Abstract
Background
Red meat intake has been associated with risk of colorectal cancer (CRC), potentially mediated through heterocyclic amines. The metabolic efficiency of N-acetyltransferase 2 (NAT2) required for the metabolic activation of such amines is influenced by genetic variation. The interaction between red meat intake, NAT2 genotype, and CRC has been inconsistently reported.
Methods
We used pooled individual-level data from the Colon Cancer Family Registry (CCFR) and the Genetics and Epidemiology of Colorectal Cancer Consortium (GECCO). Red meat intake was collected by each study. We inferred NAT2 phenotype based on polymorphism at rs1495741, highly predictive of enzyme activity. Interaction was assessed using multiplicative interaction terms in multivariate-adjusted models.
Results
From 11 studies, 8,290 CRC cases and 9,115 controls were included. The highest quartile of red meat intake was associated with increased risk of CRC compared to the lowest quartile (OR 1.41, 95%CI 1.29 – 1.55). However, a significant association was observed only for studies with retrospective diet data, not for studies with diet prospectively assessed before cancer diagnosis. Combining all studies, high red meat intake was similarly associated with CRC in those with a rapid/intermediate NAT2 genotype (OR 1.38, 95%CI 1.20 – 1.59) as with a slow genotype (OR 1.43, 95%CI 1.28 – 1.61) (p- interaction=0.9).
Conclusion
We found that high red meat intake was associated with increased risk of CRC only from retrospective case-control studies and not modified by NAT2 enzyme activity.
Impact
Our results suggest no interaction between NAT2 genotype and red-meat intake in mediating risk of CRC.
INTRODUCTION
Colorectal cancer (CRC) is a leading cause of morbidity and mortality. The National Cancer Institute (NCI) estimates there were 142,820 new cases and 50,830 deaths related to CRC in the United States in 2013(1). The past few decades have witnessed a substantial increase in understanding of the mechanisms of colorectal carcinogenesis. Genome wide association studies (GWAS) have highlighted associations of several single nucleotide polymorphisms (SNPs) in the development of these cancers (2–8). Yet, known genetic variants explain only a fraction of the disease risk, suggesting contribution from the as yet unidentified genetic risk factors, environment and gene-environment interactions (9).
The role of diet in the pathogenesis of CRC has been of particular substantial interest. Epidemiologic data supports an association between greater intake of red meat and increased risk of CRC (10–16). However the mechanism behind this association is not completely understood. One hypothesis relates to the formation of heterocyclic amines through the cooking process, and subsequent breakdown of these amines (13; 15–17). A key enzyme in the metabolic activation of heterocyclic amines is N-acetyltransferase 2 (NAT2)(18). Common genetic variants in NAT2 are key determinants of enzyme activity, with individuals widely classified according to NAT2 phenotype as slow, intermediate or rapid acetylators (19; 20).
Although some studies have suggested that individuals who are rapid acetylators exhibit a stronger association between red meat intake and CRC (12; 21–26), other studies have failed to confirm this association (27–31). This could in part be due to the small sample sizes of the replication studies, variation in assessment of red meat intake, incomplete adjustment for confounders, or different methods in estimating NAT2 enzymatic activity. However, as the mechanism behind the association between CRC and red meat intake is not completely understood, a large adequately powered study to examine a gene-environment interaction between NAT2 genotype and red meat intake might shed light on the carcinogenesis mechanism(s) and suggest potential avenues for disease prevention. Therefore, we used the strengths of a large international consortium of case-control and nested case-control studies within prospective cohorts to examine the potential interaction between NAT2 genotype and red meat intake, in relation to risk of CRC.
MATERIALS AND METHODS
Study Sample
This study included 8,290 cases of colorectal cancer and 9,115 controls from the Colon Cancer Family Registry (CCFR) and 10 studies within the Genetics and Epidemiology of Colorectal Cancer Consortium (GECCO). Details of the included studies are described in previous publications from this consortium (9; 32; 33). In brief, each study contributed CRC cases confirmed by review of medical records, pathology reports, or death certificates. All studies were approved by their respective institutional review boards. Six studies used a prospective nested case-control design while five studies were retrospective case-control studies.
Genotyping and quality control
Informed consent was obtained from the participants to provide blood or buccal cells for genotyping. The genotyping platform varied between the different studies. Cases and controls from the Diet, Activity, and Lifestyle Study (DALS), Darmkrebs: Chancen der Verhutung durch Screening (DACHS), Prostate, Lung, Colorectal, and Ovarian (PLCO), Women’s Health Initiative (WHI) Set 2, and VITamins And Lifestyle study (VITAL) were genotyped using the Illumina CytoSNP BeadChip platform. WHI Set 1 was genotyped using the Illumina 550K and 550K duo platforms; PLCO Set 1 was genotyped using Illumina 610 K and 550 K platforms, Ontario Familial Colorectal Cancer Registry (OFCCR) was genotyped using Affymetrix GeneChip Human Mapping 100K and 500K Array Set, Colon Cancer Family Registry (CCFR) using the Illumina 1M, 1Mduo, and 1M-Omni platforms, and Nurses’ Health Study (NHS), Health Professionals Follow-up Study (HPFS), and Physicians’ Health Study (PHS) were genotyped using the Illumina Human OmniExpress platform. Samples were excluded for call rates ≤ 97%, duplicates, unexpected relative pairs, gender discrepancy, heterozygosity, or being an outlier on principal component analysis (PCA).
Inferring NAT2 phenotype categories
NAT2 phenotype was inferred using a single tag SNP, rs1495741, on chromosome 8(34). Information from this SNP alone is in strong agreement with that from a 7-SNP panel and infers the NAT2 slow phenotype with 99% sensitivity and 95% specificity (34). Furthermore, rs1495741 genotype correlates well with NAT2 activity in hepatocytes (34). For studies which did not directly measure rs1495741(DALS2, PLCO2, WHI2, DACHS1, VITAL, OFCCR, PMH-CCFR), rs1495741 was imputed (mean imputation Rsq=0.99, ranging from 0.97–1.00). The best genotype call was used to determine NAT2 phenotype. The GG, AG, and AA genotypes were classified as rapid, intermediate, and slow enzyme activity, respectively.
Red meat intake and other covariates
Total red meat intake from all participating studies was assessed as number of servings per day. Additional variables collected by the studies included: referent age, sex, smoking status (ever or never), use of aspirin or non-steroid anti-inflammatory drugs (NSAIDs) (use at referent time), and body mass index (in kilogram/square meter) as a continuous variable. Additional dietary covariates were included based on association with CRC in prior studies and included: total calcium intake, total folate intake, and number of servings per day of fruits or vegetables (9; 35–38). As previously described, a multi-step harmonization process was used to combine data across the studies (9).
Statistical Analysis
All statistical analyses were performed at the central GECCO coordinating center. In a minimally adjusted model, regression models adjusted for age, sex, study site, and the first three principal components from EIGENSTRAT to account for population sub-structure (39). The primary analysis was to estimate the interaction between NAT2 genotype, red meat intake and risk of CRC. For this, we compared the NAT2 rapid (GG) or intermediate (AG) with the slow (AA) phenotype, as well as the NAT2 rapid/intermediate with the slow phenotype. We used study- and sex-specific quartiles of red meat intake modeled as indicator variables, with the lowest quartile of intake as the referent category. In sensitivity analyses, we examined the association with red meat intake when modeled as a dichotomous exposure (above or below study- and sex-specific medians) and as a continuous variable using study- and sex-specific quartiles taking on the values 1–4. The interaction between NAT2 activity and red meat intake was examined by stratifying subjects by inferred NAT2 enzyme activity into rapid/intermediate and slow categories. We tested the significance of multiplicative interaction using likelihood ratio tests comparing nested models with and without interaction terms between quartiles of red meat intake and NAT2 slow, intermediate, or rapid phenotype. We tested for the significance of additive interaction using the logistic regression methods outlined by Lundberg et al. (40) and Andersson et al. (41) to calculate the relative excess risk due to interaction (RERI)(42) for red meat intake (above vs. below study- and sex-specific medians) and NAT2 phenotype (intermediate/rapid vs. slow). In a sensitivity analysis, we repeated the regression analysis using an extended model that additionally adjusted for the demographic and dietary covariates described above.
As the association of red meat with CRC reported in prior publications has generally appeared stronger in retrospective case-control studies compared with studies within prospective cohorts, we examined if the association between red meat, NAT2, and CRC varied by study design. We also estimated associations according to tumor location (proximal colon vs. distal colon or rectum). Tumor location was classified using International Classification of Diseases, 9th edition codes as proximal (153.0, 153.1, 153.4, 153.6) or distal (153.2, 153.3, 153.7, 154.0, 154.1) tumors. Two hundred and sixty cases could not be classified as one of the two locations.
RESULTS
Table 1 presents the baseline characteristics of the study sample according to case-control status. The mean age was 64 years and just over half were women. There was no difference in age or sex between CRC cases and controls. Consistent with previously reported associations, CRC cases had a greater BMI, were more likely to have smoked, and less likely to use aspirin or NSAIDs. Total calcium intake and the number of servings per day of fruits and vegetables waere lower in cases compared with controls. The median daily intake of red meat across the studies was 0.64 servings per day (range 0 – 8).
Table 1.
Characteristics of colorectal cancer cases and controls
| Characteristics a | Cases (N=8,290) | Controls (N=9,115) | P c |
|---|---|---|---|
| Female (N)† | 4619 | 5053 | -- |
|
| |||
| Male (N) † | 3671 | 4062 | 0.71 |
|
| |||
| Age (years) | 64.0 (10.2) | 64.4 (9.4) | 0.004 |
|
| |||
| BMI (kg/m2) | 27.3 (4.9) | 26.5 (4.5) | <0.0001 |
|
| |||
| Ever smoked (%) | 57.5 | 53.9 | <0.0001 |
|
| |||
| Aspirin use (%)b | 23.0 | 29.8 | <0.0001 |
|
| |||
| Non-aspirin NSAID use (%)b | 13.4 | 16.9 | <0.0001 |
|
| |||
| Total calcium (mg/day) | 703.5 (636.6) | 831.5 (703.3) | <0.0001 |
|
| |||
| Total folate (DFEs) | 440.9 (374.4) | 490.3 (376.2) | <0.0001 |
|
| |||
| Fruit (servings/day) | 1.7 (1.4) | 1.9 (1.5) | <0.0001 |
|
| |||
| Vegetable (servings/day) | 2.2 (1.8) | 2.5 (1.9) | <0.0001 |
| Red neat (servings/day) | 0.78 (0.61) | 0.73 (0.60) | <0.0001 |
| Processed meat (servings/day) | 0.45 (0.45) | 0.37 (0.41) | <0.0001 |
Abbreviations: BMI, body mass index; NSAID, non-steroidal anti-inflammatory drug; DFEs, dietary folate equivalents
Values are mean (SD) or percentages
Use at referent time
P values calculated using t-tests for continuous variables or χ2 tests for dichotomous variables
Cases and controls were matched on age and gender
Main associations – red meat and NAT2
For the pooled analysis, adjusting for age, sex, and study site, higher intake of red meat was associated with an increased risk of CRC. Compared to the lowest quartile of red meat intake, the highest quartile was associated with an increased risk of CRC (adjusted odds ratio (OR) 1.41, 95% confidence interval (CI) 1.29 – 1.55) (Table 2). Estimates were attenuated after adjustment for smoking status, BMI, aspirin use, NSAID use, and dietary factors (OR for Q4 vs. Q1 1.29, 95% CI 1.15 – 1.44). Red meat intake modeled as a dichotomous or continuous variable similarly was associated with risk of CRC (data not shown). NAT2 enzyme activity inferred by genotype was not associated with risk of CRC. Compared with genotypes associated with slow acetylation, genotypes associated with intermediate or rapid acetylation were not associated with risk of CRC (OR 1.04, 95% CI 0.98 – 1.11) (Table 2).
Table 2.
Association between red meat intake, NAT2 genotype, and colorectal cancer
| Red Meat Intake | |||||
|---|---|---|---|---|---|
| Red meat intake | Cases / Controls | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 |
| Main model† | 8290 / 9115 | 1.0 | 1.15 (1.06 – 1.25) | 1.29 (1.19 – 1.41) | 1.41 (1.29 – 1.55) |
| Expanded model‡ | 5207 / 6141 | 1.0 | 1.15 (1.03 – 1.28) | 1.17 (1.05 – 1.31) | 1.29 (1.15 – 1.44) |
| NAT2 genotype|| | Slow | Intermediate / Rapid | |||
| Main model† | 8290 / 9115 | 1.0 | 1.04 (0.98 – 1.11) | ||
Main model adjusted for age, gender, and study site
Expanded model adjusted for age, gender, study site, smoking status (ever or never), aspirin use (yes or no), NSAID use (yes or no), body mass index (in kg/m2), quartiles of dietary calcium, folate, and number of servings of fruits and vegetables per day
NAT2 was genotyped using rs1495741 SNP. Individuals with AA, AG, and GG genotype was classified as slow, intermediate, or rapid acetylators
Interactions
Table 3 presents the association between red meat intake and risk of CRC according to NAT2 genotype. The association between the highest quartile of red meat intake and risk of CRC was similar for persons with the slow NAT2 genotype (OR 1.43, 95% CI 1.28 – 1.61) as for those with the intermediate or rapid genotype (OR 1.38, 95% CI 1.20 – 1.59). From the expanded model adjusting for demographic and dietary variables, the association between red meat intake and CRC was not modified by NAT2 genotype. There were no significant interactions on either the multiplicative (p=0.99) or additive scale (p=0.97).
Table 3.
Association between red meat intake and colorectal cancer according to NAT2 genotype in the full cohort
| Red Meat Intake | |||||
|---|---|---|---|---|---|
| Cases / Controls | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | |
| Main model† | |||||
| Slow NAT2|| | 4906/5488 | 1.0 | 1.15 (1.03 – 1.28) | 1.30 (1.17 – 1.46) | 1.43 (1.28 – 1.61) |
| Rapid / intermediate NAT2|| | 3384 / 3627 | 1.0 | 1.15 (1.11 – 1.46) | 1.27 (1.11 – 1.46) | 1.38 (1.20 – 1.59) |
| Expanded model‡ | |||||
| Slow NAT2|| | 3102 / 3707 | 1.0 | 1.13 (0.98 – 1.30) | 1.15 (1.00 – 1.32) | 1.30 (1.13 – 1.50) |
| Rapid / intermediate NAT2|| | 2105 / 2434 | 1.0 | 1.16 (0.98 – 1.38) | 1.22 (1.02 – 1.45) | 1.27 (1.06 – 1.51) |
Main model adjusted for age, gender, and study site
Expanded model adjusted for age, gender, study site, smoking status (ever or never), aspirin use (yes or no), NSAID use (yes or no), body mass index (in kg/m2), quartiles of dietary calcium, folate, and number of servings of fruits and vegetables per day
NAT2 was genotyped using rs1495741 SNP. Individuals with AA, AG, and GG genotype was classified as slow, intermediate, or rapid acetylators
Analysis by study design
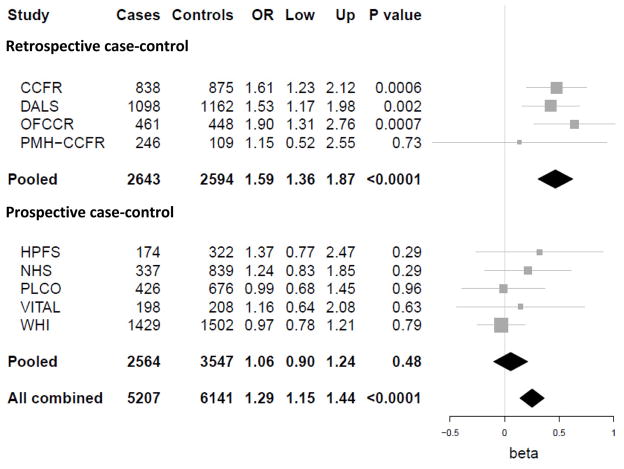
As prior reports of the association of red meat and CRC based on the subjects in our analysis appeared to show a stronger association from retrospective case-control studies compared with prospective cohorts, we estimated associations stratified according to study design. From the analysis of 3,091 cases and 4,209 controls derived from case-control studies nested within prospective cohorts, the highest quartile of red meat intake was not significantly associated with risk of CRC (OR 1.06, 95% CI 0.93 – 1.22). In contrast, using the 5,199 cases and 4,906 controls from retrospective case-control studies, the highest quartile of red meat intake was associated with a significant risk of CRC (OR 1.75, 95% CI 1.55 – 1.98), and these risk estimates were significantly different (Figure 1). We observed no significant interaction between inferred NAT2 phenotype, red meat intake and CRC risk within either the retrospective case-control studies (p=0.88) or those where diet was prospectively ascertained prior to cancer diagnosis (p=0.64) (Table 4).
Figure 1. Red meat intake and risk of colorectal cancer, stratified by study design.
Adjusted for age, gender, study site, smoking status (ever or never), aspirin use (yes or no), NSAID use (yes or no), body mass index (in kg/m2), quartiles of dietary calcium, folate, and number of servings of fruits and vegetables per day
Table 4.
Association between red meat intake and colorectal cancer risk according to study design
| Red Meat Intake | |||||
|---|---|---|---|---|---|
| Cases / Controls | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | |
| Retrospective case-control | |||||
| Slow NAT2|| | 3057 / 2950 | 1.0 | 1.23 (1.07 – 1.42) | 1.44 (1.24 – 1.66) | 1.78 (1.53 – 2.08) |
| Rapid / intermediate NAT2|| | 2142 / 1956 | 1.0 | 1.29 (1.09 – 1.52) | 1.58 (1.33 – 1.88) | 1.69 (1.40 – 2.05) |
| Prospective case-control (nested) | |||||
| Slow NAT2|| | 1849 / 2538 | 1.0 | 0.94 (0.75 – 1.18) | 0.93 (0.74 – 1.17) | 0.99 (0.79 – 1.25) |
| Rapid / intermediate NAT2|| | 1242 / 1671 | 1.0 | 0.97 (0.79 – 1.20) | 0.92 (0.74 – 1.13) | 1.04 (0.84 – 1.29) |
Main model adjusted for age, gender, and study site
NAT2 was genotyped using rs1495741 SNP. Individuals with AA, AG, and GG genotype was classified as slow, intermediate, or rapid acetylators
Associations by tumor site
Stratifying by tumor site, higher red meat intake was associated with both proximal (OR for Q4 vs. Q1 1.41, 95% CI 1.25 – 1.59) and distal CRC (OR for Q4 vs. Q1 1.50, 95% CI 1.35 – 1.68). However, for both proximal and distal CRC, the association between red meat and cancer risk was similar in those with slow NAT2 phenotype compared to those with intermediate or rapid acetylation. Among those with slow NAT 2 phenotype, compared to individuals with the lowest quartile of intake, those in the highest quartile of intake had elevated risk of proximal (OR 1.52, 95% CI 1.30 – 1.78) or distal CRC (OR 1.45, 95% CI 1.26 – 1.67) (Table 5). However, this elevated risk was similar in those with intermediate or rapid phenotype for either proximal (OR 1.25, 95% CI 1.03 – 1.51) (p-interaction=0.59) or distal CRC (OR 1.58, 95% CI 1.33 – 1.87) (p-interaction=0.90).
Table 5.
Association between red meat intake and colorectal cancer risk according to tumor site
| Red Meat Intake | |||||
|---|---|---|---|---|---|
| Cases / Controls | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | |
| Proximal colorectal cancer | |||||
| Slow NAT2|| | 2002 / 5488 | 1.0 | 1.25 (1.08 – 1.45) | 1.31 (1.12 – 1.52) | 1.52 (1.30 – 1.78) |
| Rapid / intermediate NAT2|| | 1365 / 3627 | 1.0 | 1.27 (1.06 – 1.51) | 1.41 (1.17 – 1.68) | 1.25 (1.03 – 1.51) |
| Distal colorectal cancer | |||||
| Slow NAT2|| | 2743 / 5488 | 1.0 | 1.11 (0.67 – 1.26) | 1.33 (1.17 – 1.52) | 1.45 (1.26 – 1.67) |
| Rapid / intermediate NAT2|| | 1915 / 3627 | 1.0 | 1.13 (0.67 – 1.32) | 1.24 (1.06 – 1.46) | 1.58 (1.33 – 1.87) |
Main model adjusted for age, gender, and study site
NAT2 was genotyped using rs1495741 SNP. Individuals with AA, AG, and GG genotype was classified as slow, intermediate, or rapid acetylators
DISCUSSION
In this large international study, we observed that higher intake of red meat is associated with increased risk of CRC. However, this association was seen only in retrospective case-control studies and was not evident in the studies that prospectively assessed dietary data prior to cancer diagnosis. This association was similar for both proximal and distal CRC. Nonetheless, the association between red meat intake and CRC did not appear to differ according to underlying NAT2 genotype irrespective of study design.
Prior epidemiologic evidence supports the association between red meat intake and CRC (10–16; 43). A report from the American Institute of Cancer Research estimated a 29% increase in risk of CRC with every 100g/day intake of red meat (44). In particular, cooking of red meat and the level of doneness associated with cooking have been associated with increased CRC risk (10–16; 43; 45). Of note, within this large pooled analysis of multiple study populations, the association between red meat intake and CRC was observed only in retrospective case-control studies. There are a few potential reasons for this apparently discrepant result. First, a true biological association between red meat intake and CRC may be weak or non-existent, with significant associations reported by retrospective case-control studies largely due to recall bias. Second, a true association between red meat intake and CRC may be mediated by recent intake. In general, the lag between the assessment of meat intake and incident CRC is typically prolonged in prospective cohorts. Moreover, most prospective cohorts did not update information on meat intake over follow-up, leading to misclassification of exposure and biasing associations toward the null. Nonetheless, a recent meta-analysis of prospective studies has concluded that red meat intake is associated with risk of CRC (46). Furthermore, selection bias that may occur in case-control studies should not influence the assessment of potential gene-environmental interactions (47).
One long-standing hypothesis linking red meat with cancer suggests that cooking meat at high temperatures results in the formation of heterocyclic amines (48). This process is mediated by several enzymes, perhaps most prominently NAT2, which metabolically activates heterocyclic amines to allow the formation of DNA-adducts that subsequently cause DNA damage. Thus, inter-individual variation in the activity of NAT2 may influence susceptibility to this exposure to heterocyclic amines. While variation in NAT2 enzyme activity was first described in the context of neurotoxicity related to isoniazid use for tuberculosis (49), several genetic polymorphisms in the coding region of exon 2 of the NAT2 gene have been studied as modifiers of enzyme activity (19). Several different genetic panels have been used to classify NAT2 genotype and inferred phenotype, most commonly a 7-SNP panel that includes four SNPs that directly influence NAT2 activity and three SNPs that aid in the classification of the inferred phenotype. However, in two independent cohorts, Garcia-Closas et al. demonstrated that a single tag SNP (rs1495741) demonstrated strong correlation with the 7-SNP panel, with only rare misclassification for the intermediate phenotype and none for rapid or slow acetylators (34). The association between rs1495741 genotype and NAT2 phenotype was additionally validated by measuring NAT2 catalytic activity in cryopreserved human hepatocytes with strong correlation between measured activity and rs1495741 genotypes (34). Prior studies had also demonstrated that the rapid acetylator phenotype of NAT2 has been associated with a higher level of such DNA adducts compared to the slow acetylator phenotype (50).
Several studies have examined the interaction between NAT2 and meat intake on the risk of CRC. However, the results have been inconsistent. In a case-control study nested within the prospective Nurses’ Health study, Chan et al. demonstrated a three-fold increase in risk of CRC with higher red meat intake among rapid but not slow acetylators (12). In contrast, Wang et al. identified no association between NAT2 and red meat intake on colorectal neoplasia (30). In the Multiethnic Cohort Study, the strongest association between red meat intake and CRC risk was seen among the rapid NAT2 acetylators; however this interaction was not statistically significant (31). Similarly, in the Ontario Cancer Registry, both red meat and well-done meat intake were associated with CRC but this effect was independent of the NAT2 genotype (51). Several other published studies have either supported (21–26) or refuted this interaction (27–29). There are several possible reasons for the variation in our findings as well as those of prior studies. First, cohorts varied in their assessment and definition of red meat intake as well as availability of data on cooking methods and processing. Second, it is well recognized that the distribution of genetic polymorphisms for various xenobiotic metabolizing enzymes vary across ethnicity. Thus, an interaction observed in one ethnic group may not be consistently observed in other populations.
Our findings of the absence of an association of CRC with NAT 2 acetylation status across the range of average intake of red-meat may suggest that heterocyclic amine exposure may not play a relevant role in CRC pathogenesis. However, it is still plausible that heterocyclic amines truly influence CRC risk but the range of variation in heterocyclic amine exposure associated with NAT2 acetylation status is narrow, resulting in low statistical power to detect a gene-environment interaction even within a sample size as large as the present study. Alternately, a threshold effect, rather than continuous dose-response for exposure to heterocyclic amines may exist which we were not powered to detect. Finally, polymorphisms in other enzymes involved in heterocyclic amine metabolism may interact with NAT2 acetylation status and/or influence susceptibility to CRC.
There are considerable strengths to our study. First, our large collaboration of pooled studies resulted in a sample size substantially greater than most of the prior studies that have examined this association. Not only does this confer a greater statistical power to define gene-environment interactions, but it also allows for more robust and generalizable findings. Second, we were able to adjust for a spectrum of biologically important covariates, ensuring the independent significance of red meat intake.
We acknowledge several limitations to our study. First, we examined polymorphism in only one enzyme involved in the metabolic activation of heterocyclic amines. Polymorphisms in other enzymes in this pathway (NAT1, CYP1A1, CYP1A2, CYP1B1, AHR, and GSTM1) may modify the association between red meat intake and CRC. However, among the various polymorphisms examined, the largest body of evidence and the most heterogeneous results has been for the interaction between NAT2 and red meat intake. Furthermore, for some of the other enzymes, the association between genetic polymorphisms and enzyme activity is less well understood. Secondly, within the studies included in the consortium, there were differences in dietary methods of ascertainment of red meat intake. Some studies had much greater detail on food consumption, while others were more limited. Third, uniform information on cooking techniques was not available across all studies. Consequently, we were unable to specifically examine the association with well-done red meat intake that may have a stronger correlation with heterocyclic amine exposure. Another limitation is the referent time of dietary exposure varied from study to study and the most relevant time point associated with the disease process may not have been well captured in all studies. Finally, a subgroup of the entire cohort did not have full information on all relevant covariates and could not be included in our expanded model. However, as the magnitudes of the association between red meat intake and colorectal cancer in our main model and expanded regression model are comparable, we believe our results to be generalizable.
In conclusion, in the largest study to examine the association between red meat intake, NAT2 genotype and CRC, we demonstrated that higher red meat intake is associated with increased risk of CRC primarily in retrospective but not prospective case-control studies. The effect was similar for both proximal colon cancer and distal colorectal cancer. However, irrespective of tumor site or study design, the association between red meat intake and CRC was independent of NAT2 genotype.
Acknowledgments
Funding
A. Ananthakrishnan is supported by a grant from the National Institutes of Health (K23 DK097142).
M. Du, T. A Harrison, L. Hsu, M. Thornquist, and U. Peters are affiliated with GECCO, which is supported by the following grants from the National Cancer Institute, National Institutes of Health and U.S. Department of Health and Human Services (U01 CA137088; R01 CA059045; R25 CA094880 to M Du).
G Casey, J.L. Hopper, M.A. Jenkins, and P.A. Newcomb are affiliated with CCFR which is supported by the National Institutes of Health (UM1 CA167551) and through cooperative agreements with members of the Colon Cancer Family Registry and P.I.s. This genome wide scan was supported by the National Cancer Institute, National Institutes of Health by U01 CA122839 and R01 CA143237 to GC. The content of this manuscript does not necessarily reflect the views or policies of the National Cancer Institute or any of the collaborating centers in the CCFRs, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government or the CFR. The following Colon CFR centers contributed data to this manuscript and were supported by National Institutes of Health: Australasian Colorectal Cancer Family Registry (U01/U24 CA097735) Ontario Registry for Studies of Familial Colorectal Cancer (U01/U24 CA074783) Seattle Colorectal Cancer Family Registry (U01/U24 CA074794)
H. Brenner, J. Chang-Claude, and M. Hoffmeister are affiliated with DACHS which was supported by grants from the German Research Council (Deutsche Forschungsgemeinschaft, BR 1704/6-1, BR 1704/6-3, BR 1704/6-4 and CH 117/1-1), and the German Federal Ministry of Education and Research (01KH0404 and 01ER0814).
J.D. Potter and M.L. Slattery are affiliated with DALS which was supported by National Institutes of Health (R01 CA48998 to M.L. Slattery).
A.T. Chan, C.S. Fuchs, K. Wu, E.L. Giovannucci, J. Ma, and S. Ogino are affiliated with HPFS, NHS, and PHS which are supported by the National Institutes of Health. HPFS is supported by the National Institutes of Health (P01 CA 055075, UM1 CA167552, R01 CA137178, R01 CA151993, and P50 CA 127003), NHS by the National Institutes of Health (R01 CA137178, R01 CA151993, P01 CA 087969 and P50 CA 127003,) and PHS by the National Institutes of Health (R01 CA042182).
S. Gallinger is affiliated with the OFCCR which is supported by funding from the National Institutes of Health through funding allocated to the Ontario Registry for Studies of Familial Colorectal Cancer (U01 CA074783); see CCFR section above. As subset of ARCTIC, OFCCR is supported by a GL2 grant from the Ontario Research Fund, the Canadian Institutes of Health Research, and the Cancer Risk Evaluation (CaRE) Program grant from the Canadian Cancer Society Research Institute.
S.I. Berndt and R. B. Hayes are affiliated with the PLCO which is supported by the Intramural Research Program of the Division of Cancer Epidemiology and Genetics and supported by contracts from the Division of Cancer Prevention, National Cancer Institute, NIH, DHHS. Additionally, a subset of control samples were genotyped as part of the Cancer Genetic Markers of Susceptibility (CGEMS) Prostate Cancer GWAS (Yeager, M et al. Nat Genet 2007 May;39(5):645-9), Colon CGEMS pancreatic cancer scan (PanScan) (Amundadottir, L et al. Nat Genet. 2009 Sep;41(9):986-90 and Petersen, GM et al Nat Genet. 2010 Mar;42(3):224-8), and the Lung Cancer and Smoking study. The prostate and PanScan study datasets were accessed with appropriate approval through the dbGaP online resource (http://cgems.cancer.gov/data/) accession numbers phs000207.v1.p1 and phs000206.v3.p2, respectively, and the lung datasets were accessed from the dbGaP website (http://www.ncbi.nlm.nih.gov/gap) through accession number phs000093.v2.p2. Funding for the Lung Cancer and Smoking study was provided by National Institutes of Health (NIH), Genes, Environment and Health Initiative (GEI) Z01 CP 010200, NIH U01 HG004446, and NIH GEI U01 HG 004438. For the lung study, the GENEVA Coordinating Center provided assistance with genotype cleaning and general study coordination, and the Johns Hopkins University Center for Inherited Disease Research conducted genotyping.
P. A. Newcomb is affiliated with PMH which is supported by the National Institutes of Health (R01 CA076366 to P.A. Newcomb).
E. White is affiliated with VITAL which is supported by the National Institutes of Health (K05 CA154337).
D. Duggan is affiliated with TGEN and funded through a subaward with GECCO (R01 CA059045).
The WHI program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through contracts HHSN268201100046C, HHSN268201100001C, HHSN268201100002C, HHSN268201100003C, HHSN268201100004C, and HHSN271201100004C.
DACHS: We thank all participants and cooperating clinicians, and Ute Handte-Daub, Renate Hettler-Jensen, Utz Benscheid, Muhabbet Celik and Ursula Eilber for excellent technical assistance.
GECCO: The authors would like to thank all those at the GECCO Coordinating Center for helping bring together the data and people that made this project possible.
HPFS, NHS and PHS: We would like to acknowledge Patrice Soule and Hardeep Ranu of the Dana Farber Harvard Cancer Center High-Throughput Polymorphism Core who assisted in the genotyping for NHS, HPFS, and PHS under the supervision of Dr. Immaculata Devivo and Dr. David Hunter, Qin (Carolyn) Guo and Lixue Zhu who assisted in programming for NHS and HPFS, and Haiyan Zhang who assisted in programming for the PHS. We would like to thank the participants and staff of the Nurses’ Health Study and the Health Professionals Follow-Up Study, for their valuable contributions as well as the following state cancer registries for their help: AL, AZ, AR, CA, CO, CT, DE, FL, GA, ID, IL, IN, IA, KY, LA, ME, MD, MA, MI, NE, NH, NJ, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, VA, WA, WY. In addition, this study was approved by the Connecticut Department of Public Health (DPH) Human Investigations Committee. Certain data used in this publication were obtained from the DPH. The authors assume full responsibility for analyses and interpretation of these data.
PLCO: The authors thank Drs. Christine Berg and Philip Prorok, Division of Cancer Prevention, National Cancer Institute, the Screening Center investigators and staff or the Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial, Mr. Tom Riley and staff, Information Management Services, Inc., Ms. Barbara O’Brien and staff, Westat, Inc., and Drs. Bill Kopp, Wen Shao, and staff, SAIC-Frederick. Most importantly, we acknowledge the study participants for their contributions to making this study possible. The statements contained herein are solely those of the authors and do not represent or imply concurrence or endorsement by NCI.
PMH: The authors would like to thank the study participants and staff of the Hormones and Colon Cancer study.
WHI: The authors thank the WHI investigators and staff for their dedication, and the study participants for making the program possible. A full listing of WHI investigators can be found at: https://cleo.whi.org/researchers/Documents%20%20Write%20a%20Paper/WHI%20Investigator%20Short%20List.pdf
Footnotes
Financial conflicts of interest: None
References
- 1.Society AC. Cancer Facts & Figures 2013. Atlanta: American Cancer Society; 2013. [Google Scholar]
- 2.Cheng I, Kocarnik JM, Dumitrescu L, Lindor NM, Chang-Claude J, et al. Pleiotropic effects of genetic risk variants for other cancers on colorectal cancer risk: PAGE, GECCO and CCFR consortia. Gut. 2013 doi: 10.1136/gutjnl-2013-305189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fernandez-Rozadilla C, Cazier JB, Tomlinson I, Brea-Fernandez A, Lamas MJ, et al. A genome-wide association study on copy-number variation identifies a 11q11 loss as a candidate susceptibility variant for colorectal cancer. Human genetics. 2013 doi: 10.1007/s00439-013-1390-4. [DOI] [PubMed] [Google Scholar]
- 4.Jiao S, Hsu L, Berndt S, Bezieau S, Brenner H, et al. Genome-wide search for gene-gene interactions in colorectal cancer. PloS one. 2012;7:e52535. doi: 10.1371/journal.pone.0052535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peters U, Jiao S, Schumacher FR, Hutter CM, Aragaki AK, et al. Identification of Genetic Susceptibility Loci for Colorectal Tumors in a Genome-Wide Meta-analysis. Gastroenterology. 2013;144:799–807. e24. doi: 10.1053/j.gastro.2012.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Theodoratou E, Montazeri Z, Hawken S, Allum GC, Gong J, et al. Systematic meta-analyses and field synopsis of genetic association studies in colorectal cancer. Journal of the National Cancer Institute. 2012;104:1433–57. doi: 10.1093/jnci/djs369. [DOI] [PubMed] [Google Scholar]
- 7.Wang H, Haiman CA, Burnett T, Fortini BK, Kolonel LN, et al. Fine-mapping of genome-wide association study-identified risk loci for colorectal cancer in African Americans. Human molecular genetics. 2013;22:5048–55. doi: 10.1093/hmg/ddt337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peters U, Hutter CM, Hsu L, Schumacher FR, Conti DV, et al. Meta-analysis of new genome-wide association studies of colorectal cancer risk. Human genetics. 2012;131:217–34. doi: 10.1007/s00439-011-1055-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hutter CM, Chang-Claude J, Slattery ML, Pflugeisen BM, Lin Y, et al. Characterization of gene-environment interactions for colorectal cancer susceptibility loci. Cancer research. 2012;72:2036–44. doi: 10.1158/0008-5472.CAN-11-4067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alexander DD, Weed DL, Cushing CA, Lowe KA. Meta-analysis of prospective studies of red meat consumption and colorectal cancer. European journal of cancer prevention : the official journal of the European Cancer Prevention Organisation. 2011;20:293–307. doi: 10.1097/CEJ.0b013e328345f985. [DOI] [PubMed] [Google Scholar]
- 11.Brevik A, Joshi AD, Corral R, Onland-Moret NC, Siegmund KD, et al. Polymorphisms in base excision repair genes as colorectal cancer risk factors and modifiers of the effect of diets high in red meat. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2010;19:3167–73. doi: 10.1158/1055-9965.EPI-10-0606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chan AT, Tranah GJ, Giovannucci EL, Willett WC, Hunter DJ, Fuchs CS. Prospective study of N-acetyltransferase-2 genotypes, meat intake, smoking and risk of colorectal cancer. International journal of cancer. Journal international du cancer. 2005;115:648–52. doi: 10.1002/ijc.20890. [DOI] [PubMed] [Google Scholar]
- 13.Joshi AD, Corral R, Siegmund KD, Haile RW, Le Marchand L, et al. Red meat and poultry intake, polymorphisms in the nucleotide excision repair and mismatch repair pathways and colorectal cancer risk. Carcinogenesis. 2009;30:472–9. doi: 10.1093/carcin/bgn260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Larsson SC, Wolk A. Meat consumption and risk of colorectal cancer: a meta-analysis of prospective studies. International journal of cancer. Journal international du cancer. 2006;119:2657–64. doi: 10.1002/ijc.22170. [DOI] [PubMed] [Google Scholar]
- 15.Wang J, Joshi AD, Corral R, Siegmund KD, Marchand LL, et al. Carcinogen metabolism genes, red meat and poultry intake, and colorectal cancer risk. International journal of cancer. Journal international du cancer. 2012;130:1898–907. doi: 10.1002/ijc.26199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Williams CD, Satia JA, Adair LS, Stevens J, Galanko J, et al. Associations of red meat, fat, and protein intake with distal colorectal cancer risk. Nutrition and cancer. 2010;62:701–9. doi: 10.1080/01635581003605938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sugimura T, Sato S. Mutagens-carcinogens in foods. Cancer research. 1983;43:2415s–21s. [PubMed] [Google Scholar]
- 18.Turesky RJ, Lang NP, Butler MA, Teitel CH, Kadlubar FF. Metabolic activation of carcinogenic heterocyclic aromatic amines by human liver and colon. Carcinogenesis. 1991;12:1839–45. doi: 10.1093/carcin/12.10.1839. [DOI] [PubMed] [Google Scholar]
- 19.Hein DW, Doll MA, Fretland AJ, Leff MA, Webb SJ, et al. Molecular genetics and epidemiology of the NAT1 and NAT2 acetylation polymorphisms. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2000;9:29–42. [PubMed] [Google Scholar]
- 20.Walker K, Ginsberg G, Hattis D, Johns DO, Guyton KZ, Sonawane B. Genetic polymorphism in N-Acetyltransferase (NAT): Population distribution of NAT1 and NAT2 activity. Journal of toxicology and environmental health. Part B, Critical reviews. 2009;12:440–72. doi: 10.1080/10937400903158383. [DOI] [PubMed] [Google Scholar]
- 21.Chen J, Stampfer MJ, Hough HL, Garcia-Closas M, Willett WC, et al. A prospective study of N-acetyltransferase genotype, red meat intake, and risk of colorectal cancer. Cancer research. 1998;58:3307–11. [PubMed] [Google Scholar]
- 22.da Silva TD, Felipe AV, de Lima JM, Oshima CT, Forones NM. N-Acetyltransferase 2 genetic polymorphisms and risk of colorectal cancer. World journal of gastroenterology : WJG. 2011;17:760–5. doi: 10.3748/wjg.v17.i6.760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lilla C, Verla-Tebit E, Risch A, Jager B, Hoffmeister M, et al. Effect of NAT1 and NAT2 genetic polymorphisms on colorectal cancer risk associated with exposure to tobacco smoke and meat consumption. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2006;15:99–107. doi: 10.1158/1055-9965.EPI-05-0618. [DOI] [PubMed] [Google Scholar]
- 24.Ognjanovic S, Yamamoto J, Maskarinec G, Le Marchand L. NAT2, meat consumption and colorectal cancer incidence: an ecological study among 27 countries. Cancer causes & control : CCC. 2006;17:1175–82. doi: 10.1007/s10552-006-0061-3. [DOI] [PubMed] [Google Scholar]
- 25.Tiemersma EW, Voskuil DW, Bunschoten A, Hogendoorn EA, Witteman BJ, et al. Risk of colorectal adenomas in relation to meat consumption, meat preparation, and genetic susceptibility in a Dutch population. Cancer causes & control : CCC. 2004;15:225–36. doi: 10.1023/B:CACO.0000024263.44973.92. [DOI] [PubMed] [Google Scholar]
- 26.Welfare MR, Cooper J, Bassendine MF, Daly AK. Relationship between acetylator status, smoking, and diet and colorectal cancer risk in the north-east of England. Carcinogenesis. 1997;18:1351–4. doi: 10.1093/carcin/18.7.1351. [DOI] [PubMed] [Google Scholar]
- 27.Barrett JH, Smith G, Waxman R, Gooderham N, Lightfoot T, et al. Investigation of interaction between N-acetyltransferase 2 and heterocyclic amines as potential risk factors for colorectal cancer. Carcinogenesis. 2003;24:275–82. doi: 10.1093/carcin/24.2.275. [DOI] [PubMed] [Google Scholar]
- 28.Sorensen M, Autrup H, Olsen A, Tjonneland A, Overvad K, Raaschou-Nielsen O. Prospective study of NAT1 and NAT2 polymorphisms, tobacco smoking and meat consumption and risk of colorectal cancer. Cancer letters. 2008;266:186–93. doi: 10.1016/j.canlet.2008.02.046. [DOI] [PubMed] [Google Scholar]
- 29.Tiemersma EW, Kampman E, Bueno de Mesquita HB, Bunschoten A, van Schothorst EM, et al. Meat consumption, cigarette smoking, and genetic susceptibility in the etiology of colorectal cancer: results from a Dutch prospective study. Cancer causes & control : CCC. 2002;13:383–93. doi: 10.1023/a:1015236701054. [DOI] [PubMed] [Google Scholar]
- 30.Wang H, Yamamoto JF, Caberto C, Saltzman B, Decker R, et al. Genetic variation in the bioactivation pathway for polycyclic hydrocarbons and heterocyclic amines in relation to risk of colorectal neoplasia. Carcinogenesis. 2011;32:203–9. doi: 10.1093/carcin/bgq237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nothlings U, Yamamoto JF, Wilkens LR, Murphy SP, Park SY, et al. Meat and heterocyclic amine intake, smoking, NAT1 and NAT2 polymorphisms, and colorectal cancer risk in the multiethnic cohort study. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2009;18:2098–106. doi: 10.1158/1055-9965.EPI-08-1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hiraki LT, Qu C, Hutter CM, Baron JA, Berndt SI, et al. Genetic predictors of circulating 25-hydroxyvitamin d and risk of colorectal cancer. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2013;22:2037–46. doi: 10.1158/1055-9965.EPI-13-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Newcomb PA, Baron J, Cotterchio M, Gallinger S, Grove J, et al. Colon Cancer Family Registry: an international resource for studies of the genetic epidemiology of colon cancer. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2007;16:2331–43. doi: 10.1158/1055-9965.EPI-07-0648. [DOI] [PubMed] [Google Scholar]
- 34.Garcia-Closas M, Hein DW, Silverman D, Malats N, Yeager M, et al. A single nucleotide polymorphism tags variation in the arylamine N-acetyltransferase 2 phenotype in populations of European background. Pharmacogenetics and genomics. 2011;21:231–6. doi: 10.1097/FPC.0b013e32833e1b54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cho E, Smith-Warner SA, Spiegelman D, Beeson WL, van den Brandt PA, et al. Dairy foods, calcium, and colorectal cancer: a pooled analysis of 10 cohort studies. Journal of the National Cancer Institute. 2004;96:1015–22. doi: 10.1093/jnci/djh185. [DOI] [PubMed] [Google Scholar]
- 36.Giovannucci E, Stampfer MJ, Colditz GA, Hunter DJ, Fuchs C, et al. Multivitamin use, folate, and colon cancer in women in the Nurses’ Health Study. Annals of internal medicine. 1998;129:517–24. doi: 10.7326/0003-4819-129-7-199810010-00002. [DOI] [PubMed] [Google Scholar]
- 37.Terry P, Baron JA, Bergkvist L, Holmberg L, Wolk A. Dietary calcium and vitamin D intake and risk of colorectal cancer: a prospective cohort study in women. Nutrition and cancer. 2002;43:39–46. doi: 10.1207/S15327914NC431_4. [DOI] [PubMed] [Google Scholar]
- 38.Koushik A, Hunter DJ, Spiegelman D, Beeson WL, van den Brandt PA, et al. Fruits, vegetables, and colon cancer risk in a pooled analysis of 14 cohort studies. Journal of the National Cancer Institute. 2007;99:1471–83. doi: 10.1093/jnci/djm155. [DOI] [PubMed] [Google Scholar]
- 39.Price AL, Patterson NJ, Plenge RM, Weinblatt ME, Shadick NA, Reich D. Principal components analysis corrects for stratification in genome-wide association studies. Nature genetics. 2006;38:904–9. doi: 10.1038/ng1847. [DOI] [PubMed] [Google Scholar]
- 40.Lundberg M, Fredlund P, Hallqvist J, Diderichsen F. A SAS program calculating three measures of interaction with confidence intervals. Epidemiology. 1996;7:655–6. [PubMed] [Google Scholar]
- 41.Andersson T, Alfredsson L, Kallberg H, Zdravkovic S, Ahlbom A. Calculating measures of biological interaction. European journal of epidemiology. 2005;20:575–9. doi: 10.1007/s10654-005-7835-x. [DOI] [PubMed] [Google Scholar]
- 42.Rothman KJ, Greenland S, Walker AM. Concepts of interaction. American journal of epidemiology. 1980;112:467–70. doi: 10.1093/oxfordjournals.aje.a113015. [DOI] [PubMed] [Google Scholar]
- 43.Huxley RR, Ansary-Moghaddam A, Clifton P, Czernichow S, Parr CL, Woodward M. The impact of dietary and lifestyle risk factors on risk of colorectal cancer: a quantitative overview of the epidemiological evidence. International journal of cancer. Journal international du cancer. 2009;125:171–80. doi: 10.1002/ijc.24343. [DOI] [PubMed] [Google Scholar]
- 44.Research WCRFAIfC. Food, nutrition, physical activity, and the prevention of cancer: a global perspective. 2007. [Google Scholar]
- 45.Navarro A, Munoz SE, Lantieri MJ, del Pilar Diaz M, Cristaldo PE, et al. Meat cooking habits and risk of colorectal cancer in Cordoba, Argentina. Nutrition. 2004;20:873–7. doi: 10.1016/j.nut.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 46.Chan DS, Lau R, Aune D, Vieira R, Greenwood DC, et al. Red and processed meat and colorectal cancer incidence: meta-analysis of prospective studies. PloS one. 2011;6:e20456. doi: 10.1371/journal.pone.0020456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morimoto LM, White E, Newcomb PA. Selection bias in the assessment of gene-environment interaction in case-control studies. American journal of epidemiology. 2003;158:259–63. doi: 10.1093/aje/kwg147. [DOI] [PubMed] [Google Scholar]
- 48.Cross AJ, Ferrucci LM, Risch A, Graubard BI, Ward MH, et al. A large prospective study of meat consumption and colorectal cancer risk: an investigation of potential mechanisms underlying this association. Cancer research. 2010;70:2406–14. doi: 10.1158/0008-5472.CAN-09-3929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hughes HB, Biehl JP, Jones AP, Schmidt LH. Metabolism of isoniazid in man as related to the occurrence of peripheral neuritis. American review of tuberculosis. 1954;70:266–73. doi: 10.1164/art.1954.70.2.266. [DOI] [PubMed] [Google Scholar]
- 50.Ambrosone CB, Abrams SM, Gorlewska-Roberts K, Kadlubar FF. Hair dye use, meat intake, and tobacco exposure and presence of carcinogen-DNA adducts in exfoliated breast ductal epithelial cells. Archives of biochemistry and biophysics. 2007;464:169–75. doi: 10.1016/j.abb.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 51.Cotterchio M, Boucher BA, Manno M, Gallinger S, Okey AB, Harper PA. Red meat intake, doneness, polymorphisms in genes that encode carcinogen-metabolizing enzymes, and colorectal cancer risk. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2008;17:3098–107. doi: 10.1158/1055-9965.EPI-08-0341. [DOI] [PMC free article] [PubMed] [Google Scholar]