Abstract
Blastic plasmacytoid dendritic cell neoplasm (BPDCN) is a rare disease of controversial origin recently recognized as a neoplasm deriving from plasmacytoid dendritic cells (pDCs). Nevertheless, it remains an orphan tumor with obscure biology and dismal prognosis. To better understand the pathobiology of BPDCN and discover new targets for effective therapies, the gene expression profile (GEP) of 25 BPDCN samples was analyzed and compared with that of pDCs, their postulated normal counterpart. Validation was performed by immunohistochemistry (IHC), whereas functional experiments were carried out ex vivo. For the first time at the molecular level, we definitely recognized the cellular derivation of BPDCN that proved to originate from the myeloid lineage and in particular, from resting pDCs. Furthermore, thanks to an integrated bioinformatic approach we discovered aberrant activation of the NF-kB pathway and suggested it as a novel therapeutic target. We tested the efficacy of anti-NF-kB-treatment on the BPDCN cell line CAL-1, and successfully demonstrated by GEP and IHC the molecular shutoff of the NF-kB pathway. In conclusion, we identified a molecular signature representative of the transcriptional abnormalities of BPDCN and developed a cellular model proposing a novel therapeutic approach in the setting of this otherwise incurable disease.
INTRODUCTION
Blastic plasmacytoid dendritic cell neoplasm (BPDCN) is a rare disease, included among acute myeloid leukemias in the 2008 WHO classification.1
Its histogenesis has been the object of controversy for a long time and its pathobiology still remains poorly understood. Along its history, this malignancy has been erroneously named blastic NK/T-cell lymphoma or agranular CD4+/CD56+ hematodermic neoplasm/tumour.2 Finally, in 2008, based on the immunophenotype, BPDCN has been classified as a neoplasm deriving from precursors of plasmacytoid dendritic cells(pDCs).3–5
The disease presents a male-to-female ratio of 3.5:1 and a mean and median age at diagnosis of 57.5 and 66.0 years, respectively. Most patients display skin, bone marrow and lymph node involvement and show a very aggressive clinical course.6–9 In fact, despite an initial response to chemotherapy, the disease regularly relapses, the median overall survival ranging from 12 to 14 months.6,10,11 To date, no standardized therapeutic approach has been established and the optimal therapy remains to be defined. Interestingly, although the disease is recognized among acute myeloid leukemias and acute myeloid leukemia-like regimes are often adopted, recent reports have suggested a potential role for acute lymphoid leukemia (ALL)-like protocols.12–16
The refinement of therapeutic strategies in BPDCN would require extensive molecular studies. However, the rarity of this malignancy and its recent recognition as a distinct clinicopathological entity have hampered the collection of large sample series, most reports in the literature being based on single cases. Accordingly, only few studies have so far focused on BPDCN genetics, documenting a complex karyotype with deletions on chromosomes 5q21 or 5q34 (72%), 12p13 (64%), 13q13–q21 (64%), 6q23-qter (50%), 15q (43%) and 9 (28%)17–19 and sporadic genetic alterations affecting RB1, LATS2, CDKN1B, CDKN2A, and TP53 gene.20–23
All data were generated by analyzing a limited number of patients and neither functional consequences of such abnormalities has so far been determined nor have functional experiments been carried out.
Finally, only one study explored the global gene expression profile (GEP) of the disease20 by comparing the molecular signature of BPDCN with that of cutaneous acute myelomonocytic leukemias, indicating that the two diseases, sometimes difficult to distinguish, effectively rely on different molecular patterns. Due to the specific aim of the study, the authors did not match BPDCN GEP to any normal counterpart.
In the present study based on an international effort, we collected a significant number of cases and performed a global GEP analysis of BPDCN aiming to: (1) molecularly define the cellular counterpart of BPDCN and its relationship with other leukemias; (2) identify genes and cellular programs deregulated in the tumor; and (3) delineate novel potential therapeutic targets.
MATERIALS AND METHODS
Case collection
We collected 27 BPDCN cases at diagnosis from untreated patients. All the cases were reviewed by a panel of at least three expert hematopathologists (CA, FF, PPP, MP, LC and SAP) according to the WHO Classification criteria1 and then selected for subsequent molecular analyses based on the presence of at least 80% of neoplastic cells and good RNA preservation. Two cases not matching eligibility requirements were excluded from the study.
Among the selected cases, 19 were represented by formalin-fixed paraffin-embedded (FFPE) tissue samples, 6 by frozen tissue samples. Twenty-two cases were represented by cutaneous biopsies and three by lymph nodes. The main clinicopathological features are summarized in Supplementary Table 1.
We used as control eight plasmacytoid dendritic cell (pDC) samples isolated from the peripheral blood of healthy donors (see below).
For gene expression analysis, 14 BPDCN (six frozen and eight FFPE) and four pDCs samples were randomly selected and included in a training set. The remaining 11 BPDCN (all FFPE) and four pDCs samples represented the test set (see Supplementary Information). Both training and test set were studied by Illumina whole genome expression profiling.
Furthermore, the six BPDCN frozen samples, belonging to the training set, were also analyzed by Affymetrix microarray platform, aiming to explore the cellular derivation of the tumor. For more details, see Supplementary Information.
Immunohistochemical validation studies were performed on an independent validation set including 10 BPDCN samples, one hyperplastic lymph node presenting pDCs aggregates, a pool of pDCs isolated from peripheral blood of healthy donors and the CAL-1 cell line, all included in paraffin.
Informed consent was obtained from each patient in accordance with the guidelines of the Institutional Review Board of the Department of Experimental, Diagnostic, and Specialty Medicine of the University of Bologna and the Declaration of Helsinki. The study design is schematically represented in Supplementary Figure 1.
Affymetrix gene expression profiling
For GEP data generation by the Affymetrix platform and following analysis of cellular derivation see Supplementary Information.
DASL gene expression profiling
For the GEP data generation by the Illumina platform and following analysis of target prediction therapy see Supplementary Information.
Isolation of human pDCs
Peripheral blood mononuclear cells were obtained from the buffy coat of healthy donors (provided by the Blood Transfusion Center of S. Orsola-Malpighi Hospital, Bologna, Italy) and separated by Ficoll gradient density centrifugation using Lympholyte-H. (Cedarlane Laboratories, Euroclone, Pero (MI), Italy). PDCs were isolated using the Diamond pDC isolation kit (Miltenyi Biotec, Auburn, CA, USA) according to the manufacturer’s instructions. For determination of purity, a fraction of isolated pDCs, stained for CD303 (BDCA-2)-APC and CD123-PE was analyzed by flow cytometry. The purity of pDCs isolated from the samples was >95%.
Cytoinclusion
Briefly, 1 × 106 pDCs and 5 × 106 CAL-1 cells were centrifuged, washed in PBS, fixed in 10% buffered formalin and then included in paraffin as reported.24,25
Immunohistochemical staining
All the samples of the validation set (see above) underwent immunohistochemistry (IHC) as detailed in Supplementary Information.
Cell culturing and experimental therapeutics
Cell line and reagents
CAL-1, a BPDCN cell line26, was cultured in complete RPMI 1640 medium containing 10% fetal bovine serum (Lonza, Milan, Italy) to a final concentration of 1 × 106/ml. CAL-1 cells were separately incubated with Bortezomib (VELCADE, Millennium Pharmaceuticals, Cambridge, MA, USA) cytarabine (ARA-C, AOSP, Bologna, Italy) and BMS-345541 (Sigma-Aldrich, St Louis, MO, USA). Cell viability, death and proliferation were measured as reported in Supplementary Information.
Bortezomib effect on NF-kB signaling
CAL-1 cells were incubated with Bortezomib and then analyzed by GEP (DASL Illumina assay, San Diego, CA, USA) and IHC. In parallel CAL-1 cells were also incubated with BMS-345541 and evaluated by IHC as detailed in Supplementary Information.
RESULTS
BPDCNs mirror myeloid resting pDCs and are distinct from other acute leukemias
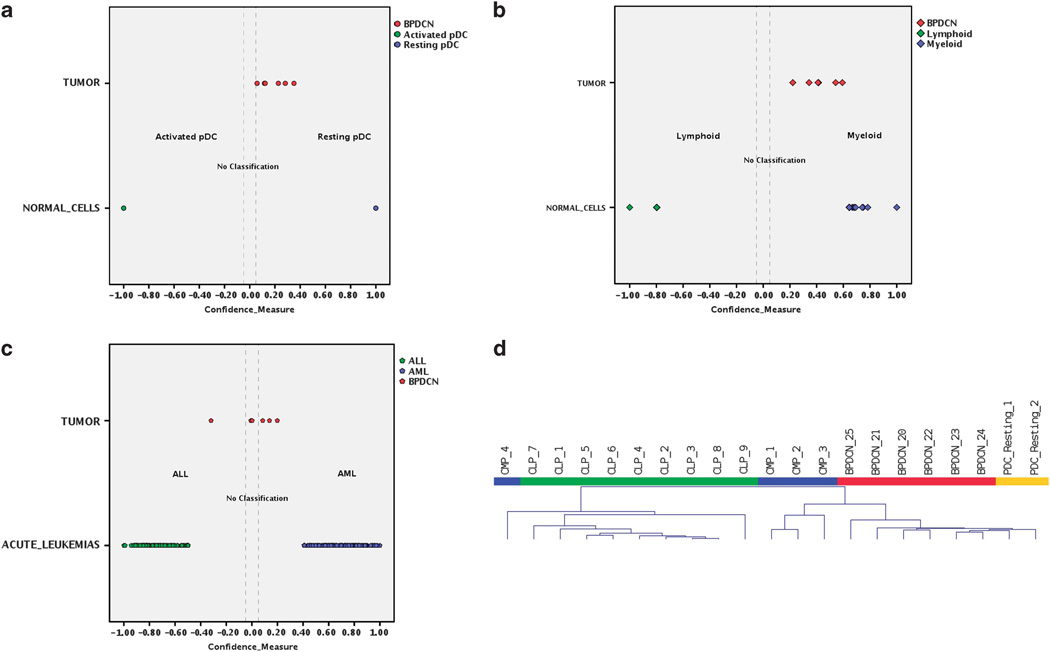
First, we defined the cellular counterpart of BPDCN at gene expression level. To address this issue, we studied the molecular profiles of the six BPDCN cases, for which frozen material was available, and those of pDCs resting and activated with Interleukin-2. Comparing the profiles of the two pDCs groups by supervised analysis, we recognized 724 genes differentially expressed upon activation (Supplementary Table 2). Based on these genes, we generated a cell type classifier using a support vector machine algorithm and classified the six BPDCN cases. We found that BPDCN gene expression was consistently closer to that of resting pDCs (Figure 1a; Supplementary Table 3). This was then confirmed by the lack of expression of interferon-related genes in BPDC (see below).
Figure 1.
Cellular derivation of BPDCN. (a) Cell type classification is used to measure the relatedness of six BPDCNs to pDCs resting, pDCs activated, MPs, LPs, acute myeloid leukemias and ALLs. Scatterplots show the distribution of BPDCNs, normal cells and acute leukemia samples according to their discriminant scores in classification steps: pDCs resting vs pDCs activated (a), MPs vs LPs (b) and acute myeloid leukemias vs ALLs (c). Each case is represented in different color according to its sample type. Cases with discriminant score minor than 0.05 in absolute value falls in no classification area and are considered as unclassified. (d) Unsupervised hierarchical clustering of BPDCN (red), resting pDCs (orange), MPs (blue) and LPs (green) samples based on the expression of top 2500 genes with higher s.d. BPDCN and pDC clustered together in the main branch, close to most MPs, while LPs and a few MPs clustered in the second branch.
Second, as pDCs can derive from both the myeloid and the lymphoid lineage, we analyzed the molecular profiles of a series of myeloid precursor and lymphoid precursor cells and identified 368 genes differentially expressed in the two compartments (Supplementary Table 4). Thereafter, based on these genes, we generated a cell type classifier and classified the six BPDCN cases according to the myeloid vs lymphoid precursor signature. In keeping with their postulated myeloid origin, BPDCN cases proved to be significantly more related to the myeloid precursors at the molecular level (Figure 1b; Supplementary Table 5). Hierarchical clustering then showed that BPDCN was closer to resting pDC than to MPs or LPs (Figure 1d).
In order to test the consistency of BPDCN classification within AML, we developed a cell type classifier able to discriminate AML from ALL and applied it to BPDCN. We found that BPDCN could not be clearly assigned to either of the two categories. In fact, although 4/6 samples appeared to be more related to AML (while two were closer to ALL), the scores were not significant (Figure 1c; Supplementary Table 6). BPDCN shared with AML and ALL, respectively, two different sets of deregulated genes, each consisting of 49 members. The former set referred to biological processes associated with hematopoietic differentiation, the latter to cellular programs involved in cellular adhesion and communication (Supplementary Table 7).
Together, these results indicate that BPDCN is a malignancy with correspondence to myeloid-derived resting pDCs but with distinct profile from other acute leukemias.
BPDCNs differ from non-neoplastic pDCs for several specific genes and cellular programs
We first analyzed data generated by DASL Illumina platform. We applied unsupervised algorithms to evaluate whether BPDCNs and pDCs could form distinct groups, by focusing on the training set of cases. The principal component analysis showed that neoplastic and non-neoplastic samples were distinct (Figure 2a) without significant sub-grouping. The following unsupervised hierarchical clustering drew similar results; tumors and controls segregated into distinct groups according to the similarities among samples. However, tumors appeared further divided into two sub-groups, one presenting more evident differential expression in comparison with pDCs (Figure 2b). These apparent sub-groups, nevertheless, did not correspond to any clinicopathological finding (biopsy site, age, gender and treatment response). The unique distinguishing feature was the enrichment of one group in frozen vs FFPE samples. Therefore, we specifically investigated whether significant differences occurred based on the examined tissue (cryopreserved vs FFPE). Indeed, supervised comparison of the two groups failed to identify any single gene differentially expressed, indicating the non-specificity of the clustering and the reliability of both the sources.
Figure 2.
Unsupervised hierarchical clustering of the BPDCN training set. (a) Principal component analysis well discriminates between tumors (blue) and controls (red). (b) Unsupervised hierarchical clustering was performed on 14 BPDCN samples (tumors) and four pDCs resting (controls), according to the expression of 5049 genes. In the heat-map, each row represents a gene and each column represents a sample. The color scale illustrates the relative expression level of a gene across all samples: red represents an expression level above the mean, blue represents expression lower than the mean. BPDCN cases (blue) roughly clustered together, scattering among normal pDCs (red).
In the next step, we used a supervised approach to uncover genes differentially expressed between tumor and control samples, and applied relatively stringent parameters. We identified a molecular signature of 142 genes able to group tumors and controls in two well-defined categories (Figures 3a and b). Among these 142 genes, 126 (89%) were upregulated, while 16 (11%) were downregulated (Supplementary Table 8) in the tumors. We then performed the hierarchical clustering using these 142 discriminatory genes in the test set, that properly clustered according to the cell type (tumors vs controls) (Figure 3c). To further validate the discriminant power of this molecular signature, we developed a cell type classifier using a support vector machine algorithm, and applied it to the test set. Remarkably, it recognized each sample as tumor or control, with an overall accuracy of 100% (Supplementary Table 9).
Figure 3.
Supervised analysis of BPDCN. (a) The Volcano plot shows the results of t-test for differential expression in BPDCNs vs pDCs. A total of 142 differentially expressed genes, highlighted in red, were selected according to filtering parameters P-value <0.05, multiple testing correction according to Benjamini–Hochberg and fold change >2. (b) Supervised analysis of BPDCN training set samples based on the identified signature of 142 genes, well distinguished controls in red from tumors in blue. The genes downregulated are reported in blue and the genes upregulated in red. (c) The signature of 142 genes correctly classified all the 11 BPDCN of the test set according to support vector machine algorithm. Hierarchical clustering could clearly separate BPDCN cases (blue) from pDCs (red). In the matrix, each row represents a gene and each column represents a sample. The color scale illustrates the relative expression level of a gene across all samples: red represents an expression level above the mean, blue represents expression lower than the mean. (d) The signature of 142 genes revealed a significant enrichment in selected functional categories according to Gene Ontology (the top ranked cellular pathways according to P-value are shown in the pie chart).
Among the 142 genes, someones retained potential pathogenetic relevance, including BCL2 (anti-apoptotic factor), CCND1 (cell cycle regulator) and IRF4 (interferon-regulatory factor). Interestingly, when looking for cellular programs specifically over-represented in BPDCN, to better infer possible pathogenetic mechanisms, we found alterations of molecules such as integrin (ITGB5), laminin (LAMA4), dermatopontin (DPT), angiopoietin (ANGPT2) and collagen (COL1A1), most of them encoding for transmembrane proteins (Supplementary Figure 2) and suggesting deregulation of cellular adhesion, intercellular communication and vascular development programs (Figure 3d).
To validate GEP data, we tested by IHC BCL2 and IRF4 in the validation test samples. Interestingly, consistently with GEP, we observed a strong positivity for the BCL2 (Figure 4) and IRF4 (not shown) proteins in BPDCNs and CAL-1 cells, while the normal counterparts turned out to be constantly negative.
Figure 4.
BCL2 expression in BPDCN. (a) Gene expression analysis reported BCL2 to be significantly overexpressed in tumors (blue box) compared with controls (red box). The two blue points represent two tumor outliers. (b) Immunohistochemical assay confirmed the BCL2 negativity of normal pDCs isolated from blood or packed in aggregates in the lymph node (as far as the latter was concerned, pDC aggregates were confirmed by immunostaining for CD123 and BDCA-2, showed in the insets) and the marked BCL2 positivity of BPDCN samples and CAL-1 cell line (Olympus BX41 microscope, Olympus CAMEDIA C-7070 camera; original magnification ×400, colors balanced after acquisition with Adobe Photoshop).
NF-kB pathway is a candidate therapeutic target in BPDCN
We analyzed the GEP of BPDCN to identify a potential rationale for the introduction of pathway-specific targeted therapies. In particular, we performed an integrated genomic approach of target therapy prediction by using four different platforms: Gene set Enrichment Analysis, Ingenuity Pathway Analysis, Metacore and C-Map. Of them, 3/4, found NF-kB pathway aberrantly activated in BPDCNs, indicating it as a suitable therapeutic target (Supplementary Figure 3). We applied IHC in the validation set samples to assess the activation status of the NF-kB pathway according to the nuclear translocation of key components. All the BPDCN cases and the CAL-1 cell line displayed signs of canonical NF-kB pathway activation with stronger nuclear positivity for p50 and c-Rel, whereas pDCs, both from peripheral blood and lymph nodes, had only slight or undetectable nuclear expression of these same factors (Figure 5). Consistently with NF-kB activation in BPDCN following the canonical pathway, mediators of the non-canonical NF-kB pathway, namely p52 and RelB, were not expressed.
Figure 5.
Immunohistochemical validation of NF-kB pathway activation in BPDCN. Immunohistochemical assay reported an evident nucleocytoplasmatic positivity for p50 (c, d), c-Rel (g, h) and RelA (k, l) in CAL-1 cell line and BPDCN patients, as expected upon NF-kB pathway activation (black arrows indicate example of nucleocytoplasmatic positivity). On the contrary, pDCs isolated from blood (a, e, i, m, q) or packed in aggregates in the lymph node (b, f, j, n, r: black arrows indicate positive internal controls for nucleocytoplasmatic positivity) reveal a weak positivity confined to the cytoplasm. The latter finding is observed also at the determination of p52 (o, p) and RelB (s, t), in both BPDCN patients, CAL-1 cell line and normal pDCs. (Olympus BX41 microscope, Olympus CAMEDIA C-7070 camera; original magnification ×400, colors balanced after acquisition with Adobe Photoshop).
Subsequently to the confirmation of the NF-kB pathway activation in BPDCNs, we aimed to assess whether its inhibition could represent an effective therapeutic strategy. To this purpose, we treated CAL-1 cells with increasing concentrations of Bortezomib (0–100 nm), a proteasome inhibitor with well-known anti-NF-kB properties, approved for the treatment of diverse hematological malignancies. Furthermore, CAL-1 cells were treated with cytarabine, a conventional chemotherapy agent currently employed in the treatment of BPDCN, at increasing concentrations (0–1000 nm). Untreated CAL-1 cells were used as controls.
After 24 h, CAL-1 cells registered a reduction of viability by 50% with 30 nm Bortezomib establishing, after repeated experiments, the IC50 at 30 nm. On the contrary, cytarabine treatment did not induce significant decrease in CAL-1 cell viability at 24 and 48 h even using the highest drug concentration (1000 nm) (Supplementary Figure 4).
On the basis of these results, CAL-1 cells appeared to be highly responsive to Bortezomib while proving refractory to cytarabine treatment.
To better define the sensitivity of CAL-1 cell line to Bortezomib, we incubated CAL-1 cells with Bortezomib (0–50 nm) for 24 h and analyzed the cell death rate (Figure 6).
Figure 6.
Bortezomib induces cell death in CAL-1 cell line. CAL-1 cells were incubated with increasing concentrations of Bortezomib, collected at 24 h and stained with Annexin-V-FITC and propidium iodide (PI) to detect early and late apoptotic cells. As shown in the histogram, CAL-1 cells rapidly died after low doses of Bortezomib. In parallel, CAL-1 cells treated with Bortezomib were stained for BrdU and subjected to flow cytometry analysis. Data are represented in the histogram as percentage of BrdU positive in the total population. Bortezomib induced increases in BrdU incorporation but the cells were gradually arrested in G0/G1 phase as shown by the analysis of cell cycle progression (Navios Flow Cytometers, Beckman Coulter).
Untreated CAL-1 cells showed 15.6% of cell death, whereas CAL-1 cells treated with Bortezomib displayed a dose-dependent increase of cell death with 63% of cells dying at 24 h with 50 nm of Bortezomib (CAL-1 + 50 nm Bortezomib vs control, P = 0.0001 with the Z-test for two sample proportions).
We further investigated the effect of Bortezomib on CAL-1 cell proliferative capability by treating cells with escalating concentrations of Bortezomib (0–50 nm) and analyzing the cellular proliferation through the BrdU assay.
The analysis of cell cycle phase distribution demonstrated that Bortezomib led to an increase in the number of cells in G1 phase at 24 h post treatment. At that time point, more than 40% of cells arrested into the G0/G1 cell cycle phase following 50 nm Bortezomib administration (Figure 6).
Consistently with BrdU incorporation, Bortezomib blocked the CAL-1 cell line proliferation, hampering the cells to enter in S phase.
These results collectively confirmed that the BPDCN cell line CAL-1 was highly sensitive to Bortezomib treatment both in terms of proliferation inhibition and cell death induction.
As Bortezomib displays a wide range of activities, we decided to verify whether it specifically targeted NF-kB signaling in CAL-1 cell line. We treated CAL-1 cells for 6 h with Bortezomib at IC50 (30 nm) and then investigated the possible effects at both transcriptional and protein level. First, after treatment with Bortezomib, IHC demonstrated a clear reduction of RELA nuclear localization, which was consistent with quenching of NF-kB activation. Similarly, western blot showed a marked decrease in RELA phosphorylation (Figures 7a and b). The statistical significance of differences in signal intensities at western blot (measured by means of ChemiDoc-It instrument) was confirmed by densitometric analysis performed using a dedicated software (ImageJ).
Figure 7.
Bortezomib inhibits NF-kB canonical pathway in CAL-1 cell line. (a) IHC showed nuclear positivity for RelA in CAL-1 cell line untreated (Crtl). After Bortezomib and BMS-345541 administration, the CAL-1 cells became RelA negative in the nuclei. (b) RELA (p65) expression and phosphorylation by whole cellular extracts of CAL-1 cell line were investigated at 6 h after exposure to Bortezomib (Velcade) 150 nm and BMS-345541 7 µm by western blot. Phospho-RELA was significantly reduced after treatment with bortezomib and completely abolished after BMS-345541. Total RELA was, conversely, increased after bortezomib administration likely due to its stabilization through IkB. Beta-actin served as control for protein loading. Signal intensities in single blots were measured by ChemiDoc-It imaging system. Results presented here have been confirmed in two additional experiments. (c) CAL-1 cells were incubated with 30 nm Bortezomib (IC50) for 6 h and then analyzed by GEP, which revealed a substantial modification of NF-kB signature after Bortezomib administration. CAL-1 cells pre- and post-treatment are shown as ‘CRTL’ and ‘Bortez’, respectively. The genes upregulated are colored in red and the downregulated in blue. (d) Gene set Enrichment Analysis enrichment plot shows that NF-kB signaling is significantly enriched in BPDCN vs pDCs and CAL-1 untreated vs treated. In the enrichment plot the x axis shows the rank order of genes from the most upregulated to the most downregulated between BPDCNs and pDCs and between CAL-1 cell line before and after Bortezomib treatment, respectively. The barcode indicates the position of NF-kB signaling genes in the ranking list. The y axis shows the distribution of the running enrichment score generated by walking down the list of ranked genes. (e) Gene Ontology analysis reported that after Bortezomib administration, the most deregulated pathways according to P-value are involved in cellular communication and response to external stimuli. (f) A support vector machine-based cell classifier classified treated and untreated CAL-1 according to their similarity to normal pDC or primary BPDCN based on the expression of genes modulated by bortezomib.
Consistent with that, GEP indicated a global modification of genes involved in the NF-kB pathway, as indicated by Gene set Enrichment Analysis (Figures 7c and d), while Gene Ontology analysis revealed the most enriched biological processes modified by Bortezomib, were involved into organelle, cytoplasm and cytoskeleton organization (Figure 7e). Noteworthy, based on the expression of genes modulated by bortezomib (Figure 7c) a support vector machine algorithm classified treated CAL-1 cells as non-neoplastic pDC, whereas untreated cells, as expected, corresponded to primary BPDCN (Figure 7f; Supplementary Table 10). This analysis indicated that treatment with bortezomib could revert the malignant phenotype of CAL-1 cells.
To further confirm that NF-kB inhibition can be sufficient to induce apoptosis and cell cycle modifications in BPDCN, we treated CAL-1 cell line with increasing concentrations of the IKK inhibitor BMS-345541. For the latter, we experimentally calculated an IC50 of 4 um (Supplementary Figure 5). After incubating CAL-1 with 4 um of BMS-345541 for 6 h, we confirmed by IHC a reduction of RELA staining, with the same trend as the one observed after Bortezomib (Figure 7a). Similarly, western blot documented that complete de-phosphorylation of RELA (Figure 7b).
DISCUSSION
This paper addressed some critical points about the cell of origin and the pathogenesis of BPDCN based on gene expression profiling in a relatively extensive panel of cases. In fact, thanks to an international effort, we could enroll a significant number of patients, if we consider the rarity of this tumor, representing only the 0.7% of cutaneous leukemias in western countries.27 This work significantly differs from the few previously published ones since it (1) establishes the cellular counterpart of BPDCN, recognizing it as a clinical entity, possibly in between myeloid and lymphoid acute leukemias, and (2) represents the first analysis performed on the whole transcriptome of BPDCN compared with its normal counterpart, providing the first rationale, to our knowledge, for a molecular-targeted therapy in BPDCN, and offering evidence of its effectiveness ex vivo.
We challenge the obscure ontogenesis of BPDCN unraveling its relatedness to myeloid-derived pDCs in resting state. The origin of BPDCN has been a topic of persistent debate for many years.28,29 In 2008, the precursors of pDCs were indicated as their putative normal counterpart but based only on immunohistochemical evidences.1,3,4 Until now, no study has investigated more in depth its effective derivation, eventually confirming this assumption. As we aimed to better clarify the molecular pathogenesis of the disease by gene expression profiling (GEP), at the outset we tried to define the proper normal counterpart. We found a strict similarity with resting rather than activated pDCs. Further, we demonstrated that BPDCN is indeed more related to myeloid than lymphoid precursors, providing the first molecular evidence that BPDCN stems from resting pDCs of myeloid origin. This is not trivial. In fact, the origin of pDCs has remained unclear for a long time. Only recently, it was demonstrated that pDCs could originate from both lymphoid and myeloid precursors with different functional properties.30–32 This new scenario has then opened a debate on the origin of BPDCN, also in the light of possible therapeutic implications. In fact, despite BPDCN is currently included among acute myeloid leukemias, the therapeutic strategies applied to cure ALLs appeared particularly effective in BPDCN as well.12–14 In this regard, we also tested the relationships among the three conditions (AML, ALL and BPDCN). Intriguingly, despite the clear myeloid origin (based on GEP), BPDCN did not appear so closely associated with AML, rather presenting with an ambiguous molecular profile partially related to either AML and ALL. Based on that, it would be definitely warranted on the one hand to randomly compare AML-like vs ALL-like treatments in this setting, and on the other hand to comprehensively investigate the possible occurrence of specific genetic lesions (characteristic of myeloid or lymphoid malignancies) in BPDCN.
We then analyzed the GEP of BPDCN patients compared with their closest normal counterpart, the myeloid resting pDCs, aiming to identify deregulated genes and cellular programs. We identified in a training set of cases a molecular signature consisting of 142 genes discriminating between tumors and controls. Notably, the robustness of the signature was validated in an independent test set and was used to develop a molecular classifier that successfully distinguished BPDCNs and pDCs.
Further, to verify whether the transcriptional deregulation caused a parallel abnormal protein production, we fruitfully performed IHC tests on an additional panel of cases.
A careful investigation of the 142 discriminant genes possibly provided several insights into the functional alterations of BPDCN, revealing an extensive deregulation of functions typically impaired in malignant cells, such as cell adhesion, matrix remodeling, vascular development and proliferation. In particular, were reported alterations of molecules such as integrin, laminin, dermatopontin, angiopoietin and collagen that promotes tumor invasiveness, activation of the vascular development and cellular aggregation (Supplementary Figure 2).33–38
These alterations could possibly explain the histological features of BPDCN, with tumor cells tightly packed (increased cell–cell adhesion), the high aggressiveness of the process (matrix invasion, migration and proliferation) and the scarce response to chemotherapy (apoptosis alteration).
Overall, a substantial impairment in cell cycle regulation appeared, as commonly observed during tumorigenesis. In particular, the alteration of G1/S transition seems to be crucial for BPDCN oncogenesis21 and, to this regard, the GEP of BPDCN revealed the upregulation of genes involved in cell cycle division, including CyclinD1, a master regulator of cell cycle progression and BCL2, a cell death inhibitor, that may contribute to tumor cell survival and chemo resistance.39,40 It should be noted that under physiological conditions, pDCs are consistently BCL2 negative, allowing a considerable rate of apoptosis.41,42 Due to the potential pathogenetic relevance, we further investigated the possible genetic determinants of BCL2 deregulation. However, we failed to identify genetic lesions at the BCL2 locus (not shown. Data on high throughput sequencing of BPDCN will be presented apart). Therefore, different mechanisms (for example, epigenetic, transcriptional regulation and so on) should be explored.
Second, to assess possible correlations between gene expression deregulation and primary genetic lesions, we matched our messenger RNA expression data with previous cytogenetic findings. We found some key regulatory genes (for example, DAPK2, ZNF346, SYK) downregulated in the correspondence of the chromosomes 9 and 5, frequently altered in BPDCN, as previously reported in CGH studies.21–23 Conversely, we identified a significant enrichment in genes on the cytobands chr2q33, chr3p26, chr3q13, chr11p15, chr2p13 and chr1q32 never reported before (not shown). These findings further highlight the evidence that BPDCN patients have a complex and quite heterogeneous genetics, definitely warranting a better definition in future studies.
As we defined pathways and cellular functions affected in BPDCN, we sought for possible therapeutic targets. We thus applied an integrated bioinformatic approach for target prediction and 3/4 systems recognized an aberrant NF-kB pathway activation, frequent in cancers.43–45
The NF-kB family consists of five closely related DNA-binding proteins working as various homodimers and heterodimers: RelA, c-Rel and p50 belonging to the canonical pathway, and RelB and p52 involved in the alternative pathway. The canonical pathway can be rapidly activated by a plethora of stimuli (for example, cytokines and antigens) after which, the released NF-kB molecules translocate into the nucleus to regulate the expression of a wide range of genes, particularly those involved in cell proliferation, adhesion and migration.
As NF-kB molecules shifted into nuclei upon activation, we used IHC to assess both the canonical and the alternative signaling activation in BPDCNs.
IHC revealed a marked nuclear expression of c-Rel and p50 proteins in tumor samples and in the BPDCN-derived cell line CAL-1, whrereas pDCs presented with a consistently cytoplasmic staining. This prompted us to verify whether NF-kB inhibition may represent an effective therapeutic strategy in BPDCN. So far, the most successful way to inhibit NF-kB pathway has consisted in blocking proteasome degradation and hence preventing NF-kB activation. We then tested Bortezomib, a well-known proteasome inhibitor currently employed in the treatment of multiple myeloma and some non-Hodgkin lymphomas.46–48 Bortezomib turned out to be more effective than cytarabine (one of the drugs most used in this setting), rapidly inducing BPDCN cell death ex vivo.49 In particular, it blocked cell cycle progression, arresting the cells in the G1/G0 phase and finally leading them to death. Of note, proteasome inhibitors can act as anti-neoplastic agents through different mechanisms but we confirmed by GEP and IHC that Bortezomib in BPDCN cell line specifically inhibits NF-kB signaling. In addition, we showed that a selective NF-kB inhibitor, such as the IkkB inhibitor BMS-345541 induced cell death and pathway inhibition in a comparable manner. The evidence of a so striking effect of these drugs on BPDCN cells—that were, on the contrary, resistant to cytarabine certainly opens new questions and perspectives. To this regard, the exact mechanisms underlying NF-kB activation needs to be better investigated as a paraphysiological stimulation might trigger the pathway. However, this possibility would be associated to a global activation of the cells that appeared, by contrast, to be resting. Therefore, a concomitant blockage of the physiological maturation would be expected. On the other hand, in some cancers, the constitutive activation of NF-kB is clearly caused by genetic alterations of genes encoding NF-kB members, as well as their inhibitors or positive regulators.50–55 Hence, studies properly addressing this issue are needed. Second, as BPDCN is characterized by a dismal prognosis and no effective treatment is known beside stem cell transplantation, clinical trials exploring the possible effectiveness of proteasome inhibitors and other NF-kB inhibitors might be designed. In this regard, however, it should be underlined that due to the rarity of the disease and the current high rate of misdiagnoses,14,56 dedicated national and international efforts are warranted.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Dr Guarguaglini at the pharmacy-compounding center of S. Orsola-Malpighi Hospital of Bologna, Italy for providing Bortezomib. This work was supported by AIRC (IG10519 and 5xMille10007, Prof Pileri), AIRC (IG 2013 N.14355, Prof Piccaluga), Centro Interdipartimentale per la Ricerca sul Cancro ‘G. Prodi’, BolognAIL, RFO (Prof Pileri, Prof Piccaluga), FIRB Futura 2011 (RBFR12D1CB; Prof Piccaluga), Fondazione Cassa di Risparmio in Bologna, Fondazione della Banca del Monte e Ravenna, Progetto Strategico di Ateneo 2006 (Prof Pileri and Dr Piccaluga) and by MIUR (PRIN 2011, Prof Facchetti and Prof Pileri).
AIRC 5XMILLE CONSORTIUM ‘GENETICS-DRIVEN TARGETED MANAGEMENT OF LYMPHOID MALIGNANCIES’
Robin Foà, Sabina Chiaretti, Filippo Berardelli, Brunangelo Falini, Enrico Tiacci, Giorgio Inghirami, Roberto Piva, Gianluca Gaidano, Davide Rossi, Stefano Pileri, Pier Paolo Piccaluga.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Supplementary Information accompanies this paper on the Leukemia website (http://www.nature.com/leu)
REFERENCES
- 1.Facchetti FJD, Petrella T. Blastic plasmacytoid dendritic cell neoplasms. In: Swerdlow SH, Campo E, Harris NL, et al., editors. WHO Classification of Tumors of Haematopoietic and Lymphoid Tissues. Lyon, France: IARC; 2008. pp. 145–147. [Google Scholar]
- 2.Assaf C, Gellrich S, Whittaker S, Robson A, Cerroni L, Massone C, et al. CD56-positive haematological neoplasms of the skin: a multicentre study of the Cutaneous Lymphoma Project Group of the European Organisation for Research and Treatment of Cancer. J Clin Pathol. 2007;60:981–989. doi: 10.1136/jcp.2006.042135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grouard G, Rissoan MC, Filgueira L, Durand I, Banchereau J, Liu YJ. The enigmatic plasmacytoid T cells develop into dendritic cells with interleukin (IL)-3 and CD40-ligand. J Exp Med. 1997;185:1101–1111. doi: 10.1084/jem.185.6.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cella M, Facchetti F, Lanzavecchia A, Colonna M. Plasmacytoid dendritic cells activated by influenza virus and CD40L drive a potent TH1 polarization. Nat Immunol. 2000;1:305–310. doi: 10.1038/79747. [DOI] [PubMed] [Google Scholar]
- 5.Soumelis V, Liu YJ. From plasmacytoid to dendritic cell: morphological and functional switches during plasmacytoid pre-dendritic cell differentiation. Eur J Immunol. 2006;36:2286–2292. doi: 10.1002/eji.200636026. [DOI] [PubMed] [Google Scholar]
- 6.Feuillard J, Jacob MC, Valensi F, Maynadie M, Gressin R, Chaperot L, et al. Clinical and biologic features of CD4(+)CD56(+) malignancies. Blood. 2002;99:1556–1563. doi: 10.1182/blood.v99.5.1556. [DOI] [PubMed] [Google Scholar]
- 7.Garnache-Ottou F, Feuillard J, Saas P. Plasmacytoid dendritic cell leukaemia/lymphoma: towards a well defined entity? Br J Haematol. 2007;136:539–548. doi: 10.1111/j.1365-2141.2006.06458.x. [DOI] [PubMed] [Google Scholar]
- 8.Facchetti F, Ungari M, Marocolo D, Lonardi S, Vermi W. Blastic plasmacytoid dendritic cell neoplasm. Hematol Meet Rep. 2009;3:1–3. [Google Scholar]
- 9.Bendriss-Vermare N, Chaperot L, Peoc’h M, Vanbervliet B, Jacob MC, Briere F, et al. In situ leukemic plasmacytoid dendritic cells pattern of chemokine receptors expression and in vitro migratory response. Leukemia. 2004;18:1491–1498. doi: 10.1038/sj.leu.2403452. [DOI] [PubMed] [Google Scholar]
- 10.Petrella T, Bagot M, Willemze R, Beylot-Barry M, Vergier B, Delaunay M, et al. Blastic NK-cell lymphomas (agranular CD4+CD56+ hematodermic neoplasms): a review. Am J Clin Pathol. 2005;123:662–675. [PubMed] [Google Scholar]
- 11.Chen J, Zhou J, Qin D, Xu S, Yan X. Blastic plasmacytoid dendritic cell neoplasm. J Clin Oncol. 2011;29:e27–e29. doi: 10.1200/JCO.2010.30.5441. [DOI] [PubMed] [Google Scholar]
- 12.Tsagarakis NJ, Kentrou NA, Papadimitriou KA, Pagoni M, Kokkini G, Papadaki H, et al. Acute lymphoplasmacytoid dendritic cell (DC2) leukemia: results from the Hellenic Dendritic Cell Leukemia Study Group. Leuk Res. 2010;34:438–446. doi: 10.1016/j.leukres.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 13.Jegalian AG, Facchetti F, Jaffe ES. Plasmacytoid dendritic cells: physiologic roles and pathologic states. Adv Anat Pathol. 2009;16:392–404. doi: 10.1097/PAP.0b013e3181bb6bc2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pagano L, Valentini CG, Pulsoni A, Fisogni S, Carluccio P, Mannelli F, et al. Blastic plasmacytoid dendritic cell neoplasm with leukemic presentation: an Italian multicenter study. Haematologica. 2013;98:239–246. doi: 10.3324/haematol.2012.072645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pileri A, Delfino C, Grandi V, Agostinelli C, Pileri SA, Pimpinelli N. Blastic plasmacytoid dendritic cell neoplasm (BPDCN): the cutaneous sanctuary. G Ital Dermatol Venereol. 2012;147:603–608. [PubMed] [Google Scholar]
- 16.Piccaluga PP, Paolini S, Sapienza MR, Pileri SA. Blastic plasmacytoid dendritic cell neoplasm: is it time to redefine the standard of care? Expert Rev Hematol. 2012;5:353–355. doi: 10.1586/ehm.12.33. [DOI] [PubMed] [Google Scholar]
- 17.Petrella T, Dalac S, Maynadie M, Mugneret F, Thomine E, Courville P, et al. CD4+ CD56+ cutaneous neoplasms: a distinct hematological entity? Groupe Francais d’Etude des Lymphomes Cutanes (GFELC) Am J Surg Pathol. 1999;23:137–146. doi: 10.1097/00000478-199902000-00001. [DOI] [PubMed] [Google Scholar]
- 18.Leroux D, Mugneret F, Callanan M, Radford-Weiss I, Dastugue N, Feuillard J, et al. CD4(+), CD56(+) DC2 acute leukemia is characterized by recurrent clonal chromosomal changes affecting 6 major targets: a study of 21 cases by the Groupe Francais de Cytogenetique Hematologique. Blood. 2002;99:4154–4159. doi: 10.1182/blood.v99.11.4154. [DOI] [PubMed] [Google Scholar]
- 19.Reichard KK, Burks EJ, Foucar MK, Wilson CS, Viswanatha DS, Hozier JC, et al. CD4(+) CD56(+) lineage-negative malignancies are rare tumors of plasmacytoid dendritic cells. Am J Surg Pathol. 2005;29:1274–1283. doi: 10.1097/01.pas.0000172194.32918.5c. [DOI] [PubMed] [Google Scholar]
- 20.Dijkman R, van Doorn R, Szuhai K, Willemze R, Vermeer MH, Tensen CP. Gene-expression profiling and array-based CGH classify CD4+CD56+ hematodermic neoplasm and cutaneous myelomonocytic leukemia as distinct disease entities. Blood. 2007;109:1720–1727. doi: 10.1182/blood-2006-04-018143. [DOI] [PubMed] [Google Scholar]
- 21.Jardin F, Callanan M, Penther D, Ruminy P, Troussard X, Kerckaert JP, et al. Recurrent genomic aberrations combined with deletions of various tumour suppressor genes may deregulate the G1/S transition in CD4+CD56+ haematodermic neoplasms and contribute to the aggressiveness of the disease. Leukemia. 2009;23:698–707. doi: 10.1038/leu.2008.359. [DOI] [PubMed] [Google Scholar]
- 22.Wiesner T, Obenauf AC, Cota C, Fried I, Speicher MR, Cerroni L. Alterations of the cell-cycle inhibitors p27(KIP1) and p16(INK4a) are frequent in blastic plasmacytoid dendritic cell neoplasms. J Invest Dermatol. 2009;130:1152–1157. doi: 10.1038/jid.2009.369. [DOI] [PubMed] [Google Scholar]
- 23.Lucioni M, Novara F, Fiandrino G, Riboni R, Fanoni D, Arra M, et al. Twenty-one cases of blastic plasmacytoid dendritic cell neoplasm: focus on biallelic locus 9p21.3 deletion. Blood. 2011;118:4591–4594. doi: 10.1182/blood-2011-03-337501. [DOI] [PubMed] [Google Scholar]
- 24.Biddolph SC, Gatter K. Lymphocytes: a practical approach. In: Rowland-Jones SL, McMichael AJ, editors. Immunohistochemistry of Lymphoid Organs. Oxford UOUP: 2000. pp. 27–54. [Google Scholar]
- 25.Agostinelli C, Paterson JC, Gupta R, Righi S, Sandri F, Piccaluga PP, et al. Detection of LIM domain only 2 (LMO2) in normal human tissues and haematopoietic and non-haematopoietic tumours using a newly developed rabbit monoclonal antibody. Histopathology. 2012;61:33–46. doi: 10.1111/j.1365-2559.2012.04198.x. [DOI] [PubMed] [Google Scholar]
- 26.Maeda T, Murata K, Fukushima T, Sugahara K, Tsuruda K, Anami M, et al. A novel plasmacytoid dendritic cell line, CAL-1, established from a patient with blastic natural killer cell lymphoma. Int J Hematol. 2005;81:148–154. doi: 10.1532/ijh97.04116. [DOI] [PubMed] [Google Scholar]
- 27.Ng AP, Lade S, Rutherford T, McCormack C, Prince HM, Westerman DA. Primary cutaneous CD4+/CD56+ hematodermic neoplasm (blastic NK-cell lymphoma): a report of five cases. Haematologica. 2006;91:143–144. [PubMed] [Google Scholar]
- 28.Rathinam C, Sauer M, Ghosh A, Rudolph C, Hegazy A, Schlegelberger B, et al. Generation and characterization of a novel hematopoietic progenitor cell line with DC differentiation potential. Leukemia. 2006;20:870–876. doi: 10.1038/sj.leu.2404157. [DOI] [PubMed] [Google Scholar]
- 29.Panoskaltsis N. Dendritic cells in MDS and AML - cause, effect or solution to the immune pathogenesis of disease? Leukemia. 2005;19:354–357. doi: 10.1038/sj.leu.2403634. [DOI] [PubMed] [Google Scholar]
- 30.Sathe P, Vremec D, Wu L, Corcoran L, Shortman K. Convergent differentiation: myeloid and lymphoid pathways to murine plasmacytoid dendritic cells. Blood. 2013;121:11–19. doi: 10.1182/blood-2012-02-413336. [DOI] [PubMed] [Google Scholar]
- 31.Ishikawa F, Niiro H, Iino T, Yoshida S, Saito N, Onohara S, et al. The developmental program of human dendritic cells is operated independently of conventional myeloid and lymphoid pathways. Blood. 2007;110:3591–3660. doi: 10.1182/blood-2007-02-071613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang GX, Lian ZX, Kikuchi K, Moritoki Y, Ansari AA, Liu YJ, et al. Plasmacytoid dendritic cells of different origins have distinct characteristics and function: studies of lymphoid progenitors versus myeloid progenitors. J Immunol. 2005;175:7281–7287. doi: 10.4049/jimmunol.175.11.7281. [DOI] [PubMed] [Google Scholar]
- 33.Sprenger CC, Drivdahl RH, Woodke LB, Eyman D, Reed MJ, Carter WG, et al. Senescence-induced alterations of laminin chain expression modulate tumorigenicity of prostate cancer cells. Neoplasia. 2008;10:1350–1361. doi: 10.1593/neo.08746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hung WY, Huang KH, Wu CW, Chi CW, Kao HL, Li AF, et al. Mitochondrial dysfunction promotes cell migration via reactive oxygen species-enhanced beta5-integrin expression in human gastric cancer SC-M1 cells. Biochim Biophys Acta. 2012;1820:1102–1110. doi: 10.1016/j.bbagen.2012.04.016. [DOI] [PubMed] [Google Scholar]
- 35.Yamatoji M, Kasamatsu A, Kouzu Y, Koike H, Sakamoto Y, Ogawara K, et al. Dermatopontin: a potential predictor for metastasis of human oral cancer. Int J Cancer. 2012;130:2903–2911. doi: 10.1002/ijc.26328. [DOI] [PubMed] [Google Scholar]
- 36.Tamamura R, Nagatsuka H, Siar CH, Katase N, Naito I, Sado Y, et al. Comparative analysis of basal lamina type IV collagen alpha chains, matrix metalloproteinases-2 and -9 expressions in oral dysplasia and invasive carcinoma. Acta Histochem. 2013;115:113–119. doi: 10.1016/j.acthis.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 37.Hatfield KJ, Hovland R, Oyan AM, Kalland KH, Ryningen A, Gjertsen BT, et al. Release of angiopoietin-1 by primary human acute myelogenous leukemia cells is associated with mutations of nucleophosmin, increased by bone marrow stromal cells and possibly antagonized by high systemic angiopoietin-2 levels. Leukemia. 2007;22:287–293. doi: 10.1038/sj.leu.2404985. [DOI] [PubMed] [Google Scholar]
- 38.Van Driessche A, Gao L, Stauss HJ, Ponsaerts P, Van Bockstaele DR, Berneman ZN, et al. Antigen-specific cellular immunotherapy of leukemia. Leukemia. 2005;19:1863–1871. doi: 10.1038/sj.leu.2403930. [DOI] [PubMed] [Google Scholar]
- 39.Galteland E, Sivertsen EA, Svendsrud DH, Smedshammer L, Kresse SH, Meza-Zepeda LA, et al. Translocation t(14;18) and gain of chromosome 18//BCL2: effects on BCL2 expression and apoptosis in B-cell non-Hodgkin’s lymphomas. Leukemia. 2005;19:2313–2323. doi: 10.1038/sj.leu.2403954. [DOI] [PubMed] [Google Scholar]
- 40.Wang M, Sun L, Qian J, Han X, Zhang L, Lin P, et al. Cyclin D1 as a universally expressed mantle cell lymphoma-associated tumor antigen for immunotherapy. Leukemia. 2009;23:1320–1328. doi: 10.1038/leu.2009.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reed JC, Zha H, Aime-Sempe C, Takayama S, Wang HG. Structure-function analysis of Bcl-2 family proteins. Regulators of programmed cell death. Adv Exp Med Biol. 1996;406:99–112. [PubMed] [Google Scholar]
- 42.Willis S, Day CL, Hinds MG, Huang DC. The Bcl-2-regulated apoptotic pathway. J Cell Sci. 2003;116:4053–4056. doi: 10.1242/jcs.00754. [DOI] [PubMed] [Google Scholar]
- 43.Turco MC, Romano MF, Petrella A, Bisogni R, Tassone P, Venuta S. NF-[kappa]B//Rel-mediated regulation of apoptosis in hematologic malignancies and normal hematopoietic progenitors. Leukemia. 2003;18:11–17. doi: 10.1038/sj.leu.2403171. [DOI] [PubMed] [Google Scholar]
- 44.Karin M. Nuclear factor-kappaB in cancer development and progression. Nature. 2006;441:431–436. doi: 10.1038/nature04870. [DOI] [PubMed] [Google Scholar]
- 45.Sarkar FH, Li Y, Wang Z, Kong D. NF-kappaB signaling pathway and its therapeutic implications in human diseases. Int Rev Immunol. 2008;27:293–319. doi: 10.1080/08830180802276179. [DOI] [PubMed] [Google Scholar]
- 46.Laubach JP, Mahindra A, Mitsiades CS, Schlossman RL, Munshi NC, Ghobrial IM, et al. The use of novel agents in the treatment of relapsed and refractory multiple myeloma. Leukemia. 2009;23:2222–2232. doi: 10.1038/leu.2009.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Satou Y, Nosaka K, Koya Y, Yasunaga Ji, Toyokuni S, Matsuoka M. Proteasome inhibitor, bortezomib, potently inhibits the growth of adult T-cell leukemia cells both in vivo and in vitro. Leukemia. 2004;18:1357–1363. doi: 10.1038/sj.leu.2403400. [DOI] [PubMed] [Google Scholar]
- 48.Shah JJ, Orlowski RZ. Proteasome inhibitors in the treatment of multiple myeloma. Leukemia. 2009;23:1964–1979. doi: 10.1038/leu.2009.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hirai M, Kadowaki N, Kitawaki T, Fujita H, Takaori-Kondo A, Fukui R, et al. Bortezomib suppresses function and survival of plasmacytoid dendritic cells by targeting intracellular trafficking of Toll-like receptors and endoplasmic reticulum homeostasis. Blood. 2011;117:500–509. doi: 10.1182/blood-2010-05-284737. [DOI] [PubMed] [Google Scholar]
- 50.Hartmann S, Gesk S, Scholtysik R, Kreuz M, Bug S, Vater I, et al. High resolution SNP array genomic profiling of peripheral T cell lymphomas, not otherwise specified, identifies a subgroup with chromosomal aberrations affecting the REL locus. Br J Haematol. 2010;148:402–412. doi: 10.1111/j.1365-2141.2009.07956.x. [DOI] [PubMed] [Google Scholar]
- 51.Martinez-Delgado B, Cuadros M, Honrado E, Ruiz de la Parte A, Roncador G, Alves J, et al. Differential expression of NF-[kappa]B pathway genes among peripheral T-cell lymphomas. Leukemia. 2005;19:2254–2263. doi: 10.1038/sj.leu.2403960. [DOI] [PubMed] [Google Scholar]
- 52.Compagno M, Lim WK, Grunn A, Nandula SV, Brahmachary M, Shen Q, et al. Mutations of multiple genes cause deregulation of NF-kappaB in diffuse large B-cell lymphoma. Nature. 2009;459:717–721. doi: 10.1038/nature07968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Annunziata CM, Davis RE, Demchenko Y, Bellamy W, Gabrea A, Zhan F, et al. Frequent engagement of the classical and alternative NF-kappaB pathways by diverse genetic abnormalities in multiple myeloma. Cancer Cell. 2007;12:115–130. doi: 10.1016/j.ccr.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yamamoto M, Ito T, Shimizu T, Ishida T, Semba K, Watanabe S, et al. Epigenetic alteration of the NF-kappaB-inducing kinase (NIK) gene is involved in enhanced NIK expression in basal-like breast cancer. Cancer Sci. 2010;101:2391–2397. doi: 10.1111/j.1349-7006.2010.01685.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Garg A, Aggarwal BB. Nuclear transcription factor-kappaB as a target for cancer drug development. Leukemia. 2002;16:1053–1068. doi: 10.1038/sj.leu.2402482. [DOI] [PubMed] [Google Scholar]
- 56.Piccaluga PP, Fuligni F, De Leo A, Bertuzzi C, Rossi M, Bacci F, et al. Molecular profiling improves classification and prognostication of nodal peripheral T-cell lymphomas: results of a phase iii diagnostic accuracy study. J Clin Oncol. 2013;31:3019–3025. doi: 10.1200/JCO.2012.42.5611. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.