V.T. participated in study concept and design.
C.M., S.B., M.F., A.D., C.Z. and V.T. participated in interpretation of results.
C.M., S.D.C, S.B., M.F., A.D.,C.Z. and V.T. participated in acquisition of data.
C.M., A.F. and M.C. participated in statistical analysis.
S.R., B.S., P.B., G.T., A.M., D.B., T.H., A.T., Ch. Me. and F.M. performed laboratory testing, acquired laboratory and clinical data used in the study.
C.M., A.D. and V.T. obtained funding, drafted the article and all authors participated in critical revision and approved the final version of the manuscript.
C.M. and V.T. are the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data.
References
- 1.Seshasai SR, Kaptoge S, Thompson A, Di Angelantonio E, Gao P, Sarwar N, Whincup PH, Mukamal KJ, Gillum RF, Holme I, et al. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med. 2011;364(9):829–841. doi: 10.1056/NEJMoa1008862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes. 1988;37(12):1595–1607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- 3.DeFronzo RA. Insulin resistance, lipotoxicity, type 2 diabetes and atherosclerosis: the missing links. The Claude Bernard Lecture 2009. Diabetologia. 2010;53(7):1270–1287. doi: 10.1007/s00125-010-1684-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ford ES. Risks for all-cause mortality, cardiovascular disease, and diabetes associated with the metabolic syndrome: a summary of the evidence. Diabetes Care. 2005;28(7):1769–1778. doi: 10.2337/diacare.28.7.1769. [DOI] [PubMed] [Google Scholar]
- 5.Ausk KJ, Boyko EJ, Ioannou GN. Insulin resistance predicts mortality in nondiabetic individuals in the U.S. Diabetes Care. 2010;33(6):1179–1185. doi: 10.2337/dc09-2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prudente S, Morini E, Trischitta V. Insulin signaling regulating genes: effect on T2DM and cardiovascular risk. Nat Rev Endocrinol. 2009;5(12):682–693. doi: 10.1038/nrendo.2009.215. [DOI] [PubMed] [Google Scholar]
- 7.Scott RA, Lagou V, Welch RP, Wheeler E, Montasser ME, Luan J, Mägi R, Strawbridge RJ, Rehnberg E, Gustafsson S, et al. Large-scale association analyses identify new loci influencing glycemic traits and provide insight into the underlying biological pathways. Nat Genet. 2012;44(9):991–1005. doi: 10.1038/ng.2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Manning AK, Hivert MF, Scott RA, Grimsby JL, Bouatia-Naji N, Chen H, Rybin D, Liu CT, Bielak LF, Prokopenko I, et al. A genome-wide approach accounting for body mass index identifies genetic variants influencing fasting glycemic traits and insulin resistance. Nat Genet. 2012;44(6):659–669. doi: 10.1038/ng.2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pizzuti A, Frittitta L, Argiolas A, Baratta R, Goldfine ID, Bozzali M, Ercolino T, Scarlato G, Iacoviello L, Vigneri R, et al. A polymorphism (K121Q) of the human glycoprotein PC-1 gene coding region is strongly associated with insulin resistance. Diabetes. 1999;48(9):1881–1884. doi: 10.2337/diabetes.48.9.1881. [DOI] [PubMed] [Google Scholar]
- 10.Bacci S, Di Paola R, Menzaghi C, Di Fulvio P, Di Silvestre S, Pellegrini F, Baratta R, Marucci A, Mastroianno S, Fini G, et al. ENPP1 Q121 variant, increased pulse pressure and reduced insulin signaling, and nitric oxide synthase activity in endothelial cells. Arterioscler Thromb Vasc Biol. 2009;29(10):1678–1683. doi: 10.1161/ATVBAHA.109.189191. [DOI] [PubMed] [Google Scholar]
- 11.Di Paola R, Caporarello N, Marucci A, Dimatteo C, Iadicicco C, Del Guerra S, Prudente S, Sudano D, Miele C, Parrino C, et al. ENPP1 affects insulin action and secretion: evidences from in vitro studies. PLoS One. 2011;6(5):e19462. doi: 10.1371/journal.pone.0019462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Almind K, Inoue G, Pedersen O, Kahn CR. A common amino acid polymorphism in insulin receptor substrate-1 causes impaired insulin signaling. Evidence from transfection studies. J Clin Invest. 1996;97(11):2569–2575. doi: 10.1172/JCI118705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Porzio O, Federici M, Hribal ML, Lauro D, Accili D, Lauro R, Borboni P, Sesti G. The Gly972-->Arg amino acid polymorphism in IRS-1 impairs insulin secretion in pancreatic beta cells. J Clin Invest. 1999;104(3):357–364. doi: 10.1172/JCI5870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hribal ML, Federici M, Porzio O, Lauro D, Borboni P, Accili D, Lauro R, Sesti G. The Gly-->Arg972 amino acid polymorphism in insulin receptor substrate-1 affects glucose metabolism in skeletal muscle cells. J Clin Endocrinol Metab. 2000;85(5):2004–2013. doi: 10.1210/jcem.85.5.6608. [DOI] [PubMed] [Google Scholar]
- 15.Marchetti P, Lupi R, Federici M, Marselli L, Masini M, Boggi U, Del Guerra S, Patanè G, Piro S, Anello M, et al. Insulin secretory function is impaired in isolated human islets carrying the Gly(972)-->Arg IRS-1 polymorphism. Diabetes. 2002;51(5):1419–1424. doi: 10.2337/diabetes.51.5.1419. [DOI] [PubMed] [Google Scholar]
- 16.Federici M, Pandolfi A, De Filippis EA, Pellegrini G, Menghini R, Lauro D, Cardellini M, Romano M, Sesti G, Lauro R, et al. G972R IRS-1 variant impairs insulin regulation of endothelial nitric oxide synthase in cultured human endothelial cells. Circulation. 2004;109(3):399–405. doi: 10.1161/01.CIR.0000109498.77895.6F. [DOI] [PubMed] [Google Scholar]
- 17.Prudente S, Hribal ML, Flex E, Turchi F, Morini E, De Cosmo S, Bacci S, Tassi V, Cardellini M, Lauro R, et al. The functional Q84R polymorphism of mammalian Tribbles homolog TRB3 is associated with insulin resistance and related cardiovascular risk in Caucasians from Italy. Diabetes. 2005;54(9):2807–2811. doi: 10.2337/diabetes.54.9.2807. [DOI] [PubMed] [Google Scholar]
- 18.Andreozzi F, Formoso G, Prudente S, Hribal ML, Pandolfi A, Bellacchio E, Di Silvestre S, Trischitta V, Consoli A, Sesti G. TRIB3 R84 variant is associated with impaired insulin-mediated nitric oxide production in human endothelial cells. Arterioscler Thromb Vasc Biol. 2008;28(7):1355–1360. doi: 10.1161/ATVBAHA.108.162883. [DOI] [PubMed] [Google Scholar]
- 19.Liew CW, Bochenski J, Kawamori D, Hu J, Leech CA, Wanic K, Malecki M, Warram JH, Qi L, Krolewski AS, et al. The pseudokinase tribbles homolog 3 interacts with ATF4 to negatively regulate insulin exocytosis in human and mouse beta cells. J Clin Invest. 2010;120(8):2876–2888. doi: 10.1172/JCI36849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prudente S, Morini E, Marselli L, Baratta R, Copetti M, Mendonca C, Andreozzi F, Chandalia M, Pellegrini F, Bailetti D, et al. Joint effect of insulin signaling genes on insulin secretion and glucose homeostasis. J Clin Endocrinol Metab. 2013;98(6):E1143–E1147. doi: 10.1210/jc.2012-4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bacci S, Prudente S, Copetti M, Spoto B, Rizza S, Baratta R, Di Pietro N, Morini E, Di Paola R, Testa A, et al. Joint effect of insulin signaling genes on cardiovascular events and on whole body and endothelial insulin resistance. Atherosclerosis. 2013;226(1):140–145. doi: 10.1016/j.atherosclerosis.2012.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Menzaghi C, Bacci S, Salvemini L, Mendonca C, Palladino G, Fontana A, De Bonis C, Marucci A, Goheen E, Prudente S, et al. Serum resistin, cardiovascular disease and all-cause mortality in patients with type 2 diabetes. PLoS One. 2013;8(6):e64729. doi: 10.1371/journal.pone.0064729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Testa A, Spoto B, Tripepi G, Mallamaci F, Malatino L, Fatuzzo P, Maas R, Boeger R, Zoccali C. The GLU298ASP variant of nitric oxide synthase interacts with asymmetric dimethyl arginine in determining cardiovascular mortality in patients with end-stage renal disease. J Hypertens. 2005;23(10):1825–1830. doi: 10.1097/01.hjh.0000182528.59687.d1. [DOI] [PubMed] [Google Scholar]
- 24.De Cosmo S, Copetti M, Lamacchia O, Fontana A, Massa M, Morini E, Pacilli A, Fariello S, Palena A, Rauseo A, et al. Development and Validation of a Predicting Model of All-Cause Mortality in Patients With Type 2 Diabetes Mellitus. Diabetes Care. 2013 doi: 10.2337/dc12-1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Doria A, Wojcik J, Xu R, Gervino EV, Hauser TH, Johnstone MT, Nolan D, Hu FB, Warram JH. Interaction between poor glycemic control and 9p21 locus on risk of coronary artery disease in type 2 diabetes. JAMA. 2008;300(20):2389–2397. doi: 10.1001/jama.2008.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Olkin I, Sampson A. Comparison of meta-analysis versus analysis of variance of individual patient data. Biometrics. 1998;54(1):317–322. [PubMed] [Google Scholar]
- 27.Giovannucci E, Harlan DM, Archer MC, Bergenstal RM, Gapstur SM, Habel LA, Pollak M, Regensteiner JG, Yee D. Diabetes and cancer: a consensus report. Diabetes Care. 2010;33(7):1674–1685. doi: 10.2337/dc10-0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morris AP, Voight BF, Teslovich TM, Ferreira T, Segrè AV, Steinthorsdottir V, Strawbridge RJ, Khan H, Grallert H, Mahajan A, et al. Large-scale association analysis provides insights into the genetic architecture and pathophysiology of type 2 diabetes. Nat Genet. 2012;44(9):981–990. doi: 10.1038/ng.2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coronary Artery Disease Genetics C. A genome-wide association study in Europeans and South Asians identifies five new loci for coronary artery disease. Nature genetics. 2011;43(4):339–344. doi: 10.1038/ng.782. [DOI] [PubMed] [Google Scholar]
- 30.Sebastiani P, Solovieff N, Dewan AT, Walsh KM, Puca A, Hartley SW, Melista E, Andersen S, Dworkis DA, Wilk JB, et al. Genetic signatures of exceptional longevity in humans. PLoS One. 2012;7(1):e29848. doi: 10.1371/journal.pone.0029848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beekman M, Blanché H, Perola M, Hervonen A, Bezrukov V, Sikora E, Flachsbart F, Christiansen L, De Craen AJ, Kirkwood TB, et al. Genome-wide linkage analysis for human longevity: Genetics of Healthy Aging Study. Aging Cell. 2013;12(2):184–193. doi: 10.1111/acel.12039. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.