Abstract
Prostate cancer is the most frequently diagnosed cancer in American men. We have previously demonstrated that Src mediates androgen-independent proliferation in prostate cancer. We sought to investigate the Src-mediated oncogenic pathways and tumor biology using AZD0530, a novel Src family kinase/Abl dual-kinase inhibitor that is entering phase II clinical trials. We show that while both Src and Abl are expressed in all prostate cancer cell lines, Src but not Abl is activated in the prostate. Furthermore, Src activation is inhibited by AZD0530 in a rapid and dose-dependent manner. We show that Src mediates cell proliferation in DU145 and PC3 cells at the G1 phase of cell cycle. Src inhibition resulted in decreased binding of β-catenin to the promoters of G1 phase cell cycle regulators cyclin D1 and c-Myc. C-Myc may also be regulated at the protein level by extracellular signal-regulated kinase 1/2 and GSK3β. Cell motility factors focal adhesion kinase, p130CAS and paxillin activation in DU145 and PC3 cells were also inhibited. Administration of AZD0530 in mice reduced orthotopic DU145 xenograft growth by 45%. We have further delineated the Src-mediated oncogenic growth and migration pathways in prostate cancer and established mechanistic rationale for Src inhibition as novel therapy in the treatment of prostate cancer.
Keywords: Src, FAK, prostate cancer, AZD0530, proliferation, migration
Introduction
Prostate cancer is the second leading cause of cancer death in men in the United States (Jemal et al., 2008). Though prostate cancer growth is hormonally regulated, antiandrogen therapy inevitably results in disease progression with uncontrolled growth and metastasis. An important mediator of this process is Src, a prototypical non-receptor tyrosine kinase (Lee et al., 2001; Desai et al., 2006).
The role of Src in human malignancies has not been fully appreciated in part because of the lack of frequent mutations in human cancers. Nevertheless, Src over-expression and activation are associated with numerous types of cancers (Biscardi et al., 2000; Yeatman, 2004; Zhu et al., 2007). Increasing evidence connects Src activity to prostate carcinogenesis. Src activity is required for androgen-independent activation of androgen receptor mediated by neuropeptide (Lee et al., 2001; Desai et al., 2006), epidermal growth factor (Guo et al., 2006) and interleukin-8 (Lee et al., 2004). Src and focal adhesion kinase (FAK), a Src substrate, are also involved in interleukin-8-induced migration of LNCaP. The application of pan-Src inhibitor 4-amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo[3,4-d]pyrimidine (PP2) leads to significant suppression of androgen-independent growth and migration of LNCaP (Lee et al., 2001, 2004), as well as migration of PC3 and DU145 (Slack et al., 2001). Dasatinib, a Src family kinase (SFK)/Abl dual-inhibitor, inhibits cell adhesion and migration of DU145 (Nam et al., 2005b). Besides growth (Lee et al., 2001, 2004; Kotha et al., 2006), survival (Unni et al., 2004; Nam et al., 2005a; Kotha et al., 2006) and metastasis (Lee et al., 2004; Nam et al., 2005b), Src is also implicated in angiogenesis (Gray et al., 2005) and neuroendocrine differentiation (Bang et al., 1994). Overall, these studies suggest that Src plays pleiotropic roles in prostate cancer, often in a cell context-dependent manner and that Src is a promising target for intervention.
Src is an integrator of divergent signals. In prostate cancer cells, Src is activated by growth factors, cytokines, chemokines and gastrin-releasing peptide. Src activation leads to the activation of FAK and Etk (endothelial/epithelial tyrosine kinase), kinases consistently activated or overexpressed in prostate cancer cells (Rovin et al., 2002; Guo et al., 2006). The pleiotropic effects of Src activity are almost certainly due to the multiple signal pathways engaged by Src and its accompanying kinases. Src is able to channel phosphorylation signals through Ras/Raf/extracellular signal-regulated kinase (ERK) 1/2 and in certain cells, phosphatidylinositol 3-kinase (PI3K)/AKT pathways. Somewhat selective to SFKs is their ability to activate signal and transducer of transcription (STAT) 3 and β-catenin, which leads to the activation of c-Myc (Bowman et al., 2001; Furstoss et al., 2002; Farkas et al., 2005) and consequently cyclin D1 (Steiner et al., 1998; Taj et al., 2001; Devi et al., 2002). Although STAT3 was shown to regulate cyclin D1 levels in prostate cancer, it remains unclear whether Src is an upstream regulator of STAT3 in prostate cancer cells, as some Src inhibitors do not diminish STAT3 activation and STAT3 can also be activated by Janus family kinases (Nam et al., 2005b; Kotha et al., 2006). The role of β-catenin activation in prostate carcinogenesis has been extensively documented (de la Taille et al., 2003; Chen et al., 2004; Cronauer et al., 2005; Verras and Sun, 2006). The importance of c-Myc activation in prostate cancer is underscored by recent reports that c-Myc overexpression in mice prostate epithelial tissues gives rise to malignant lesions and that a significant fraction of prostate cancers amplify region 8q24 encompassing c-Myc (Bromann et al., 2004; Dong, 2006). What is not well understood is the role of Src in the activation of β-catenin and c-Myc in prostate cancer. As for signaling pathways of migration and invasion in prostate cancer, it was shown that Src, through activated FAK, phosphorylates p130CAS (CRK-associated substrate) and upregulates matrix metalloproteinase (MMP)-9 (Hauck et al., 2002; Planas-Silva et al., 2006). In summary, Src transmits multiple signals including Ras/Raf/ERK1/2, PI3K/AKT, β-catenin/c-Myc/cyclin D1 and FAK/p130CAS/MMP-9 to induce growth, survival and migration in various types of cancer cells. Whether Src directly mediates cellular changes in prostate cancer through these signals remain unclear.
Recent interest in Src as a target for molecule-specific therapy has led to the development of small molecule inhibitors (Golas et al., 2003; Lombardo et al., 2004; Lee and Gautschi, 2006). Dasatinib inhibits PC3 growth (Lombardo et al., 2004; Park et al., 2008) and DU145 migration (Nam et al., 2005b). Although Dasatinib is shown to inhibit growth in vitro in prostate cancer and has been suggested to inhibit proliferation through Lyn not Src, its mechanism of action in inhibiting cell proliferation remains unclear. AZD0530 (AstraZeneca, Alderley Park, UK), a 5-, 7-substituted anilinoquinazo-line, is another novel SFK/Abl dual-inhibitor (Figure 1a) (Hennequin et al., 2006; Lee and Gautschi, 2006). An oral compound with clinical therapeutic potential and low toxicity in phase I trials, it is highly specific with most kinases having in vitro kinase IC50 values greater than 10μM. AZD0530 has antimigratory and modest antiproliferative effects in vitro in breast cancer (Hiscox et al., 2006). AZD0530 has not, however, been previously studied in prostate cancer nor have Src-mediated signal pathways inhibited by this compound been defined. This study provides the first characterizations of the molecular and biological effects of AZD0530 in prostate cancer.
Figure 1.
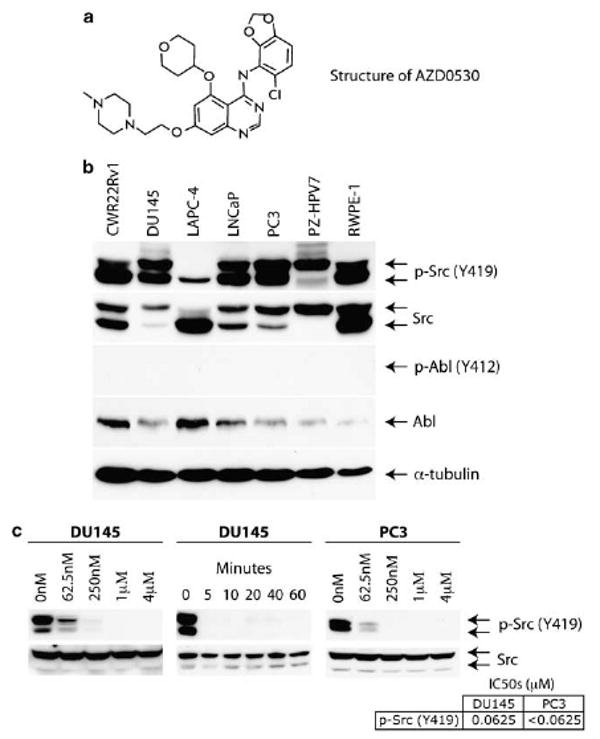
AZD0530 inhibits Src activation through inhibition of Y419 phosphorylation. (a) The chemical structure of AZD0530. (b) Commonly used cell lines were harvested and probed with Abl, p-Abl, phospho-Src Y419 and Src antibodies demonstrating relative increased ratio of activated-to-total Src in DU145 and PC3 cells. (c) Src autophosphorylation in DU145 and PC3 cells were inhibited in a dose-dependent manner by AZD0530 following 30-min treatment (left and right, respectively) or rapid manner by 1 μM of AZD0530 (middle).
We show in this study that Src inhibition leads to growth suppression and cell cycle arrest in prostate cancer, which is accompanied by inactivation of ERK1/2 and AKT, activation of GSK3β and downregulation of β-catenin, c-Myc and cyclin D1. Focal adhesion kinase and p130CAS phosphorylation are also attenuated as Src activity is inhibited, leading to significantly reduced cell migration. We also extended the analysis of AZD0530 as an antitumor agent in vivo. Using DU145 as our orthotopic mouse model, we show that AZD0530 is an inhibitor of growth in vivo. These studies provide important information regarding this small molecule inhibitor and set the stage for NCI approved phase II trials, using AZD0530 in advanced prostate cancer.
Results
Src is expressed and activated in prostate cancer cell lines
Autophosphorylation of Src at tyrosine 419 (Y419) is a surrogate marker of its activity (Bjelfman et al., 1990). Src is expressed in LNCaP, DU145 and PC3 cell lines and increased Src activity correlates with more aggressive phenotypes (Bang et al., 1994; Lee et al., 2001, 2004; Nam et al., 2005b; Kotha et al., 2006). Src activity and expression levels in CWR22Rv1, LAPC-4 and immortalized normal prostate epithelial cell lines such as RWPE-1 and PZ-HPV7, however, have not previously been characterized. We therefore sought to compare and contrast the relative Src activation and expression levels in these cell lines. As Abl is also an AZD0530 target, we sought to characterize Abl in prostate cell lines as well.
Two Src isoforms were detected (Figure 1b) and were confirmed as Src through transfection experiments with wild-type Src cDNA constructs (data not shown). Src is expressed and activated in prostate cell lines. Notably, DU145 and PC3, cell lines with higher rates of proliferation and increased cell motility demonstrate an increased activated-to-total Src ratio when compared to other phenotypically less aggressive cell lines. Accounting for α-tubulin levels, immortalized cells express more Src but have lower activated-to-total Src ratio than cancer cells. Although Abl is expressed in all prostate cell lines, it is not activated.
Our analysis with other SFKs suggests that Src is the predominant species expressed in prostate cancer cell lines (data not shown). As AZD0530 inhibits all SFKs at comparable concentrations, our data apply to all members. For simplicity, we will describe our results in the context of Src.
AZD0530 is a potent and rapid inhibitor of cellular Src activation
We were interested in characterizing cellular Src inhibition by AZD0530. In DU145 and PC3, AZD0530 inhibited Src activation in a dose-dependent manner (Figure1c, left, right). Src inhibition by AZD0530 was also rapid, within 5 min of treatment (Figure 1c, center).
AZD0530 inhibits growth and induces cell cycle arrest of prostate cancer
Src is involved in prostate cancer cell proliferation. We were therefore interested in the effectiveness of AZD0530 against mitogenesis. Single treatment with AZD0530 resulted in dose-dependent decrease of the number of cells in all cell lines (Figure 2a). LAPC-4, which has the smallest relative active-to-total Src ratio, is the most resistant against AZD0530 among prostate cancer cell lines. Immortalized nonmalignant cell lines PZ-HPV7 and RWPE-1 are also on average more resistant to Src inhibition than cancer cell lines. Figure 2b shows the kinetics and the dose–responses of growth inhibition for DU145 and PC3 cells. These analyses substantiate the growth inhibitory effects of AZD0530 on prostate cancer cells.
Figure 2.
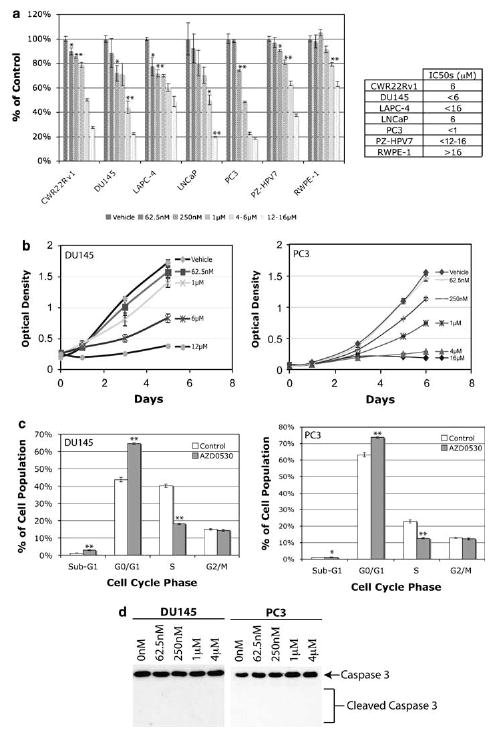
AZD0530 inhibits cell proliferation at G0/G1-S transition. (a) Single administration of AZD0530 inhibited cell proliferation in a dose-dependent manner in 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide assay showing immortalized cells on average being more resistant than malignant cell lines. (b) AZD0530 inhibited DU145 and PC3 proliferation in a dose-dependent manner over time. (c) AZD0530 induced G1/S cell cycle arrest but not apoptosis in DU145 and PC3 cells. (d) AZD0530 did not induce apoptosis in DU145 and PC3 cells after 2 days, as shown by the lack of caspase 3 cleavage. Columns, mean; bars, standard error; *P<0.05 (n=3); **P<0.01 (n=3).
Although AZD0530 decreased the number of prostate cancer cells over time, it was unclear whether this is secondary to apoptosis or decreased cell proliferation. Studies of other Src inhibitors on DU145 induced apoptosis and cell cycle arrest at the G0/G1 phase of the cell cycle (Nam et al., 2005a; Kotha et al., 2006). We thus sought to clarify the effects of AZD0530 using DU145 and PC3 as our models (Figure 2c). AZD0530 treatment of DU145 and PC3 respectively increased the proportion of G0/G1 cells by 21 and 11% and concurrently decreased S cells by 22 and 10% in the cell cycle, respectively. The fraction of apoptotic cells (sub-G1) is very low in both treated and untreated DU145 and PC3 samples. Furthermore, there is no significant caspase 3 cleavage following AZD0530 treatment (Figure 2d). Thus, the decreased numbers of cells is not due to apoptosis but to cell cycle arrest at G1/S.
Inhibition of c-Myc and cyclin D1 expression and downregulation of β-catenin by AZD0530
The effect of AZD0530 on G1/S transition prompted us to study its effect on c-Myc, an Src target gene (Barone and Courtneidge, 1995) and cyclin D1, the rate limiting factor for cellular proliferation (Quelle et al., 1993; Albanese et al., 1995; Watanabe et al., 1996). Both were downregulated upon AZD0530 treatment (Figure 3a). Since c-Myc is more resistant to AZD0530 than Src, we sought to examine the kinetics of AZD0530 on c-Myc. Although both c-Myc and phospho-Src levels decrease after AZD0530 treatment, they both rebound over time, with relatively small changes in phospho-Src levels corresponding to larger changes seen in c-Myc. Cyclin D1 and c-Myc in DU145 cells are more sensitive to Src inhibition than PC3 cells. We further show corresponding reductions in transcripts (Figure 3b).
Figure 3.
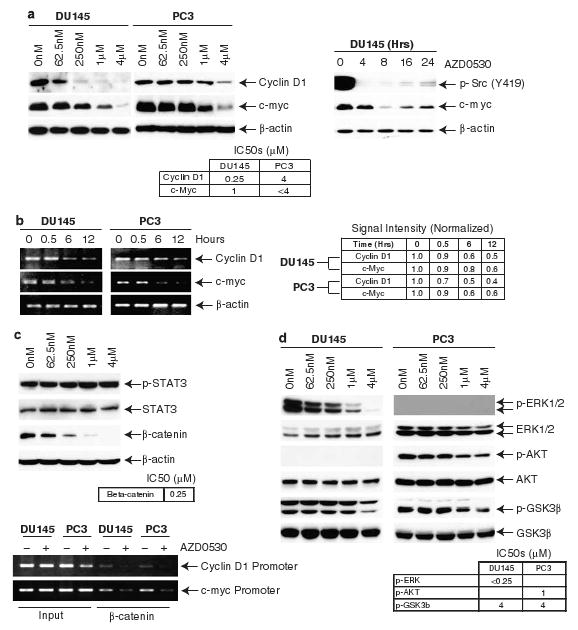
AZD0530 inhibits cell proliferation through β-catenin, ERK1/2 and GSK3β-mediated cyclin D1 and c-myc regulation. (a) DU145 and PC3 cells treated with AZD0530 shows dose-dependent decreases in levels of cyclin D1 and c-Myc. Corresponding rebound of phospho-Src and c-Myc is seen over time. (b) Cyclin D1 and c-Myc transcript levels decreased following 4 μM AZD0530 treatment. (c) AZD0530 treatment did not inhibit signal and transducer of transcription 3 activation after 30 min but downregulated β-catenin after 24 h (top). DU145 and PC3 treated with 4 μM of AZD0530 for 12 h shows decreased binding of β-catenin to cyclin D1 and c-Myc promoter regions (bottom). (d) AZD0530 inhibited ERK1/2 and GSK3β phosphorylation in DU145 and AKT and GSK3β phosphorylation in PC3 after 30 min.
To study the upstream effectors that regulate c-Myc and cyclin D1 transcription, we noted that both STAT3 and β-catenin are Src targets and key transcriptional factors of c-Myc and cyclin D1 (Morin, 1999; Prathapam et al., 2006). We previously showed that Src enhances STAT3 tyrosine phosphorylation through Etk (Tsai et al., 2000). Other studies also showed that STAT3 mediates signals between Src and cyclin D1 in some prostate cancer cells (Gao et al., 2005; Kotha et al., 2006). Since PC3 does not express STAT3 (Yuan et al., 2005), we focused on DU145. In DU145, AZD0530 treatment does not affect STAT3 phosphorylation, indicating its activation via a Src-independent pathway (Figure 3c, top).
We then turned our attention to β-catenin. β-catenin is a Src substrate (Roura et al., 1999) and its synthesis is activated by Src (Karni et al., 2005). It has also been shown to mediate both c-Myc and cyclin D1 transcription (Morin, 1999; Prathapam et al., 2006). The protein level of β-catenin is highly sensitive to AZD0530 treatment (Figure 3c, top). Furthermore, AZD0530 treatment results in decreased binding of β-catenin to both cyclin D1 and c-Myc promoters (Figure 3c, bottom). These data taken together suggest that Src mediates cell cycle progression by the induction of c-Myc and cyclin D1 transcription through increased β-catenin expression.
Inhibition of ERK1/2 and GSK3β phosphorylation by AZD0530
In addition to transcriptional regulation by β-catenin, c-Myc protein is regulated by ERK1/2 and AKT-GSK3β. GSK3β phosphorylation of c-Myc at T58 leads to ubiquitin-mediated proteosomal degradation, whereas ERK1/2-mediated phosphorylation at S62 stabilizes c-Myc (Dominguez-Sola and Dalla-Favera, 2004; Sears, 2004). GSK3β in turn is inactivated by AKT and ERK1/2 (Cross et al., 1995; Cheng et al., 2005; Kim et al., 2007). Likewise, GSK3β negatively regulates the stability of β-catenin. We therefore wondered if AZD0530 inhibits ERK1/2 and AKT. ERK1/2 was inhibited by AZD0530 treatment, whereas AKT is constitutively inactive DU145 (Figure 3d). The lack of AKT activity is consistent with the presence of intact phosphatase and tensin homolog pathway in DU145, which diminishes PI3K-mediated AKT activation. This may also account for the lack of regulation of survival pathway by AZD0530 in DU145. ERK1/2 in PC3 cells on the other hand is not constitutively activated. AKT in PC3, however, is inhibited by AZD0530. AZD0530 treatment results in the removal of the inhibitory phosphorylation of GSK3β at S9 in both cell lines (Figure 3d) and therefore increased GSK3β activity, although at a higher concentration than that of β-catenin and ERK1/2 inhibition seen in DU145. These results suggest that in DU145 and PC3, ERK1/2 and AKT contribute to Src-mediated stabilization of c-Myc, respectively. Furthermore, GSK3β is not responsible for Src-mediated stabilization of c-Myc and β-catenin in DU145.
AZD0530 is an inhibitor of cell migration
Src is an integral part of cell migration signaling pathway. We were therefore interested in whether AZD0530 effectively inhibits cell migration. We show that AZD0530 inhibits DU145 and PC3 migration in the Boyden chamber in a dose-dependent manner (Figure 4a).
Figure 4.
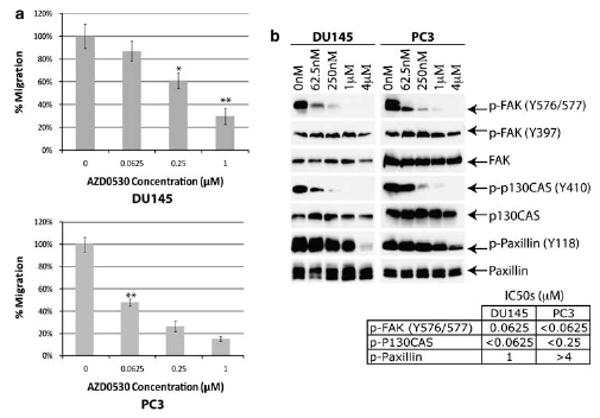
AZD0530 inhibits cell migration through Src-mediated FAK activation. (a) DU145 (top) and PC3 (bottom) treated with AZD0530 shows dose-dependent decrease in cell migration. (b) Paxillin, p130CAS and p-FAK (Y576/577) phosphorylation were inhibited in DU145 and PC3, following AZD0530 treatment for 30 min. Columns, mean; bars, standard error; *P<0.05 (n=3); **P<0.01 (n=3).
Src and FAK are known to cross-activate, and enhanced migratory activity is linked to increased FAK expression and activation (Schaller, 2001; Slack et al., 2001). Although autophosphorylation of FAK Y397 is necessary for its activity, Src phosphorylation of FAK Y576/Y577 is important in enhancing downstream signaling pathways (Parsons, 2003). AZD0530 treatment inhibited phosphorylation of Y576/577 but not Y397 (Figure 4b), indicating that AZD0530 targets Src but not FAK.
P130CAS is also an Src substrate involved in the formation of focal adhesion complexes. As shown in Figure 4b, p130CAS phosphorylation is inhibited by AZD0530. Furthermore, phosphorylation of paxillin, an adaptor protein and an Src-FAK substrate important in recruiting other proteins to the focal adhesion complex, is also inhibited by AZD0530, although at a higher of AZD0530 concentration than Src or FAK. This may reflect the fact that AZD0530 does not inhibit FAK autokinase activity (as reflected by the same level of Y397 phosphorylation), which continues to phosphorylate paxillin.
AZD0530 is a promising inhibitor of prostate cancer growth in orthotopic SCID mice model
Utilizing the data we gathered regarding the effects of AZD0530 on in vitro growth and migration of prostate cancer cells, we tested its efficacy in vivo using orthotopically implanted DU145 in mice as our xenograft model. Xenograft mice receiving daily AZD0530 starting 2 days after the implantation have on average 45% smaller (P<0.01) tumor than control mice (Figure 5a). Src inhibition in vivo by AZD0530 was verified by immunohistochemistry (Figure 5b). Since AZD0530 treatment started shortly after implantation, the decreased tumor size xenograft mice treated with AZD0530 is consistent with the in vitro data of growth inhibition versus apoptosis. This is significant, as previous studies with other Src inhibitors revealed mostly inhibitory effects on metastasis rather than growth. We were unable to study the effect of AZD0530 on metastasis, as orthotopically implanted DU145 does not metastasize to any significant extent.
Figure 5.
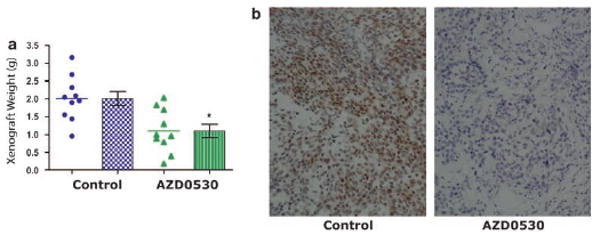
AZD0530 inhibits tumor growth in vivo. (a) 25 mg/kg of AZD0530 was administered orally daily starting 2 days after orthotopic injection of 2 million DU145 cells. Mice were euthanized after 54 days. Established tumors were harvested and weighed. (b) Immunohistochemical analysis of tumor samples from (a) using specific phospho-Src Y419 antibody as described. Dots and triangles, tumor samples; columns, mean; bars, standard error; *P<0.05 (n=10).
Discussion
Src is involved in prostate cancer growth and migration (Lee et al., 2001, 2004; Nam et al., 2005b; Kotha et al., 2006). We previously reviewed Src's role (Chang et al., 2007) in prostate cancer and wished to further characterize it. We therefore utilized AZD0530 to facilitate identification of Src-driven cell proliferation and migration signaling pathways in prostate cancer. Our data provide further understanding to foster correlative studies and translational research initiatives.
We found an association between higher relative Src activation and aggressive cell phenotypes. There are two Src isoforms and their expression levels are cell line-dependent. The origin of these isoforms is presently unclear. Also interesting is that cells with the lowest activated/total Src ratios (LAPC-4, PZ-HPV7, RWPE-1) also express the most Src. A possible explanation is that highly active Src is polyubiquitinated and thus quickly degraded (Hakak and Martin, 1999).
In our studies with AZD0530, we see a temporal sequence of its effects. As AZD0530 inhibits Src, changes to phosphorylation signals downstream occurred within minutes. The inhibited phosphorylation of FAK, p130CAS and paxillin quickly decreased cell migration. Taking into account the time it takes for transcriptional inhibition and protein degradation, changes in cyclin D1 and c-Myc levels are relatively late events seen hours post-treatment. Finally, consistent with cell doubling times, cell cycle changes and differential proliferation rates are observed days post-treatment.
We inhibited cell migration and proliferation using AZD0530. Although mechanistic studies of cell migration signaling did not reveal significant mechanistic differences between DU145 and PC3, they appear to regulate cell proliferation through c-Myc and cyclin D1 in different ways. Both cyclin D1 and c-Myc levels are more responsive to AZD0530 in DU145 than PC3. This is attributable to ERK1/2 being active and sensitive to AZD0530 in DU145 but not PC3. Since PC3 has no constitutively active ERK1/2, it alternatively regulates cyclin D1 and c-Myc through the Src–Ras–AKT–GSK3β pathway (Diehl et al., 1998; Morin, 1999; Daaka, 2002). Interestingly, β-catenin but not GSK3β is affected in a dose-dependent manner by AZD0530 in DU145. Possible explanations of this finding include Src-mediated β-catenin synthesis (Karni et al., 2005) and phosphorylation (Bjelfman et al., 1990), thus resulting in increased stability (Roura et al., 1999). Common to both cell lines, however, is that Src does not regulate cyclin D1 and c-Myc through STAT3.
Although our studies show that AZD0530 inhibits cell proliferation and migration through various signaling factors, they have relatively higher IC50 values than Src autophosphorylation. Dose-dependent inhibition demonstrated in these assays suggests that Src contributes to their regulation. Nonspecific AZD0530 inhibition, however, cannot be excluded. Nevertheless, there are alternative explanations for these findings. Since phosphorylation status of proteins are dynamic systems dependent on the summative velocities of kinases and phosphatases, partial inhibition of kinase activity may not be sufficient to allow phosphorylation status changes if phosphatase velocity remains less than kinase velocity. Furthermore, as shown through c-Myc in DU145 (Figure 3a), the accuracy of determining IC50 values is dependent on the timing of the assay if Src is not the sole regulator of the factor in question, as 1 μM of AD0530 decreases c-Myc levels by 90% at 8 h but 50% at 24 h. Actual c-Myc AZD0530 IC50 is therefore less than 1 μM. Another explanation is that very little Src may be required to activate downstream signals. Individual kinases, such as ERK1/2, have been shown to display cooperative kinetics, which cumulatively in a signal-transduction chain is ultrasensitive to activation, akin to an on-off switch response (Li and Qian, 2003). In other words, very low initial activation of the upstream factor in a signal-transduction chain can be amplified and lead to changes downstream. Our kinetics study of c-Myc, which is regulated by ERK1/2, supports this hypothesis as very small amount of Src activation correlates with a relatively large rise in c-Myc levels (Figure 3a). The combination of ultrasensitivity and dose-dependent residual Src activity at μM AZD0530 concentrations (data not shown) suggests that the effect of Src inhibition on downstream factors decreases exponentially with increasing AZD0530, and therefore increases the IC50 values of Src downstream factors. Extrapolating this further, we can see how the IC50 values of transcription/translation and cell proliferation and migration involving a multitude of factors, many of which are not regulated by Src, can be significantly higher than Src.
The complexity of linking dose inhibition of Src phosphorylation with linear dose inhibition of other molecules and biological events is evident in the dosing and temporal data we present. Although AZD0530 may have other effects, we show it essential to the pathways and events presented. The decreased in vivo tumor growth correlates with significant inhibition of Src autophosphorylation by immunohistochemistry, demonstrating biological and translational relevance. AZD0530 represents an oral drug of low toxicity potentially of high value in the targeted therapy of prostate cancer. The mechanistic differences between the two androgen-independent prostate cancer cell lines DU145 and PC3 highlight the importance of an individualized, pharmacogenomic approach to patients. Studies such as ours are important in linking disease, detailed oncogenic pathway analysis and a targeted therapy in vitro and in vivo. These data have direct translational application to prostate cancer patients entering clinical trials using AZD0530.
Materials and methods
Cells and reagents
LNCaP, DU145, PC3, RWPE-1, PZ-HPV7 were obtained from American Type Culture Collection (Manassas, VA, USA). LAPC-4 was provided by Dr Sawyers (Department of Medicine, University of California at Los Angeles, Los Angeles, CA, USA). CWR22Rv1 was provided by Dr Pretlow (Department of Pathology, Case Western Reserve University, Cleveland, OH, USA). Cell cultures were maintained in RPMI-1640 (Life Technologies Inc., Rockville, MD, USA) with 10% (LNCaP, RWPE-1), 5% (DU145, PC3, CWR22Rv1) fetal bovine serum, Dulbecco's Modified Eagle's Medium with 10% fetal bovine serum (LAPC-4) or keratino-cyte serum-free medium with 5 ng/ml human recombinant epidermal growth factor and 50 μg/ml bovine pituitary extract (PZ-HPV7) supplemented with 100 U/ml penicillin and 100 μg/ml streptomycin at 37 °C with 5% CO2. Polyclonal antibodies to AKT, p-AKT (S473), caspase 3, ERK1/2, p-FAK (Y576/577), p-GSK3α/β (S21/9), p-p130CAS (Y410), paxillin, p-paxillin (Y118), p-Src (Y419) and STAT3 were obtained from Cell Signaling Technologies (Cambridge, MA, USA). Polyclonal antibodies to FAK and cyclin D1 were obtained from Upstate Biotechnology (Lake Placid, NY, USA) and Santa Cruz Biotechnology (Santa Cruz, CA, USA), respectively. Monoclonal antibodies to p-ERK1/2 (T202/Y204) and p-STAT3 (Y705) were obtained from Cell Signaling Technologies. Monoclonal antibodies to c-Myc and α-tubulin were obtained from Santa Cruz Biotechnology. Monoclonal antibodies to p-FAK (Y397) and p130CAS were obtained from BD Biosciences (San Jose, CA, USA). Monoclonal antibodies to c-Myc and β-catenin were obtained from Santa Cruz Biotechnology. Monoclonal antibodies to GSK3β, Src and β-actin were obtained from Cell Signaling Technologies, Upstate Biotechnology and Sigma-Aldrich (St Louis, MO, USA), respectively. AZD0530 was obtained from AstraZeneca International (Alderley Park, UK). 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide was obtained from Sigma-Aldrich. The DAKO Envision + Kit was obtained from DAKO North American Inc. (Carpinteria, CA, USA). Diff-Quick set was purchased from Dade Behring Inc. (Newark, DE, USA). Dimethyl sulfoxide was obtained from Fisher Scientific (Pittsburgh, PA, USA). DNase-free RNase was obtained from Fermentas (Hanover, MD, USA). Fibronectin was obtained from Roche Applied Science (Indianapolis, IN, USA). Propidium iodide was obtained from Boehringer Mannheim Corporation (Indianapolis).
Boyden chamber cell migration assay
Cell migration assay was performed as described previously and performed in triplicates (Evans et al., 1991). Lower wells of the microchamber were filled with 50 μg/ml of fibronectin in 0.1% BSA phenol-red free RPMI-1640 media as chemoattractant. Both chambers contained varying concentrations of AZD0530 (0–2 μM). Cells were allowed to migrate for 4 h followed by Diff-Quick stain and counted as an average of five fields.
Cell cycle analysis
Cells were plated in triplicate in 60 mm dishes followed by AZD0530 (1 μM) treatment for 48 (DU145) and 72 (PC3) hours, accounting for slower proliferation rate in PC3 cells. Growth media were removed and saved. Cells were washed with phosphate-buffered saline (PBS) and the wash saved with the growth media. Remaining cells were trypsinized and placed together with growth media and PBS. Cells were pelleted and resuspended in 75% ethanol followed by overnight storage at −20 °C. Cells were centrifuged, washed with PBS, resuspended in PBS containing 10 μg/ml DNase-free RNase, and incubated in 37 °C for 45 min. Final propidium iodide concentration of 0.05 mg/ml was added and incubated at room temperature for 20 min. Cell clumps were filtered. Cell DNA content was measured on Coulter Epics XL flow cytometer (Beckman Coulter, Miami, FL, USA) and cell cycle phase was analysed using Phoenix Multicycle (Phoenix Flow Systems, San Diego, CA, USA).
Chromatin immunoprecipitation assay
Chromatin immunoprecipiation assay was performed as described previously (Vinall et al., 2006). Primers for cyclin D1 and c-Myc promoter regions are as follows: 5′-GCTCTCCACTTGCCCCTTTTA-3′ (c-Myc, forward), 5′-GTTCCCAATTTCTCAGCC-3 (c-Myc, reverse), 5′-GGGAGGAATTCACCCTGAAA-3′ (cyclin D1, forward), 5′-CCTGCCCCAAATTAAGAAAA-3′ (cyclin D1, reverse).
3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide cell proliferation assay
Cells were seeded overnight 2000 cells per well in triplicate in 96-well plates followed by single treatment of AZD0530 (62.5 nM–16 μM). On post-treatment days 1, 3 and 5, growth medium was removed followed by addition of 0.2 ml dimethyl sulfoxide per well and continuous shaking of plates at 200 rotations per minute for 15 min. Colorimetric measurement was performed at 450 nm.
Orthotopic mouse model
Severe combined immunodeficiency (CB17) mice 4 weeks of age were obtained from Harlan Sprague-Dawley and housed in pathogen-free conditions. Mice were placed in anesthesia with 2% isoflurane air. Two million DU145 cells were mixed with Matrigel in 1:1 ratio by volume and injected into a lateral lobe of the prostate as previously described (Stephenson et al., 1992). Twenty-five milligrams per kilogram of AZD0530 dissolved in 0.5% hydroxypropyl methylcellulose (Sigma-Aldrich), 0.1% Tween 80 (Sigma-Aldrich) was orally given daily 2 days post-operation. Mice were euthanized 54 days post-operation and tumors harvested. Animal housing and experimental conditions were in compliance with the protocol approved by the Institutional Animal Care and Use Committee at the University of California, Davis.
Reverse transcription–PCR
The Versagene RNA purification kit (Qiagen USA, Valencia, CA, USA) was used for mRNA extraction as per the manufacturer's instructions. RNA was reverse transcribed to cDNA using oligo-dT primers and Moloney murine leukemia virus reverse transcriptase (Promega, Madison, WI, USA) as per the manufacturer's instructions. Reverse transcriptase products were used as templates for PCR. The primers are as follows: 5′-ACCGAGGAGAATGTCAAGAGGC-3′ (c-Myc, forward), 5′-CGTCGTTTCCGCAACAAGTC-3′ (c-Myc, reverse), 5′-TGTTTGCAAGCAGGACTTTG-3′ (cyclin D1, forward), 5′-TCATCCTGGCAATGTGAGAA-3′ (cyclin D1, reverse).
Statistics
Data were analysed using Statview version 5.1 (SAS, Cary, NC, USA).
Western blotting
Western blotting was performed as described previously (Qiu et al., 1998). Membranes were incubated overnight in 4 1C with primary antibodies in 5% non-fat milk tris-buffered saline Tween-20 followed by wash and 1-h room temperature incubation with respective horseradish peroxidase-conjugated secondary antibodies. Antibody-epitope binding was detected using SuperSignal West Pico Chemiluminescent Substrate (Pierce, Rockford, IL, USA).
Acknowledgments
This study was supported by National Institutes of Health Grant KO8 DK60748-01 (CPE), RO1 DK52659 (H-JK) and Department of Defense Grant PC040161 (CPE). The contents of this report are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health or the Department of Defense.
References
- Albanese C, Johnson J, Watanabe G, Eklund N, Vu D, Arnold A, et al. Transforming p21ras mutants and c-Ets-2 activate the cyclin D1 promoter through distinguishable regions. J Biol Chem. 1995;270:23589–23597. doi: 10.1074/jbc.270.40.23589. [DOI] [PubMed] [Google Scholar]
- Bang YJ, Pirnia F, Fang WG, Kang WK, Sartor O, Whitesell L, et al. Terminal neuroendocrine differentiation of human prostate carcinoma cells in response to increased intracellular cyclic AMP. Proc Natl Acad Sci USA. 1994;91:5330–5334. doi: 10.1073/pnas.91.12.5330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barone MV, Courtneidge SA. Myc but not Fos rescue of PDGF signalling block caused by kinase-inactive Src. Nature. 1995;378:509–512. doi: 10.1038/378509a0. [DOI] [PubMed] [Google Scholar]
- Biscardi JS, Ishizawar RC, Silva CM, Parsons SJ. Tyrosine kinase signalling in breast cancer: epidermal growth factor receptor and c-Src interactions in breast cancer. Breast Cancer Res. 2000;2:203–210. doi: 10.1186/bcr55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjelfman C, Meyerson G, Cartwright CA, Mellstrom K, Hammerling U, Pahlman S. Early activation of endogenous pp60src kinase activity during neuronal differentiation of cultured human neuroblastoma cells. Mol Cell Biol. 1990;10:361–370. doi: 10.1128/mcb.10.1.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman T, Broome MA, Sinibaldi D, Wharton W, Pledger WJ, Sedivy JM, et al. Stat3-mediated Myc expression is required for Src transformation and PDGF-induced mitogenesis. Proc Natl Acad Sci USA. 2001;98:7319–7324. doi: 10.1073/pnas.131568898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromann PA, Korkaya H, Courtneidge SA. The interplay between Src family kinases and receptor tyrosine kinases. Oncogene. 2004;23:7957–7968. doi: 10.1038/sj.onc.1208079. [DOI] [PubMed] [Google Scholar]
- Chang YM, Kung HJ, Evans CP. Nonreceptor tyrosine kinases in prostate cancer. Neoplasia. 2007;9:90–100. doi: 10.1593/neo.06694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Shukeir N, Potti A, Sircar K, Aprikian A, Goltzman D, et al. Up-regulation of Wnt-1 and beta-catenin production in patients with advanced metastatic prostate carcinoma: potential pathogenetic and prognostic implications. Cancer. 2004;101:1345–1356. doi: 10.1002/cncr.20518. [DOI] [PubMed] [Google Scholar]
- Cheng JQ, Lindsley CW, Cheng GZ, Yang H, Nicosia SV. The Akt/PKB pathway: molecular target for cancer drug discovery. Oncogene. 2005;24:7482–7492. doi: 10.1038/sj.onc.1209088. [DOI] [PubMed] [Google Scholar]
- Cronauer MV, Schulz WA, Ackermann R, Burchardt M. Effects of WNT/beta-catenin pathway activation on signaling through T-cell factor and androgen receptor in prostate cancer cell lines. Int J Oncol. 2005;26:1033–1040. doi: 10.3892/ijo.26.4.1033. [DOI] [PubMed] [Google Scholar]
- Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378:785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- Daaka Y. Mitogenic action of LPA in prostate. Biochim Biophys Acta. 2002;1582:265–269. doi: 10.1016/s1388-1981(02)00180-4. [DOI] [PubMed] [Google Scholar]
- de la Taille A, Rubin MA, Chen MW, Vacherot F, de Medina SG, Burchardt M, et al. Beta-catenin-related anomalies in apoptosis-resistant and hormone-refractory prostate cancer cells. Clin Cancer Res. 2003;9:1801–1807. [PubMed] [Google Scholar]
- Desai SJ, Ma AH, Tepper CG, Chen HW, Kung HJ. Inappropriate activation of the androgen receptor by nonsteroids: involvement of the Src kinase pathway and its therapeutic implications. Cancer Res. 2006;66:10449–10459. doi: 10.1158/0008-5472.CAN-06-2582. [DOI] [PubMed] [Google Scholar]
- Devi GR, Oldenkamp JR, London CA, Iversen PL. Inhibition of human chorionic gonadotropin beta-subunit modulates the mitogenic effect of c-myc in human prostate cancer cells. Prostate. 2002;53:200–210. doi: 10.1002/pros.10151. [DOI] [PubMed] [Google Scholar]
- Diehl JA, Cheng M, Roussel MF, Sherr CJ. Glycogen synthase kinase-3beta regulates cyclin D1 proteolysis and subcellular localization. Genes Dev. 1998;12:3499–3511. doi: 10.1101/gad.12.22.3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez-Sola D, Dalla-Favera R. PINning down the c-Myc oncoprotein. Nat Cell Biol. 2004;6:288–289. doi: 10.1038/ncb0404-288. [DOI] [PubMed] [Google Scholar]
- Dong JT. Prevalent mutations in prostate cancer. J Cell Biochem. 2006;97:433–447. doi: 10.1002/jcb.20696. [DOI] [PubMed] [Google Scholar]
- Evans CP, Walsh DS, Kohn EC. An autocrine motility factor secreted by the Dunning R-3327 rat prostatic adenocarcinoma cell subtype AT2.1. Int J Cancer. 1991;49:109–113. doi: 10.1002/ijc.2910490120. [DOI] [PubMed] [Google Scholar]
- Farkas A, Szatmari E, Orbok A, Wilhelm I, Wejksza K, Nagyoszi P, et al. Hyperosmotic mannitol induces Src kinase-dependent phosphorylation of beta-catenin in cerebral endothelial cells. J Neurosci Res. 2005;80:855–861. doi: 10.1002/jnr.20521. [DOI] [PubMed] [Google Scholar]
- Furstoss O, Dorey K, Simon V, Barila D, Superti-Furga G, Roche S. c-Abl is an effector of Src for growth factor-induced c-myc expression and DNA synthesis. EMBO J. 2002;21:514–524. doi: 10.1093/emboj/21.4.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao L, Zhang L, Hu J, Li F, Shao Y, Zhao D, et al. Down-regulation of signal transducer and activator of transcription 3 expression using vector-based small interfering RNAs suppresses growth of human prostate tumor in vivo. Clin Cancer Res. 2005;11:6333–6341. doi: 10.1158/1078-0432.CCR-05-0148. [DOI] [PubMed] [Google Scholar]
- Golas JM, Arndt K, Etienne C, Lucas J, Nardin D, Gibbons J, et al. SKI-606, a 4-anilino-3-quinolinecarbonitrile dual inhibitor of Src and Abl kinases, is a potent antiproliferative agent against chronic myelogenous leukemia cells in culture and causes regression of K562 xenografts in nude mice. Cancer Res. 2003;63:375–381. [PubMed] [Google Scholar]
- Gray MJ, Zhang J, Ellis LM, Semenza GL, Evans DB, Watowich SS, et al. HIF-1alpha, STAT3, CBP/p300 and Ref-1/APE are components of a transcriptional complex that regulates Src-dependent hypoxia-induced expression of VEGF in pancreatic and prostate carcinomas. Oncogene. 2005;24:3110–3120. doi: 10.1038/sj.onc.1208513. [DOI] [PubMed] [Google Scholar]
- Guo Z, Dai B, Jiang T, Xu K, Xie Y, Kim O, et al. Regulation of androgen receptor activity by tyrosine phosphorylation. Cancer Cell. 2006;10:309–319. doi: 10.1016/j.ccr.2006.08.021. [DOI] [PubMed] [Google Scholar]
- Hakak Y, Martin GS. Ubiquitin-dependent degradation of active Src. Curr Biol. 1999;9:1039–1042. doi: 10.1016/s0960-9822(99)80453-9. [DOI] [PubMed] [Google Scholar]
- Hauck CR, Hsia DA, Puente XS, Cheresh DA, Schlaepfer DD. FRNK blocks v-Src-stimulated invasion and experimental metastases without effects on cell motility or growth. EMBO J. 2002;21:6289–6302. doi: 10.1093/emboj/cdf631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennequin LF, Allen J, Breed J, Curwen J, Fennell M, Green TP, et al. N-(5-Chloro-1,3-benzodioxol-4-yl)-7-[2-(4-methylpiperazin-1-yl)ethoxy]-5- (tetrahydro-2H-pyran-4-yloxy)quinazolin-4-amine, a novel, highly selective, orally available, dual-specific c-Src/Abl kinase inhibitor. J Med Chem. 2006;49:6465–6488. doi: 10.1021/jm060434q. [DOI] [PubMed] [Google Scholar]
- Hiscox S, Morgan L, Green TP, Barrow D, Gee J, Nicholson RI. Elevated Src activity promotes cellular invasion and motility in tamoxifen resistant breast cancer cells. Breast Cancer Res Treat. 2006;97:263–274. doi: 10.1007/s10549-005-9120-9. [DOI] [PubMed] [Google Scholar]
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- Karni R, Gus Y, Dor Y, Meyuhas O, Levitzki A. Active Src elevates the expression of beta-catenin by enhancement of cap-dependent translation. Mol Cell Biol. 2005;25:5031–5039. doi: 10.1128/MCB.25.12.5031-5039.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Rath O, Kolch W, Cho KH. A hidden oncogenic positive feedback loop caused by crosstalk between Wnt and ERK pathways. Oncogene. 2007;26:4571–4579. doi: 10.1038/sj.onc.1210230. [DOI] [PubMed] [Google Scholar]
- Kotha A, Sekharam M, Cilenti L, Siddiquee K, Khaled A, Zervos AS, et al. Resveratrol inhibits Src and Stat3 signaling and induces the apoptosis of malignant cells containing activated Stat3 protein. Mol Cancer Ther. 2006;5:621–629. doi: 10.1158/1535-7163.MCT-05-0268. [DOI] [PubMed] [Google Scholar]
- Lee D, Gautschi O. Clinical development of SRC tyrosine kinase inhibitors in lung cancer. Clin Lung Cancer. 2006;7:381–384. doi: 10.3816/clc.2006.n.020. [DOI] [PubMed] [Google Scholar]
- Lee LF, Guan J, Qiu Y, Kung HJ. Neuropeptide-induced androgen independence in prostate cancer cells: roles of nonreceptor tyrosine kinases Etk/Bmx, Src, and focal adhesion kinase. Mol Cell Biol. 2001;21:8385–8397. doi: 10.1128/MCB.21.24.8385-8397.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee LF, Louie MC, Desai SJ, Yang J, Chen HW, Evans CP, et al. Interleukin-8 confers androgen-independent growth and migration of LNCaP: differential effects of tyrosine kinases Src and FAK. Oncogene. 2004;23:2197–2205. doi: 10.1038/sj.onc.1207344. [DOI] [PubMed] [Google Scholar]
- Li G, Qian H. Sensitivity and specificity amplification in signal transduction. Cell Biochem Biophys. 2003;39:45–59. doi: 10.1385/CBB:39:1:45. [DOI] [PubMed] [Google Scholar]
- Lombardo LJ, Lee FY, Chen P, Norris D, Barrish JC, Behnia K, et al. Discovery of N-(2-chloro-6-methyl- phenyl)-2-(6-(4-(2-hydroxyethyl)- piperazin-1-yl)-2-methylpyrimidin-4- ylamino)thiazole-5-carboxamide (BMS-354825), a dual Src/Abl kinase inhibitor with potent antitumor activity in preclinical assays. J Med Chem. 2004;47:6658–6661. doi: 10.1021/jm049486a. [DOI] [PubMed] [Google Scholar]
- Morin PJ. beta-catenin signaling and cancer. Bioessays. 1999;21:1021–1030. doi: 10.1002/(SICI)1521-1878(199912)22:1<1021::AID-BIES6>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Nam S, Buettner R, Turkson J, Kim D, Cheng JQ, Muehlbeyer S, et al. Indirubin derivatives inhibit Stat3 signaling and induce apoptosis in human cancer cells. Proc Natl Acad Sci USA. 2005a;102:5998–6003. doi: 10.1073/pnas.0409467102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam S, Kim D, Cheng JQ, Zhang S, Lee JH, Buettner R, et al. Action of the Src family kinase inhibitor, dasatinib (BMS-354825), on human prostate cancer cells. Cancer Res. 2005b;65:9185–9189. doi: 10.1158/0008-5472.CAN-05-1731. [DOI] [PubMed] [Google Scholar]
- Park SI, Zhang J, Phillips KA, Araujo JC, Najjar AM, Volgin AY, et al. Targeting SRC family kinases inhibits growth and lymph node metastases of prostate cancer in an orthotopic nude mouse model. Cancer Res. 2008;68:3323–3333. doi: 10.1158/0008-5472.CAN-07-2997. [DOI] [PubMed] [Google Scholar]
- Parsons JT. Focal adhesion kinase: the first ten years. J Cell Sci. 2003;116:1409–1416. doi: 10.1242/jcs.00373. [DOI] [PubMed] [Google Scholar]
- Planas-Silva MD, Bruggeman RD, Grenko RT, Stanley Smith J. Role of c-Src and focal adhesion kinase in progression and metastasis of estrogen receptor-positive breast cancer. Biochem Biophys Res Commun. 2006;341:73–81. doi: 10.1016/j.bbrc.2005.12.164. [DOI] [PubMed] [Google Scholar]
- Prathapam T, Tegen S, Oskarsson T, Trumpp A, Martin GS. Activated Src abrogates the Myc requirement for the G0/G1 transition but not for the G1/S transition. Proc Natl Acad Sci USA. 2006;103:2695–2700. doi: 10.1073/pnas.0511186103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu Y, Robinson D, Pretlow TG, Kung HJ. Etk/Bmx, a tyrosine kinase with a pleckstrin-homology domain, is an effector of phosphatidylinositol 3′-kinase and is involved in interleukin 6-induced neuroendocrine differentiation of prostate cancer cells. Proc Natl Acad Sci USA. 1998;95:3644–3649. doi: 10.1073/pnas.95.7.3644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quelle DE, Ashmun RA, Shurtleff SA, Kato JY, Bar-Sagi D, Roussel MF, et al. Overexpression of mouse D-type cyclins accelerates G1 phase in rodent fibroblasts. Genes Dev. 1993;7:1559–1571. doi: 10.1101/gad.7.8.1559. [DOI] [PubMed] [Google Scholar]
- Roura S, Miravet S, Piedra J, Garcia de Herreros A, Dunach M. Regulation of E-cadherin/Catenin association by tyrosine phosphorylation. J Biol Chem. 1999;274:36734–36740. doi: 10.1074/jbc.274.51.36734. [DOI] [PubMed] [Google Scholar]
- Rovin JD, Frierson HF, Jr, Ledinh W, Parsons JT, Adams RB. Expression of focal adhesion kinase in normal and pathologic human prostate tissues. Prostate. 2002;53:124–132. doi: 10.1002/pros.10114. [DOI] [PubMed] [Google Scholar]
- Schaller MD. Biochemical signals and biological responses elicited by the focal adhesion kinase. Biochim Biophys Acta. 2001;1540:1–21. doi: 10.1016/s0167-4889(01)00123-9. [DOI] [PubMed] [Google Scholar]
- Sears RC. The life cycle of C-myc: from synthesis to degradation. Cell Cycle. 2004;3:1133–1137. [PubMed] [Google Scholar]
- Slack JK, Adams RB, Rovin JD, Bissonette EA, Stoker CE, Parsons JT. Alterations in the focal adhesion kinase/Src signal transduction pathway correlate with increased migratory capacity of prostate carcinoma cells. Oncogene. 2001;20:1152–1163. doi: 10.1038/sj.onc.1204208. [DOI] [PubMed] [Google Scholar]
- Steiner MS, Anthony CT, Lu Y, Holt JT. Antisense c-myc retroviral vector suppresses established human prostate cancer. Hum Gene Ther. 1998;9:747–755. doi: 10.1089/hum.1998.9.5-747. [DOI] [PubMed] [Google Scholar]
- Stephenson RA, Dinney CP, Gohji K, Ordonez NG, Killion JJ, Fidler IJ. Metastatic model for human prostate cancer using orthotopic implantation in nude mice. J Natl Cancer Inst. 1992;84:951–957. doi: 10.1093/jnci/84.12.951. [DOI] [PubMed] [Google Scholar]
- Taj MM, Tawil RJ, Engstrom LD, Zeng Z, Hwang C, Sanda MG, et al. Mxi1, a Myc antagonist, suppresses proliferation of DU145 human prostate cells. Prostate. 2001;47:194–204. doi: 10.1002/pros.1063. [DOI] [PubMed] [Google Scholar]
- Tsai YT, Su YH, Fang SS, Huang TN, Qiu Y, Jou YS, et al. Etk, a Btk family tyrosine kinase, mediates cellular transformation by linking Src to STAT3 activation. Mol Cell Biol. 2000;20:2043–2054. doi: 10.1128/mcb.20.6.2043-2054.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unni E, Sun S, Nan B, McPhaul MJ, Cheskis B, Mancini MA, et al. Changes in androgen receptor nongenotropic signaling correlate with transition of LNCaP cells to androgen independence. Cancer Res. 2004;64:7156–7168. doi: 10.1158/0008-5472.CAN-04-1121. [DOI] [PubMed] [Google Scholar]
- Verras M, Sun Z. Roles and regulation of Wnt signaling and beta-catenin in prostate cancer. Cancer Lett. 2006;237:22–32. doi: 10.1016/j.canlet.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Vinall RL, Tepper CG, Shi XB, Xue LA, Gandour-Edwards R, de Vere White RW. The R273H p53 mutation can facilitate the androgen-independent growth of LNCaP by a mechanism that involves H2 relaxin and its cognate receptor LGR7. Oncogene. 2006;25:2082–2093. doi: 10.1038/sj.onc.1209246. [DOI] [PubMed] [Google Scholar]
- Watanabe G, Howe A, Lee RJ, Albanese C, Shu IW, Karnezis AN, et al. Induction of cyclin D1 by simian virus 40 small tumor antigen. Proc Natl Acad Sci USA. 1996;93:12861–12866. doi: 10.1073/pnas.93.23.12861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeatman TJ. A renaissance for SRC. Nat Rev Cancer. 2004;4:470–480. doi: 10.1038/nrc1366. [DOI] [PubMed] [Google Scholar]
- Yuan ZL, Guan YJ, Chatterjee D, Chin YE. Stat3 dimerization regulated by reversible acetylation of a single lysine residue. Science. 2005;307:269–273. doi: 10.1126/science.1105166. [DOI] [PubMed] [Google Scholar]
- Zhu S, Bjorge JD, Fujita DJ. PTP1B contributes to the oncogenic properties of colon cancer cells through Src activation. Cancer Res. 2007;67:10129–10137. doi: 10.1158/0008-5472.CAN-06-4338. [DOI] [PubMed] [Google Scholar]


