Abstract
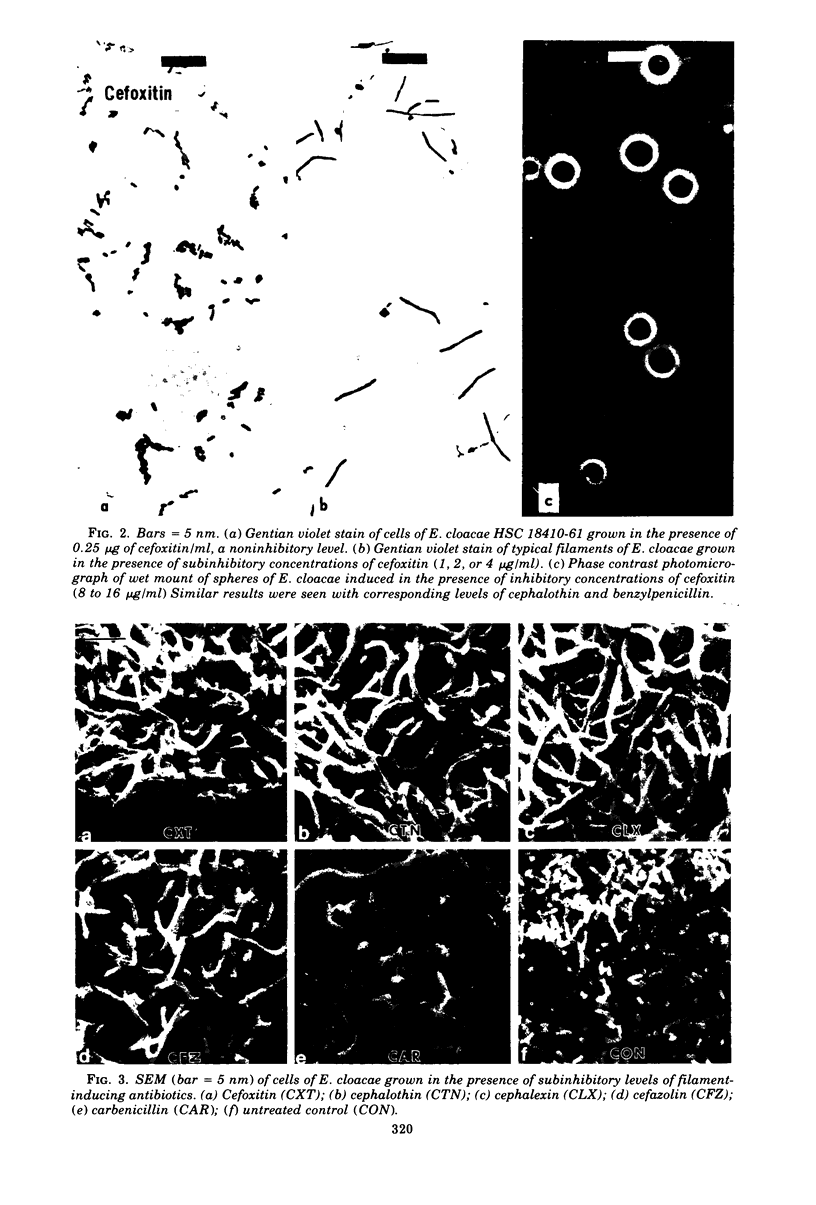
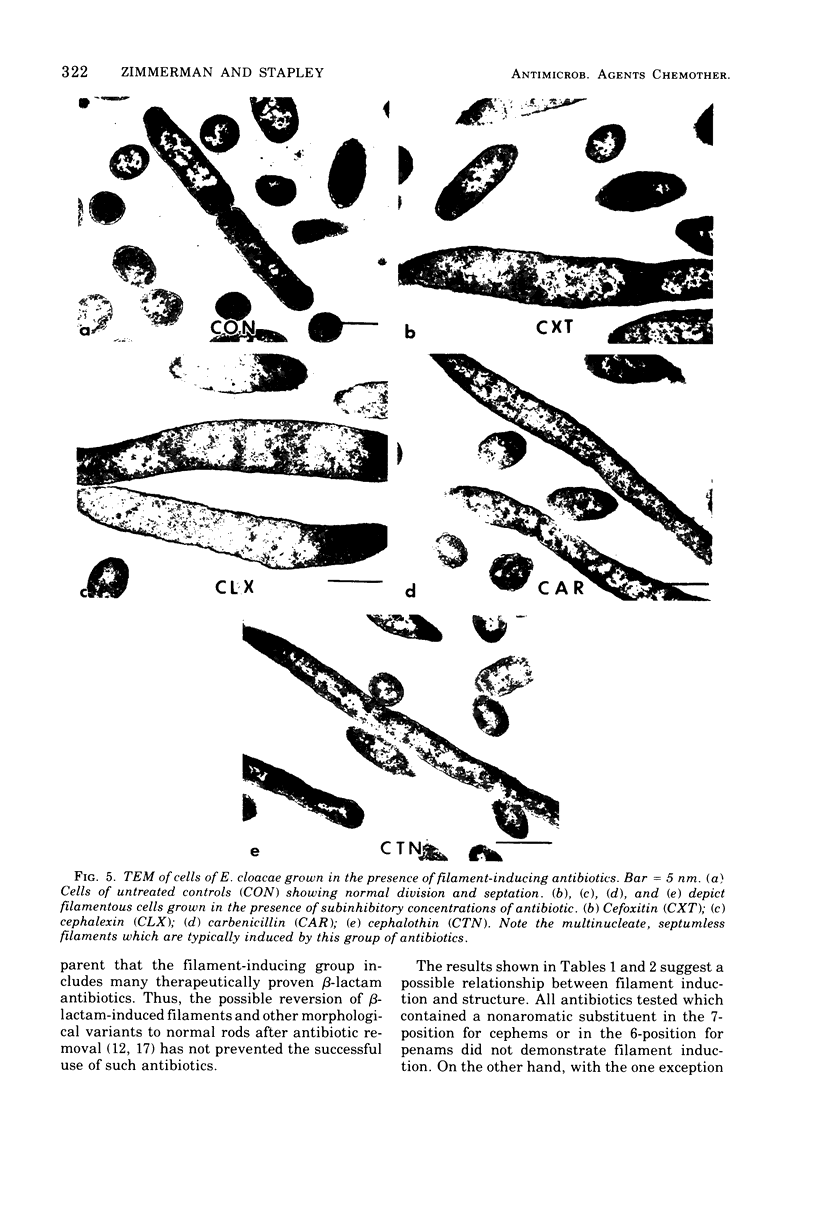
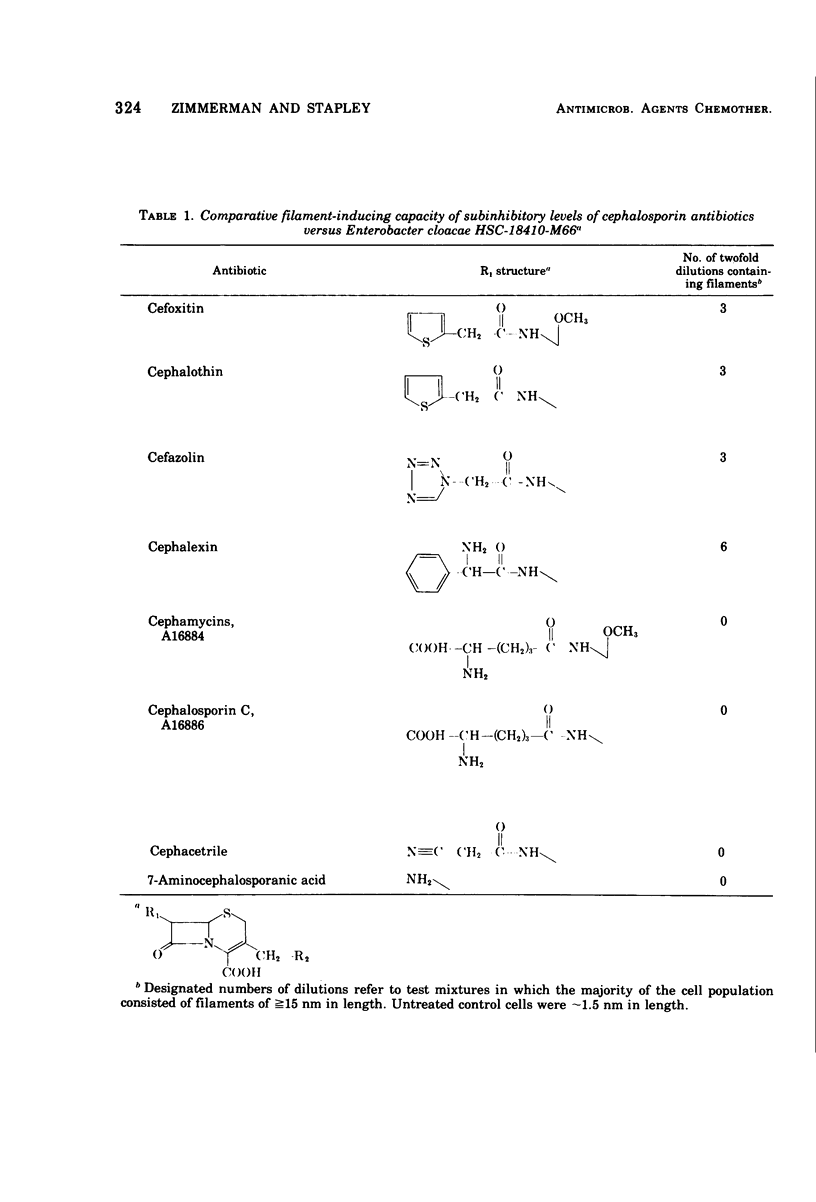
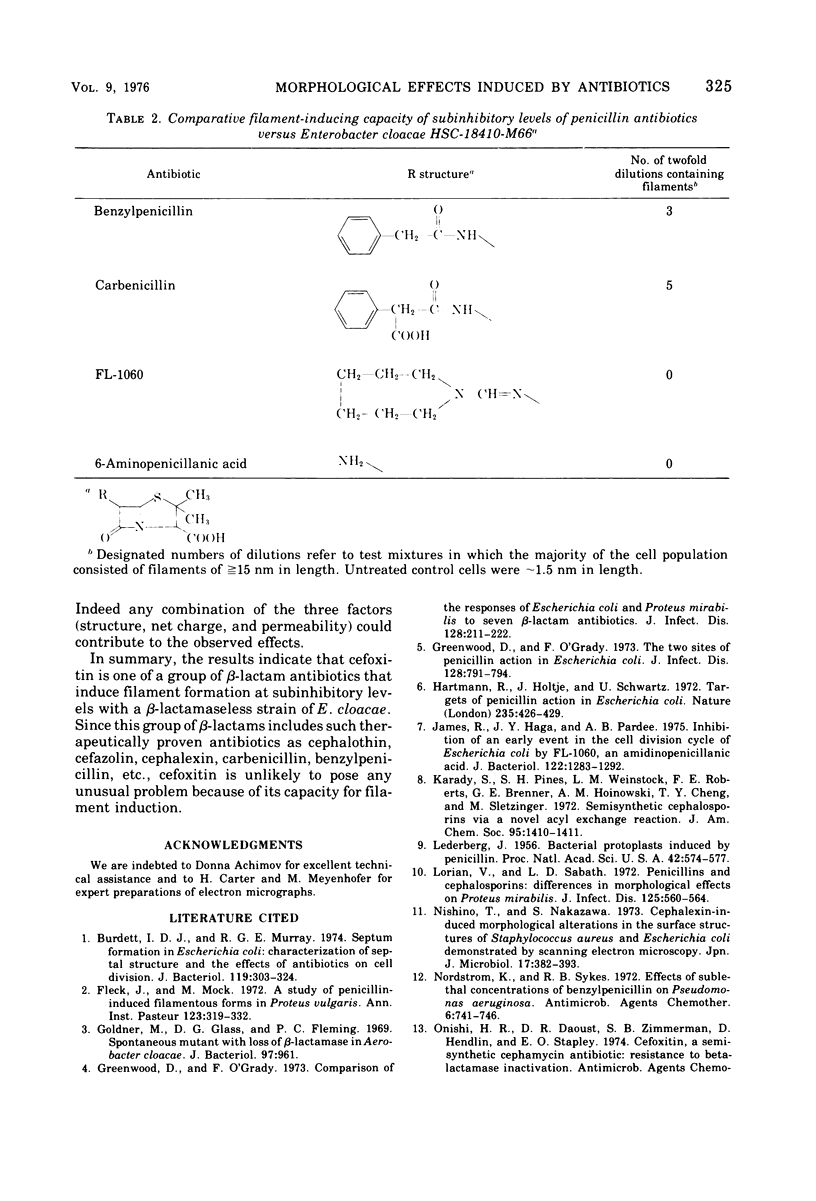
Cefoxitin, a new semisynthetic cephamycin antibiotic, induced filament formation at subinhibitory concentrations with a β-lactamaseless strain of Enterobacter cloacae (HSC 18410 M66). The extent of filament induction by cefoxitin was similar to that seen with cephalothin, cefazolin, and benzylpenicillin. Filament induction by cefoxitin was markedly less than that seen with cephalexin, carbenicillin, ticarcillin, cephradine, and cephapirin. Antibiotics which failed to induce filaments at any level tested included cephaloridine, cephacetrile, cephalosporin C, the cephamycins, 6-aminopenicillanic acid, 7-aminocephalosporanic acid, A16884, A16886, and FL-1060. Those antimicrobial agents tested which lacked an aromatic substituent in the 7-position (for cephems) or in the 6-position (for penams) did not induce filaments. These observations suggest a possible relationship between filament induction of the test organism and the molecular nature of constituents in the 7- or 6-position of β-lactams.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burdett I. D., Murray R. G. Septum formation in Escherichia coli: characterization of septal structure and the effects of antibiotics on cell division. J Bacteriol. 1974 Jul;119(1):303–324. doi: 10.1128/jb.119.1.303-324.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleck J., Mock M. Etude de la forme filamenteuse de Proteus vulgaris P 18 induite par la pénicilline. Ann Inst Pasteur (Paris) 1972 Sep;123(3):319–332. [PubMed] [Google Scholar]
- Goldner M., Glass D. G., Fleming P. C. Spontaneous mutant with loss of beta-lactamase in Aerobacter cloacae. J Bacteriol. 1969 Feb;97(2):961–961. doi: 10.1128/jb.97.2.961-.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood D., O'Grady F. Comparison of the responses of Escherichia coli and proteus mirabilis to seven beta-lactam antibodies. J Infect Dis. 1973 Aug;128(2):211–222. doi: 10.1093/infdis/128.2.211. [DOI] [PubMed] [Google Scholar]
- Greenwood D., O'Grady F. The two sites of penicillin action in Escherichia coli. J Infect Dis. 1973 Dec;128(6):791–794. doi: 10.1093/infdis/128.6.791. [DOI] [PubMed] [Google Scholar]
- Hartmann R., Höltje J. V., Schwarz U. Targets of penicillin action in Escherichia coli. Nature. 1972 Feb 25;235(5339):426–429. doi: 10.1038/235426a0. [DOI] [PubMed] [Google Scholar]
- James R., Haga J. Y., Pardee A. B. Inhibition of an early event in the cell division cycle of Escherichia coli by FL1060, an amidinopenicillanic acid. J Bacteriol. 1975 Jun;122(3):1283–1292. doi: 10.1128/jb.122.3.1283-1292.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karady S., Pines S. H., Weinstock L. M., Roberts F. E., Brenner G. S., Hoinowski A. M., Cheng T. Y., Sletzinger M. Semisynthetic cephalosporins via a novel acyl exchange reaction. J Am Chem Soc. 1972 Feb 23;94(4):1410–1411. doi: 10.1021/ja00759a090. [DOI] [PubMed] [Google Scholar]
- Lederberg J. BACTERIAL PROTOPLASTS INDUCED BY PENICILLIN. Proc Natl Acad Sci U S A. 1956 Sep;42(9):574–577. doi: 10.1073/pnas.42.9.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorain V., Sabath L. D. Penicillins and cephalosporins: differences in morphologic effects on Proteus mirabilis. J Infect Dis. 1972 May;125(5):560–564. doi: 10.1093/infdis/125.5.560. [DOI] [PubMed] [Google Scholar]
- May J. R., Roberts D. E., Ingold A., Want S. V. Osmotically stable L forms of Haemophilus influenzae and their significance in testing sensitivity to penicillins. J Clin Pathol. 1974 Jul;27(7):560–564. doi: 10.1136/jcp.27.7.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordström K., Sykes R. B. Effects of sublethal concentrations of benzylpenicillin on Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1974 Dec;6(6):741–746. doi: 10.1128/aac.6.6.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onishi H. R., Zimmerman S. B., Stapley E. O. Observations on the mode of action of cefoxitin. Ann N Y Acad Sci. 1974 May 10;235(0):406–425. doi: 10.1111/j.1749-6632.1974.tb43280.x. [DOI] [PubMed] [Google Scholar]
- Perkins R. L., Miller M. A. Scanning electron microscopy of morphological alterations in Proteus mirabilis induced by cephalosporins and semisynthetic penicillins. Can J Microbiol. 1973 Feb;19(2):251–255. doi: 10.1139/m73-038. [DOI] [PubMed] [Google Scholar]
- Prior R. B., Warner J. F. Morphological alterations of Pseudomonas aeruginosa by ticarcillin: a scanning electron microscope study. Antimicrob Agents Chemother. 1974 Dec;6(6):853–855. doi: 10.1128/aac.6.6.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell A. D. Effects of some beta-lactam antibiotics on the morphology of some beta-lactamase and non-beta-lactamase producing strains of gram negative bacteria. J Appl Bacteriol. 1973 Jun;36(2):357–359. doi: 10.1111/j.1365-2672.1973.tb04115.x. [DOI] [PubMed] [Google Scholar]
- Suginaka H., Blumberg P. M., Strominger J. L. Multiple penicillin-binding components in Bacillus subtilis, Bacillus cereus, Staphylococcus aureus, and Escherichia coli. J Biol Chem. 1972 Sep 10;247(17):5279–5288. [PubMed] [Google Scholar]