Glucose transporters play a pivotal role in glucose homeostasis. The fission yeast high-affinity glucose transporter Ght5 is regulated with regard to transcription and localization via CaMKK and TOR pathways. These results clarify the evolutionarily conserved mechanisms underlying glucose homeostasis that prevent hyperglycemia in humans.
Abstract
Hexose transporters are required for cellular glucose uptake; thus they play a pivotal role in glucose homeostasis in multicellular organisms. Using fission yeast, we explored hexose transporter regulation in response to extracellular glucose concentrations. The high-affinity transporter Ght5 is regulated with regard to transcription and localization, much like the human GLUT transporters, which are implicated in diabetes. When restricted to a glucose concentration equivalent to that of human blood, the fission yeast transcriptional regulator Scr1, which represses Ght5 transcription in the presence of high glucose, is displaced from the nucleus. Its displacement is dependent on Ca2+/calmodulin-dependent kinase kinase, Ssp1, and Sds23 inhibition of PP2A/PP6-like protein phosphatases. Newly synthesized Ght5 locates preferentially at the cell tips with the aid of the target of rapamycin (TOR) complex 2 signaling. These results clarify the evolutionarily conserved molecular mechanisms underlying glucose homeostasis, which are essential for preventing hyperglycemia in humans.
INTRODUCTION
Glucose is the fundamental source of carbon and energy in eukaryotes, and its uptake and utilization are therefore vital to drive various energy-consuming cellular processes. Because glucose is a hydrophilic molecule that cannot pass through the cell membrane, cellular incorporation of glucose requires a transporter (Boles and Hollenberg, 1997; Özcan and Johnston, 1999; Uldry and Thorens, 2004; Manolescu et al., 2007). Hexose transporter proteins, which mediate the transport of hexoses, including glucose, belong to the major facilitator superfamily and comprise 12 transmembrane segments with both amino and carboxy termini on the cytosolic side of the membrane (Mueckler et al., 1985; Hresko et al., 1994). Some transmembrane segments are predicted to form a pore through which hexose molecules pass (Mueckler and Makepeace, 2006). Although hexose transporter proteins have been identified in a wide variety of organisms from humans to bacteria, their amino acid sequences are fairly diverse. Overall structure and several specific motifs, some of which are believed to be involved in substrate recognition and/or determination of membrane topology, are well conserved (Seatter et al., 1998; Sato and Mueckler, 1999; Manolescu et al., 2007). Multiple putative hexose transporter genes are found in the genome of a single species. For example, 13, 8, and 20 such genes are present in the genomes of humans, the fission yeast Schizosaccharomyces pombe, and the budding yeast Saccharomyces cerevisiae, respectively (Özcan and Johnston, 1999; Heiland et al., 2000; Wood et al., 2002; Uldry and Thorens, 2004; Manolescu et al., 2007).
Although the physiologic relevance of hexose transporter multiplication is not well understood, it appears important for ensuring cellular hexose homeostasis in various environments. Each transporter protein has a different affinity for glucose and/or related monosaccharides. For example, the Michaelis–Menten constant (Km) for glucose transport of human GLUT3, which is a high-affinity glucose transporter expressed in neurons, is reported to be 1 mM, whereas that of GLUT2, a low-affinity glucose transporter in liver and intestine, is reported to be much higher, 11 mM (Colville et al., 1993a, b; Brown, 2000; Uldry and Thorens, 2004). Similarly, in budding yeast, there are two types of glucose transporters: high-affinity transporters, expressed in glucose-limited environments, and low-affinity transporters, expressed in glucose-rich environments (Reifenberger et al., 1995; Özcan and Johnston, 1999; Maier et al., 2002). Some transporters also facilitate diffusion of saccharides other than glucose. The human GLUT2 transporter mediates fructose transport, as well as that of glucose. Thus the types and combinations of expressed hexose transporters might be a key factor determining the rates of cellular hexose uptake and the modes of hexose utilization. For proper levels of hexose uptake, expression levels and activity of each transporter must be modulated according to changes in environmental hexose concentrations and/or the physiologic requirement for hexose in tissues. An understanding of the mechanisms involved in controlling the expression and activity of the transporters is therefore important.
We explored the cellular mechanism enabling efficient uptake and utilization of low glucose concentrations comparable to that of human blood (∼0.1%), using the fission yeast S. pombe, for which laboratory media normally contain high glucose concentrations (2–3%). We predicted that S. pombe mutant cells defective in this mechanism might serve as genetically tractable models for hyperglycemia in humans, as these mutant cells supposedly fail to use glucose completely, leaving higher glucose concentrations in extracellular fluid (i.e., growth medium) than wild-type (WT) cells. When transferred from high-glucose (111 mM, 2%) to low-glucose (4.4 mM, 0.08%) medium, S. pombe WT cells transiently stop proliferation and then resume division at a rate similar to that in high glucose (Pluskal et al., 2011; Saitoh and Yanagida, 2014). Distinct phosphosignaling cascades appear to be required for proliferation and/or size control of fission yeast cells under low-glucose conditions. One cascade involves Sds23-mediated down-regulation of the 2A-type protein phosphatases (PP2As) Ppa1and Ppa2 and the PP2A-like PP6 phosphatase, Ppe1. Another is the Ca2+/calmodulin-dependent kinase kinase (CaMKK), Ssp1, which genetically interacts with Sds23. A third cascade involves an evolutionarily conserved phosphatidylinositol 3-kinase–like protein kinase complex, TORC2, which contains the target of rapamycin (TOR) kinase (Hanyu et al., 2009; Ikai et al., 2011).
In this study, we comprehensively analyzed eight hexose transporters encoded by ght1+–ght8+ genes in fission yeast. These eight transporters appear to be generated by gene duplication after fission yeast species were evolutionally established and are thus closely related at the amino acid sequence level. Despite the high similarity in their sequences, the eight transporters do not appear to function redundantly. Among them, ght5+ is essential and sufficient for cell proliferation under low-glucose conditions. Unexpectedly, we found that the phosphatase inhibitor Sds23 and the CaMKK, Ssp1, regulated the expression level of the ght5+ gene via nucleocytoplasmic shuttling of a zinc-finger protein, Scr1, whereas TORC2 regulated cytoplasmic trafficking of Ght5 to the cell surface under low-glucose conditions. Our findings shed light on the molecular mechanism in fission yeast that modulates glucose uptake and consumption, depending on the glucose concentration in the cellular environment, which is presumed to be crucial for maintaining glucose homeostasis in multicellular organisms.
RESULTS
S. pombe has eight hexose transporters
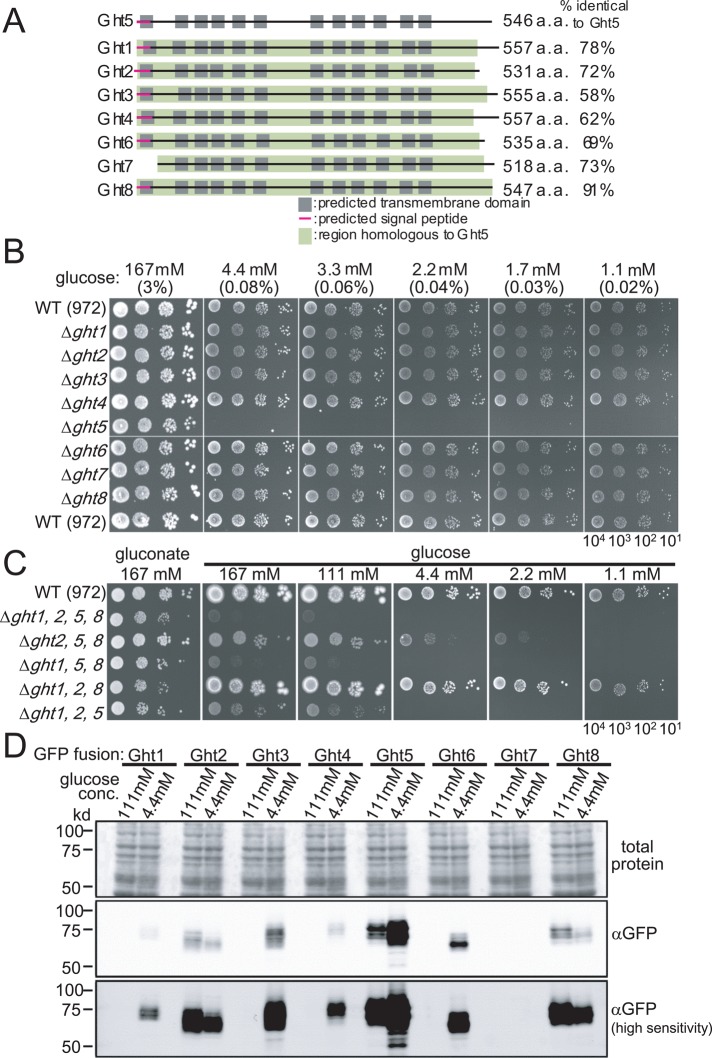
The S. pombe genome contains eight coding regions for different hexose transporters (Heiland et al., 2000; PomBase database, www.pombase.org/), designated Ght1–8. Their sequence features are summarized in Figure 1A, with the locations of predicted transmembrane domains, amino-terminal signal peptides, and homologous regions indicated. The total number of amino acids and the proportion of amino acids identical to those in Ght5 (percentage identity) are also shown. As described later, Ght5 is the major transporter in S. pombe. Ght7 does not have a signal peptide. Its expression is extremely low, and it is virtually absent except during meiotic sporulation (Mata et al., 2007). To comprehensively characterize these transporters, we constructed eight deletion mutants (Δght1–Δght8), each having one of the eight ght genes deleted, and eight green fluorescent protein (GFP)-fusion strains (ght1-GFP–ght8-GFP), each having one of the ght genes replaced with a modified version in which we fused GFP to the 3′-end of the gene. All genes remained under the control of their respective native promoters. No functional abnormalities could be detected in any of the GFP-fused strains (Materials and Methods).
FIGURE 1:
Among eight hexose transporters, only Ght5 is essential for cell division under low-glucose conditions. (A) Eight putative hexose transporters in the S. pombe genome are schematically drawn. Transmembrane domains are represented by the gray boxes, and signal peptides, predicted with SignalP 4.0 (Petersen et al., 2011), are indicated by magenta lines. In each transporter, the region homologous to Ght5 is represented by a green box, and the proportions of residues identical to those in Ght5 are indicated (percentage identical to Ght5). (B) Aliquots (104 cells) of WT and deletion strains lacking each ght (Δght1–Δght8) were diluted serially 10-fold, spotted onto YES solid media containing the indicated concentrations (mM and percentage) of glucose, and incubated for 3 d at 33°C. (C) Aliquots of 104 cells of WT, the Δght1 Δght2 Δght5 Δght8 quadruple-deletion strain (Δght1,2,5,8), and triple-deletion strains lacking three of the four ght genes were diluted serially 10-fold, spotted onto YES solid media containing the indicated concentrations of glucose (potassium gluconate for control; see the text), and incubated for 4 d at 33°C. (D) The protein level of each Ght hexose transporter was measured by immunoblot. Cells in which one of ght genes was C-terminally fused with GFP at its own locus and expressed under the native promoter were cultured in high-glucose (111 mM) or low-glucose (4.4 mM) medium. Total protein extracts were prepared after 6-h incubation, and the levels of GFP-fused Ght transporters were examined by immunoblot using an antibody against GFP (middle and bottom). Normal- and high-sensitivity (enhanced) images of the immunoblot are shown. The pattern of total proteins stained with Ponceau S is also shown as a loading control (top).
Ght5 transporter is essential and sufficient for cell division under low-glucose conditions
All eight transporter genes are nonessential in YES rich culture medium containing a high glucose concentration (3%, 167 mM; Kim et al., 2010). To identify whether ght+ genes become essential for cell proliferation under low-glucose conditions, we examined colony-forming abilities of ght deletion mutants on solid YES medium containing a series of glucose concentrations, 1.1–167 mM (0.02–3%; Figure 1B). Only one strain, Δght5, failed to form colonies on low-glucose medium (1.1–4.4 mM), whereas the other ght deletion mutants and the WT strain (972) formed colonies on both high- and low-glucose media. The Ght5 transporter thus has an indispensable role in cell division under low-glucose conditions.
A series of strains simultaneously lacking multiple ght genes was then constructed, and their colony formation abilities were examined on solid media with various glucose concentrations (Figure 1C). Quadruple-deletion mutant cells lacking ght1+, ght2+, ght5+, and ght8+ hardly grew, even on high (167 mM)-glucose medium, although they could form colonies on medium containing gluconate, a molecule generated by glucose oxidation, which undergoes glycolysis via the pentose phosphate pathway. We suspected that these four genes are involved in glucose transport, and the others may be involved in transport of other saccharides. Indeed, the Ght3 and Ght4 transporters are reportedly required for cell proliferation with gluconate, suggesting that they have high affinity for gluconate and that Ght6 has higher affinity for fructose than glucose (Heiland et al., 2000).
The triple-deletion mutant lacking ght1+, ght5+, and ght8+ genes also failed to form colonies in high glucose (111 and 167 mM, 2 and 3%). In contrast, other triple mutants could form colonies, at least on high-glucose media (167 mM), suggesting that transport in high glucose concentrations might be redundantly mediated by Ght1, Ght5, and Ght8. Ght5 alone was virtually sufficient for glucose uptake under low-glucose conditions (1.1–4.4 mM, 0.02–0.08%), as the colonies formed by Δght1, Δght2, Δght8 triple-deletion mutant cells in which the ght5+ gene is intact were nearly identical in size to the WT.
Ght5 and other transporters change their levels in response to low glucose
To determine the intracellular abundance of these transporter proteins, we performed immunoblot analysis using the ght1- to ght8-GFP strains, in which the expression of the GFP-fused ght+ gene is under the control of its native promoter. Total protein extracts were obtained from cells cultivated in synthetic EMM2 containing 111 or 4.4 mM glucose for 6 h at 33°C, separated by 10% SDS–PAGE, and followed by the detection of Ght proteins using anti-GFP antibodies (Figure 1D).
The level of GFP-tagged Ght5 was already significant in 111 mM glucose and further increased after cultivation in low glucose, displaying the highest intensity among the eight GFP-tagged Ght proteins. In sharp contrast, the levels of two transporters, Ght2 and Ght8, were decreased in cells cultured in low glucose. The Ght protein levels thus differed, and some were altered by lowering the glucose concentration. Elevated expression and high abundance of Ght5 are consistent with an essential role under low-glucose conditions.
The Ght5 transcript was most abundant under low-glucose conditions
mRNA levels of these hexose transporters were determined by reverse transcription-quantitative PCR (RT-qPCR; Figure 2A). Results were consistent with changes in the protein levels; levels of five mRNAs (ght1+, ght3+, ght4+, ght5+, and ght6+) were increased in low-glucose medium (2–6 h), whereas levels of two mRNAs (ght2+ and ght8+) were slightly decreased. Thus, whereas transcript levels of ght8+, ght5+, and ght2+ were relatively abundant in high glucose, the transcript level of ght5+ became highest in low glucose; in low-glucose conditions, ght5+ mRNA was approximately three times more abundant than that of ght3+.
FIGURE 2:
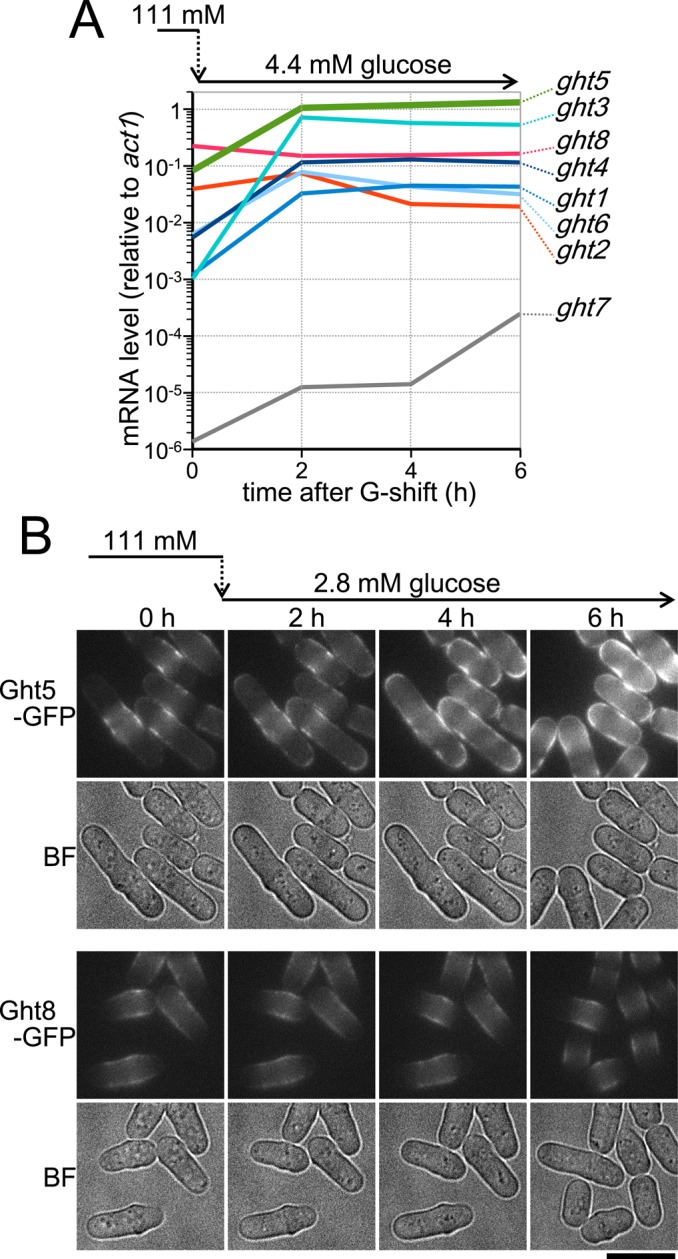
Expression and protein localization of eight Ght transporters are differentially regulated in response to the glucose concentration in the medium. (A) Time course of ght mRNA levels was examined in WT cells growing in low-glucose (4.4 mM) medium. Cells growing exponentially in high-glucose (111 mM) EMM2 were transferred to fresh EMM2 with 4.4 mM glucose at time 0 and cultivated at 33°C. Total RNA samples were obtained every 2 h, and the mRNA copy number of each ght gene was measured by RT-qPCR. The copy number of the act1+ gene was also measured and used for normalization. Levels (shown on logarithmic scale) of ghts increasing in low glucose are indicated in bluish colors, whereas those of ghts decreasing are in reddish colors. Levels of ght5, which was abundantly expressed under both glucose conditions, are shown in green. Levels of ght7, shown in gray, were very low regardless of the glucose concentration. (B) Time-lapse images of GFP-fused Ght5 (top) and Ght8 (bottom). Cells harboring Ght5-GFP or Ght8-GFP were cultivated at 26°C in a microfluidic perfusion chamber with a continuous supply of medium (Pluskal et al., 2011), and the glucose concentration was switched from 111 to 2.8 mM at time 0 h. GFP fluorescence and brightfield (BF) microscopy images were obtained at 2-h intervals. Bar, 10 μm. See also Supplemental Figure S1.
Under low-glucose conditions, newly synthesized Ght5-GFP proteins preferentially localized at the cell tip
We examined intracellular localization of GFP-tagged hexose transporters. Figure 2B shows time-course changes of representative transporters, Ght5-GFP and Ght8-GFP, after the shift to low-glucose medium. As described earlier, Ght5 and Ght8 expression levels increased and decreased, respectively, under low-glucose conditions. Cells of the ght5-GFP or ght8-GFP strains were cultivated in a microfluidic perfusion chamber (ONIX Perfusion System; CellASIC, Hayward, CA) with a continuous supply of medium in which the glucose concentration was reduced from 111 to 2.8 mM (0.05%) at time 0 (Pluskal et al., 2011). Consistent with changes in expression levels, the fluorescence of Ght5-GFP became stronger during incubation in low-glucose medium, whereas that of Ght8 decreased slightly. As predicted from their function, these transporters were predominantly observed on the surface but not on the whole cell surface. Ght8-GFP located preferentially in the middle regions of the cells and was hardly detected at the cell tips before and after the shift to low glucose; the fluorescent region became narrower after the shift.
In sharp contrast, whereas Ght5-GFP was less prominent at the cell tips and relatively enriched near the cell equator under high-glucose conditions (time 0 h), its fluorescence was intensified at the cell tip regions during incubation in low-glucose medium. After 4–6 h of incubation in low glucose, Ght5-GFP appeared to be saturated at the cell tips, expanding across the entire cell surface. Localizations of other Ght transporters tagged with GFP were then examined (Supplemental Figure S1). Similar to Ght5, the low glucose–induced transporters Ght1, Ght3, and Ght4 became enriched at the cell tips during growth in low-glucose medium, whereas the low glucose–repressed Ght2 was localized near the cell equator, similar to Ght8. These observations suggested that, under low-glucose conditions, the newly synthesized Ght5 molecules, as well as the other transporters, such as Ght1, 3, and 4, were incorporated into the cell membrane preferentially at the cell tips.
Glucose consumption is diminished in ∆ght5, ∆sds23, ssp1-837, and ∆tor1 mutants only under low-glucose conditions
Fission yeast ssp1+ and sds23+ genes, which encode, respectively, a CaMKK and an inhibitor of PP2A phosphatases (Ppa1 and Ppa2) and PP6 phosphatase (Ppe1), are required for cell proliferation under low-glucose conditions (Hanyu et al., 2009), as well as quiescence in G0 phase under nitrogen-starved conditions (Shimanuki et al., 2007; Sajiki et al., 2009). These gene products are also required for cell size reduction upon limitation of either glucose or nitrogen (Yanagida et al., 2011). The sds23+ and ssp1+ genes are functionally similar; Sds23 is a high-copy suppressor of ssp1 mutants and vice versa (Hanyu et al., 2009). Hence these gene products are predicted to function in synergistic pathways. A signaling pathway through TORC2 is also required for cell adaptation to low glucose, as disruption of the tor1+ gene encoding the catalytic subunit of TORC2 prevents cell-size reduction upon glucose limitation (Ikai et al., 2011). However, it is unknown how these signaling pathways enable fission yeast cells to adapt to and proliferate rapidly in low-glucose environments.
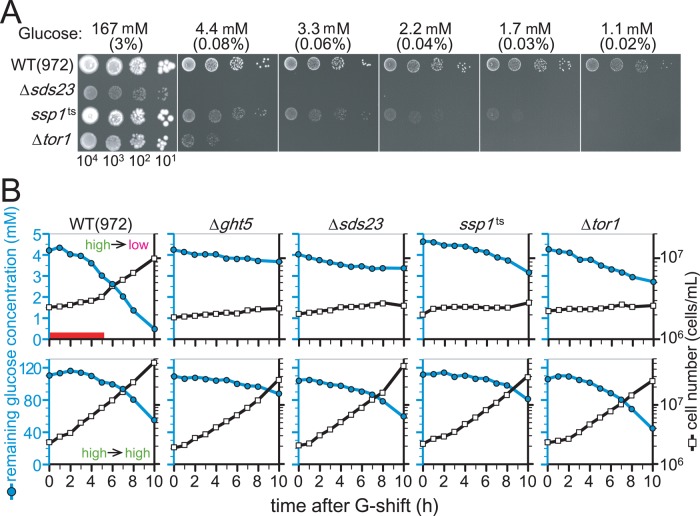
Colony formation by mutant cells harboring a temperature sensitive (ts) allele of the ssp1+ gene (ssp1-837) and cells lacking the sds23+ gene (Δsds23) was severely diminished on media containing low glucose concentrations at a semipermissive temperature for the mutants, 33°C (Figure 3A). Similarly, cells lacking the tor1+ gene (Δtor1) also failed to form colonies on low-glucose media (Figure 3A).
FIGURE 3:
Δsds23, ssp1-837, and Δtor1 mutant cells slowly consume glucose under low glucose concentrations. (A) Aliquots of 104 cells of WT, Δsds23, ssp1-837 (ssp1ts), and Δtor1 strains were serially diluted 10-fold, spotted onto YES solid medium containing the indicated concentrations of glucose, and incubated at 33°C for 3 d. (B) Glucose consumption and cell proliferation were examined in the WT and indicated mutant strains. Cells growing at permissive temperatures were transferred to fresh EMM2 supplied with 4.4 mM (top) or 111 mM (bottom) glucose at time 0 and cultivated at 33°C. The glucose concentration remaining in the media (blue filled circles, left axis) and cell density (open square, right axis) were measured every hour. See the text.
To examine glucose consumption by WT and mutant cells (∆ght5, ∆sds23, ssp1-837, or ∆tor1), we made time-course measurements of glucose concentrations in media in which cells grow; WT and mutant cells initially cultivated in high-glucose (111 mM) medium were transferred to high-glucose (111 mM) and low-glucose (4.4 mM) media at time 0 and incubated at 33°C. Figure 3B shows changes in the cell number (open rectangles, right axis) and remaining glucose levels (blue filled circles, left axis). As reported previously (Pluskal et al., 2011; Saitoh and Yanagida, 2014), when WT cells were transferred from high-glucose to low-glucose medium (top left), cell division transiently declined for a period equivalent to one to two divisions (3–5 h, indicated by the red bar), during which the cells presumably adapted to low-glucose conditions, and then resumed growing. WT cells then proliferated in low-glucose (4.4 mM) medium with a doubling time similar to that in high-glucose (111 mM) medium. After the shift to high glucose (bottom left), no such temporary arrest was observed in WT cells.
In contrast, cell numbers of all the ∆ght5, ∆sds23, ssp1-837, and ∆tor1 mutant cells failed to increase in low glucose (top), and glucose consumption was strikingly delayed in mutant cultures compared with WT. Among mutant strains, ∆ght5 had the lowest glucose consumption, followed by, in order, ∆sds23, ssp1-837, and ∆tor1. In high glucose (bottom), cell numbers of all mutants increased like WT, although glucose consumption was slightly reduced in ∆ght5 cells. Delayed consumption in low glucose explains the loss of proliferative ability of these mutant cells in low glucose.
The rate of cellular glucose uptake is enhanced under low-glucose conditions
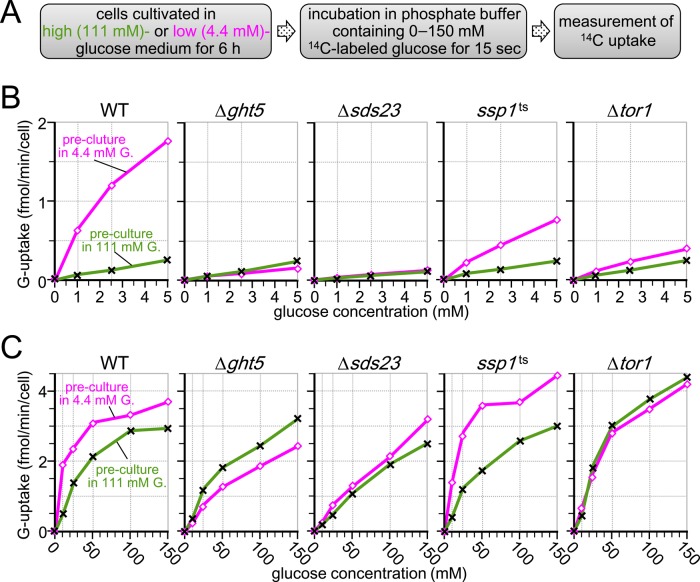
To gain insight into the mechanism required for cells to resume active division under low-glucose conditions, we measured rates of glucose uptake in S. pombe cells previously cultured in medium containing high or low glucose concentrations. The procedure for measurement is schematized in Figure 4A. WT and mutant cells were initially cultivated in EMM2 containing either 111 or 4.4 mM glucose for 6 h at 33°C before the measurements, and then rates of glucose uptake were measured by incubating cells in 0.15 M phosphate buffer containing C14-radiolabeled glucose at concentrations ranging from 0 to 5 mM or 0 to 150 mM (Figure 4, B and C, respectively), as previously described (Özcan et al., 1993; Walsh et al., 1994; Lichtenberg-Fraté et al., 1997; also see Materials and Methods).
FIGURE 4:
Glucose uptake is enhanced in cells preadapted to low glucose in a manner dependent on Sds23, Ssp1, and Tor1. (A) Procedure for measurement. (B, C) Rates of radiolabeled-glucose uptake were measured in the WT and indicated mutant cells. Cells were cultivated in EMM2 containing 111 (crosses) or 4.4 mM (open diamonds) glucose for 6 h at 33°C before measurement and then suspended in 0.15 M potassium phosphate (pH 4.5) buffer. Radiolabeled glucose ([14C]glucose) solutions were added to the cell suspension at final concentrations ranging from 0 to 5 mM (B) or 0 to 150 mM (C). Rates of glucose uptake were determined by measuring radioactivity incorporated into the cells during 15-s incubation at 33°C.
WT cells precultured in 4.4 mM glucose medium showed markedly higher (∼10-fold) rates of glucose uptake in low glucose concentrations (1–5 mM) than WT cells precultured in 111 mM glucose medium (Figure 4B, left; open rectangles, 4.4 mM glucose preculture; crosses, 111 mM glucose preculture). We conclude that previous acclimation of WT cells to low glucose greatly enhanced the ability of cells to take up low glucose concentrations, which might be essential for acclimatization of cells to low-glucose conditions. Rates of glucose uptake of WT cells in high glucose concentration are shown in Figure 4C (left). The rate was higher in cells precultured in 4.4 mM glucose than in those precultured in 111 mM glucose, but the difference was small (only 30% difference at 150 mM glucose).
Enhanced glucose uptake rates in low-glucose conditions are abolished in Δght5, Δtor1, ssp1-837, and Δsds23 mutants
Glucose uptake rates were measured for mutant strains precultured in either high- or low-glucose medium (Figure 4, B and C). Unlike WT, rates of incorporation at low glucose concentrations greatly declined in Δght5 cells precultured in 4.4 mM glucose and became virtually identical to those of cells precultured in 111 mM glucose. These findings indicated that the Ght5 transporter was required for enhanced glucose uptake under low-glucose conditions. Even in the high-glucose range (5–150 mM), rates of glucose uptake for ∆ght5 cells precultured in 4.4 mM glucose were still lower than those for cells precultured in 111 mM glucose. Thus preculture in low glucose failed to increase the glucose uptake efficiency of Δght5 mutant cells across a range of glucose concentrations.
Results with three other mutants, ∆tor1, ∆sds23, and ssp1-837, were similar to those obtained for ∆ght5. Uptake of low (1–5 mM) glucose was markedly slower in these mutants than in WT cells previously cultured in low glucose. The phenotype of ssp1-837 was somewhat “leaky,” possibly due to residual activity of the ts mutant Ssp1 protein at the semirestrictive temperature (33°C). Results with ∆sds23 and ∆tor1 were virtually identical to those of ∆ght5, suggesting that Ght5 is not fully functional in these mutants.
TORC2 (Tor1) is required for proper localization of Ght5 on the cell surface
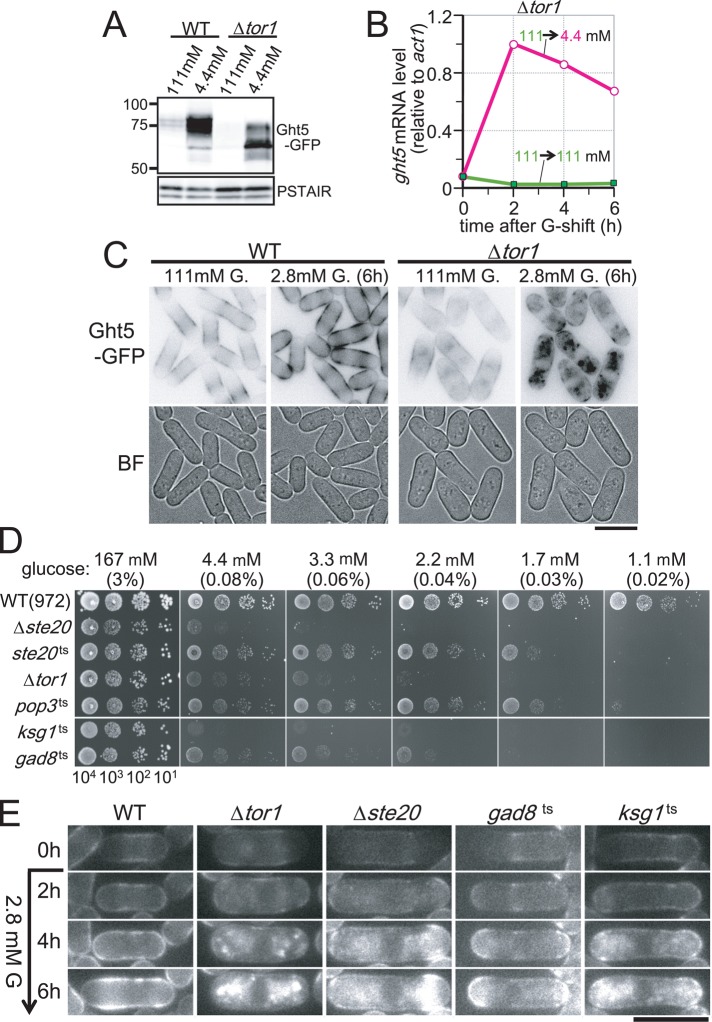
The foregoing results prompted us to examine the hypothesis that these protein kinases and the phosphatase inhibitor affect expression and/or protein behavior of Ght5 in low glucose. For this purpose, we determined the level of Ght5-GFP by immunoblot analysis using antibodies against GFP. The protein level of Ght5-GFP in WT and ∆tor1 cells cultivated in 111 mM glucose medium and 4.4 mM glucose medium is shown in Figure 5A. Like WT cells, Ght5 protein increased in ∆tor1 mutant cells under low-glucose conditions, although the protein band position was significantly altered; Ght5 might be modified differently (e.g., hypophosphorylated) or partially cleaved in mutant cells. Consistently, the level of Ght5 mRNA was elevated in Δtor1 cells after cells were transferred to low-glucose (4.4 mM) medium (Figure 5B).
FIGURE 5:
The TORC2 signaling pathway is required for proper trafficking of Ght5 to the cell surface. (A) Immunoblotting for Ght5-GFP in WT and Δtor1. Cells were cultivated in fresh EMM2 containing either 111 or 4.4 mM glucose at 33°C for 6 h before sample preparation. The blot with anti-PSTAIR antibody, which recognizes S. pombe Cdc2, is shown as the loading control. (B) Time course of ght5+ mRNA levels was examined in the Δtor1 strain. Exponentially growing cells were transferred to fresh EMM2 containing 111 (filled rectangles) or 4.4 mM (open circles) glucose and cultivated at 33°C. The mRNA level of ght5+ relative to that of act1+ was measured by RT-qPCR at 2-h intervals. (C) Intracellular localization of Ght5 was examined in Δtor1 and WT control cells. Cells harboring Ght5-GFP were cultivated at 33°C in a microfluidic perfusion chamber with continuous supply of medium, and the glucose concentration was switched from 111 to 2.8 mM. GFP and brightfield (BF) microscopy images of the cells before medium switching and after 6-h cultivation in 2.8 mM glucose are shown. Black and white is reversed in GFP images. Bar, 10 μm. (D) Aliquots of 104 cells of WT and TORC2-related mutant strains were serially diluted 10-fold, spotted onto YES medium plates containing the indicated concentrations of glucose, and incubated at 30°C for 3 d. The ste20+, pop3+, and tor1+ genes encode components of TORC2, whereas the gad8+ gene encodes an AGC-family kinase, which is phosphorylated and activated by TORC2 and a PDK-related kinase encoded by ksg1+ (Niederberger and Schweingruber, 1999; Matsuo et al., 2003, 2007; Hayashi et al., 2007). (E) Time-lapse images of Ght5 localization in WT and TORC2-related mutant cells. WT and indicated mutant cells harboring Ght5-GFP were cultivated at semipermissive temperatures in a microfluidic perfusion chamber with a continuous supply of medium, and the glucose concentration was switched from 111 to 2.8 mM at time 0 h. GFP fluorescence images at 2-h intervals are shown. Bar, 10 μm. See also Supplemental Figure S2.
Intracellular localization of Ght5 was then examined in Δtor1 mutant cells. In WT cells, Ght5-GFP fluorescence expanded from the middle region (0 h) to the tips of the cell surface 6 h after the shift to low glucose. In ∆tor1 mutant cells, however, GFP fluorescence at the surface was diminished after 6-h cultivation under low-glucose conditions; instead, Ght5 accumulated in the cytoplasm, forming aberrant aggregates (Figure 5C). Note that under high-glucose (111 mM) conditions, Ght5 localized at the cell surface of the middle region in the mutant cells, although some accumulation in the cytoplasm was also observed. These findings suggested that Tor1 was required for proper localization of Ght5, especially under low-glucose conditions.
Because the tor1+ gene encodes a kinase comprising the multiprotein complex TORC2, a phosphosignaling pathway through the TORC2 complex might be important for proper trafficking of the Ght5 transporter to the cell surface. Consistent with this supposition, mutant strains defective in the TORC2-signaling pathway, such as ste20, pop3, gad8, and ksg1 mutants, also failed to proliferate on low-glucose solid media (Figure 5D). The genes ste20+ and pop3+ encode conserved TORC2 subunits homologous to mammalian Rictor and LST8, respectively (Hayashi et al., 2007; Matsuo et al., 2007). The genes gad8+ and ksg1+ encode an Akt-related AGC family kinase and a PDK-like kinase, respectively, both of which function in the TORC2 signaling cascade; Gad8 kinase is activated through phosphorylation by TORC2 and Ksg1 kinases (Niederberger and Schweingruber, 1999; Matsuo et al., 2003; Ikeda et al., 2008; Tatebe et al., 2010). It may be of note that Ksg1 was reported to interact genetically and physically with a protein kinase C (Graub et al., 2003), suggesting that Ksg1 is regulated through the Ca2+ signaling pathway. We examined localization of Ght5 in these mutants grown in low-glucose culture medium. Cytoplasmic accumulation of Ght5 was observed not only in Δtor1cells, but also in Δste20, gad8, and ksg1 mutant cells under low-glucose (2.8 mM) conditions at semipermissive temperatures (34.5°C for the gad8 mutant and 33°C for the other strains), suggesting that the failure of proper Ght5 exocytosis to the cell surface is why these TORC2 mutants cannot support cell division under low-glucose conditions (Figure 5E).
In contrast to TORC2, another TOR-containing complex, TORC1, is likely to be dispensable for translocation of Ght5 to the surface in low glucose; ts mutations in TORC1-specific subunits (tor2-L2048S and mip1-310; Shinozaki-Yabana et al., 2000; Hayashi et al., 2007) did not diminish colony formation on low-glucose medium at the semipermissive temperature nor perturb Ght5 localization on the cell surface (Supplemental Figure S2, A and B).
Ssp1 and Sds23 are required for elevated expression of Ght5 in low-glucose conditions
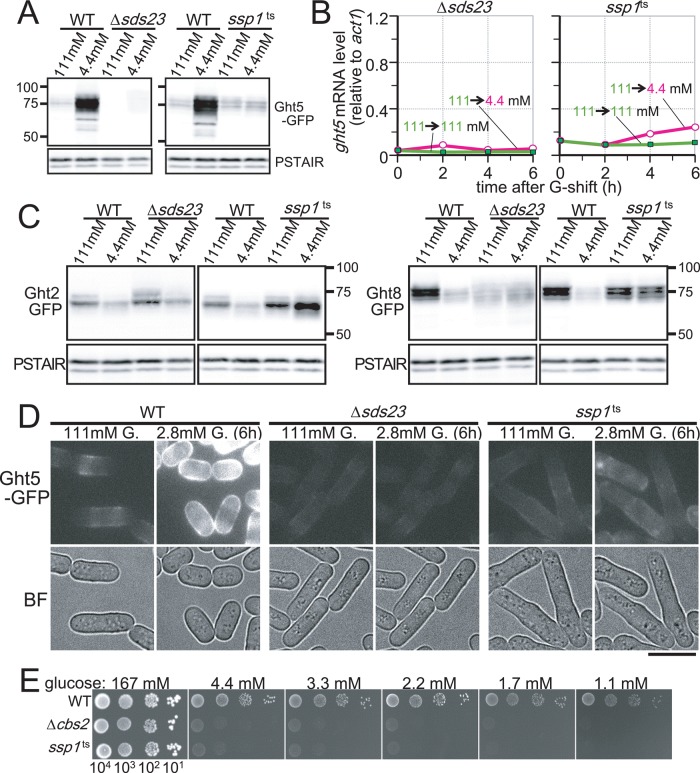
We then examined how Sds23 and Ssp1 regulate Ght5 function. Unexpectedly, immunoblot analysis using anti-GFP antibody showed that the protein level of Ght5-GFP was markedly reduced in Δsds23 and also in the ssp1-837 mutant cells under low-glucose (4.4 mM) conditions (Figure 6A). The protein level of Ght5-GFP was nearly negligible in ∆sds23 mutant cells in both high- and low-glucose conditions, whereas a small amount of Ght5-GFP was detected in the ssp1-873 mutant. Consistent with reduced levels of Ght5 protein, levels of ght5+ mRNA were also low in these mutant cells under both low-glucose (4.4 mM) and high-glucose (111 mM) conditions (Figure 6B). In contrast to Ght5, protein levels of Ght2-GFP and Ght8-GFP in Δsds23 and ssp1ts were comparable to those in the WT (Figure 6C).
FIGURE 6:
Sds23 and Ssp1 are required for elevated expression of Ght5 under low glucose. (A) Immunoblotting for Ght5-GFP in WT, Δsds23, and ssp1-837 (ssp1ts) mutant strains. WT and mutant cells were cultivated in EMM2 containing either 111 or 4.4 mM glucose for 6 h at 33°C before sample preparation. Blots with anti-PSTAIR antibody are shown as the loading control. (B) Time course of ght5+ mRNA levels was examined in the Δsds23 and the ssp1ts strains. Exponentially growing cells were transferred to fresh EMM2 containing 111 (filled rectangles) or 4.4 mM (open circles) glucose and cultivated at 33°C. mRNA levels of ght5+ relative to those of act1+ were measured by RT-qPCR at 2-h intervals. (C) Immunoblotting for Ght2- and Ght8-GFP in WT, Δsds23, and ssp1-837 (ssp1ts) mutant strains. WT and mutant cells were cultivated in EMM2 containing either 111 or 4.4 mM glucose for 6 h at 33°C before sample preparation. Blots with anti-PSTAIR antibody are shown as the loading control. (D) Intracellular localization of Ght5 was examined in Δsds23 and ssp1ts mutants, as well as in WT control. Cells harboring Ght5-GFP were cultivated at 33°C in a microfluidic perfusion chamber with a continuous supply of medium, and the glucose concentration was switched from 111 to 2.8 mM. GFP and brightfield (BF) microscopy images of the cells before medium switching and after 6-h cultivation in 2.8 mM glucose are shown. All GFP images here were processed under the same conditions. Bar, 10 μm. (E) Aliquots of 104 cells of the WT, Δcbs2, and ts ssp1-837 mutant strains were serially diluted 10-fold, spotted onto YES medium plates containing the indicated concentrations of glucose, and incubated at 33°C for 3 d. See also Supplemental Figure S3.
Intracellular localization of Ght5-GFP protein was observed in mutant cells cultured in the microfluidic chamber with a continuous supply of medium in which the glucose concentration was switched from 111 to 2.8 mM for 6 h (Figure 6D). In sharp contrast to the vigorously dividing WT cells with strong GFP fluorescence, only faint GFP fluorescence was detected in both the Δsds23- and the ssp1-837 mutant cells, which scarcely divided during the 6-h incubation in the low-glucose (2.8 mM) medium. These results suggested that Ssp1 and Sds23 played an essential role in ght5+ gene expression.
Ssp1 was previously reported to be homologous to mammalian CaMKK, which phosphorylates an AMP-activated kinase (AMPK), as well as Ca2+/calmodulin-dependent kinase (CaMK; Hanyu et al., 2009). Although fission yeast harbors two genes encoding AMPK (ssp2+ and ppk9+), only one gene (cbs2+) encodes the γ-subunit binding to both AMPKs (Hanyu et al., 2009). Thus we examined whether AMPK was also involved in ght5+ gene expression by testing whether cells lacking cbs2+ (Δcbs2) could form colonies on low-glucose media. Δcbs2 cells did not form colonies on low-glucose medium, implying that AMPK is also involved in ght5+ expression (Figure 6E). Consistently, fluorescence signals of Ght5-GFP in Δcbs2 were as faint as those in ssp1ts, even under low-glucose conditions (Supplemental Figure S3).
Scr1 is a transcriptional repressor for ght5+ that is regulated by Sds23 and Ssp1
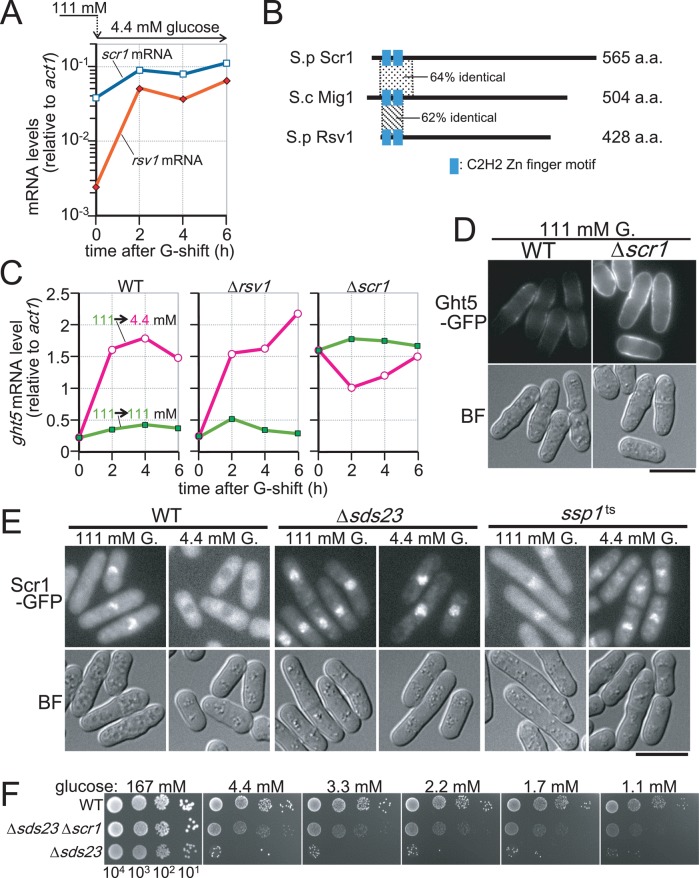
As described earlier, Ght5 expression is regulated in response to glucose concentrations, and signaling pathways involving PP2A-like phosphatases and CaMKK play important roles in this regulation. These findings raised the question of how these phosphatases and kinases control ght5+ gene transcription. To address this question, we searched for transcription factors regulating ght5+ expression and found two candidate transcription factors, Rsv1 and Scr1. Our microarray analysis revealed that limiting glucose increased the mRNA levels of ∼500 genes, which include the rsv1+ and scr1+ genes (Saitoh and Yanagida, 2014). mRNA levels of these genes after the shift to low-glucose medium were measured by RT-qPCR (Figure 7A). Expression of rsv1+ markedly (>20-fold) increased after the shift, whereas that of scr1+ was relatively high before the shift (time = 0 h) and further (approximately threefold) increased during cultivation in low-glucose medium. Rsv1 and Scr1 are proteins with C2H2-type zinc finger motifs that are moderately homologous to budding yeast Mig1 (Figure 7B). Rsv1 is reportedly essential for maintaining cell viability during a stationary phase induced by glucose starvation (Hao et al., 1997), whereas Scr1 is involved in transcriptional regulation of fbp1+ and gld1+ genes, which are required for gluconeogenesis and assimilation of nonfermentable glycerol, respectively (Hirota et al., 2006; Matsuzawa et al., 2010).
FIGURE 7:
Nuclear exclusion of Scr1, which represses transcription of ght5+ in high glucose, requires the presence of Sds23 and Ssp1. (A) Levels of rsv1+ (filled diamonds) and scr1+ (open squares) mRNAs relative to that of act1+ were determined by RT-qPCR in the cells cultivated in 4.4 mM glucose medium for 0, 2, 4, and 6 h after the shift from 111 mM glucose. (B) S. pombe Scr1 and Rsv1 proteins are schematically presented together with their S. cerevisiae homologue, Mig1p. The length of each protein is indicated at the right. Although Scr1 and Rsv1 are the closest fission yeast homologues to Mig1p, their sequence similarities, which are represented as percentage identity to Mig1p, are limited to small regions surrounding C2H2-type zinc finger motifs, whose positions are indicated by the filled boxes. (C) Time course of ght5+ mRNA levels was examined in WT, Δrsv1, and Δscr1 strains. Exponentially growing cells were transferred to fresh EMM2 containing 111 (filled rectangles) or 4.4 mM (open circles) glucose and cultivated at 33°C. The mRNA levels of ght5+ relative to those of act1+ were measured by RT-qPCR at 2-h intervals. (D) Micrographs of the WT and the Δscr1 cells expressing Ght5-GFP under the native promoter. Exponentially growing cells in EMM2 containing 111 mM glucose at 33°C were harvested by centrifugation and resuspended in a small amount of the same medium. Ght5-GFP fluorescence and brightfield (BF) microscopy images showing cell shape were obtained immediately without fixation. Fluorescence images here were processed under the same conditions. Bar, 10 μm. (E) Intracellular localization of Scr1-GFP in WT, Δsds23, and ssp1-537 cells. Cells expressing Scr1-GFP were transferred from EMM2 with 111 mM glucose to EMM2 with either 111 or 4.4 mM glucose and cultivated for 4 h before sampling. Scr1-GFP fluorescence and BF microscopy images were obtained immediately without fixation. Bar, 10 μm. (F) Aliquots of 104 cells of WT, Δsds23 Δscr1 double-mutant, and Δsds23 mutant strains were serially diluted 10-fold, spotted onto YES medium plates containing the indicated concentrations of glucose, and incubated at 30°C for 3 d. See also Supplemental Figure S4.
We first examined how Ght5 expression levels were affected by deletion of the rsv1+ and scr1+ genes. Cells lacking rsv1+ (Δrsv1) or scr1+ (Δscr1) were cultivated in medium with 111 or 4.4 mM glucose, and the levels of ght5+ mRNA were measured every 2 h by RT-qPCR (Figure 7C). The ght5+ expression profile in Δrsv1 was nearly identical to that in WT, suggesting that Rsv1 is unlikely to be a regulator of ght5+ expression. In contrast, ght5+ mRNA levels were greatly enhanced in Δscr1 cells under high-glucose conditions, whereas these levels remained low in WT cells, suggesting that Scr1 is a transcriptional repressor of ght5+ in high-glucose conditions. Consistent with the constitutive elevation in the transcript level, abundant Ght5 protein was expressed in Δscr1 cells constitutively, regardless of glucose concentration (Figure 7D); strong fluorescence signals of Ght5-GFP were detected on the surface of Δscr1 cells in high-glucose (111 mM) medium, but the fluorescence intensity was relatively weak in WT cells.
We then examined the intracellular localization of Scr1. Cells in which the genomic copy of the scr1+ gene was replaced with the GFP-tagged scr1+ under the native promoter were cultivated in liquid EMM2 with 111 or 4.4 mM glucose medium for 4 h (Figure 7E). Consistent with the assumption that Scr1 functions as a repressor specifically under high-glucose conditions, Scr1-GFP was located in the nuclei of WT cells growing in high-glucose (111 mM) medium, whereas it appeared to be excluded from the nucleus and localized predominantly in the cytoplasm in low-glucose (4.4 mM) medium. Exclusion of Scr1 from the nucleus under low-glucose conditions depended on the presence of Sds23 and Ssp1; in both Δsds23 and ssp1ts mutants, the Scr1 protein was localized in the nucleus even under low-glucose conditions (Figure 7E). Especially in Δsds23 cells, Scr1 located exclusively in the nucleus, regardless of the glucose concentration (e.g., the cytoplasmic GFP signal in 4.4 mM glucose was hardly observed). Taken together, these findings strongly suggest that transcription of the ght5+ gene is repressed in the presence of high glucose concentrations by the zinc finger protein Scr1, exclusion of which from the nucleus increases the abundance of ght5+ mRNA under low-glucose conditions. The signaling pathway involving the Sds23 inhibitor and the Ssp1 kinase appeared to promote the export of Scr1 from the nucleus in response to a glucose limitation. Defects in this pathway appeared to cause constitutive accumulation of the Scr1 repressor in the nucleus, resulting in diminished expression of Ght5 under low-glucose conditions. Consistent with this hypothesis, deletion of the scr1+ gene alleviated the defect in colony formation of the Δsds23 mutant on low-glucose medium (Figure 7F).
DISCUSSION
The present study provides evidence that S. pombe cells use dramatically distinct mechanisms for glucose uptake under high-glucose (111–167 mM, or 2–3%) and low-glucose (<4.4 mM, or 0.08%) conditions, suggesting that large-scale “remodeling” occurs in cellular metabolic pathways and division control in response to changes in the extracellular glucose concentration. First, mRNA and protein levels and distributions of each of the eight transporters were differentially regulated, depending on the glucose concentration (see also Saitoh and Yanagida, 2014). Whereas the GFP-tagged Ght2 and Ght8 transporters were plentiful at the non-tip regions of the plasma membrane in both high and low glucose, the abundance of the major glucose transporter, Ght5, and related transporters (Ght1, Ght3, and Ght4) was greatly increased, particularly in cell tip regions under low glucose. These large-scale changes in transcription, synthesis, and localization of glucose transporters are hallmarks of cell remodeling upon glucose shift. Second, the rate of glucose uptake in WT cells was greatly enhanced if cells were previously treated with low glucose, but this ability was lost in some mutant cells, such as ∆ght5, ∆sds23, ssp1ts, and ∆tor1 (Figure 4). Under low-glucose conditions, a specific group of genes thus became necessary for WT cells to grow and divide. Third, we showed that a zinc finger transcription factor, Scr1, repressed ght5+ gene expression, and the derepression of Ght5 under low-glucose conditions required the Ssp1 kinase and the Sds23 phosphatase inhibitor; Ssp1 and Sds23 were needed to exclude Scr1 from nucleus in low-glucose conditions. Fourth, TORC2 and related protein kinases (Gad8/Akt and Ksg1/PDK1) were required for Ght5 to localize properly at the plasma membrane. Taken together, these results provide a novel view that a series of large-scale changes, such as de novo synthesis of Ght5 and its preferential localization at the cell tip, are induced in S. pombe during cellular adaptation to low-glucose conditions. Calcium- and phosphatase-mediated regulations leading to the displacement of the transcriptional repressor Scr1 from the nucleus are needed to induce such changes. In addition, TORC2 signaling is essential for proper trafficking of Ght5 to the plasma membrane. These changes seem to optimize the rate of glucose uptake in a wide range of environmental glucose conditions; highly efficient glucose uptake might be achieved by Ght5 localizing along the entire plasma membrane in low-glucose conditions, whereas restricted distribution of the transporters to the non-tip regions may reduce the uptake rate in the presence of high-glucose concentrations.
Although glucose is a fundamental source of energy in eukaryotes, abundant glucose is not always available in the natural environment. Thus the mechanism allowing for rapid cell division under a broad range of glucose concentrations should be vital for cell survival in nature. In our previous study (Pluskal et al., 2011), we found that WT S. pombe cells have increased cell numbers at nearly an identical division rate (3.5 h/division) in both 111 and 4.4 mM glucose-containing media, although the average cell sizes were largely different. We supposed that reduced cell size might contribute, at least to some extent, to rapid cell proliferation under low-glucose conditions. In the present study, we show that the mode of glucose uptake is substantially altered, and such alterations appear to contribute significantly to cellular adaptation to glucose-limited environments. Elevated expression and subsequent cell tip localization of the Ght5 transporter should greatly enhance the cellular ability to incorporate dilute glucose, ensuring cell proliferation, and presumably longevity, under low-glucose conditions. Because Ght5 is a high-affinity glucose transporter (Heiland et al., 2000), this change seems to be an efficient way for cells to respond to a low-glucose environment.
In mammalian muscle skeletal cells, the abundance of the GLUT4 glucose transporter on the cell surface is regulated via two distinct pathways (Watson and Pessin, 2006; Ojuka et al., 2012). On insulin stimulation, vesicles storing GLUT4 in the cytoplasm are targeted to the cell surface by exocytosis (Bryant et al., 2002; Dugani and Klip, 2005). This vesicle trafficking to the cell surface is regulated by Akt kinase, which is phosphorylated and activated by TORC2 and PDK1 kinase (Scheid et al., 2002; Matsuo et al., 2003; Sarbassov et al., 2005). In addition, independently of insulin, contractile activity during exercise increases the transcriptional level of GLUT4 via the Ca2+ signaling pathway, presumably involving CaMKK, CaMK, and AMPK (Ojuka et al., 2002; Wright et al., 2004; Witczak et al., 2007). These molecules are essential for S. pombe cell proliferation under low-glucose conditions, and surprising parallels between the mechanisms regulating the abundance and localization of hexose transporters seem to exist in skeletal muscle and fission yeast, although S. pombe does not secret insulin. Of note, 4.4 mM (0.08%) glucose is actually equivalent to the amount present in human blood.
As in skeletal muscle cells, CaMKK and AMPK, which are involved in calcium signaling, are required to increase the level of Ght5 in S. pombe cells. Here we show that CaMKK-like Ssp1, Cbs2 (the γ subunit of AMPK; Hanyu et al., 2009), and the PP2A-, PP6-inhibitor Sds23 are required for up-regulation of ght5+ transcription in low glucose. An evolutionarily conserved mechanism involving calcium signaling may thus exist for transcriptional regulation of hexose transporters. Ssp1 contains calmodulin-binding motifs and a conserved basic stretch, resembling mammalian CaMKK (Hanyu et al., 2009). Ssp1 was originally identified as an extragenic suppressor of ppe1 phosphatase and sts5 mutants, both of which interact genetically with pck1+, encoding protein kinase C (Shimanuki et al., 1993; Matsusaka et al., 1995; Toda et al., 1996). The Ca2+ signaling cascade might therefore activate ght5+ expression under low-glucose conditions via Ssp1. On the other hand, Sds23 was originally identified as a multicopy suppressor of mutants defective in type 1 phosphatase (PP1), encoded by the dis2+ and sds22+ genes (Ishii et al., 1996), suggesting that Sds23 somehow enhances PP1 activity. Of interest, Sds23 binds the regulatory subunits of PP2A-like phosphatases and inhibits their activity (Hanyu et al., 2009). Thus PP1 and PP2A may be functionally opposed, and Sds23 may simultaneously down-regulate PP2A and up-regulate PP1. The balance between these phosphatases might be important in the expression level of ght5+. The mutual genetic interaction between ssp1+ and sds23+ suggests that Ssp1 and Sds23 synergistically act at the level of the ght5+ transcription upon glucose limitation.
We show that the C2H2-type zinc finger Scr1 plays a pivotal role in transcriptional regulation of the Ght5 hexose transporter, and the behavior of the Scr1 repressor is greatly affected by Ssp1 and Sds23. Scr1 interacts with the corepressors (Tup11 and Tup12), and its nuclear exclusion occurs upon carbon-source switch from fermentable glucose to nonfermentable glycerol (Hirota et al., 2006; Matsuzawa et al., 2012). Scr1 and Tup proteins remodel chromatin at the fbp1+ gene locus to keep it transcriptionally inactive under high-glucose conditions, and the transcription of fbp1+ occurs upon the removal of these molecules under low-glucose nonfermentable conditions. Our findings indicated that Scr1 also functions as a ght5+ transcription repressor under high-glucose conditions. S. pombe Scr1 is similar to Aspergillus CREA (Tanaka et al., 1998), which is required for carbon catabolite repression (Dowzer and Kelly, 1989), and also to budding yeast Mig1, which is regulated by the AMPK-like Snf1 kinase in response to glucose concentrations and is involved in the repression of genes related to carbon metabolism (Celenza and Carlson, 1986; De Vit et al., 1997). In mammalian skeletal muscle cells, Scr1-like transcriptional repressors have not been reported. It is noteworthy that fission yeast contains another Scr1-like C2H2-zinc finger protein, called Rsv1. Rsv1 is required for cell survival in the stationary phase after complete depletion of glucose (Hao et al., 1997), although it does not seem to be involved in transcriptional regulation of ght5+. In sharp contrast to Scr1, which was excluded from the nucleus, Rsv1 accumulated in the nucleus in low-glucose conditions (Supplemental Figure S4). Therefore Rsv1 might repress the expression of genes other than ght5+ under low-glucose conditions. Rsv1 and Scr1 might be complementary regulators in response to a change in glucose availability.
Under low-glucose conditions, there were a large number of hexose transporters located on the cell surface. Of interest, the transporters were not evenly distributed across the whole cell surface but tended to be concentrated near the cell equator under high glucose, whereas newly synthesized transporters appeared to be loaded preferentially near the cell tips under low glucose. Thus there might be a mechanism to allocate hexose transporters to specific regions of the cell membrane. As previously reported, the membrane-bound DYRK-family protein kinase Pom1 is enriched on the membrane near the cell tips. The phosphorylation status of Pom1, based on its autophosphorylation and dephosphorylation by PP1 (Dis2), regulates Pom1 binding to the cell membrane for preferential accumulation near the tips (Martin and Berthelot-Grosjean, 2009; Moseley et al., 2009; Hachet et al., 2011). Similarly, the phosphorylation status of hexose transporters might regulate their affinity to the membrane or yet-unidentified membrane proteins that anchor Ght transporters near the tips. Further studies are required to uncover factors switching between the cell-tip and cell-equator localization of the transporters depending on glucose availability.
TORC2 is essential for the distribution of Ght5 on the cell surface under low-glucose conditions. S. pombe Gad8 and Ksg1, which are believed to be homologues of Akt and PDK1, respectively (Niederberger and Schweingruber, 1999; Matsuo et al., 2003; Ikeda et al., 2008; Cybulski and Hall, 2009; Tatebe et al., 2010), are also essential. TOR kinase forms two evolutionarily conserved complexes—rapamycin-sensitive TORC1 and the less sensitive TORC2 (Loewith et al., 2002; Wullschleger et al., 2006). TOR kinase regulates various aspects of cell growth, metabolism, and autophagy in response to environmental cues such as nutrients and growth factors. Although TORC1 is known to be activated in the presence of amino acids via leucyl-tRNA synthetase (Han et al., 2012) and to regulate endocytotic internalization of amino acid permease in response to changes in nitrogen source (Merhi and Andre, 2012; O’Donnell, 2012), it was hitherto unclear whether TORC2 contributes to the cellular response to nutritional status. Our findings demonstrate that the TORC2 signaling cascade is an integral part of the cellular response to low glucose. How the TORC2 pathway regulates Ght5 localization remains to be elucidated, but we speculate that TORC2 and/or related kinases phosphorylate Ght5 so that it becomes enriched near the cell tips under low glucose. It is also possible that the TORC2 pathway regulates vesicle trafficking machinery, localizing Ght5 on the plasma membrane.
In summary, we show that large-scale remodeling and translocation of hexose transporters is the major outcome of extracellular glucose reduction. Displacement of nuclear Scr1 repressing ght5+ transcription requires Ssp1/CaMKK, whereas trafficking of Ght5 from the cytoplasm to the plasma membrane requires TORC2 and Gad8/Akt signaling. Although the means by which cells sense the extracellular glucose concentration remains unknown, we suspect that the Ca2+ signaling pathway involving Ssp1/CaMKK may play a central role in the glucose- sensing mechanism. Although Ssp1 normally occurs throughout the cytoplasm, environmental stressors, such as hyperosmotic pressure, cause it to migrate immediately to the plasma membrane (Rupe˘s et al., 1999; Freitag et al., 2014). Thus the findings in this study, together with those of previous studies, reveal a phosphosignaling pathway from the plasma membrane to the nucleus. On reduction of extracellular glucose, this signaling cascade involving Ssp1/CaMKK activates to exclude Scr1 from the nucleus, up-regulating expression of Ght5.
MATERIALS AND METHODS
General techniques and materials
For cultivation of fission yeast, YES (rich medium) and EMM2 (minimal medium), which have been previously described (Moreno et al., 1991), were used with modified glucose concentrations as indicated. Unless otherwise stated, WT cells were cultivated at 33°C, and mutant cells were cultured at their permissive temperatures, 26°C for the ssp1-837 mutant and 30°C for the Δsds23 mutant. For C-terminal gene tagging and gene disruption, we used the PCR-mediated method (Krawchuk and Wahls, 1999). The Δght1, Δght3, Δght4, Δght6, and Δght7 strains were originally obtained from Bioneer Corporation (Daejeon, Korea) and subsequently back-crossed against WT for the removal of auxotroph markers. The Δght2, Δght5, and Δght8 strains were constructed by disrupting the corresponding ght genes from the WT genome. Gene deletion in each Δght strain was confirmed by PCR.
Total RNA preparation and RT-qPCR
Total RNA samples were prepared using the hot phenol extraction method described previously (Lyne et al., 2003) and purified using a High Pure RNA Isolation Kit (Roche Diagnostics, Mannheim, Germany). A ReverTra Ace qPCR RT Kit (TOYOBO, Osaka, Japan) was used for reverse transcription. qPCR was performed and analyzed using LightCycler Nano system with FastStart Essential DNA Green Master kit (Roche Diagnostics). The copy number of ght mRNA relative to act1 was calculated from a standard curve drawn using serial dilutions of genomic DNA containing the same number of DNA fragments of ght+ and act1+ as a template. The PCR primer sequences are available upon request.
Measurement of the glucose uptake rate
The rate of glucose uptake was measured as previously described (Walsh et al., 1994) with modifications. Briefly, cells were suspended in 0.15 M KH2PO4 buffer (pH 4.5) at a concentration of 8 × 107/ml after washing. An aliquot of the cell suspension (25 μl) was preincubated at 33°C and mixed with an equal volume of 2× concentrated radioactive glucose solution. After 15-s incubation at 33°C, the cells were transferred into 10 ml of ice-cold KH2PO4 buffer containing 500 mM nonlabeled glucose and then collected on a glass fiber filter. The amount of glucose incorporated into the cells was determined by liquid scintillation counting.
Measurement of glucose consumption
Exponentially growing cells in EMM2 containing 111 mM glucose were transferred to fresh EMM2 containing 4.4 or 111 mM glucose at time 0 and cultured at 33°C. An aliquot of the culture was obtained at each time point and stored at 4°C after cell removal by centrifugation. The amount of glucose remaining in the medium was measured using a Glucose HK Assay Kit (Sigma-Aldrich, St. Louis, MO).
Preparation of cell extract and immunoblotting
Cells were collected after 6-h cultivation in EMM2 containing 111 or 4.4 mM glucose at 33°C, washed once in distilled water, and stored at −80°C until use. Protein extracts were prepared by vortexing the cells with glass beads in ice-cold lysis buffer containing 50 mM Tris-HCl (pH 7.5), 150 mM NaCl, 5 mM EDTA, 10% glycerol, 1% Nonidet P-40, 20 mM β-glycerophosphate, 10 mM p-nitrophenyl phosphate, 10 mM NaF, 0.1 mM sodium orthovanadate, and 1 mM dithiothreitol. Proteinase inhibitor cocktail (Nacalai Tesque, Kyoto, Japan) and 2 mM phenylmethylsulfonyl fluoride were added to the extracts to prevent protein degradation. Before separation in SDS–PAGE, the extract was mixed with one-third volume of 4× lithium dodecyl sulfate (LDS) sample solution (0.99 M Tris-HCl, pH 8.5, 40% glycerol, 2 mM EDTA, 8% LDS, 10% β-mercaptoethanol, and a trace amount of bromophenol blue) and incubated on ice for 2 h. The anti-GFP monoclonal antibody was purchased from Roche Diagnostics.
Microscope image acquisition
Fluorescence microscopy was performed using a Olympus IX81 microscope (Olympus, Tokyo, Japan) equipped with a 100× objective lens (numerical aperture [NA] 1.35) or a Leica ASMDW Live Cell Imaging System (Leica Microsystems, Wetzlar, Germany) equipped with a 100× objective lens (NA 1.4) and the ONIX microfluidic perfusion system (CellASIC, Hayward, CA). For live-cell analysis, cells growing in EMM2 at the permissive temperature were loaded into the microfluidic perfusion chamber and cultured with a continuous medium supply. For the first 3 h, EMM2 with 111 mM glucose was supplied, and then the medium was switched to that with 2.8 mM glucose. Images were obtained by a charge-coupled device camera and processed using ImageJ software (National Institutes of Health, Bethesda, MD).
Supplementary Material
Acknowledgments
We are indebted to Yukinobu Nakaseko (Kyoto University, Kyoto, Japan), Kenichi Sajiki, and Takeshi Hayashi for their help in gene identification. Shigeaki Saitoh was supported by a grant from the Ishibashi Foundation for the Promotion of Science, and Grants-in-Aid for Scientific Research (C) and Young Scientists (B) from the Japan Society for the Promotion of Science. We acknowledge generous support from the CREST program of the Japan Science and Technology Corporation, the Okinawa Institute of Science and Technology Promotion Corporation (OIST), and the MEXT-Supported Program for the Strategic Research Foundation at Private University from the Ministry of Education, Culture, Sports, Science and Technology, Japan.
Abbreviations used:
- AMPK
adenosine monophosphate–activated kinase
- CAMK
Ca2+/calmodulin-dependent kinase
- CaMKK
Ca2+/calmodulin-dependent kinase kinase
- LDS
lithium dodecyl sulfate
- PP
protein phosphatase
- RT-qPCR
reverse transcription-quantitative PCR
- TOR
target of rapamycin
- ts
temperature sensitive
- WT
wild type.
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E14-11-1503) on November 19, 2014.
S. Saitoh, A.M., L.U., F.M., and S. Soejima performed the experiments. S. Saitoh and M.Y. analyzed the results, participated in experimental design, and wrote the manuscript.
The authors declare that they have no conflict of interest.
REFERENCES
- Boles E, Hollenberg CP. The molecular genetics of hexose transport in yeasts. FEMS Microbiol Rev. 1997;21:85–111. doi: 10.1111/j.1574-6976.1997.tb00346.x. [DOI] [PubMed] [Google Scholar]
- Brown GK. Glucose transporters: structure, function and consequences of deficiency. J Inherit Metab Dis. 2000;23:237–246. doi: 10.1023/a:1005632012591. [DOI] [PubMed] [Google Scholar]
- Bryant NJ, Govers R, James DE. Regulated transport of the glucose transporter GLUT4. Nat Rev Mol Cell Biol. 2002;3:267–277. doi: 10.1038/nrm782. [DOI] [PubMed] [Google Scholar]
- Celenza JL, Carlson M. A yeast gene that is essential for release from glucose repression encodes a protein kinase. Science. 1986;233:1175–1180. doi: 10.1126/science.3526554. [DOI] [PubMed] [Google Scholar]
- Colville CA, Seatter MJ, Gould GW. Analysis of the structural requirements of sugar binding to the liver, brain and insulin-responsive glucose transporters expressed in oocytes. Biochem J. 1993a;294:753–760. doi: 10.1042/bj2940753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colville CA, Seatter MJ, Jess TJ, Gould GW, Thomas HM. Kinetic analysis of the liver-type (GLUT2) and brain-type (GLUT3) glucose transporters in Xenopus oocytes: substrate specificities and effects of transport inhibitors. Biochem J. 1993b;290:701–706. doi: 10.1042/bj2900701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cybulski N, Hall MN. TOR complex 2: a signaling pathway of its own. Trends Biochem Sci. 2009;34:620–627. doi: 10.1016/j.tibs.2009.09.004. [DOI] [PubMed] [Google Scholar]
- De Vit MJ, Waddle JA, Johnston M. Regulated nuclear translocation of the Mig1 glucose repressor. Mol Biol Cell. 1997;8:1603–1618. doi: 10.1091/mbc.8.8.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowzer CE, Kelly JM. Cloning of the creA gene from Aspergillus nidulans: a gene involved in carbon catabolite repression. Curr Genet. 1989;15:457–459. doi: 10.1007/BF00376804. [DOI] [PubMed] [Google Scholar]
- Dugani CB, Klip A. Glucose transporter 4: cycling, compartments and controversies. EMBO Rep. 2005;6:1137–1142. doi: 10.1038/sj.embor.7400584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitag SI, Wong J, Young PG. Genetic and physical interaction of Ssp1 CaMKK and Rad24 14-3-3 during low pH and osmotic stress in fission yeast. Open Biol. 2014;4:130127. doi: 10.1098/rsob.130127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graub R, Hilti N, Niederberger C, Schweingruber ME. Ksg1, a homologue of the phosphoinositide-dependent protein kinase 1, controls cell wall integrity in Schizosaccharomyces pombe. J Basic Microbiol. 2003;43:473–482. doi: 10.1002/jobm.200310287. [DOI] [PubMed] [Google Scholar]
- Hachet O, Berthelot-Grosjean M, Kokkoris K, Vincenzetti V, Moosbrugger J, Martin SG. A phosphorylation cycle shapes gradients of the DYRK family kinase Pom1 at the plasma membrane. Cell. 2011;145:1116–1128. doi: 10.1016/j.cell.2011.05.014. [DOI] [PubMed] [Google Scholar]
- Han JM, Jeong SJ, Park MC, Kim G, Kwon NH, Kim HK, Ha SH, Ryu SH, Kim S. Leucyl-tRNA synthetase is an intracellular leucine sensor for the mTORC1-signaling pathway. Cell. 2012;149:410–424. doi: 10.1016/j.cell.2012.02.044. [DOI] [PubMed] [Google Scholar]
- Hanyu Y, Imai KK, Kawasaki Y, Nakamura T, Nakaseko Y, Nagao K, Kokubu A, Ebe M, Fujisawa A, Hayashi T, et al. Schizosaccharomyces pombe cell division cycle under limited glucose requires Ssp1 kinase, the putative CaMKK, and Sds23, a PP2A-related phosphatase inhibitor. Genes Cells. 2009;14:539–554. doi: 10.1111/j.1365-2443.2009.01290.x. [DOI] [PubMed] [Google Scholar]
- Hao Z, Furunobu A, Nagata A, Okayama H. A zinc finger protein required for stationary phase viability in fission yeast. J Cell Sci. 1997;110:2557–2566. doi: 10.1242/jcs.110.20.2557. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Hatanaka M, Nagao K, Nakaseko Y, Kanoh J, Kokubu A, Ebe M, Yanagida M. Rapamycin sensitivity of the Schizosaccharomyces pombe tor2 mutant and organization of two highly phosphorylated TOR complexes by specific and common subunits. Genes Cells. 2007;12:1357–1370. doi: 10.1111/j.1365-2443.2007.01141.x. [DOI] [PubMed] [Google Scholar]
- Heiland S, Radovanovic N, Hofer M, Winderickx J, Lichtenberg H. Multiple hexose transporters of Schizosaccharomyces pombe. J Bacteriol. 2000;182:2153–2162. doi: 10.1128/jb.182.8.2153-2162.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota K, Hoffman CS, Ohta K. Reciprocal nuclear shuttling of two antagonizing Zn finger proteins modulates Tup family corepressor function to repress chromatin remodeling. Eukaryot Cell. 2006;5:1980–1989. doi: 10.1128/EC.00272-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hresko RC, Kruse M, Strube M, Mueckler M. Topology of the Glut 1 glucose transporter deduced from glycosylation scanning mutagenesis. J Biol Chem. 1994;269:20482–20488. [PubMed] [Google Scholar]
- Ikai N, Nakazawa N, Hayashi T, Yanagida M. The reverse, but coordinated, roles of Tor2 (TORC1) and Tor1 (TORC2) kinases for growth, cell cycle and separase-mediated mitosis in Schizosaccharomyces pombe. Open Biol. 2011;1:110007. doi: 10.1098/rsob.110007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda K, Morigasaki S, Tatebe H, Tamanoi F, Shiozaki K. Fission yeast TOR complex 2 activates the AGC-family Gad8 kinase essential for stress resistance and cell cycle control. Cell Cycle. 2008;7:358–364. doi: 10.4161/cc.7.3.5245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii K, Kumada K, Toda T, Yanagida M. Requirement for PP1 phosphatase and 20S cyclosome/APC for the onset of anaphase is lessened by the dosage increase of a novel gene sds23+ EMBO J. 1996;15:6629–6640. [PMC free article] [PubMed] [Google Scholar]
- Kim DU, Hayles J, Kim D, Wood V, Park HO, Won M, Yoo HS, Duhig T, Nam M, Palmer G, et al. Analysis of a genome-wide set of gene deletions in the fission yeast Schizosaccharomyces pombe. Nat Biotechnol. 2010;28:617–623. doi: 10.1038/nbt.1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krawchuk MD, Wahls WP. High-efficiency gene targeting in Schizosaccharomyces pombe using a modular, PCR-based approach with long tracts of flanking homology. Yeast. 1999;15:1419–1427. doi: 10.1002/(SICI)1097-0061(19990930)15:13<1419::AID-YEA466>3.0.CO;2-Q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenberg-Fraté H, Naschen T, Heiland S, Höfer M. Properties and heterologous expression of the glucose transporter GHT1 from Schizosaccharomyces pombe. Yeast. 1997;13:215–224. doi: 10.1002/(SICI)1097-0061(19970315)13:3<215::AID-YEA80>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Loewith R, Jacinto E, Wullschleger S, Lorberg A, Crespo JL, Bonenfant D, Oppliger W, Jenoe P, Hall MN. Two TOR complexes, only one of which is rapamycin sensitive, have distinct roles in cell growth control. Mol Cell. 2002;10:457–468. doi: 10.1016/s1097-2765(02)00636-6. [DOI] [PubMed] [Google Scholar]
- Lyne R, Burns G, Mata J, Penkett CJ, Rustici G, Chen D, Langford C, Vetrie D, Bähler J. Whole-genome microarrays of fission yeast: characteristics, accuracy, reproducibility, and processing of array data. BMC Genomics. 2003;4:27. doi: 10.1186/1471-2164-4-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier A, Volker B, Boles E, Fuhrmann GF. Characterisation of glucose transport in Saccharomyces cerevisiae with plasma membrane vesicles (countertransport) and intact cells (initial uptake) with single Hxt1, Hxt2, Hxt3, Hxt4, Hxt6, Hxt7 or Gal2 transporters. FEMS Yeast Res. 2002;2:539–550. doi: 10.1111/j.1567-1364.2002.tb00121.x. [DOI] [PubMed] [Google Scholar]
- Manolescu AR, Witkowska K, Kinnaird A, Cessford T, Cheeseman C. Facilitated hexose transporters: new perspectives on form and function. Physiology (Bethesda) 2007;22:234–240. doi: 10.1152/physiol.00011.2007. [DOI] [PubMed] [Google Scholar]
- Martin SG, Berthelot-Grosjean M. Polar gradients of the DYRK-family kinase Pom1 couple cell length with the cell cycle. Nature. 2009;459:852–856. doi: 10.1038/nature08054. [DOI] [PubMed] [Google Scholar]
- Mata J, Wilbrey A, Bähler J. Transcriptional regulatory network for sexual differentiation in fission yeast. Genome Biol. 2007;8:R217. doi: 10.1186/gb-2007-8-10-r217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo T, Kubo Y, Watanabe Y, Yamamoto M. Schizosaccharomyces pombe AGC family kinase Gad8p forms a conserved signaling module with TOR and PDK1-like kinases. EMBO J. 2003;22:3073–3083. doi: 10.1093/emboj/cdg298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo T, Otsubo Y, Urano J, Tamanoi F, Yamamoto M. Loss of the TOR kinase Tor2 mimics nitrogen starvation and activates the sexual development pathway in fission yeast. Mol Cell Biol. 2007;27:3154–3164. doi: 10.1128/MCB.01039-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsusaka T, Hirata D, Yanagida M, Toda T. A novel protein kinase gene ssp1+ is required for alteration of growth polarity and actin localization in fission yeast. EMBO J. 1995;14:3325–3338. doi: 10.1002/j.1460-2075.1995.tb07339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzawa T, Fujita Y, Tohda H, Takegawa K. Snf1-like protein kinase Ssp2 regulates glucose derepression in Schizosaccharomyces pombe. Eukaryot Cell. 2012;11:159–167. doi: 10.1128/EC.05268-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzawa T, Ohashi T, Hosomi A, Tanaka N, Tohda H, Takegawa K. The gld1+ gene encoding glycerol dehydrogenase is required for glycerol metabolism in Schizosaccharomyces pombe. Appl Microbiol Biotechnol. 2010;87:715–727. doi: 10.1007/s00253-010-2586-3. [DOI] [PubMed] [Google Scholar]
- Merhi A, Andre B. Internal amino acids promote Gap1 permease ubiquitylation via TORC1/Npr1/14-3-3-dependent control of the Bul arrestin-like adaptors. Mol Cell Biol. 2012;32:4510–4522. doi: 10.1128/MCB.00463-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno S, Klar A, Nurse P. Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol. 1991;194:795–823. doi: 10.1016/0076-6879(91)94059-l. [DOI] [PubMed] [Google Scholar]
- Moseley JB, Mayeux A, Paoletti A, Nurse P. A spatial gradient coordinates cell size and mitotic entry in fission yeast. Nature. 2009;459:857–860. doi: 10.1038/nature08074. [DOI] [PubMed] [Google Scholar]
- Mueckler M, Caruso C, Baldwin SA, Panico M, Blench I, Morris HR, Allard WJ, Lienhard GE, Lodish HF. Sequence and structure of a human glucose transporter. Science. 1985;229:941–945. doi: 10.1126/science.3839598. [DOI] [PubMed] [Google Scholar]
- Mueckler M, Makepeace C. Transmembrane segment 12 of the Glut1 glucose transporter is an outer helix and is not directly involved in the transport mechanism. J Biol Chem. 2006;281:36993–36998. doi: 10.1074/jbc.M608158200. [DOI] [PubMed] [Google Scholar]
- Niederberger C, Schweingruber ME. A Schizosaccharomyces pombe gene, ksg1, that shows structural homology to the human phosphoinositide-dependent protein kinase PDK1, is essential for growth, mating and sporulation. Mol Gen Genet. 1999;261:177–183. doi: 10.1007/s004380050955. [DOI] [PubMed] [Google Scholar]
- O’Donnell AF. The running of the Buls: control of permease trafficking by a-arrestins Bul1 and Bul2. Mol Cell Biol. 2012;32:4506–4509. doi: 10.1128/MCB.01176-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojuka EO, Goyaram V, Smith JA. The role of CaMKII in regulating GLUT4 expression in skeletal muscle. Am J Physiol Endocrinol Metab. 2012;303:E322–E331. doi: 10.1152/ajpendo.00091.2012. [DOI] [PubMed] [Google Scholar]
- Ojuka EO, Jones TE, Nolte LA, Chen M, Wamhoff BR, Sturek M, Holloszy JO. Regulation of GLUT4 biogenesis in muscle: evidence for involvement of AMPK and Ca2+ Am J Physiol Endocrinol Metab. 2002;282:E1008–1013. doi: 10.1152/ajpendo.00512.2001. [DOI] [PubMed] [Google Scholar]
- Özcan S, Freidel K, Leuker A, Ciriacy M. Glucose uptake and catabolite repression in dominant HTR1 mutants of Saccharomyces cerevisiae. J Bacteriol. 1993;175:5520–5528. doi: 10.1128/jb.175.17.5520-5528.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Özcan S, Johnston M. Function and regulation of yeast hexose transporters. Microbiol Mol Biol Rev. 1999;63:554–569. doi: 10.1128/mmbr.63.3.554-569.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen TN, Brunak S, von Heijne G, Nielsen H. SignalP 4.0: discriminating signal peptides from transmembrane regions. Nat Methods. 2011;8:785–786. doi: 10.1038/nmeth.1701. [DOI] [PubMed] [Google Scholar]
- Pluskal T, Hayashi T, Saitoh S, Fujisawa A, Yanagida M. Specific biomarkers for stochastic division patterns and starvation-induced quiescence under limited glucose levels in fission yeast. FEBS J. 2011;278:1299–1315. doi: 10.1111/j.1742-4658.2011.08050.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reifenberger E, Freidel K, Ciriacy M. Identification of novel HXT genes in Saccharomyces cerevisiae reveals the impact of individual hexose transporters on glycolytic flux. Mol Microbiol. 1995;16:157–167. doi: 10.1111/j.1365-2958.1995.tb02400.x. [DOI] [PubMed] [Google Scholar]
- Rupe˘s I, Jia Z, Young PG. Ssp1 promotes actin depolymerization and is involved in stress response and new end take-off control in fission yeast. Mol Biol Cell. 1999;10:1495–1510. doi: 10.1091/mbc.10.5.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitoh S, Yanagida M. Does a shift to limited glucose activate checkpoint control in fission yeast. FEBS Lett. 2014;588:2373–2378. doi: 10.1016/j.febslet.2014.04.047. [DOI] [PubMed] [Google Scholar]
- Sajiki K, Hatanaka M, Nakamura T, Takeda K, Shimanuki M, Yoshida T, Hanyu Y, Hayashi T, Nakaseko Y, Yanagida M. Genetic control of cellular quiescence in S. pombe. J Cell Sci. 2009;122:1418–1429. doi: 10.1242/jcs.046466. [DOI] [PubMed] [Google Scholar]
- Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- Sato M, Mueckler M. A conserved amino acid motif (R-X-G-R-R) in the Glut1 glucose transporter is an important determinant of membrane topology. J Biol Chem. 1999;274:24721–24725. doi: 10.1074/jbc.274.35.24721. [DOI] [PubMed] [Google Scholar]
- Scheid MP, Marignani PA, Woodgett JR. Multiple phosphoinositide 3-kinase-dependent steps in activation of protein kinase B. Mol Cell Biol. 2002;22:6247–6260. doi: 10.1128/MCB.22.17.6247-6260.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seatter MJ, De la Rue SA, Porter LM, Gould GW. QLS motif in transmembrane helix VII of the glucose transporter family interacts with the C-1 position of D-glucose and is involved in substrate selection at the exofacial binding site. Biochemistry. 1998;37:1322–1326. doi: 10.1021/bi972322u. [DOI] [PubMed] [Google Scholar]
- Shimanuki M, Chung SY, Chikashige Y, Kawasaki Y, Uehara L, Tsutsumi C, Hatanaka M, Hiraoka Y, Nagao K, Yanagida M. Two-step, extensive alterations in the transcriptome from G0 arrest to cell division in Schizosaccharomyces pombe. Genes Cells. 2007;12:677–692. doi: 10.1111/j.1365-2443.2007.01079.x. [DOI] [PubMed] [Google Scholar]
- Shimanuki M, Kinoshita N, Ohkura H, Yoshida T, Toda T, Yanagida M. Isolation and characterization of the fission yeast protein phosphatase gene ppe1+ involved in cell shape control and mitosis. Mol Biol Cell. 1993;4:303–313. doi: 10.1091/mbc.4.3.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinozaki-Yabana S, Watanabe Y, Yamamoto M. Novel WD-repeat protein Mip1p facilitates function of the meiotic regulator Mei2p in fission yeast. Mol Cell Biol. 2000;20:1234–1242. doi: 10.1128/mcb.20.4.1234-1242.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka N, Ohuchi N, Mukai Y, Osaka Y, Ohtani Y, Tabuchi M, Bhuiyan MS, Fukui H, Harashima S, Takegawa K. Isolation and characterization of an invertase and its repressor genes from Schizosaccharomyces pombe. Biochem Biophys Res Commun. 1998;245:246–253. doi: 10.1006/bbrc.1998.8406. [DOI] [PubMed] [Google Scholar]
- Tatebe H, Morigasaki S, Murayama S, Zeng CT, Shiozaki K. Rab-family GTPase regulates TOR complex 2 signaling in fission yeast. Curr Biol. 2010;20:1975–1982. doi: 10.1016/j.cub.2010.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toda T, Niwa H, Nemoto T, Dhut S, Eddison M, Matsusaka T, Yanagida M, Hirata D. The fission yeast sts5+ gene is required for maintenance of growth polarity and functionally interacts with protein kinase C and an osmosensing MAP-kinase pathway. J Cell Sci. 1996;109:2331–2342. doi: 10.1242/jcs.109.9.2331. [DOI] [PubMed] [Google Scholar]
- Uldry M, Thorens B. The SLC2 family of facilitated hexose and polyol transporters. Pflugers Arch. 2004;447:480–489. doi: 10.1007/s00424-003-1085-0. [DOI] [PubMed] [Google Scholar]
- Walsh MC, Smits HP, Scholte M, van Dam K. Affinity of glucose transport in Saccharomyces cerevisiae is modulated during growth on glucose. J Bacteriol. 1994;176:953–958. doi: 10.1128/jb.176.4.953-958.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson RT, Pessin JE. Bridging the GAP between insulin signaling and GLUT4 translocation. Trends Biochem Sci. 2006;31:215–222. doi: 10.1016/j.tibs.2006.02.007. [DOI] [PubMed] [Google Scholar]
- Witczak CA, Fujii N, Hirshman MF, Goodyear LJ. Ca2+/calmodulin-dependent protein kinase kinase-alpha regulates skeletal muscle glucose uptake independent of AMP-activated protein kinase and Akt activation. Diabetes. 2007;56:1403–1409. doi: 10.2337/db06-1230. [DOI] [PubMed] [Google Scholar]
- Wood V, Gwilliam R, Rajandream MA, Lyne M, Lyne R, Stewart A, Sgouros J, Peat N, Hayles J, Baker S, et al. The genome sequence of Schizosaccharomyces pombe. Nature. 2002;415:871–880. doi: 10.1038/nature724. [DOI] [PubMed] [Google Scholar]
- Wright DC, Hucker KA, Holloszy JO, Han DH. Ca2+ and AMPK both mediate stimulation of glucose transport by muscle contractions. Diabetes. 2004;53:330–335. doi: 10.2337/diabetes.53.2.330. [DOI] [PubMed] [Google Scholar]
- Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–484. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Yanagida M, Ikai N, Shimanuki M, Sajiki K. Nutrient limitations alter cell division control and chromosome segregation through growth-related kinases and phosphatases. Philos Trans R Soc Lond B Biol Sci. 2011;366:3508–3520. doi: 10.1098/rstb.2011.0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.