Abstract
Ultraviolet (UV) radiation-induced immunosuppression has been linked with the risk of skin carcinogenesis. Approximately, two million new cases of skin cancers, including melanoma and non-melanoma, diagnosed each year in the USA and therefore have a tremendous bad impact on public health. Dietary phytochemicals are promising options for the development of effective strategy for the prevention of photodamaging effects of UV radiation including the risk of skin cancer. Grape seed proanthocyanidins (GSPs) are such phytochemicals. Dietary administration of GSPs with AIN76A control diet significantly inhibits UV-induced skin tumor development as well as suppression of immune system. UV-induced suppression of immune system is commonly determined using contact hypersensitivity (CHS) model which is a prototype of T cell-mediated immune response. We present evidence that inhibition of UV-induced suppression of immune system by GSPs is mediated through: (i) the alterations in immunoregulatory cytokines, interleukin (IL)-10 and IL-12, (ii) DNA repair, (iii) stimulation of effector T cells, and (iv) DNA repair-dependent functional activation of dendritic cells in mouse model. These information have important implications for the use of GSPs as a dietary supplement in chemoprevention of UV-induced immunosuppression as well as photocarcinogenesis.
INTRODUCTION
Natural bioactive phytochemicals have been used for the prevention and treatment of many diseases for centuries. There is a renewed interest in natural phytochemicals as promising options for the development of more effective treatment or preventative strategies for various types of diseases including the cancers of different organs. Full realization of this potential, however, requires an improved knowledge of the therapeutic effects of these phytochemicals. For this purpose, the exact identification of the components of the plant, or the dietary agents derived from the plants that are responsible for the therapeutic activities is required at the first place. Second, the elucidation of the molecular and cellular targets of these components as well as identification of the specific mechanisms that play a role in reducing the risk of disease is needed.
Solar ultraviolet radiation (UVR) can act as a tumor initiator, tumor promoter and co-carcinogen (1, 2). Exposure of the skin to UVR has multiple effects in the skin which includes the generation of oxidative stress, inflammatory mediators, DNA damage and suppression of immune system (3, 4). The immunosuppressive effects of UV radiation, particularly UVB spectrum (290–320 nm), are well recognized. UVR-induced suppression of immune system contributes to the pathogenesis of UV radiation-induced skin cancer including both melanoma and non-melanoma in laboratory animals as well as in humans (5, 6). The immunosuppressive effect of UV radiation on skin cancer risk is supported by the following observations: Chronically immunosuppressed patients living in regions of intense sun exposure experience an exceptionally high rate of skin cancer (7). The incidence of skin cancers, particularly squamous cell carcinoma (SCC), is increased among organ transplant recipients. This increased frequency of SCC is associated with a long-term immunosuppressive therapy (8–11). In contrast, the animals with an enhanced immune response exhibit a lower risk of skin cancer development on chronic UV exposure (12). These investigations suggest that protection from UVR-induced immunosuppression may be an important strategy in the management of skin carcinogenesis. The development of new and effective strategies for the prevention of skin cancers is an urgent need as it has been reported that approximately 2 million new cases of nonmelanoma skin cancers diagnosed each year in the USA alone and therefore have a tremendous impact on public health and healthcare expenditures.
There has been a great interest in the use of various plant products for the prevention of UV-induced cutaneous photodamage. Phytochemicals show promising options for their use as anti-photodamaging effects on the skin. The photoprotective effects of phytochemicals are due to their anti-inflammatory, anti-oxidative and immunomodulatory properties. Among numerous well studied phytochemicals, grape seed proanthocyanidins (GSPs) have been extensively studied for their cutaneous anti-photodamaging effects in vitro and in vivo animal models. As UVR-induced immunosuppression plays a critical role in skin diseases and in particular skin cancers, the investigations on this aspect of GSPs were summarized and discussed here in more detail. Also, the extensive work on the photoprotective effect of GSPs was conducted in the research laboratory of Dr. Katiyar, that work is discussed here predominantly.
GRAPE SEED PROANTHOCYANIDINS (GSPs), SOURCE AND COMPOSITION
Grapes (Vitis vinifera) are rich source of polyphenolic components with approximately 60% to 70% of these polyphenols being found in the grape seeds. Seeds contain a larger fraction of proanthocyanidins, which are composed of dimers, trimers, tetramers and oligomers of monomeric catechins or epicatechins (13–15). The GSPs are commercially available from the Kikkoman Corporation (Japan and USA) and the chemical composition has been described (16, 17). Briefly, GSPs contain approximately 89% proanthocyanidins, with dimers (6.6%), trimers (5.0%), tetramers (2.9%) and oligomers (74.8%). GSPs are stable for at least 2 years when refrigerated at 4°C. In most of the in vivo investigations, GSPs were used as a diet in supplementation with control AIN76A diet, and was commercially prepared in pellet form.
DIETARY INTAKE OF GSPs INHIBIT UVR-INDUCED IMMUNOSUPPRESSION: USE OF CONTACT HYPERSENSITIVITY (CHS) AS A MODEL
It is known that exposure of the skin to UVR results in suppression of immune system. Both UVA (320–400 nm) and UVB (290–320 nm) radiation suppress the immune systems of mice and humans; however, the effect of UVB spectrum is considered to be more immunosuppressive than the UVA fraction of UV spectrum. The chemopreventive effect of dietary GSPs on UV-induced immunosuppression was evaluated using C3H/HeN mouse model. To assess the effect of UVR-induced immune suppression, usually contact hypersensitivity (CHS) model is used, which is considered as a prototype of T-cell mediated immune response. CHS represents a special form of the delayed-type hypersensitivity response. It is induced by epicutaneous application of contact allergens or skin contact sensitizer, such as 2,4-dinitrofluorobenzene (DNFB) (17, 18). The CHS response in those mice that did not receive GSPs and that were UVB (180 mJ/cm2) irradiated was significantly lower (P<0.001) than those mice that were not UVR irradiated, confirming the immunosuppressive effect of the UV irradiation. In contrast, the mice that received GSPs in diet (0.5% or 1.0% GSPs, w/w) and UV irradiated resulted in significant prevention (P<0.001) of UVB-induced suppression of CHS (17). These studies indicate that supplementation of the diet with GSPs at a concentration of 0.5 or 1.0% is capable of protecting mice from UVB-induced immunosuppression. However, the administration of dietaryGSPs does not prevent UVB-induced suppression of CHS response in mice which were exposed to abnormally higher dose of UVR (e.g., 1000 mJ/cm2).
ROLE OF IMMUNOMODULATORY CYTOKINES IN GSPs-MEDIATED CHS RESPONSE
It has been recognized that skin exposure to UVB stimulates keratinocytes to release immunosuppressive soluble mediators, including the increase of interleukin (IL)-10 in the skin and regional lymph nodes (19). These soluble mediators then enter the circulatory system and can thus suppress the immune system in a systemic manner. Importantly, the exposure of the skin to UV radiation triggers the release of prostaglandins (PGs), such as PGE2, PGD3 and PGF2α, which are produced from arachidonic acid by the action of cyclooxygenases (COX), and in particular COX-2. Among the PG metabolites, PGE2, which is abundantly produced by keratinocytes in UV-exposed skin (20, 21), is the major and most effective metabolite generated by COX-2 activity and is considered to be a potent mediator of inflammatory responses. Additionally, PGE2 plays a key role in UV-induced immunosuppression. PGE2 enhances the production of IL-10 by epidermal keratinocytes as well as activated macrophages while decreases the levels of IL-12. IL-12 actsas an immunostimulatory cytokine (22,23). Therefore the effect of GSPs on UVB-caused changes on the immunoregulatory cytokines was determined. GSPs inhibit UVB-induced increases in IL-10 production both in the skin and in the draining lymph nodes while increases the production of IL-12 in UVB-exposed mice. The inhibition of immunosuppressive cytokine IL-10 production and stimulation of IL-12 by GSPs may have a crucial role in prevention of UVB-induced immunesuppression in mice. Importantly, GSPs also inhibit the levels of COX-2 expression and PGE2 production in UV-exposed skin and that may play a crucial role in the inhibition of IL-10 production and upregulation of IL-12 in UV exposed mice (24). Intraperitoneal injection of IL-10 inhibits the ability of mice to be sensitized to trinitrophenyl-coupled spleen cells for a delayed type hypersensitivity response (23). Intraperitoneal injection of IL-10 into sensitized mice before challenge resulted in a significant suppression of ear swelling or CHS response, suggesting that IL-10 has the ability to block the effector phase of CHS in vivo. Moreover, the administration of neutralizing antibodies to IL-10 inhibited the ability of UV radiation to suppress sensitization to alloantigens (22). In agreement with these observations, it seems that prevention of UVB-induced immunosuppression by dietary GSPs may be mediated, at least in part, through the inhibition of IL-10 production in mice. Further, the outcome of such studies not only provides substantial insight into the intricate roles of PGE2 in UVB-induced immunosuppression but also provides a rationale for considering the development of new strategies using the inhibitors of PGE2, such as GSPs, alone or in combination with other drugs/agents for the prevention or treatment of nonmelanoma skin cancers.
Multiple investigations suggest that IL-12 plays a role in vivo in the induction phase of the CHS response. CHS appears to be a Th1 type cell-mediated immune response (25) and epidermal Langerhans cells, a type of antigen presenting cells, have been reported to be an additional source of IL-12 production (26). After UV exposure, the antigen presenting cells in the skin migrate to the regional lymph nodes and initiate sensitization. The intake of GSPs-supplemented diet increases the production of IL-12 in the draining lymph nodes of mice which is the site of T cell development. It may be the possibility that the number of cells that migrate from the skin to the regional lymph nodes is higher in the UVB irradiated mice that received GSPs in the diet than those that did not. IL-12 regulates the development and function of T-cells particularly the development of Th1 type cells by stimulating the production of IFN-γ (27–29). Intraperitoneal injection of recombinant IL-12 prevents UV-induced immune-suppression and overcomes UV-induced hapten-specific tolerance (30, 31). Dietary GSPs increase IL-12 production in T-cell specific areas of the draining lymph nodes and thus alter the immune response in favor of the development of Th1 type cells. The dietary GSPs-induced shift in the cytokine balance appears to be a potential mechanism by which GSPs inhibit UVB-induced immune suppression in mice (17). Intraperitoneal injection of anti-IL-12 antibody was resulted in the UVB-induced suppression of the CHS response to DNFB in GSPs-fed mice and thus suggested that GSPs-induced IL-12 production is important for protection of immune system in UV-exposed mice. These studies indicate that inhibition of immunosuppressive cytokine IL-10 production and induction of IL-12 may play a crucial role in the inhibition of UVB-induced suppression of CHS response by GSPs in mice. This observation was further supported by the experiments in which dietary GSPs do not inhibit UVB-induced immunosuppression in IL-12-deficient mice and that treatment of these mice with rIL-12 restores the inhibitory effect (32). Together, these observations suggest that dietary GSPs have the capability to protect the skin from UVB-induced photodamage, and further suggest that the protection from UVB-induced immunosuppression by GSPs may be associated with the prevention from UV radiation-induced skin tumor development in mice (16).
GSPs STIMULATE THE REPAIR OF UV-INDUCED DNA DAMAGE
UV irradiation induces DNA damage predominantly the formation of cyclobutane pyrimidine dimers (CPDs) in the UV-exposed skin site (Fig. 1). UV-induced CPDs have been recognized as a molecular trigger for the suppression of immune responses (33, 34). Reduction or repair of CPDs through application of DNA repair enzymes reverses this process. DNA damage in antigen presenting cells impairs their capacity to present antigen, which in turn results in a lack of sensitization (35). CPD-positive antigen presenting cells have been identified in the draining lymph nodes of UV-irradiated mice (31, 36). Thus UV-induced DNA damage is one of the earliest molecular events in the development of immune suppression. Supplementation of GSPs with AIN76A control diet significantly reduced the levels of CPD+ cells in UVB-exposed mouse skin; however, GSPs did not significantly reduce UVB-induced CPD+ cells in the skin of IL-12-deficient mice, suggesting that IL-12 is required for the repair of CPDs by GSPs (37). This piece of observation suggests that GSPs prevent UVB-induced immunosuppression through rapid repair of UV-induced DNA damage in the form of CPDs.
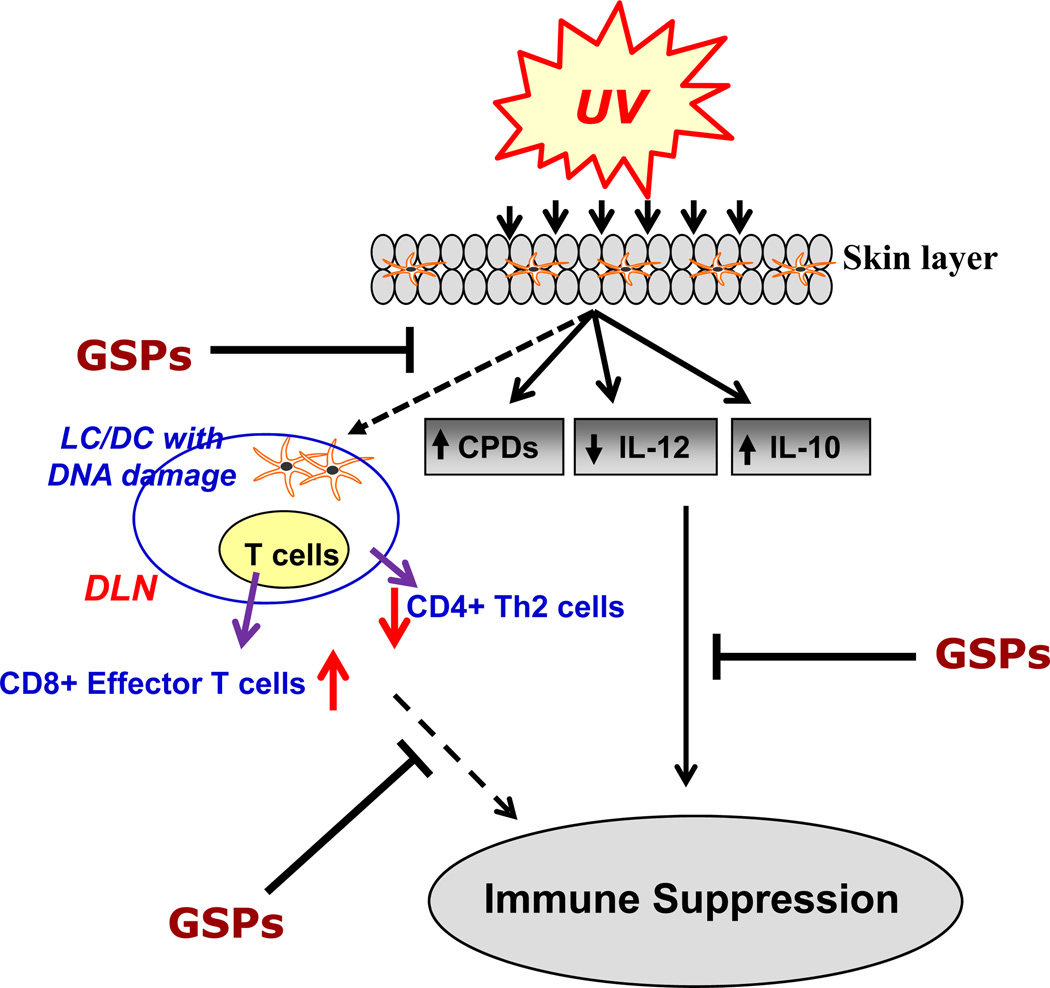
Figure 1.
UV irradiation causes increases in IL-10 and decreases in IL-12 production in the skin and draining lymph nodes. Additionally, UV radiation causes DNA damage in antigen presenting cells (LC/DC), which migrates to lymph nodes and results in increase of CD4+ Th2 cells while decrease in CD8+ effector T cells in UV-irradiated mice. Regular intake of dietary GSPs results in stimulation of IL-12, reduction in IL-10 and stimulates repair of UV-induced DNA damage which resulted in elevated levels of CD8+ effector T cells while reduced levels of Th2 and Treg cells. These alterations result in chemoprevention of UV-induced immunosuppression by dietary GSPs in mice. Upward arrows (↑) indicate the enhancement or stimulation, and downward arrows (↓) indicate the reduction in levels. LC = Langerhans cells; DC = dendritic cells; DLN, draining lymph nodes.
IMMUNOMODULATORY EFFECT OF DIETARY GSPs ONCD8+ AND CD4+T CELL SUBSETS
As T cell development and activation play a role in immune system, the effect of dietary GSPs was assessed using adoptive transfer model/experiment. Using this model, efforts were made to identify the T-cell subpopulations responsible for the transfer of the GSPs-induced prevention of immunosuppression. The donor C3H/HeN mice were treated or not treated with GSPs, exposed to UVB radiation and sensitized with DNFB. The regional lymph nodes were obtained from donor mice that had been sensitized 5 days earlier by topical treatment of the UVB-exposed skin with DNFB, and the CD8+ T cells positively selected using the MACS system. Purified CD8+ T cells were injected i.v. into naïve mice, which were then challenged immediately by application of DNFB on the ear skin. Ear skin thickness was measured 24 h later (32). Those naïve mice which have received CD8+ effector T cells from GSPs-treated, UVB-exposed donor mice showed a greater CHS response than the naïve mice that received cells from UVB-exposed mice but not treated with GSPs. This observation suggests that the prevention of UVB-induced immunosuppression by GSPs is transferable to naïve mice by CD8+ T cells. Further the treatment of mice with GSPs results in activation of the CD8+ T-cell subpopulation and that these cells play a role in the enhanced CHS response in UVB-irradiated animals (32).
There are conflicting opinions as to whether the CD8+ or the CD4+ T-cell subpopulation mediates the CHS response. Studies by Hauser (38) and Kondo et al (39) suggest that CD4+ T cells are the critical effector cells for CHS response, while other investigators (40–43) provide evidence that CD8+ T cells are involved in CHS response. To explain the effect of GSPs on the CD4 T cell response, both spleens and lymph nodes were isolated from donor mice sensitized five days earlier by topical administration of DNFB onto the UVB-exposed backs of mice that were treated with or without GSPs. Single-cell suspensions were prepared and CD4+ T cells were isolated by positive selection. Naïve mice were injected i.v. with the purified CD4+ T cells (8 × 106 cells/mouse) and, in this case, the mice were sensitized 24 h later by application of DNFB onto the abdominal skin prior to challenge by application of DNFB on the ear skin five days later and the change in ear skin thickness was measured 24 h later. Those naïve mice that received CD4+ T cells from UVB-irradiated and DNFB-sensitized donor mice had a significantly lower CHS response (P<0.01) than those mice that were sensitized but not exposed to UVB radiation. Those naïve mice that had received CD4+ T cells from the GSPs-treated, UVB-exposed donor mice had a greater CHS response than those naïve mice that had received CD4+ T cells from UVB alone-irradiated donor mice, suggesting that prevention of UVB-induced immunosuppression by GSPs is also transferable to naïve mice by CD4+ T cells.
ANALYSIS OF CYTOKINE PRODUCTION PROFILE BY THE CD8+ T CELLS AND CD4+ T CELLS IN RESPONSE TO UVB AND GSPs TREATMENT
The functional activation of CD8+ T cells by dietary GSPs was determined by analyzing the levels of Th1 and Th2 cytokine profiles using cytokine-specific ELISA (32). The levels of IFNγ (>5-fold) and IL-2 (8-fold) were considerably higher in the supernatants of CD8+ T cells prepared from the GSPs treated, UVB-exposed mice than in the supernatants of cells from mice that were exposed to UVB but not treated with GSPs. In contrast, the Th2 cytokines were hardly detectable in the supernatants of CD8+ T cells obtained either from the GSPs-treated, UVB-exposed mice or the CD8+ T cells from the mice that were exposed to UVB but not treated with GSPs. The significantly higher levels of Th1 cytokines in the mice that were treated with GSPs suggests that the activation of CD8+ T cells by the dietary GSPs may play a significant role in the immune response in the GSPs-treated, UVB-exposed mice.
The effect of dietary GSPs was also studied and compared on the levels of Th1 and Th2 cytokine profiles of CD4+ T cells from mice from the different treatment groups. Vaid et al (32) have found that the levels of Th2 cytokines (IL-4, IL-10) in the supernatants of the CD4+ T cells from the GSPs-treated and UVB-exposed mice were significantly lower than the levels of these cytokines in the supernatants of CD4+ T cells from UVB alone-irradiated mice that were not treated with GSPs. Both IFNγ and IL-2 were detectable in the supernatants of CD4+ T cells irrespective of whether the donor mice were treated with GSPs but the levels of these Th1 cytokines were low, particularly when compared with the levels of the Th1 cytokines in the supernatants of the CD8+ effector T cells. The low levels of the Th2 cytokines in the supernatants of the CD4+ T cells in this study suggest that treatment of mice with dietary GSPs suppresses the functional activity of CD4+ T cells and thus resulted in enhanced immune activity in these mice. Thus, these studies indicate that CD8+ T cells are the critical effector cells, a finding that is in accordance with the findings of other investigators (41, 43). This property of GSPs can be used as an alternative strategy to augment the induction of CD8+ effector T cells and to diminish the development of CD4+ regulatory T cells and that may lead to the prevention of skin cancer risk in humans. Xu et al. (43) reported that CHS is mediated through CD8+ T cells, whereas CD4+ Th2 cells exhibit an inhibitory effect on CHS. This observation was supported by the findings of Gocinski and Tigelaar (41) and Anderson et al. (40) who claimed that depletion of CD4+ T cells before sensitization results in an enhanced ear skin thickness or CHS response.
DIETARY GSPs ENHANCE THE FUNCTIONAL ACTIVITY OF DENDRITIC CELLS IN UV-IRRADIATED MOUSE SKIN
UV-induced photodamage of epidermal Langerhans cells (LC) is considered to be an important mechanism or cellular target in UV-induced immune suppression (26, 44). There is evidence indicating that DNA repair mechanisms are related directly to the function of LCs in the stimulation of T cells and the induction of immune response (35, 45). Studies have identified that a reduction in cyclobutane pyrimidine dimer-positive (CPD+) LCs is correlated with increased function of the LCs as assessed by the induction of CHS and the production of IFNγ by T cells (35). The repair of CPDs in UV-exposed skin requires nucleotide excision repair (NER) mechanisms or the xeroderma pigmentosum complementation group A (XPA) gene (35, 37). It was found that dietary GSPs prevent UV-induced suppression of the CHS response to the contact sensitizer, DNFB, in wild-type mice but this effect of GSPs was not observed in XPA-deficient mice, suggesting the involvement of XPA or NER mechanisms in the prevention of UV-induced immunosuppression by GSPs in mice. To verify whether GSPs can act to enhance DNA repair in DCs, the GSPs-treated or untreated mouse bone marrow-derived dendritic cells (BM-DC) were exposed to UV radiation. The cells were harvested immediately or 24 h after UV irradiation and CPD+ cells were identified by immunocytostaining (46). It was found that treatment of UV-irradiated DC with GSPs resulted in repair of CPDs; however, GSPs were not able to repair UV-induced DNA damage in DCs obtained from XPA-deficient mice. Additionally, dietary GSPs were found to enhance the repair of UV-induced DNA damage in the form of CPDs in epidermal DCs in wild-type mice, but this effect of GSPs was not observed in the epidermal DCs of UV-exposed skin of XPA-deficient mice (46). These findings suggest that GSPs-mediated DNA repair in DCs is mediated through XPA and is an important mechanism in their prevention of UV-induced immunosuppression. Li et al (47) also have shown the role of XPA in the repair of UV-induced DNA damage. Roy et al (48) have found that GSPs induce apoptosis in JB6 C141 keratinocytes and this effect of GSPs was p53-dependent as apoptosis occurred mainly in cells expressing wild-type p53 than in cells which were p53-deficient. These findings suggest the involvement of p53 in repair of damaged DNA, and needs further studies on this aspect and its role in UV-induced immunosuppression and its prevention.
COMPARATIVE ANALYSES AND ROLE OF CYTOKINES SECRETED BY SKIN DENDRITIC CELLS
Investigations have shown that the production of certain cytokines by DCs plays a major role in their ability to stimulate specific populations of T cells. DCs can produce both IL-12 and IL-10 cytokines (49–52). UV-irradiation suppresses the production of IL-12, whereas it increases the production of IL-10 in the skin (53–55). The UV-irradiation reduction in the production of IL-12 by the DCs, suggests that this may be a mechanism by which UV radiation stimulates the development of tolerogenic DCs (56, 57). The effect of dietary GSPs on the production of various cytokines by DCs obtained from UV-exposed XPA-deficient and their wild-type counterparts was determined. Dietary GSPs enhanced the production of IL-12 and IFNγ while reducing the levels of IL-10 in DCs obtained from UVB-exposed wild-type mice. This ameliorating effect of GSPs was not found in the DCs obtained from UVB-exposed XPA-deficient mice. These findings suggest that GSPs can rescue the regulated production of IL-12 and IL-10 by the UV-damaged dendritic cells and that the modulation of cytokines in DCs is associated with the GSPs-mediated repair of the UVB-induced DNA damage. This concept was supported by analysis of the ability of GSPs to restore the function of DCs in terms of their ability to activate T-cell subpopulations. To address this issue, the DCs obtained from different treatment groups of XPA-deficient and their wild-type counterparts were co-cultured with CFSE-labeled CD4+ T cells. The results of FACS analysis revealed that DCs obtained from UVB-irradiated wild-type mice that were fed GSPs significantly stimulate T-cell proliferation whereas DCs obtained from UVB-irradiated wild-type mice that were not administered GSPs suppressed T-cell proliferation (46). These findings suggest that GSPs enhance the function of DCs in terms of their ability to enhance the proliferation ability of T cells. GSPs also enhanced the production of IFNγ while suppressing the levels of IL-10 and IL-4 by the DCs, which further supports the concept that dietary GSPs enhance the functional activity of DCs. However, under identical conditions, dietary GSPs failed to activate DCs obtained from UV-exposed XPA-deficient mice. The DCs obtained from the UVB-irradiated XPA-deficient mice that were fed GSPs were not able to stimulate the proliferation ability of T cells or the levels of IFNγ (46). These results suggest that GSPs prevent UVB-induced immunosuppression by repair of CPDs in DCs that is associated with functional activation of UVB-irradiated DCs in vivo animal model.
The studies conducted in Dr. Katiyar’s laboratory thus suggest that GSPs increase the ability of UV-irradiated DCs to stimulate T-cell activation/proliferation. It was further examined whether dietary GSPs improve the ability of UVB-exposed DCs to induce CHS responses following adoptive transfer. Transfer of DCs from UVB-irradiated wild-type mice that had been fed GSPs to naïve mice resulted in a higher CHS response to DNFB than that observed in naïve mice that received DCs from UV-exposed wild-type mice that were not fed GSPs. Under identical conditions, adoptive transfer of DCs from UVB-irradiated XPA-deficient mice that were fed GSPs to naïve mice did not induce a CHS response to the contact sensitizer, DNFB. This may be associated with the inability of GSPs to protect DCs from UV-induced DNA damage in these mice. It is to note that dendritic cells in the skin consist of different subpopulations. It is uninvestigated, and therefore, not excluded that DC subpopulations may have different sensitivities to UV induced DNA damage and they may respond differently to GSPs mediated preventive effects. These issues need further exploration.
CONCLUSION
The studies which have been summarized in this article indicate the potential effects of dietary grape seed proanthocyanidins in the prevention of UVR-induced immunosuppression in animals. These studies clearly show that the GSPs prevention of UV radiation-induced immunosuppression is mediated through: (i) alterations in immunoregulatory cytokines, (ii) stimulation of damaged DNA repair and (iii) DNA repair-dependent functional activation of dendritic cells in mice, as summarized in Fig. 1. These findings have important implications for the use of GSPs either alone or in combination with existing drugs in the chemoprevention of UV radiation-induced melanoma and non-melanoma skin cancers in humans.
Acknowledgments
The work reported from Dr. Katiyar’s laboratory was supported by the funds from National Cancer Institute/NCCAM/NIH (CA140197, CA140832) and Veterans Administration Merit Review Award (S.K.K.). The content of this article does not necessarily reflect the views or policies of the funding agencies.
REFERENCES
- 1.Donawho CK, Kripke ML. Evidence that the local effect of ultraviolet radiation on the growth of murine melanomas is immunologically mediated. Cancer Res. 1991;51:4176–4181. [PubMed] [Google Scholar]
- 2.Ziegler A, Jonason AS, Leffell DJ, Simon JA, Sharma HW, Kimmelman J, Remington L, Jacksand T, Brash DE. Sunburn and p53 in the onset of skin cancer. Nature. 1994;372:773–776. doi: 10.1038/372773a0. [DOI] [PubMed] [Google Scholar]
- 3.Katiyar SK. UV-induced immune suppression and photocarcinogenesis: Chemoprevention by dietary botanical agents. Cancer Lett. 2007;255:1–11. doi: 10.1016/j.canlet.2007.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Katiyar SK. Grape seed proanthocyanidines and skin cancer prevention: Inhibition of oxidative stress and protection of immune system. Mol. Nutr. Food Res. 2008;52:S71–S76. doi: 10.1002/mnfr.200700198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Urbach F. Incidences of nonmelanoma skin cancer. Dermatol.Clin. 1991;9:751–755. [PubMed] [Google Scholar]
- 6.Yoshikawa T, Rae V, Bruins-Slot W, vand-den-Berg JW, Taylorand JR, Streilein JW. Susceptibility to effects of UVB radiation on induction of contact hypersensitivity as a risk factor for skin cancer in humans. J. Invest. Dermatol. 1990;95:530–536. doi: 10.1111/1523-1747.ep12504877. [DOI] [PubMed] [Google Scholar]
- 7.Kinlen L, Sheil A, Peta J, Doll R. Collaborative United Kingdom–Australia study of cancer in patients treated with immunosuppressive drugs. Br. J. Med. 1979;II:1461–1466. doi: 10.1136/bmj.2.6203.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cowen EW, Billingsley EM. Awareness of skin cancer by kidney transplant patients. J. Am. Acad. Dermatol. 1999;40:697–701. doi: 10.1016/s0190-9622(99)70149-0. [DOI] [PubMed] [Google Scholar]
- 9.Otley CC, Pittelkow MR. Skin cancer in liver transplant recipients. Liver Transpl. 2000;6:253–262. doi: 10.1053/lv.2000.6352. [DOI] [PubMed] [Google Scholar]
- 10.Fortina AB, Caforio AL, Piaserico S, Alaibac M, Tona F, Feltrin G, Livi U, Peserico A. Skin cancer in heart transplant recipients: frequency and risk factor analysis. J. Heart Lung Transplant. 2000;19:249–255. doi: 10.1016/s1053-2498(99)00137-0. [DOI] [PubMed] [Google Scholar]
- 11.DiGiovanna JJ. Post transplantation skin cancer: scope of the problem, management and role for systemic retinoid chemoprevention. Transplant. Proc. 1998;30:2771–2775. doi: 10.1016/s0041-1345(98)00806-9. [DOI] [PubMed] [Google Scholar]
- 12.Beissert S, Bluestone JA, Mindt I, Voskort M, Metze D, Mehling A, Luger TA, Schwarz T, Grabbe S. Reduced UV-induced carcinogenesis in mice with a functional disruption in B7-mediated co stimulation. J. Immunol. 1999;63:6725–6731. [PubMed] [Google Scholar]
- 13.Silva RC, Rigaud J, Cheynier V, Chemina A. Procyanidin dimers and trimers from grape seeds. Phytochemistry. 1991;30:1259–1264. [Google Scholar]
- 14.Shi J, Yu J, Pohorly JE, Kakuda Y. Polyphenolics in grape seeds-biochemistry and functionality. J. Med. Food. 2003;6:291–299. doi: 10.1089/109662003772519831. [DOI] [PubMed] [Google Scholar]
- 15.Prieur C, Rigaud J, Cheynier V, Moutounet M. Oligomeric and polymeric procyanidins from grape seeds. Phytochemistry. 1994;36:781–789. [Google Scholar]
- 16.Mittal A, Elmets CA, Katiyar SK. Dietary feeding of proanthocyanidins from grape seeds prevents photocarcinogenesis in SKH-1 hairless mice: relationship to decreased fat and lipid peroxidation. Carcinogenesis. 2003;24:1379–1388. doi: 10.1093/carcin/bgg095. [DOI] [PubMed] [Google Scholar]
- 17.Sharma SD, Katiyar SK. Dietary grape-seed proanthocyanidin inhibition of ultraviolet B-induced immune suppression is associated with induction of IL-12. Carcinogenesis. 2006;27:95–102. doi: 10.1093/carcin/bgi169. [DOI] [PubMed] [Google Scholar]
- 18.Meeran SM, Mantenaand SK, Katiyar SK. Prevention of ultraviolet radiation-induced immunosuppression by (−)-epigallocatechin-3-gallate in mice is mediated through interleukin 12-dependent DNA repair. Clinical Cancer Res. 2006;12:2272–2280. doi: 10.1158/1078-0432.CCR-05-2672. [DOI] [PubMed] [Google Scholar]
- 19.Katiyar SK, Challa A, McCormick TS, Cooperand KD, Mukhtar H. Prevention of UVB-induced immunosuppression in mice by green tea polyphenol (−)-epigallocatechin-3-gallate may be associated with alterations in IL-10 and IL-12 production. Carcinogenesis. 1999;20:2117–2124. doi: 10.1093/carcin/20.11.2117. [DOI] [PubMed] [Google Scholar]
- 20.Ruzicka T, Walter JF, Printz MP. Changes in arachidonic acid metabolism in UV-irradiated hairless mouse skin. J. Invest. Dermatol. 1983;81:300–303. doi: 10.1111/1523-1747.ep12519287. [DOI] [PubMed] [Google Scholar]
- 21.Kuwamoto K, Miyauchi-Hashimoto H, Tanaka K, Eguchi N, Inui T, Urade Y, Horio T. Possible involvement of enhanced prostaglandin E2 production in the photosensitivity in xeroderma pigmentosum group A model mice. J. Invest. Dermatol. 2000;114:241–246. doi: 10.1046/j.1523-1747.2000.0883x.x. [DOI] [PubMed] [Google Scholar]
- 22.Rivas JM, Ullrich SE. The role of IL-4, IL-10, and TNF-alpha in the immune suppression induced by ultraviolet radiation. J. Leukoc. Biol. 1994;56:769–775. doi: 10.1002/jlb.56.6.769. [DOI] [PubMed] [Google Scholar]
- 23.Schwarz A, Grabbe S, Riemann H, Aragane Y, Simon M, Manon S, Andrade S, Luger TA, Zlotnik A, Schwarz T. In vivo effects of interleukin-10 on contact hypersensitivity and delayed-type hypersensitivity reactions. J. Invest. Dermatol. 1994;103:211–216. doi: 10.1111/1523-1747.ep12393073. [DOI] [PubMed] [Google Scholar]
- 24.Sharma SD, Katiyar SK SK. Dietary grape seed proanthocyanidins inhibit UVB-induced cyclooxygenase-2 expression and other inflammatory mediators in UVB-exposed skin and skin tumors of SKH-1 hairless mice. Pharm. Res. 2010;27:1092–1102. doi: 10.1007/s11095-010-0050-9. [DOI] [PubMed] [Google Scholar]
- 25.Hauser C. Cultured epidermal Langerhans cells activate effector T cells for contact sensitivity. J. Invest. Dermatol. 1990;95:436–440. doi: 10.1111/1523-1747.ep12555587. [DOI] [PubMed] [Google Scholar]
- 26.Toews GB, Bergstresser PR, Streilein JW, Sullivan S. Epidermal Langerhans cell density determines whether contact hypersensitivity or unresponsiveness follows skin painting with DNFB. J. Immunol. 1980;124:445–453. [PubMed] [Google Scholar]
- 27.Manetti R, Parronchi P, Giudizi MG, Piccinni M, Maggi E, Trinchieri G, Romagnani S. Natural killer cell stimulatory factor (interleukin-12 [IL-12]) induces T helper type 1 (Th1)-specific immune responses and inhibits the development of IL-4-producing Th cells. J. Exp. Med. 1993;177:1199–1204. doi: 10.1084/jem.177.4.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hsieh C, Macatonia SE, Tripp CS, Wolf SF, O’Garra A, Murphy KM. Development of Th1 CD4+T cells through IL-12 produced by Listeria-induced macrophages. Science. 1993;260:547–549. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- 29.Scott P. IL-12: initiation cytokine for cell-mediated immunity. Science. 1993;260:496–497. doi: 10.1126/science.8097337. [DOI] [PubMed] [Google Scholar]
- 30.Schmitt DA, Owen-Schauband L, Ullrich SE. Effect of IL-12 on immune suppression and suppressor cell induction by ultraviolet radiation. J. Immunol. 1995;154:5114–5120. [PubMed] [Google Scholar]
- 31.Schwarz A, Grabbe S, Aragane Y, Sandkuhl K, Riemann H, Luger TA, Kubin M, Trinchieriand G, Schwarz T. Interleukin-12 prevents ultraviolet B-induced local immunosuppression and overcomes UVB-induced tolerance. J. Invest. Dermatol. 1996;106:1187–1191. doi: 10.1111/1523-1747.ep12347944. [DOI] [PubMed] [Google Scholar]
- 32.Vaid M, Singh T, Li A, Katiyar N, Sharma S, Elmets CA, Xu H, Katiyar SK. Proanthocyanidins inhibit UV-induced immunosuppression through IL-12-dependent stimulation of CD8+ effector T cells and inactivation of CD4+ T cells. Cancer Prev. Res. 2010;4:238–247. doi: 10.1158/1940-6207.CAPR-10-0224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kripke ML, Cox PA, Alas LG, Yarosh DB. Pyrimidine dimers in DNA initiated systemic immunosuppression in UV-irradiated mice. Proc. Natl. Acad. Sci. USA. 1992;89:7516–7520. doi: 10.1073/pnas.89.16.7516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Applegate LA, Ley RD, Alcalay J, Kripke ML. Identification of molecular targets for the suppression of contact hypersensitivity by ultraviolet radiation. J. Exp. Med. 1989;170:1117–1131. doi: 10.1084/jem.170.4.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vink AA, Moodycliffe AM, Shreedhar V, Ullrich SE, Roza L, Yarosh DB, Kripke ML. The inhibition of antigen-presenting activity of dendritic cells resulting from UV irradiation of murine skin is restored by in vitro photorepair of cyclobutane pyrimidine dimers. Proc. Natl. Acad. Sci. USA. 1997;94:5255–5260. doi: 10.1073/pnas.94.10.5255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Katiyar SK, Vaid M, van Steeg H, Meeran SM. Green tea polyphenols prevent UV-induced immunosuppression by rapid repair of DNA damage and enhancement of nucleotide excision repair genes. Cancer Prev. Res. 2010;3:179–189. doi: 10.1158/1940-6207.CAPR-09-0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vaid M, Sharma SD, Katiyar SK. Proanthocyanidins inhibit photocarcinogenesis through enhancement of DNA repair and xeroderma pigmentosum Group A-dependent mechanism. Cancer Prev. Res. 2010;3:1621–1629. doi: 10.1158/1940-6207.CAPR-10-0137. [DOI] [PubMed] [Google Scholar]
- 38.Hauser C. Cultured epidermal Langerhans cells activate effector T cells for contact sensitivity. J. Invest.Dermatol. 1990;95:436–440. doi: 10.1111/1523-1747.ep12555587. [DOI] [PubMed] [Google Scholar]
- 39.Kondo S, Beissert S, Wang B, Fujisawa H, Kooshesh F, Stratigos A, Granstein RD, Mak TW, Sauder DN. Hyporesponsiveness in contact hypersensitivity and irritant contact dermatitis in CD4 gene targeted mouse. J. Invest. Dermatol. 1996;106:993–1000. doi: 10.1111/1523-1747.ep12338505. [DOI] [PubMed] [Google Scholar]
- 40.Anderson C, Hehr A, Robbins R, Hasan R, Athar M, Mukhtar H, Elmets CA. Metabolic requirements for induction of contact hypersensitivity to immunotoxic polyaromatic hydrocarbons. J. Immunol. 1995;155:3530–3537. [PubMed] [Google Scholar]
- 41.Gocinski BL, Tigelaar RE. Roles of CD4+ and CD8+ T cells in murine contact sensitivity revealed by in vivo monoclonal antibody depletion. J. Immunol. 1990;144:4121–4128. [PubMed] [Google Scholar]
- 42.Bour H, Peyron E, Gaucherand M, Garrigue JL, Desvignes C, Kaiserlian D, Revillard JP, Nicolas JF. Major histocompatibility complex class I-restricted CD8+ T cells and class II-restricted CD4+ T cells, respectively, mediate and regulate contact sensitivity to dinitrofluorobenzene. Eur. J.Immunol. 1995;25:3006–3010. doi: 10.1002/eji.1830251103. [DOI] [PubMed] [Google Scholar]
- 43.Xu H, DiIulio NA, Fairchild RL. T cell populations primed by hapten sensitization in contact sensitivity are distinguished by polarized patterns of cytokine production: interferon gamma-producing (Tc1) effector CD8+ T cells and interleukin (IL) 4/IL-10-producing (Th2) negative regulatory CD4+ T cells. J. Exp. Med. 1996;183:1001–1012. doi: 10.1084/jem.183.3.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cooper KD, Oberhelman L, Hamilton TA, Baadsgaard O, Terhune M, LeVee G, Anderson T, Koren H. UV exposure reduces immunization rates and promotes tolerance to epicutaneous antigens in humans: relationship to dose, CD1a-DR+ epidermal macrophage induction, and Langerhans cell depletion. Proc. Natl. Acad. Sci. USA. 1992;89:8497–8501. doi: 10.1073/pnas.89.18.8497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vink AA, Strickland FM, Bucana C, Cox PA, Roza L, Yaroshand DB, Kripke ML. Localization of DNA damage and its role in altered antigen-presenting cell function in ultraviolet-irradiated mice. J. Exp. Med. 1996;183:1491–1500. doi: 10.1084/jem.183.4.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vaid M, Singh T, Prasad R, Elmets CA, Xu H, Katiyar SK. Bioactive grape proanthocyanidins enhance immune reactivity in UV-irradiated skin through functional activation of dendritic cells in mice. Cancer Prev. Res. 2013;6:242–252. doi: 10.1158/1940-6207.CAPR-12-0320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li Z, Musich PR, Serrano MA, Dong Z, Zou Y. XPA-mediated regulation of global nucleotide excision repair by ATR is p53-dependent and occurs primarily in S-phase. PLoS ONE. 2011;6:e28326. doi: 10.1371/journal.pone.0028326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roy AM, Baliga MS, Elmets CA, Katiyar SK. Grape seed proanthocyanidins induce apoptosis through p53, Bax and caspase 3 pathways. Neoplasia. 2005;7:24–36. doi: 10.1593/neo.04412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wakkach A, Fournier N, Brun V, Breittmayer JP, Cottrez F, Groux H. Characterization of dendritic cells that induce tolerance and T regulatory 1 cell differentiation in vivo. Immunity. 2003;18:605–617. doi: 10.1016/s1074-7613(03)00113-4. [DOI] [PubMed] [Google Scholar]
- 50.Morelli AE, Thomson AW. Dendritic cells: regulators of alloimmunity and opportunities for tolerance induction. Immunological Rev. 2003;196:125–146. doi: 10.1046/j.1600-065x.2003.00079.x. [DOI] [PubMed] [Google Scholar]
- 51.Corinti S, Albanesi C, la Sala A, Pastore S, Girolomoni G. Regulatory activity of autocrine IL-10 on dendritic cell functions. J. Immunol. 2001;166:4312–4318. doi: 10.4049/jimmunol.166.7.4312. [DOI] [PubMed] [Google Scholar]
- 52.Constant SL, Bottomly K. Induction of Th1 and Th2 CD4+ T cell responses: The alternative approaches. Ann. Rev.Immunol. 1997;15:297–322. doi: 10.1146/annurev.immunol.15.1.297. [DOI] [PubMed] [Google Scholar]
- 53.Katiyar SK. Interleukin-12 and photocarcinogenesis. Toxicol. Appl. Pharmacol. 2007;224:220–227. doi: 10.1016/j.taap.2006.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schmitt DA, Owen-Schaub L, Ullrich SE. Effect of IL-12 on immune suppression and suppressor cell induction by ultraviolet radiation. J. Immunol. 1995;154:5114–5120. [PubMed] [Google Scholar]
- 55.Loser K, Apelt J, Voskort M, Mohaupt M, Balkow S, Schwarz T, Grabbe S, Beissert S. IL-10 controls ultraviolet-induced carcinogenesis in mice. J. Immunol. 2007;179:365–371. doi: 10.4049/jimmunol.179.1.365. [DOI] [PubMed] [Google Scholar]
- 56.Ullrich SE, Schmitt DA. The role of cytokines in UV-induced systemic immune suppression. J. Dermatol. Sci. 2000;23:S10–S12. doi: 10.1016/s0923-1811(99)00073-0. [DOI] [PubMed] [Google Scholar]
- 57.Ullrich SE. Mechanism involved in the systemic suppression of antigen-presenting cell function by UV irradiation. Keratinocyte-derived IL-10 modulates antigen-presenting cell function of splenic adherent cells. J. Immunol. 1994;152:3410–3416. [PubMed] [Google Scholar]