Abstract
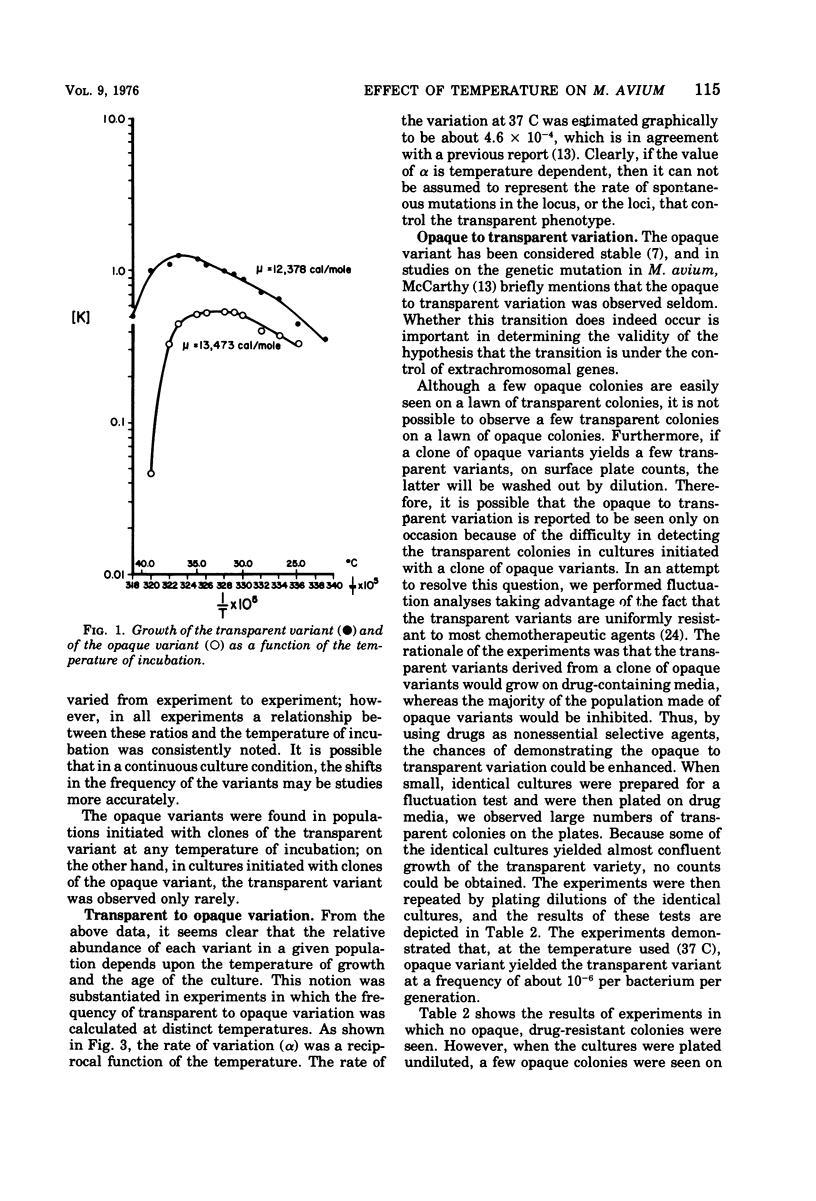
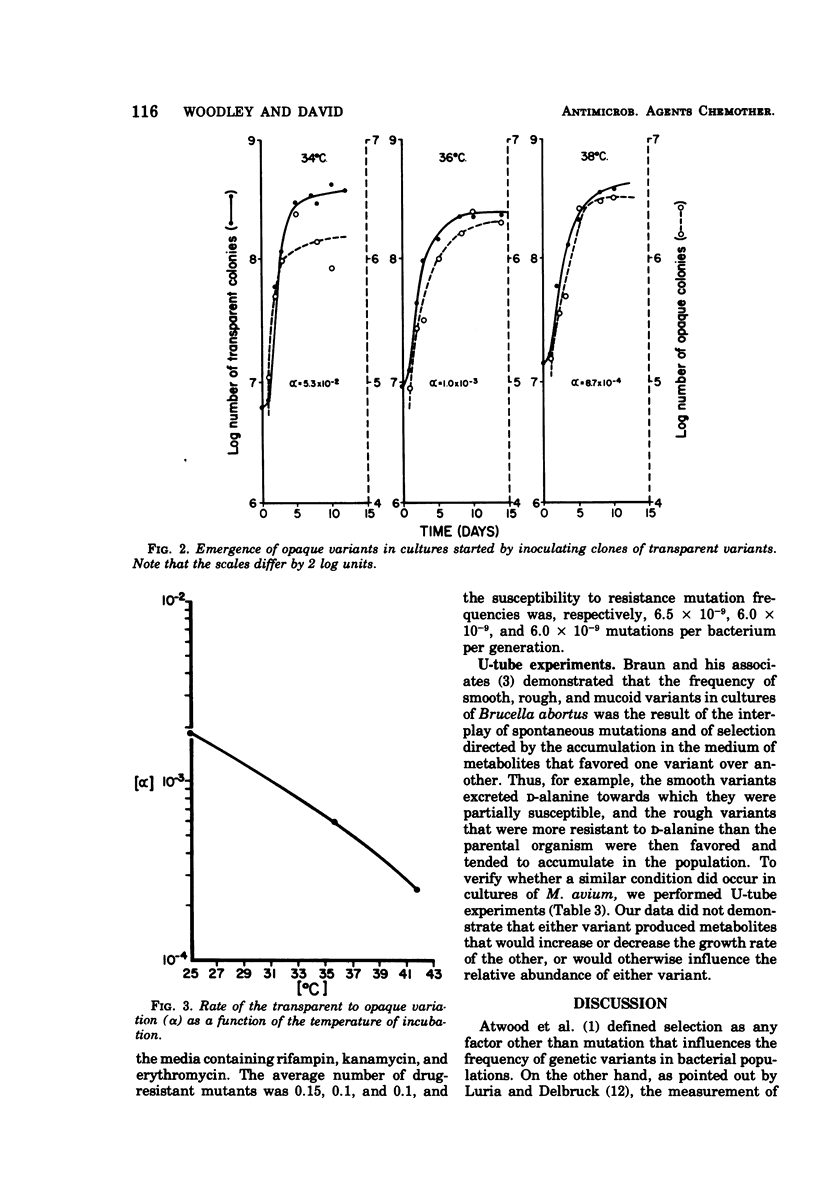
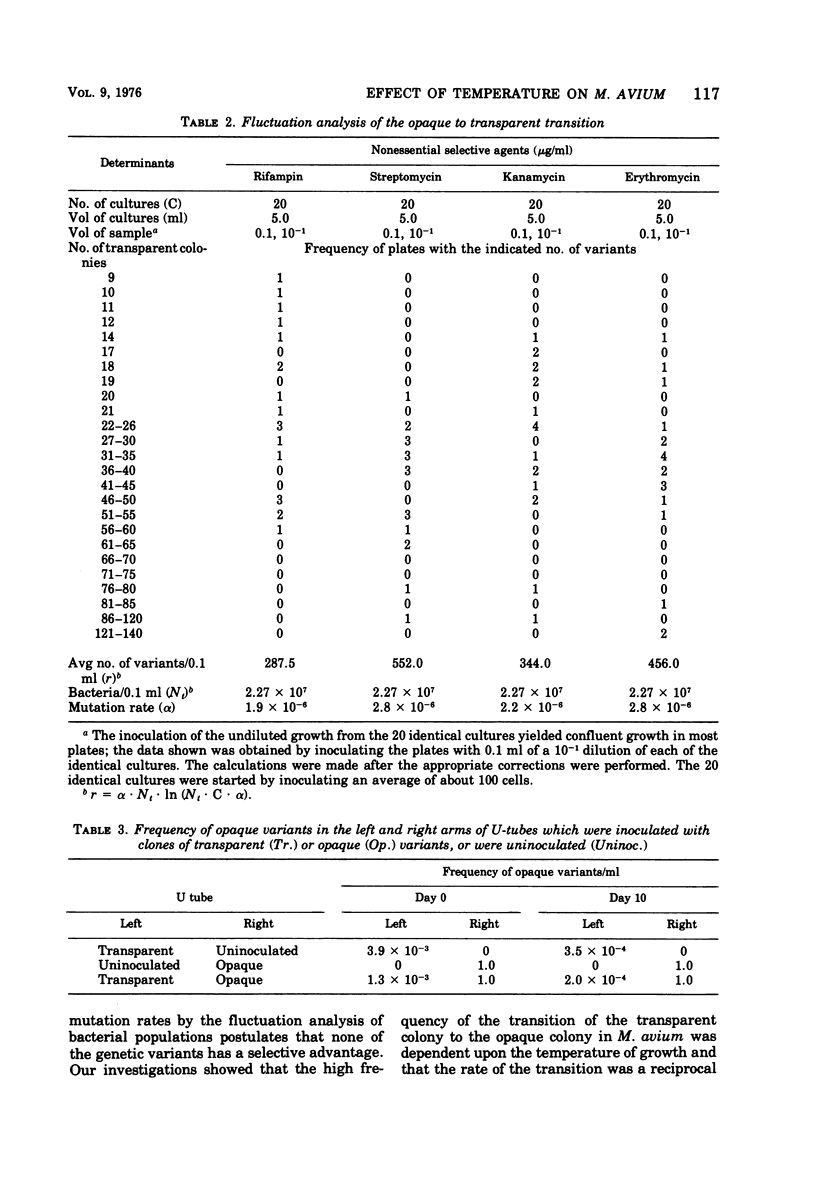
The results of drug susceptibility tests were found to be affected by changes that occur spontaneously in populations of Mycobacterium avium maintained in the laboratory. Because the transparent colony type variant was resistant to antituberculosis chemotherapeutic agents and the opaque colony type variant was usually susceptible to these agents, the transition of transparent to opaque colony type was investigated. The rate of the transition was found to be temperature dependent and, in agreement with a previous report, was found to be about 10−4 to 10−5 per generation at 37 C. Reversion was found to occur at a rate of 10−6 to 10−7 at 37 C. The mutation rate from susceptibility to resistance to rifampin, kanamycin, and erythromycin was about 10−8 to 10−9 mutations per bacterium per generation. Judged from our data, the high rate of the transparent to opaque variation was not caused either by mutator effects or by the occurrence of extrachromosomal genes in these bacteria, but could have been due to selective mechanisms still incompletely understood.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ATWOOD K. C., SCHNEIDER L. K., RYAN F. J. Selective mechanisms in bacteria. Cold Spring Harb Symp Quant Biol. 1951;16:345–355. doi: 10.1101/sqb.1951.016.01.026. [DOI] [PubMed] [Google Scholar]
- Böhme H. Genetic instability of an ultraviolet-sensitive mutant of Proteus mirabilis. Biochem Biophys Res Commun. 1967 Jul 21;28(2):191–196. doi: 10.1016/0006-291x(67)90428-7. [DOI] [PubMed] [Google Scholar]
- CANETTI G., RIST N., GROSSET J. [Measurement of sensitivity of the tuberculous bacillus to antibacillary drugs by the method of proportions. Methodology, resistance criteria, results and interpretation]. Rev Tuberc Pneumol (Paris) 1963 Feb-Mar;27:217–272. [PubMed] [Google Scholar]
- David H. L. Probability distribution of drug-resistant mutants in unselected populations of Mycobacterium tuberculosis. Appl Microbiol. 1970 Nov;20(5):810–814. doi: 10.1128/am.20.5.810-814.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David H. L. Response of Mycobacteria to ultraviolet light radiation. Am Rev Respir Dis. 1973 Nov;108(5):1175–1185. doi: 10.1164/arrd.1973.108.5.1175. [DOI] [PubMed] [Google Scholar]
- Dunbar F. P., Pejovic I., Cacciatore R., Peric-Golia L., Runyon E. H. Mycobacterium intracellulare. Maintenance of pathogenicity in relationship to lyophilization and colony form. Scand J Respir Dis. 1968;49(2):153–162. [PubMed] [Google Scholar]
- GOLDSTEIN A., SMOOT J. S. A strain of Escherichia coli with an unusually high rate of auxotrophic mutation. J Bacteriol. 1955 Nov;70(5):588–595. doi: 10.1128/jb.70.5.588-595.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUNDERSEN W. B. NEW TYPE OF STREPTOMYCIN RESISTANCE RESULTING FROM ACTION OF THE EPISOMELIKE MUTATOR FACTOR IN ESCHERICHIA COLI. J Bacteriol. 1963 Sep;86:510–516. doi: 10.1128/jb.86.3.510-516.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JYSSUM K. Observations on two types of genetic instability in Escherichia coli. Acta Pathol Microbiol Scand. 1960;48:113–120. doi: 10.1111/j.1699-0463.1960.tb04747.x. [DOI] [PubMed] [Google Scholar]
- Liberfarb R. M., Bryson V. Isolation, characterization, and genetic analysis of mutator genes in Escherichia coli B and K-12. J Bacteriol. 1970 Oct;104(1):363–375. doi: 10.1128/jb.104.1.363-375.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luria S. E., Delbrück M. Mutations of Bacteria from Virus Sensitivity to Virus Resistance. Genetics. 1943 Nov;28(6):491–511. doi: 10.1093/genetics/28.6.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy C. M., Schaefer J. O. Response of Mycobacterium avium to ultraviolet irradiation. Appl Microbiol. 1974 Jul;28(1):151–153. doi: 10.1128/am.28.1.151-153.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy C. Spontaneous and Induced Mutation in Mycobacterium avium. Infect Immun. 1970 Sep;2(3):223–228. doi: 10.1128/iai.2.3.223-228.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moehring J. M., Solotorovsky M. R. Relationship of colonial morphology to virulence for chickens of Mycobacterium avium and the nonphotochromogens. Am Rev Respir Dis. 1965 Nov;92(5):704–713. doi: 10.1164/arrd.1965.92.5.704. [DOI] [PubMed] [Google Scholar]
- Pattyn S. R., Hermans-Boveroulle M. T. Dissociation in M. avium. Pneumonologie. 1970;142(2):119–125. doi: 10.1007/BF02095206. [DOI] [PubMed] [Google Scholar]
- QUADLING C., STOCKER B. A. An environmentally-induced transition from the flagellated to the non-flagellated state in Salmonella typhimurium: the fate of parental flagella at cell division. J Gen Microbiol. 1962 Jun;28:257–270. doi: 10.1099/00221287-28-2-257. [DOI] [PubMed] [Google Scholar]
- Schaefer W. B., Davis C. L., Cohn M. L. Pathogenicity of transparent, opaque, and rough variants of Mycobacterium avium in chickens and mice. Am Rev Respir Dis. 1970 Oct;102(4):499–506. doi: 10.1164/arrd.1970.102.4.499. [DOI] [PubMed] [Google Scholar]
- Siegel E. C. Ultraviolet-sensitive mutator strain of Escherichia coli K-12. J Bacteriol. 1973 Jan;113(1):145–160. doi: 10.1128/jb.113.1.145-160.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]


