Abstract
Background
Experimental disruption of the labrum has been shown to compromise its sealing function and alter cartilage lubrication. However, it is not known whether pathological changes to the labrum secondary to femoroacetabular impingement (FAI) have a similar impact on labral function.
Questions/purposes
Does damage to the labrum occurring in association with abnormal femoral morphology affect the labral seal?
Methods
Using 10 fresh cadaveric specimens (mean age 50 years, ± 8), we measured the capacity of the central compartment of the hip (the iliofemoral joint) to maintain a seal during fluid infusion, which may help elucidate the function of the labrum during weightbearing. Specimens with and without abnormal femoral morphology (six normal-appearing specimens and four whose geometry suggested cam-type FAI) were tested in postures observed during functional activities, including simulations of normal gait, stooping, and pivoting. Each specimen with FAI morphology exhibited secondary damage of the labrum and the adjacent chondral surface, whereas specimens of normal morphology were undamaged.
Results
Average peak central compartment pressure was reduced during pivoting for specimens with the presence of labral damage secondary to FAI. When placed in pivoting positions, hips with FAI maintained lower fluid pressures within the central compartment compared with intact specimens (15 ± 3 versus 42 ± 8 kPa, respectively; effect size: 1.08 [−0.36 to 2.31]; p = 0.007). No differences in peak pressure were observed between groups (FAI versus normal) for postures simulating either gait (21 ± 6 versus 22 ± 4 kPa; p = 0.902) or stooping (9 ± 2 versus 8 ± 3 kPa; p = 0.775) with the numbers available.
Conclusions
The acetabular seal, quantified by the maximum intraarticular pressure, was reduced during pivoting; however, the seal was maintained during simulated gait and stooping.
Clinical Relevance
Because degeneration is progressive with repetitive impingement, loss of the labral seal starts to be seen during pivoting and may progress from there, but in this small-sample cadaver study that evaluated specimens in middle adulthood, the seal remains intact during simulated gait and stooping. Our study suggests that labral damage secondary to cam-type FAI may reduce the ability of the labral to provide an adequate seal of the central compartment of the hip during loading; however, the extent to which this is affected requires further investigation.
Introduction
The acetabular labrum improves hip stability by augmenting femoral head coverage [17, 22] and by providing a sealing function, which regulates the passage of synovial fluid between the central and peripheral compartments [5–8, 10, 16]. The central compartment represents the iliofemoral joint, whereas the peripheral compartment includes structures in the capsule and lateral to the labrum. The sealing function also creates functionally negative pressure in the joint, enhancing joint stability and the resistance to dislocation [5–7, 10, 16]. The presence of a pressurized layer of fluid in the central compartment provides more uniform distribution of contact stress across the joint surfaces [5]. Experimental disruption of the labrum has been shown to compromise its sealing function and alter the magnitude and distribution of articular contact forces [6], resulting in increased peak stresses and strains within the cartilaginous solid matrix [10]. The development of increased articular stresses has been shown to lead to greater friction during joint motion and may contribute to degenerative changes of the joint [23]. However, the majority of previous laboratory studies have been performed using experimental models derived from normal hips without realistic labral pathology, thus limiting insight into the potential role of labrochondral lesions in disrupting normal labral function.
Femoroacetabular impingement (FAI) has been identified as a potential contributor to labral and cartilage damage in the hip [9, 19, 21]. Although the mechanism remains unproven, it has been postulated that pathologic asphericity of the femoral head results in repetitive abutment against the acetabulum when the hip is placed in critical positions, classically internal rotation with flexion [9]. This generates increased tensile stresses within the anterior labrum, especially at the labrochondral junction, leading ultimately to separation of the labrum from the acetabular rim [20]. However, unlike the clean horizontal labral separations mimicked in experimental studies, labral damage observed clinically in patients with FAI is complex, often involving diffuse concomitant articular cartilage [18–20]. The impact on labral function of pathological changes to the labrum and chondral surfaces associated with FAI is presently unknown.
In this study, we used an experimental model of the hip to quantitatively assess whether the sealing capacity of the labrochondral complex is affected by the labral damage seen secondary to FAI. We hypothesized that the presence of labral damage would lead to reduced capacity of the hip to maintain normal fluid pressures within the central compartment during loading in functional postures.
Materials and Methods
An experimental model was developed consisting of a fresh intact cadaveric hip (hemipelvis and proximal femur) loaded in positions simulating walking, pivoting, and stooping. In addition, the model was instrumented to allow measurement of the fluid pressures with the intraarticular (central) and the extraarticular (peripheral) compartments of the intracapsular space. To allow adequate representation of both normal and dysmorphic (FAI) morphologies, 10 fresh male cadaveric hip specimens were obtained from donors of average age 50 ± 8 years (range, 42–63 years). All specimens were screened using plain radiographs and CT scans taken before testing. Specimen-specific virtual (computer) models of each specimen were prepared by reconstructing each CT scan using custom software (MIMICS 10.01; Materialise, Leuven, Belgium). Subsequent morphologic analysis of the CT reconstructions demonstrated that six specimens were of normal morphology (Fig. 1), whereas four displayed morphology typical of cam FAI (alpha angle > 55°) (Fig. 2).
Fig. 1.
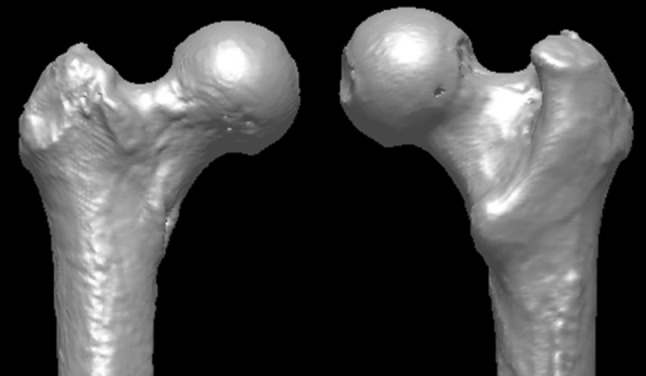
A representative CT reconstruction shows a femur exhibiting normal morphology.
Fig. 2.
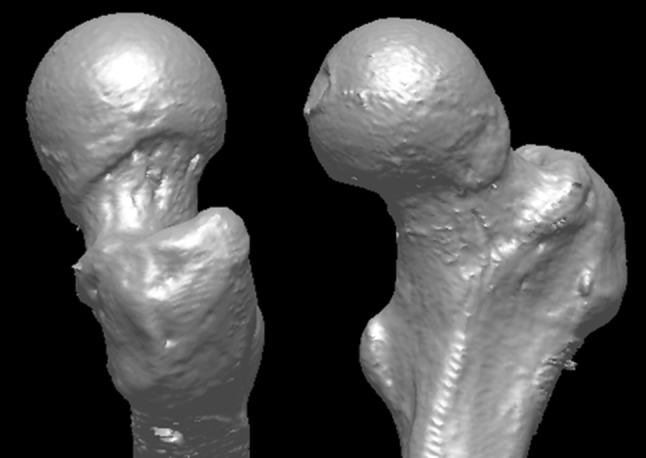
A representative CT reconstruction of a femur exhibits morphology consistent with cam FAI.
Each specimen was dissected free of overlying soft tissue, leaving both the capsule and labrum intact. The iliac wing was mounted in a polymeric cylinder in a nominal orientation of neutral flexion, adduction, and internal rotation and then cast in situ with polymethylmethacrylate (PMMA) cement. The femur was transected at the proximal third of the diaphysis and potted in a second cylinder. Each specimen was placed in a loading apparatus, which allowed angulation of the femur in all three cardinal planes (Fig. 3). Catheters inserted into the central and peripheral compartments of each hip allowed infusion of fluid and monitoring of compartment pressures. Access to the central compartment was achieved through a 5-mm porthole created in the quadrilateral plate of the ilium through to the acetabular fossa. The porthole was sealed around the catheter with cyanoacrylate and PMMA to prevent leakage during testing. A miniature pressure transducer (OMEGA Engineering, Inc, Stamford, CT, USA) was connected to the catheter in series with a syringe pump, which delivered phosphate-buffered solution (PBS) at a constant rate (0.75 cm3/sec) during testing. A second porthole, created just distal to the greater trochanter, allowed access to the peripheral compartment through the femoral neck without disruption of the joint capsule. Fluid pressures within the peripheral compartment were continuously monitored through a second pressure sensor connected to the catheter.
Fig. 3.
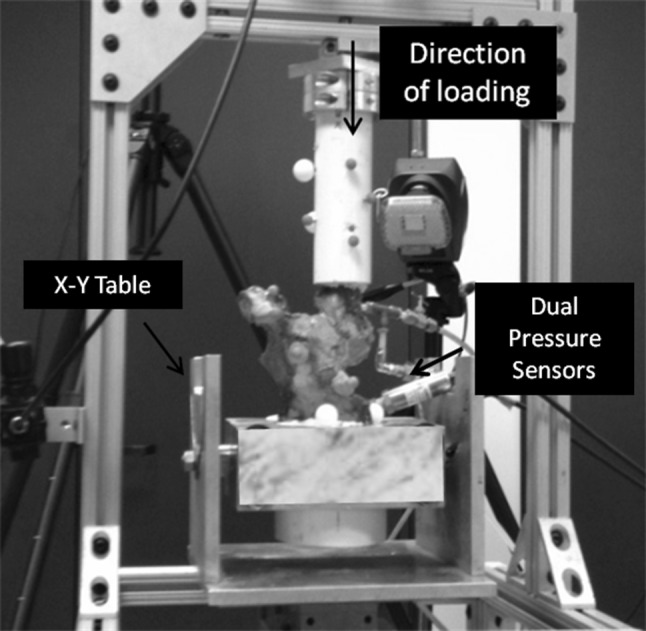
A typical cadaveric specimen is placed under axial load.
Before pressurization of the central compartment, an axial load of 0.50 body weight (374 N) was applied to the shaft of the femur through a vertical hydraulic actuator. We used a load of 0.50 body weight, because any load greater than this resulted in dislocation of the joint in certain positions during pilot testing, because the stabilizing effect of the hip musculature was absent. PBS was then introduced into the central compartment at a constant rate until leakage from the central to the peripheral compartment was indicated by a rise in pressure within the hip capsule [4]. The threshold to detect a rise in pressure in the peripheral compartment was an increase of 2 SDs above baseline, which remained increased for a window greater than 25 ms. The sealing capacity of each labrum was defined as the maximum pressure reached within the central compartment at a constant rate of infusion before the detection of fluid transport to the peripheral joint space. The pressurization measurements were repeated with each hip positioned in 10 static kinematic postures simulating sequential stages of normal gait (four postures) [15], stooping (three postures) [14], and pivoting (three postures) [14] (Table 1). The order of testing for the 10 positions was randomized between specimens. Each specimen remained in the given position for 10 to 15 minutes to allow the joint to come to a steady state before fluid infusion and to prepare for testing.
Table 1.
Three-dimensional kinematic positions used for testing
| Activity | Flexion (degrees) | Abduction (degrees) | External rotation (degrees) |
|---|---|---|---|
| Gait | |||
| Heel strike | 38 | −1 | 4 |
| Midstance | 12 | −5 | 0 |
| Heel off | −5 | −3 | 0 |
| Toe off | 0 | 0 | 0 |
| Stooping | |||
| Start | 45 | 13 | −5 |
| Middle | 70 | 5 | −15 |
| End | 105 | −5 | −25 |
| Pivoting | |||
| Start | 0 | 0 | 15 |
| Middle | −10 | −1 | 25 |
| End | −15 | 2 | 35 |
Visual observation after testing showed that each specimen with FAI morphology exhibited secondary damage of the labrum and the adjacent chondral surface, whereas specimens of normal morphology were undamaged (Fig. 4). The labral disruptions were all of partial thickness and were located at the articular junction and not the capsular margin. Classification of each lesion was based on the system previously published by one of the authors (JCM) [11]. In two specimens, diffuse Stage 4 lesions were observed, defined as a full-thickness defect with erosion of the subchondral bone, spanning the entire acetabular rim. For one specimen, a single Stage 3A chondrolabral lesion, defined as a partial-thickness defect, was located anteriorly (1–2 o’clock) on the acetabular rim, whereas for the remaining specimen, two distinct Stage 3A lesions were located at 4 o’clock and at 8 to 9 o’clock on the acetabular rim.
Fig. 4.
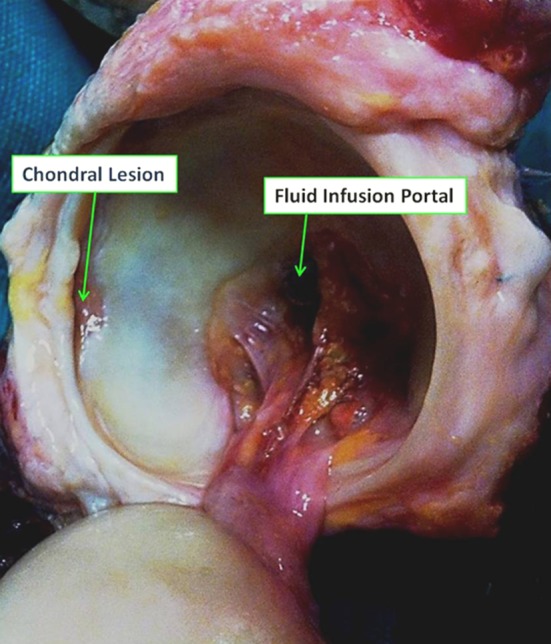
A photograph of the acetabulum of a cadaveric specimen with FAI exhibited gross labral and chondral damage observed after disarticulation.
All statistical analyses were performed using IBM SPSS Version 21.0 (IBM Corporation, Armonk, NY, USA). Sample size estimates were based on a previous study that reported hip intraarticular pressure measures between specimens with (average, 550 ± 56 kPa) and without labral resection (average, 195 ± 145 kPa) [7]. Using these values, the calculated effect size was large (effect size, 2.81; 95% confidence interval, 0.85–4.76). The minimum number of specimens needed to obtain statistical significance (α < 0.05) with 90% power is three. Average pressure measurements for each position were examined for deviation from normality using the Kolmogorov-Smirnov test. Peak pressures for the individual positions of each activity were combined to calculate an average peak pressure for gait, stoop, and pivot. Separate independent t-tests were used to compare peak central compartment pressure between specimens with and without labral damage for each of the three activities. Data are presented as mean ± SE. The level of statistical significance was set a priori at p < 0.05.
Results
Peak pressure values ranged from a minimum of 8 ± 3 kPa during stoop to a maximum of 42 ± 8 kPa during pivot for specimens with normal morphology and a minimum of 9 ± 2 kPa during stoop to a maximum of 21 ± 6 kPa during gait for specimens with labral damage (Table 2). The specimens with labral damage exhibited reduced peak pressure in the pivoting posture when compared with specimens with normal morphology (15 ± 3 versus 42 ± 8 kPa, respectively; effect size: 1.08 [−0.36 to 2.31]; p = 0.007). No differences in peak pressure were observed between groups (FAI versus normal) for postures simulating either gait (21 ± 6 versus 22 ± 4 kPa; p = 0.902) or stooping (9 ± 2 versus 8 ± 3 kPa; p = 0.775) with the numbers available.
Table 2.
Average peak central compartment pressure resistance while the joint is placed in kinematic postures (mean ± SE)
| Activity | Phase | Normal | Cam |
|---|---|---|---|
| Gait | Heel strike | 19.2 ± 10.3 | 10.7 ± 5.5 |
| Midstance | 19.2 ± 6.0 | 28.2 ± 17.5 | |
| Heel off | 28.9 ± 7.6 | 22.0 ± 13.6 | |
| Toe off | 34.4 ± 8.3 | 23.5 ± 11.9 | |
| Average | 22.0 ± 4.2 | 21.1 ± 6 | |
| Stoop | Stoop start | 20.6 ± 6.2 | 5.7 ± 2.2 |
| Stoop middle | 1.7 ± 1.1 | 6.6 ± 2.8 | |
| Stoop end | 3.6 ± 2.4 | 18.3 ± 7.6 | |
| Average | 7.5 ± 2.6 | 8.6 ± 2.4 | |
| Pivot | Pivot start | 35.7 ± 5.8 | 22.2 ± 3.0 |
| Pivot middle | 45.2 ± 14.2 | 11.4 ± 4.0 | |
| Pivot end | 49.7 ± 13.1 | 12.2 ± 5.1 | |
| Average | 42.3 ± 7.7 | 15.2 ± 2.4 |
Discussion
By regulating the flow of synovial fluid between the central and peripheral compartments of the hip during loading, the acetabular labrum contributes to hip stability and helps protect the cartilaginous surfaces of the joint [6, 7, 10, 16]. Injury to the labrum may predispose the joint to degeneration [9, 11–13], because it disrupts joint stability [3] and results in increased loads being transmitted to the solid matrices of the joint cartilage surfaces [6, 7, 16]. Abnormal femoral morphology is associated with high incidences of labral and chondral damage [1, 21]; however, the extent to which clinical chondrolabral damage resulting from FAI disrupts native labral function is unknown. In our study, we sought to determine if the sealing capacity of the labrum is affected by the presence of labral damage secondary to impingement and hypothesized that peak central compartment pressure before fluid transfer from the central compartment would be reduced for specimens with labral damage compared with uninjured specimens.
Our study is not without limitations. First, we recognize that insertion of fluid into the central compartment of the joint does not exactly recreate the native fluid mechanics of the joint. However, our experiments were primarily designed to isolate the labral sealing function of the labrum using standard, reproducible methods rather than developing a replica of the hip ex vivo. Our methods allowed us to determine the maximum pressure resistance of the labrum in response to changes in joint position, thereby providing a deeper understanding of native labral function. Second, we used PBS as joint fluid instead of synovial fluid or bovine serum. This was done to allow our data to be compared with earlier studies. We recognize that our choice of fluid will affect the absolute value of ultimate pressure resistance; however, because a consistent fluid environment was maintained for each specimen and each position, we believe that the relative changes that were observed are valid and are independent of the viscosity of the pressurizing medium. Third, we were unable to apply physiologic loads (200%–500% body weight [BW]) to the specimens during testing, because loads above 50% BW resulted in traumatic dislocation of the joint as a result of the absence of the hip musculature. Fourth, although we used positions that simulated kinematic postures incurred during dynamic joint function, we were unable to correctly simulate a loaded dynamic continuous movement during testing. Thus, our model reflects static forces. Finally, given our small sample size, we were only able to assess specimens with cam-type FAI; therefore, we cannot speculate how labral damage as a result of pincer-type or combined-type FAI may affect compartment pressures. In addition, the small sample may have not been able to account for the natural variation between specimens.
Using our model, we demonstrated that the ability of the labrum to restrict fluid transfer from the central to the peripheral compartment was less effective in hips with labral pathology secondary to FAI than in intact hips. The reduction in ultimate pressure resistance in the specimens with FAI lesions occurred in joint positions seen during pivoting maneuvers; however, the seal was maintained during positions of gait and stooping. Our results are in contrast with previous experiments, which reported complete negation of the labral seal in response to experimentally created labral disruptions [2, 7]. Both Cadet et al. [2] and Ferguson et al. [7] examined the sealing function of the labrum when the hip was held in positions close to neutral. In the present study, these positions are most similar to those simulating normal gait; however, in positions simulating gait, we found no differences between intact specimens and those with labral lesions in terms of peak pressure resistance. The lack of agreement between the previous studies and ours may be the result of a difference in methods, specifically the different joint positions, as well as the use of experimentally created labral disruption versus clinical labral damage. Arthroscopic examination of labral pathology reveals that the majority of labral tears are not clean disruptions of the tissue but are complex injuries often accompanied by adjacent cartilage damage [13, 20]. Therefore, we feel the use of specimens with clinical labral and chondral lesions may be a more realistic representation of the pathomechanics of the hip at risk, although this requires further investigation with a larger number of specimens.
Pivoting requires the hip to move through the extremes of extension and external rotation. In intact specimens, these motions resulted in the greatest pressure resistance with a 48% increase compared with gait and an 82% increase compared with stooping. However, for specimens with FAI, resistance to fluid exiting the central compartment during pivoting was reduced by 29% compared with gait and increased only 42% during stooping. We previously demonstrated that the orientation of the femoral head with respect to the acetabulum affects the sealing ability of the labrum and that the conformity of the femoroacetabular joint alters with joint position [4]. Pivoting produced posterior displacement of the femoral head, and the magnitude of the displacement was found to be inversely related to peak pressure resistance for intact specimens. We hypothesize that the asphericity of the head-neck junction observed in specimens with FAI may alter the region of contact between the femoral head and acetabulum during pivoting, resulting in increased gapping and reduced pressure resistance.
We demonstrated that the labral seal was reduced when the joint was placed in certain positions for specimens with the presence of labral damage secondary to impingement. The reduction in pressure resistance was observed during pivoting, but with the numbers available, it appeared that resistance was maintained during gait and stooping. Because degeneration is progressive with repetitive impingement, loss of the labral seal may begin during pivoting and could progress from there, but, in this group of specimens ranging in age from 42 to 63 years, the seal remains intact during gait and stooping. Our study suggests that labral damage secondary to cam-type FAI may reduce the ability of the labral to provide an adequate seal of the central compartment of the hip during loading; however, the extent to which this is affected requires further investigation.
Acknowledgments
We acknowledge Mr Michael Hogen, Mr Stephen Wallace, and Mr Andrew Moorman for their assistance with data collection and specimen dissection; Mr Jerry Alexander for his assistance in acquiring specimens; and Mr Sabir Ismaily for his assistance with capturing the motion analysis data.
Footnotes
Each author certifies that he or she, or a member of his or her immediate family, has no funding or commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research ® editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research ® neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA-approval status, of any drug or device prior to clinical use.
Each author certifies that all investigations were conducted in conformity with ethical principles of research.
This work was performed at the Institute of Orthopedic Research and Education, Houston, TX, USA.
References
- 1.Anderson SE, Siebenrock KA, Mamisch TC, Tannast M. Femoroacetabular impingement magnetic resonance imaging. Top Magn Reson Imaging. 2009;20:123–128. doi: 10.1097/RMR.0b013e3181d99459. [DOI] [PubMed] [Google Scholar]
- 2.Cadet ER, Chan AK, Vorys GC, Gardner T, Yin B. Investigation of the preservation of the fluid seal effect in the repaired, partially resected, and reconstructed acetabular labrum in a cadaveric hip model. Am J Sports Med. 2012;40:2218–2223. doi: 10.1177/0363546512457645. [DOI] [PubMed] [Google Scholar]
- 3.Crawford MJ, Dy CJ, Alexander JW, Thompson MT, Schroder S, Vega C, Patel R, Miller A, McCarthy JC, Lowe W, Noble P. The biomechanics of the hip labrum and the stability of the hip. Clin Orthop Relat Res. 2007;465:16–22. doi: 10.1097/BLO.0b013e31815b181f. [DOI] [PubMed] [Google Scholar]
- 4.Dwyer M, Jones H, Hogen M, Field RE, McCarthy J, Noble PC. The acetabular labrum regulates fluid circulation of the hip joint during functional activities. Am J Sports Med. 2014;42:812–819. doi: 10.1177/0363546514522395. [DOI] [PubMed] [Google Scholar]
- 5.Ferguson SJ, Bryant JT, Ganz R, Ito K. The acetabular labrum seal: a poroelastic finite element model. Clin Biomech. 2000;15:463–468. doi: 10.1016/S0268-0033(99)00099-6. [DOI] [PubMed] [Google Scholar]
- 6.Ferguson SJ, Bryant JT, Ganz R, Ito K. The influence of the acetabular labrum on hip joint cartilage consolidation: a poroelastic finite element model. J Biomech. 2000;33:953–960. doi: 10.1016/S0021-9290(00)00042-7. [DOI] [PubMed] [Google Scholar]
- 7.Ferguson SJ, Bryant JT, Ganz R, Ito K. An in vitro investigation of the acetabular labral seal in hip joint mechanics. J Biomech. 2003;36:171–178. doi: 10.1016/S0021-9290(02)00365-2. [DOI] [PubMed] [Google Scholar]
- 8.Field RE, Rajakulendran K. The labro-acetabular complex. J Bone Joint Surg Am. 2011;93(Suppl 2):22–27. doi: 10.2106/JBJS.J.01710. [DOI] [PubMed] [Google Scholar]
- 9.Ganz R, Parvizi J, Beck M, Leunig M, Notzli H, Siebenrock K. Femoroacetabular impingement: a cause for osteoarthritis of the hip. Clin Orthop Relat Res. 2003;417:112–120. doi: 10.1097/01.blo.0000096804.78689.c2. [DOI] [PubMed] [Google Scholar]
- 10.Greaves LL, Gilbart MK, Yung AC, Kozlowski P, Wilson DR. Effect of acetabular labral tears, repair and resection on hip cartilage strain: a 7T MR study. J Biomech. 2010;43:858–863. doi: 10.1016/j.jbiomech.2009.11.016. [DOI] [PubMed] [Google Scholar]
- 11.McCarthy JC, Noble P, Aluisio FV, Schuck M, Wright J, Lee J-A. Anatomy, pathologic features, and treatment of acetabular labral tears. Clin Orthop Relat Res. 2003;406:38–47. doi: 10.1097/00003086-200301000-00008. [DOI] [PubMed] [Google Scholar]
- 12.McCarthy JC, Noble P, Schuck M, Wright J, Lee J-A. The role of labral lesions to development of early degenerative hip disease. Clin Orthop Relat Res. 2001;393:25–37. doi: 10.1097/00003086-200112000-00004. [DOI] [PubMed] [Google Scholar]
- 13.McCarthy JC, Noble P, Schuck M, Wright J, Lee JL. The watershed labral lesion: it’s relationship to early arthritis of the hip. J Arthroplasty. 2001;16:81–87. doi: 10.1054/arth.2001.28370. [DOI] [PubMed] [Google Scholar]
- 14.Nadzadi M, Pedersen D, Yack H, Callaghan JJ, Brown T. Kinematics, kinetics, and finite element analysis of commonplace maneuvers at risk for total hip dislocation. J Biomech. 2003;36:577–591. doi: 10.1016/S0021-9290(02)00232-4. [DOI] [PubMed] [Google Scholar]
- 15.Orthoload. Available at: http://www.orthoload.com. Accessed May 5, 2012.
- 16.Safran MR, Giordano G, Lindsey DP, Gold GE, Rosenberg J, Zaffagnini S, Giori NJ. Strains across the acetabular labrum during hip motion: a cadaveric model. Am J Sports Med. 2011;39:92S–102S. doi: 10.1177/0363546511414017. [DOI] [PubMed] [Google Scholar]
- 17.Seldes RM, Tan V, Hunt J, Katz M, Winiarsky R, Fitzgerald RH. Anatomy, histologic features, and vascularity of the adult acetabular labrum. Clin Orthop Relat Res. 2001;382:232–240. doi: 10.1097/00003086-200101000-00031. [DOI] [PubMed] [Google Scholar]
- 18.Stelzeneder D, Hingsammer A, Bixby SD, Kim YJ. Can radiographic morphometric parameters for the hip be assessed on MRI? Clin Orthop Relat Res. 2013;471:989–999. doi: 10.1007/s11999-012-2654-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stelzeneder D, Mamisch TC, Kress I, Domayer SE, Werlen S, Bixby SD, Millis MB, Kim YJ. Patterns of joint damage seen on MRI in early hip osteoarthritis due to structural hip deformities. Osteoarthritis Cartilage. 2012;20:661–669. doi: 10.1016/j.joca.2012.03.014. [DOI] [PubMed] [Google Scholar]
- 20.Tamura S, Nishii T, Takao M, Sakai T, Yoshikawa H, Sugano N. Differences in the locations and modes of labral tearing between dysplastic hips and those with femoroacetabular impingement. Bone Joint J. 2013;95:1320–1325. doi: 10.1302/0301-620X.95B10.31647. [DOI] [PubMed] [Google Scholar]
- 21.Tannast M, Goricki D, Beck M, Murphy SB, Siebenrock KA. Hip damage occurs at the zone of femoroacetabular impingement. Clin Orthop Relat Res. 2008;466:273–280. doi: 10.1007/s11999-007-0061-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Won Y-Y, Chung I, Chung N, Song K. Morphological study on the acetabular labrum. Yonsei Med J. 2003;44:855–862. doi: 10.3349/ymj.2003.44.5.855. [DOI] [PubMed] [Google Scholar]
- 23.Wu J, Herzog W, Epstein M. Joint contact mechanics in the early stages of osteoarthritis. Med Eng Phys. 2000;22:1–12. doi: 10.1016/S1350-4533(00)00012-6. [DOI] [PubMed] [Google Scholar]


