Abstract
The direct application of low power argon plasma for the decontamination of pre-formed Staphylococcus aureus biofilms on various surfaces was examined. Distinct chemical/physical properties of reactive species found in argon plasmas generated at different wattages all demonstrated very potent but very different anti-biofilm mechanisms of action. An in depth analysis of results showed that: (1) the different reactive species produced in each plasma demonstrated specific antibacterial and/or anti-biofilm activity, and 2) the commonly associated etching effect could be manipulated and even controlled, depending on experimental conditions. Under optimal experimental parameters, bacterial cells in S. aureus biofilms were killed (>99.9%) by plasmas within 10 min of exposure and no bacteria nor biofilm re-growth from argon discharge gas treated biofilms was observed for 150 h. The decontamination ability of plasmas for the treatment of biofilm related contaminations on various materials was confirmed and an entirely novel layer-by-layer decontamination approach was designed and examined.
Keywords: argon plasma, decontamination, biofouling, layer-by-layer, Staphylococcus aureus, biofilms
Introduction
Staphylococci have a notable ability to adhere and subsequently colonize different surfaces with varying chemical properties. Subsequently, the attached cells not only reproduce, but also are involved in the recruitment of additional planktonic cells for surface colonization (Brooun 2000; Lynch and Abbanat 2010). This colonization and recruitment process assists in the development of mature, thick, multi-layered, and complex biofilms (Peters et al. 1982; Stoodley et al. 2002). It is these well-organized communities, i.e. biofilms, that facilitate the pathogenicity of these bacteria and serve as reservoirs for the development of pathogenic infections (von Eiff C et al. 2002; Stewart 2002; Vertes et al. 2012).
Biofilm communities are encapsulated in an extracellular polymeric matrix (EPM), composed of exopolysaccharides, and a minute quantity of proteins, minerals, metals, and nucleic acids. The EPM plays various roles in both the structure and function of biofilm communities (Lewis 2008). More specifically, the formation of the EPM provides considerable advantages, such as protection against antimicrobial agents, acquisition of new genetic traits, nutrient availability, and metabolic cooperability (Cos et al. 2010).
In response to the resistant nature and infectious attributes of biofilms, gas discharge plasma has been used and investigated for the eradication of planktonic bacteria and spores in various industrial and medical applications. Although plasmas have been proven effective against a wide range of free-living microorganisms and even spores, there are only a few reports pertaining to the use of plasmas for biofilm inactivation (Walsh et. al. 2008; Moreau et al. 2000; Traba and Liang 2011).
Gas discharge plasmas are generated by supplying energy in many different forms e.g. electrical, optical, or thermal, to a neutral gas, causing the formation of reactive species by means of excitation and ionization. Plasma results from the energy transfer from a source, usually an electric discharge, to the surrounding gas. Some of the gas molecules are raised from their energy ground state to an excited one, with a modified electron distribution. The electrons and ions are then accelerated by the electric field and through collisions with atoms and molecules within the neutral gas, an enhanced level of dissociation, excitation, and ionization is accomplished, creating new reactive species, including ultraviolet photons, along with charged particles (Laroussi and Leipoil 2004). When all of the aforementioned processes occur, a build up of charged particles, which is eventually balanced by charge carrier loss develops and steady-state plasma is achieved (Pelletier 1992; Laroussi 2002).
The argon plasma used in this study was a cool plasma generated by electrical discharge. Cool or “non-thermal plasmas” are generated when the electron temperature is much higher than the other ions or molecules whose temperature is relatively low, 40–100° C (Stoffels et. al. 2002). The attractive features of cold plasma decontaminations are (1) short treatment times, (2) efficient and effective antibacterial activity and 3) minimal temperature elevation, confirming that reactive species produced in the plasma are solely responsible for bacterial/biofilm inactivation. Such plasmas are used as an alternative for the sterilization of medical devices as well as heat-sensitive biomaterials, including biological matter (tissues and organs) (Laroussi. 2005). Plasmas have also been used for teeth whitening and cavity decontaminations (Sun et al 2010). Therefore, this technology is clean and safe for both the patient and the operator (Moisan et. al. 2002)
With the success of plasma decontaminations, many groups have focused on high power (>20k W) plasma devices, along with various combinations and mixtures of gases to completely destroy biofilms (Lerouge et. al. 2001; Min et. al 2003; Hino et. al. 2004). In these traditional plasma decontamination studies, great detail regarding the ability of plasmas to decontaminate surfaces is conveyed, however, without any concern for the mechanism in which this is accomplished. Such studies do not allow for the careful mechanistic dissections of the anti-biofilm activity of plasma. It is believed that discharge gas simply damages the chemical composition of the extracellular matrix of the biofilm, resulting in the release of bacteria from the biofilm. Therefore, the inactivation of bacterial biofilms by means of non-thermal and low power gas discharge plasmas is still relatively novel, with many unanswered questions.
The results discussed in this study begin to unravel the mechanisms of action of argon plasmas, generated under specific and controlled experimental conditions. These results provide evidence for the successful manipulation low-power plasmas in the decontamination of S. aureus biofilm contaminated materials. Plasma-mediated inactivation of bacteria in biofilms has been reported in recent publications (Conrads and Schmidt 2000; Zelaya et. al. 2012; Moisan et. al. 2001).
Materials and methods
Bacterial strains and medium
Staphylococcus aureus (methicillin resistant, ATCC 29213), a good biofilm forming S. aureus strain, was purchased from the American Type Culture Collection (ATCC, Manassas, VA). S. aureus cells were grown in tryptic soy broth (TSB) supplemented with 0.2% glucose (TSBG). For each experiment, a single bacterial colony was isolated from a tryptic soy agar plate, transferred to 10–15 ml of medium, and then incubated under orbital agitation (100–150 rpm) at 37 °C for 18–24 h.
Reagents and solutions
A LIVE/DEAD staining kit was purchased from Invitrogen Life Technologies (Carlsbad, CA) for staining bacteria within biofilms. Also, 5% 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT), in phosphate buffered saline (PBS), crystal violet (CV), ethylene bromine (EB), sodium dodecyl sulfate (SDS), and other reagents were all purchased from the Sigma Chemical Laboratory (St Louis, MO).
Growth of biofilms on different materials
For each experiment, an isolated single bacterial colony was picked from an agar plate, transferred to 10–15 ml of TSBG medium and then incubated under orbital agitation (100–150 rpm) at 37 °C for 18–24 h. This overnight culture of S. aureus was diluted in TSBG to 2×106 cells ml−1 and inoculated on the surfaces of different materials including 8-well glass chambers, polyethylene terephthalate films, polystyrene 6-well plates, and silicon wafers. S. aureus biofilms 16~20 μm in thickness were formed on all tested materials within 24 h. At the end of incubation, the biofilms were washed with PBS to remove planktonic and loosely attached bacteria.
Biofilm assays
A widely used CV staining method in combination with the MTT based viability assay was used to assess biofilm susceptibility to discharge gases. Unlike CV staining, which is used for staining bacterial cells (both live and dead) and other macromolecules such as polysaccharides, DNA, and proteins in the extracellular matrix of the biofilm, the MTT assay was designed for live bacteria by measuring the overall metabolic activity of bacterial cells in biofilms. Thus, CV staining was used for the quantification of biofilms (total biomass of biofilm) while MTT assay and EB staining were utilized to evaluate the viability of bacteria in biofilms and DNA/polysaccharides in the extracellular matrix of the biofilm.
In CV staining, biofilms were stained with 0.1% (w/v) CV for 10 min. The excess dye was removed by thoroughly rinsing the plate with water. CV dye associated with biofilms was then extracted by 33% glacial acetic acid and quantified using a microplate reader by measuring solution absorbance values at 570 nm.
In the MTT assay, biofilms were incubated with MTT at 37 °C for 10 min. After washing, the purple formazan formed inside the bacterial cells was dissolved with sodium dodecyl sulfate and then measured using a microplate reader by setting the detecting and reference wavelengths at 570 nm and 630 nm, respectively (Traba and Liang 2011).
Generation of gas discharge plasma
Discharges were generated using the Plasma Prep III device (SPI Supplies, AC 110 W) with a frequency of 13.56 MHz as described previously (Traba and Liang 2011). Bottled oxygen, nitrogen, and argon were purchased from Praxair (Keasbey, NJ) and were prepared by Cryogenic Air separation which led to a purity of >99.9%. Preformed biofilm samples were placed 8 cm away from the gas inlet and not grounded (Fig. 1). The system was first evacuated to 40 Pa at a fixed gas flow rate of 2.4 ft3 h−1. During the treatment process, discharge powers were controlled in the range between 0 and 100 W to generated plasma. The chamber pressure was maintained at 460 mTorr with a temperature of about 40 – 60°C. Due to the manufacturing settings the plasma chamber was vacuumed and therefore isolated from the environment, resulting in no or very minimal plasma chamber contamination.
Figure 1.
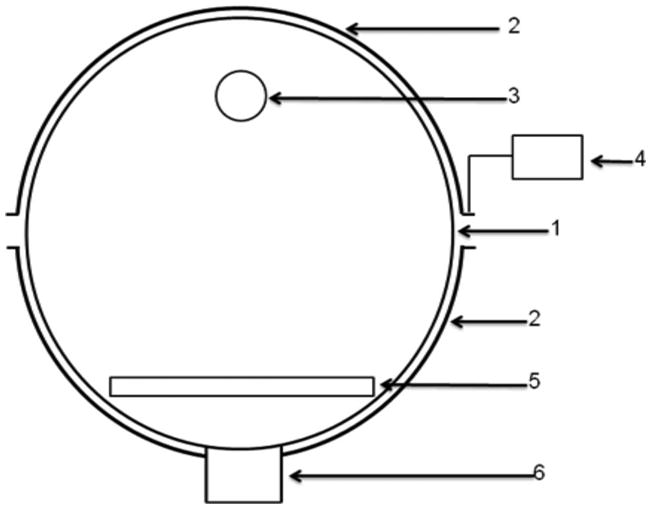
Schematic diagram of the reaction chamber. (1) Quartz reaction chamber; (2) semi-tubular electrode; (3) gas outlet; (4) RF power supply; (5) sample holder; (6) vacuum connection. The distance between the gas outlet and the sample holder is 8 cm.
Staining of live and dead bacteria in biofilms
Live and dead bacterial distributions in biofilms were studied by confocal laser scanning microscopy (CLSM) using a LIVE/DEAD staining kit as described previously (Traba et. al 2013). Biofilms grown on LabTek 8-well cover-glass chambers were washed with PBS to remove planktonic bacteria and TSBG medium. LIVE/DEAD dyes in water were then added and incubated for 15 min at room temperature. Stained live (green) and dead (red) bacteria in biofilms were visualized by CLSM according to the protocol provided by the manufacturer.
Biofilm re-growth experiments
One-day old S. aureus biofilms were treated by gas discharge plasma for the indicated time. Bacteria re-growth was conducted under different experimental conditions: (1) plasma treated biofilms were fed with fresh TSBG medium and (2) plasma treated biofilms were subjected to sonication treatment after plasma exposure with a Branson 5510 Ultrasonic Cleaner (Thomas Scientific). This treatment was conducted at 37° C for 10 min to release bacteria from biofilms the (Traba and Liang 2013). Biofilms were then fed with fresh TSBG medium containing 10% glucose, in an attempt to stimulate biofilm growth. All re-growth experiments were done at 37° C for the indicated time. Bacterial growth was measured for 150 h by monitoring absorbance changes at 570 nm in the supernatant, bacteria within the biofilm (MTT), and the biofilm (CV).
Atomic force microscopy
AFM measurements of treated biofilms on silicon wafers were performed in air at room temperature using a NSCRIPTOR dip pen nanolithography system, Nanoink. The instrument was operated in the ac (tapping) mode using P-MAN-SICC-0 AFM cantilevers (Pacific Nanotechnology, Inc.) with a nominal force constant of 40 N m−1.
Scanning electron microscopy
SEM images were obtained using an Auriga scanning electron microscope. Silicon wafers containing adhered S. aureus cells were treated with argon plasma under specific experimental conditions. These samples were attached to the SEM stage using conductive tape and coated with gold for 30 s. The applied voltage was 2 kV.
Results and discussion
Anti-biofilm activity of low-power argon plasma
The distinct chemical and physical properties of reactive species formed in argon plasma under different powers (0–100 W) can be observed through two significantly different assays: (1) viability (MTT) and (2) biomass (CV). Similar to high plasma power (>20k W) argon plasmas, the reactive species produced in the Plasma Prep III, a device capable of sustaining low power (0–100 W) argon plasmas, can cause severe cell membrane destruction with respect to planktonic bacterial cells (Traba and Liang 2011). In this study, argon plasma (0–100 W) was tested against mature one-day old biofilms from S. aureus ATCC 29213. S. aureus biofilms had normal architectural structures with proven resistance to antibiotic treatment and thicknesses of between 16 – 20 μm when cultured for 24 h in TSBG culture medium (Kharidia and Liang 2011). In the biofilms grown throughout the study, individual bacteril cells were protected and attached to one another by EPM, which is secreted by the bacteria and indicated by red arrows (Fig. 2). In the present study, 6-well plates were used in all assays, unless otherwise indicated. The purpose of using 6-well plates was to ensure complete uniformity along with reproducibility of the data, subsequently reducing standard deviations.
Figure 2.
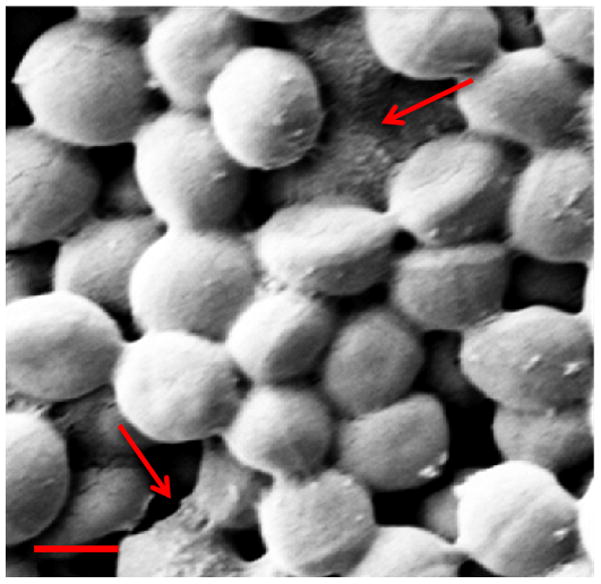
SEM image of mature one-day old S. aureus biofilms. Scale bar = 1 μm.
Up until 60 W of power for a treatment time of 10 min, the anti-biofilm activity of argon plasma was not effective, as indicated by no significant decrease in viability studies (Fig. 3). The minimal electrical power required to achieve excellent anti-biofilm activity of argon discharge gas for 10 min of treatment to preformed biofilms was at and above 80 watts (Fig. 3). Interestingly, at this electrical power (80 W), moderate biomass loss was achieved. Based on the results obtained, experiments were conducted in order to expand on the findings and to test the effectiveness and efficiency of argon discharge plasma mediated biofilm inactivation at low powers (80 – 100 W). Argon plasma generated at 100 W was excluded due to the significant and early etching of the biofilms (data not shown). Although argon plasma generated under different powers (80–90 W) all showed good antibacterial activity (Fig. 4), each plasma demonstrated its own biofilm inactivation mechanism.
Figure 3.
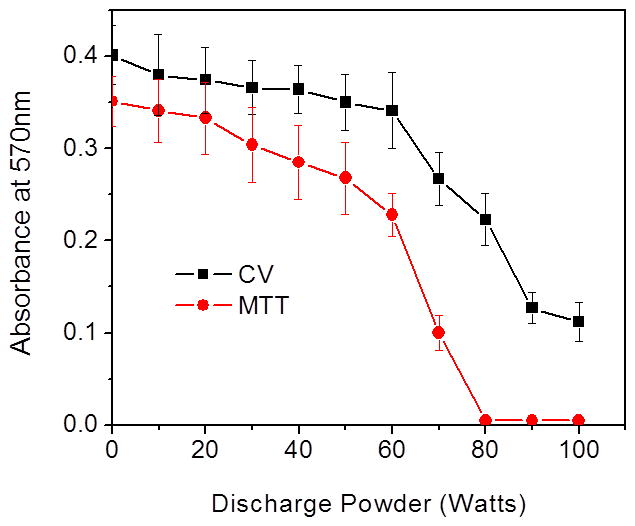
Discharge gases caused bacterial death and biomass loss in 1-day old S. aureus biofilms grown on 6-well plates. Bacterial viability and total biomass in biofilms were determined by MTT and CV staining assays, respectively. Experimental conditions: discharge gas: argon; exposure time: 10 min; power: 0–100 W. Bacterial numbers and biomass loss in biofilms were quantified by measuring absorbance changes in biofilms at OD570 nm after treatment. The data represent the means and SDs of at least three samples.
Figure 4.
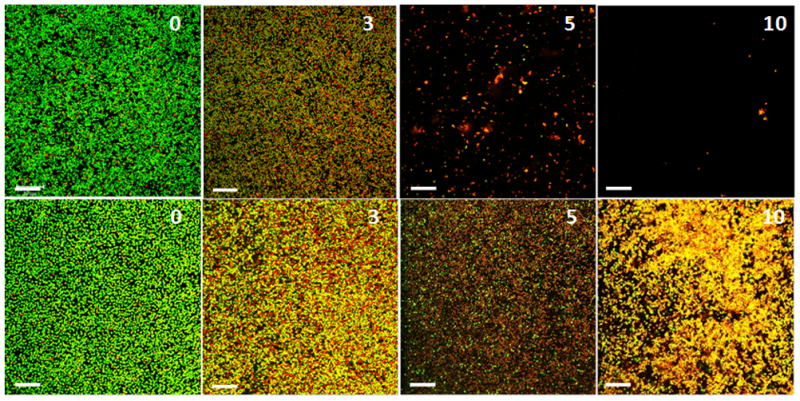
Fluorescence CLSM images of plasma mediated biofilm inactivation and removal of S. aureus biofilms grown on PET films. Top panels, argon plasma generated at 90 W and treated for the times indicated (min) on each image. Bottom panels, argon plasma generated at 80 W and treated for the times indicated (min) on each image. Biofilms were stained with Live/Dead staining kit. Live bacteria are stained green and dead bacteria are stained red, respectively. Scale bars = 20 μm
Mechanistic differences of argon plasmas generated at different powers
It has been reported that exposing various electrical powers to plasmas significantly changes the antibacterial/anti-biofilm activity of the plasma by altering the types of species generated (Traba and Liang 2013). It is likely that synergistic effects among the active agents within the chamber result in plasma being a more effective sterilization method (Perni et. al. 2007). In previous studies, involving the use of argon plasmas at low powers, the anti-biofilm activity of plasmas was maintained while minimizing the etching effect normally associated with plasma decontaminations (Traba et. al. 2013). Interestingly, when the antibacterial activity of the argon plasmas generated at 80 and 90 W was compared, both argon plasmas were capable of inducing complete bacterial death (>99.9) after treatment for 10 min (Fig. 5A).
Figure 5.
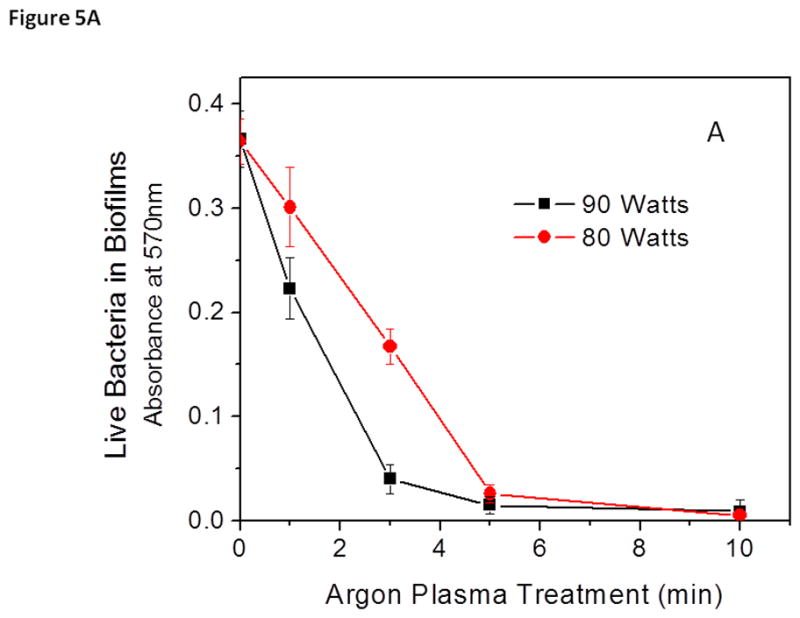
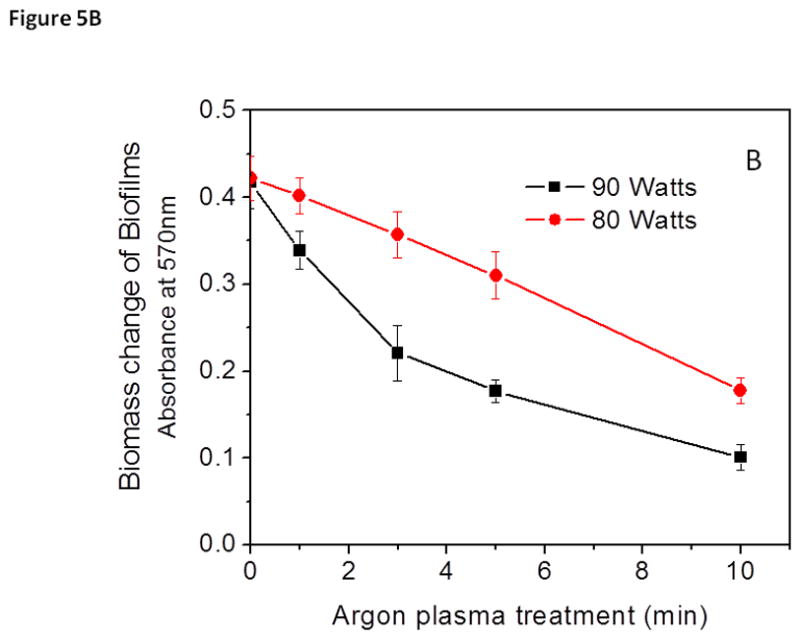
Discharge gases generated at 80 W (black) and 90 watts (red) caused bacterial death (A) and biomass loss (B) in 1-day old S. aureus biofilms. Bacterial viability and total biomass in biofilms were determined by MTT and CV staining assays, respectively. Experimental conditions: discharge gas: argon; exposure time: 0–10 min. The data represent the means and SDs of at least three samples.
Unlike the MTT results, the CV staining results showed that two completely different mechanisms governed the antibacterial/anti-biofilm activity of the discharge gases when generated at electric powers of 80 and 90 watts. The antibacterial activity of argon plasma at 80 W occurred gradually, after treatment for about 1 min (Fig. 5A). Once a significant amount of the bacteria in biofilms were killed in situ, steady removal/etching of biomass shortly followed the 80-W of argon mediated biofilm inactivation (Fig. 5B). It should be stated that although argon plasma at 80 W caused the removal of biofilms from the attached surface, the etching effect of argon plasma at 90 W was much more violent. Argon plasma generated at 90 W removed biofilm debris much more quickly and in a much more energetic manner during the 10-min treatment period (Fig. 4). Early signs of etching are noticeable after treatment for only 1 min at 90 W of argon plasma, which is consistent with plasmas generated at extreme powers (Fig. 5B). The etching of biofilms when treated with argon plasma at 80 W did not begin until the later parts of the treatment. From these results, it can be stated that argon plasma generated at 80 W demonstrated more antibacterial (bacterial lysis) activity, while the results for argon plasma at 90 W demonstrated severe etching activity, with respect to mature 1-day old S. aureus biofilms. Removal of bacteria, along with the EPM of the biofilm during treatments with argon discharge gas at 90 W resulted in the substantial decrease in viability observed in Figure 5A, as well as the significant decrease in CV absorbance (Fig. 5B).
As stated above, the mechanism of action of both argon plasmas generated at 80 and 90 W were distinct towards bacteria within biofilms. Live/Dead Z-stack CLSM images allowed visualization of these decontamination mechanisms at specific time points, subsequently aiding in understanding the antibacterial processes. In the earlier stages of biofilm treatment with argon plasma generated at 80 W, there was a gradual viability decline, which was associated with the inability of the reactive species to penetrate and travel freely throughout the biofilm. After this initial exposure, the reactive species began to compromise and eventually damage the integrity of the biofilm, exposing the bacteria to the reactive species, which then resulted in cell membrane damage and cell lysis (Fig. 6). This initial treatment of bacteria to the argon plasma at 80 W, was followed by the ability of the reactive species to essentially remove the top layer of the biofilm. Z-stack confocal images (Fig. 6) confirm that discharge gas caused damage to the protective coating/EPM, which in turn compromised the architecture of the biofilm, resulting in the exposure of bacteria at the top of the biofilms to the discharge gas. Prolonged exposure subsequently led to bacterial and EPM removal starting at the top of the biofilm and gradually working downwards.
Figure 6.
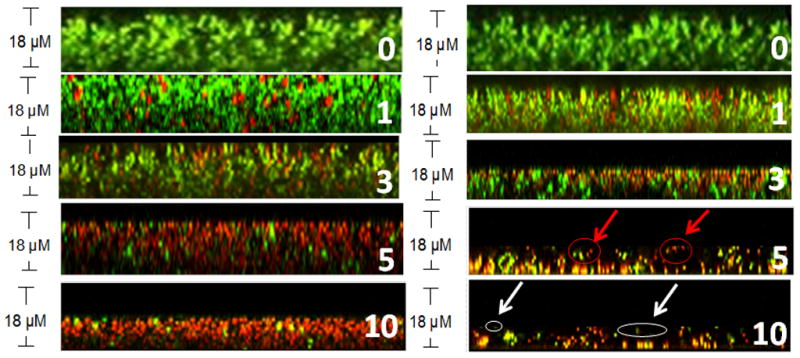
Z-Stack CLSM images of 1-day old S. aureus biofilms stained using the Live/Dead staining kit. Power: 80 W (left) and 90 W (right). The numbers in images indicate the exposure time in min.
Unlike argon discharge gas generated at 80 W, argon discharge gas at 90 W resulted in a substantial decrease in CV staining absorbance. This decrease was observed during the early stages of treatment (Fig. 5B). The diffusion and penetration of the reactive species into the biofilm caused significant biofilm erosion, eventually causing severe damage to the architecture of the biofilm, resulting in a substantial decrease in viability (Fig. 5A). Prolonged discharge gas treatment led to the chemical break down of the EPM (Fig. 6). Treatments at 90 W led to the formation of loosely attached bacterial clusters with severely damaged biofilm architectures, indicated by red circles in Figure 6. Once the integrity of the biofilm architecture was severely damaged, the EPM became unable to protect the live and dead bacteria from continuous bombardment by the reactive species, which led to the detachment of the biofilm from the substratum. These results indicate that the antibacterial activity of argon discharge gas at 90 W was primarily based on the etching effect of bacteria from the biofilm.
Layer-by-layer activity of argon plasma
In plasma, it is considered that the chemical behaviors of a gas are an important factor in determining the process feature. The results from this study indicate that argon plasma, when generated under different experimental conditions, has its own unique reactive species. This was concluded based on the different anti-biofilm mechanisms exhibited by each argon plasma. It is quite obvious that the mechanism of action with respect to either killing or etching effect is dependent on the (1) chemical composition of reactive species, (2) the concentration of the reactive species, and (3) the details of plasma generation.
As stated above, argon discharge gas has distinct mechanisms of action, which are dependent on the discharge power. Z-stack CLSM images were used to validate both the MTT and the CV staining results. At lower powers (80 W), Z-stack CLSM images of untreated 1-day old S. aureus biofilms (control groups) indicate a thickness of ~ 18 μm (Fig. 6). Control groups (0 min) were filled with live bacteria (stained green). After argon plasma treatments (80 W), gradual and methodical thickness reductions in the same biofilm were observed in a layer-by-layer approach until >99.9% of bacterial death was achieved (indicated by low MTT absorbance and a red color in CLSM images). More specifically, after treatment for 1 min the antibacterial ability of argon plasma at 80 W was observed coinciding with very little etching/biomass removal. However, in argon plasma treated groups (90 W), the etching effect was immediately present, resulting in biofilm thickness of 14–16 μm. As treatment times were increased to 3 min, biofilms treated with 80 W of argon plasma were reduced to 16–18 μm. The thickness of biofilms grown under the same conditions, when treated with argon plasma at 90 W for 3- min, were reduced to 8–10 μm, demonstrating the aggressive and early etching ability associated with argon plasma at >90 W. As treatment times were increased (5 min), a layer-by-layer anti-biofilm activity of argon plasma generated at 80 W began, starting at the top of the biofilm. Treated biofilm samples had a thickness of 14~16 μm. On the other hand, many differences were observed with argon plasma at 90 W. Although a significant number of bacteria were dead after treatment for 5 min, large chunks of the biofilm were removed from the surface. After 10 min of argon plasma (80 W), bacteria in the biofilms were completely destroyed. In order to achieve complete bacterial death, biofilm thicknesses were reduced to 9–11 μm. Upon completion of bacterial inactivation, no further treatment of S. aureus biofilms with 80 W was clearly needed, therefore treatments were stopped. On the other hand, prolonged exposure times (10 min) of argon plasma at 90 W led to biofilm thicknesses of 4–7 μm. Argon plasmas at 90 W effectively “chopped up” the biofilm and resulted in biofilm areas free of bacteria, indicated by white circles in Fig. 6.
Significant differences between antibacterial effects and the etching effects of reactive species
Under different experimental conditions, substantial changes in the chemical properties of the reactive species generated in argon plasma were achieved. In order to fully understand the effects of low power argon plasma on biofilms, treated biofilms were analyzed using Atomic Force Microscopy (AFM). AFM images of untreated biofilms depict round and circular bacteria, protected by an EPM (Fig. 7A). However, AFM images of the same biofilm treated with argon at 80 W for 10 min indicate that the bacteria still in the biofilm have lost their cell membrane integrity (round/circular shape). AFM images of treated samples show reductions in biofilm thickness, along with bacteria with deformed cell membranes (Fig. 7B). Loss of cell membrane integrity led to cell lysis and eventually bacterial death. Argon plasma at 90 W completely destroyed the bacteria, leaving no remnants of the bacterial cells. Plasma treatments also resulted in severely damaged the biofilm architecture through the significant etching of live and dead bacteria, along with biofilm debris. Samples treated under the same experimental conditions and analyzed under CLSM depicted significant reductions in biofilm thickness (Fig. 7C). The results indicate that argon plasma (80 W) mediated inactivation of biofilms is essentially a more gentle mechanism of action that argon at 90 W. AFM images show that argon discharge gas under different experimental conditions, form a variety of reactive species, which is demonstrated by the differences in bacterial shape after argon plasma treatment. Therefore, reactive species influenced either the antibacterial or etching effects associated with argon plasma mediated biofilm decontamination.
Figure 7.
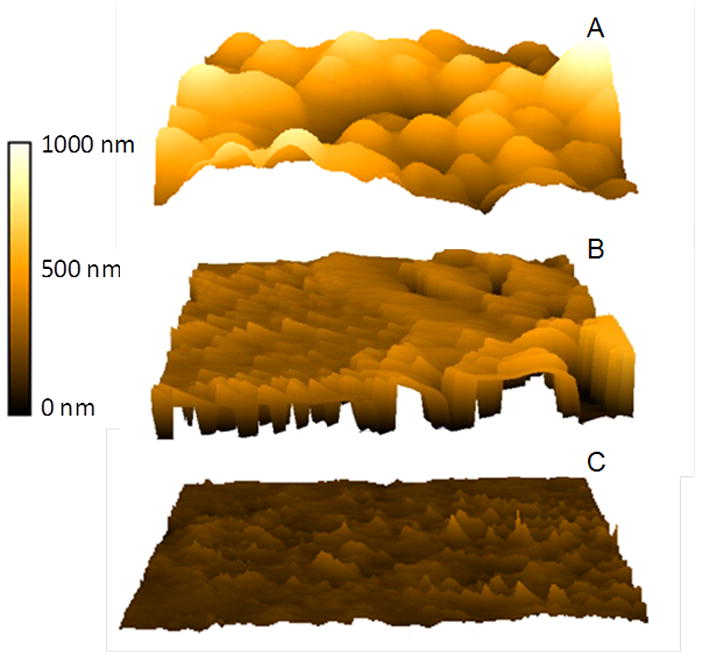
Typical AFM images of S. aureus biofilms (A) before; (B) after argon plasma (80 W) treatment for 10 min; (C) after argon plasma (90 W) treatment for 10 min. Scan distance = 5μm.
Efficiency and effectiveness of low power argon plasma decontamination
Decontamination by means of gas discharge plasma has proven to be a very efficient and effective approach to the eradication of biofilms (Traba and Liang 2011; Traba et. al. 2013). However, the question arises whether or not discharge gases at lower powers are capable of sustaining an infection free surface for long periods of times after treatment. This is because a single bacterium, if not killed during treatment, has the ability and will eventually re-form another biofilm, resulting in the re-contamination of a surface. In this study, although MTT assays indicated >99.9% bacterial death, Z-stack CLSM images showed the presence of “live” (green) bacteria in biofilms treated under optimal conditions. It was imperative to determine whether or not these “live” bacteria were capable of reproducing and ultimately re-forming the biofilm.
In order to evaluate the effectiveness of our low power argon treatment, a bacteria and biofilm re-growth experiment was conducted on treated biofilms. In these experiments, biofilms were treated with argon plasma under the specified experimental conditions as described above. After treatment, biofilms were not washed but directly fed with fresh TSBG. This TSBG contained an additional 10 g of glucose. Along with the TSB containing 10 g of glucose, both control as well as treated biofilm groups were sonicated in order to help release bacteria deep within the biofilm and subsequently stimulate bacterial growth.
Treated biofilms were incubated for an additional 150 h and bacterial growth from treated biofilms were measured by monitoring the bacterial concentration increase in the biofilm (Fig. 8A). Biofilm regrowth was estimated by measuring biomass changes in biofilms using CV staining (Fig. 8B). Untreated S. aureus biofilms continued to grow rapidly after 24 h of the extended incubation and the total biomass of biofilms increased exponentially thereafter, indicating that the sonication process did not jeopardize the viability of the bacteria. On the contrary, no bacterial or biofilm re-growth was observed in S. aureus biofilms treated with the discharge gas (80 W) for 10 min even after an extended incubation period of 150 h. These findings are consistent with the results obtained from the MTT assays (Fig. 5A) and confirm the effectiveness of low power discharge argon in biofilm inactivation after treatment for 10 min.
Figure 8.
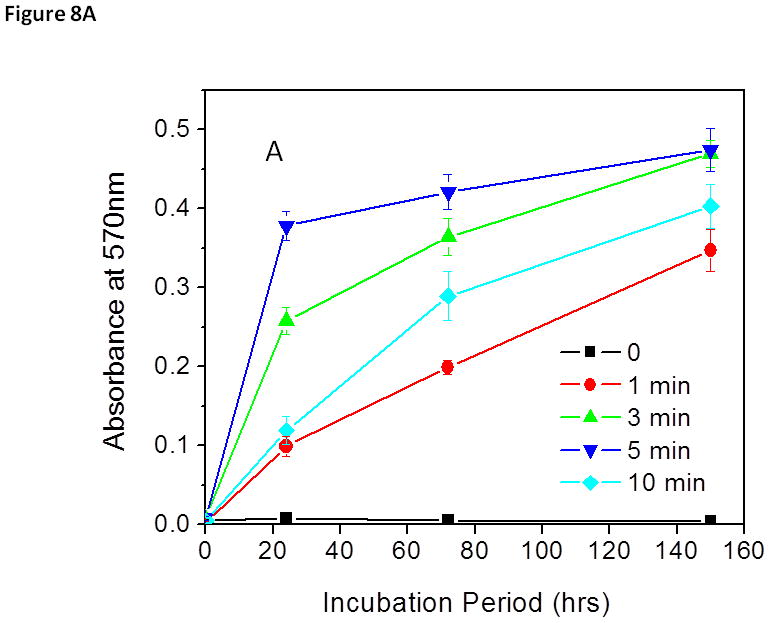
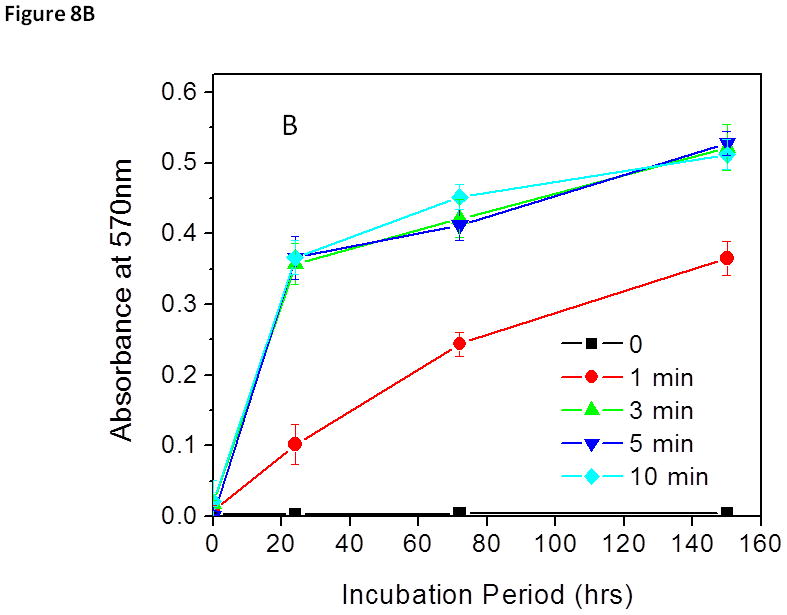
Biofilm (A) and S. aureus (B) re-growth from discharge argon treated and sonicated biofilms. Experimental conditions: power: 80 W; exposure time: 0–10 min. After treatment, biofilms were fed with fresh TSBG medium and incubated for an additional 150 h. Biofilm biomass was measured by CV staining while bacterial re-growth was quantified by measuring absorbance changes in the treated biofilm at OD570. The data represent the means and SDs of at least three samples
The question that needs to be addressed therefore is, why were there live bacteria present in all CLSM images, when MTT, along with re-growth assays showed complete bacterial death? The answer is interesting. There was a competitive binding issue between propidium iodide and SYTO 9 dyes (the nucleic acid probes found in the Live/Dead staining kit) on bacteria with severely destroyed cell membranes. These severely damaged cell membranes allowed SYTO 9 dye to stain the membrane, but prevented propidium iodide from entering the already stained bacterial membrane. These results were unexpected because SYTO 9 is only permeable to healthy cell membranes and thus bacteria with intact (live) cell membranes are stained green, while propidium iodide, a nucleic acid probe with a red fluorescence color, is cell membrane impermeable and thus only stains dead bacteria with damaged cell membranes.
Conclusions
Although the effects of discharge gas on biofilms have been reported, very few have speculated on the mechanism of action of low power argon plasmas. This study has confirmed the anti-biofilm ability of another decontamination approach using low power argon plasma on S. aureus biofilms, in which antibiotic resistance has developed. A better understanding of the mechanisms of action of an inert gas was achieved by manipulating the formation of reactive species. The MTT and CV staining results showed that different radio frequency powers influence the chemical composition, reactivity, concentration, and antibacterial activity of the species in each discharge gas. Previous experiments confirm that different reactive species can be generated under different experiment parameters for the same gas. Changes in the chemical composition of the reactive species in argon plasma significantly influenced the different killing and etching mechanisms seen at different powers. These findings have major implications for the ways in which plasmas can be generated and manipulated for argon plasma mediated biofilm inactivation. As a result, a layer-by-layer approach was devised and examined in this study. The possibility of isolating and distinguishing between the reactive species in the discharge gas generated at different electric outputs will be explored, analyzed, and linked to a certain antibacterial function.
Acknowledgments
This work was supported by NIH grant AI110924. Mr Traba is a recipient of the Robert Crooks Stanley Pre-doctoral Fellowship.
References
- Brooun A. A dose-response study of antibiotic resistance in Pseudomonas aeruginosa biofilms. Antimicrob Agents Chemother. 2000;44:640–646. doi: 10.1128/aac.44.3.640-646.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrads H, Schmidt M. Plasma generation and plasma source. Plasma Sources Sci Technol. 2000;9:441–454. [Google Scholar]
- Cos P, Toté K, Horemans T, Maes L. Biofilms: an extra hurdle for effective antimicrobial therapy. Curr Pharm Des. 2010;16:2279–2295. doi: 10.2174/138161210791792868. [DOI] [PubMed] [Google Scholar]
- Hino T, Yamauchi Y, Ono J, Hirohata Y. Enhancement of reactive species density in nitrogen plasma by mixture of helium and nitridation experiment for silicon. Vacuum. 2004;74:467–471. [Google Scholar]
- Kharidia R, Liang JF. The activity of a small lytic peptide PTP- 7 on Staphylococcus aureus biofilms. J Microbiol. 2011;49:663–668. doi: 10.1007/s12275-011-1013-5. [DOI] [PubMed] [Google Scholar]
- Laroussi M. Low temperature plasma-based sterilization: overview and state-of-the-art. Plasma Process Polym. 2005;2:391–400. [Google Scholar]
- Laroussi M. Nonthermal decontamination of biological media by atmospheric-pressure plasmas: review, analysis, and prospects. IEEE Trans Plasma Sci. 2002;30:1409–1415. [Google Scholar]
- Laroussi M, Leipold F. Evaluation of the roles of reactive species, heat, and UV radiation in the inactivation of bacterial cells by air plasmas at atmospheric pressure. Int J Mass Spectrom. 2004;223:81–86. [Google Scholar]
- Lerouge S, Wertheimer MR, Yahia LH. Plasma sterilization: a review of parameters, mechanisms, and limitations. Plasmas Polym. 2001;6:175–188. [Google Scholar]
- Lewis K. Multidrug tolerance of biofilms and persister cells. Curr Top Microbiol Immunol. 2008;322:107–131. doi: 10.1007/978-3-540-75418-3_6. [DOI] [PubMed] [Google Scholar]
- Lynch AS, Abbanat D. New antibiotic agents and approaches to treat biofilm-associated infections. Expert Opin Ther Pat. 2010;20:1373–1387. doi: 10.1517/13543776.2010.505923. [DOI] [PubMed] [Google Scholar]
- Min J, Lee CW, Gu MB. Gamma-radiation dose-rate effects on DNA damage and toxicity in bacterial cells. Radiat Environ Biophys. 2003;3:189–192. doi: 10.1007/s00411-003-0205-8. [DOI] [PubMed] [Google Scholar]
- Moisan M, Barbeau J, Crevier MC, Pelletier J, Philip N, Saoudi B. Plasma sterilization. 2002. Methods and mechanisms. Pure Appl Chem. 74:349–358. [Google Scholar]
- Moreau S, Moisan M, Barbeau J, Pelletier J, Ricard A. Using the flowing afterglow of a plasma to inactivate bacillus subtilis spores: Influence of the operating conditions. J Appl Phys. 2000;88:1166–1174. [Google Scholar]
- Moisan M, Barbeau J, Moreau S, Pelletier J, Tabrizian M, Yahia LH. Low-temperature sterilization using gas plasmas: a review of the experiments and an analysis of the inactivation mechanisms. Int J Pharm. 2001;226:1/2, 1–21. doi: 10.1016/s0378-5173(01)00752-9. [DOI] [PubMed] [Google Scholar]
- Pelletier J. Sterilization by the plasma procedure. Agressologie. 1992;33:105–110. [PubMed] [Google Scholar]
- Perni S, Shama G, Hobman JL, Lund PA, Kershaw CJ, Hidalgo-Arroyo GA, Penn CW, Deng XT, Walsh JL, Kong MG. Probing bactericidal mechanisms induced by cold atmospheric plasmas with Escherichia coli mutants. Appl Phys Lett. 2007;90:0739e02. [Google Scholar]
- Peters G, Locci R, Pulverer G. Adherence and growth of coagulase-negative staphylococci on surfaces of intravenous catheters. J Infect Dis. 1982;146:479–482. doi: 10.1093/infdis/146.4.479. [DOI] [PubMed] [Google Scholar]
- Stewart PS. Mechanisms of antibiotic resistance in bacterial biofilms. Int J Med Microbiol. 2002;292:107–113. doi: 10.1078/1438-4221-00196. [DOI] [PubMed] [Google Scholar]
- Stoffels E, Flikweert AJ, Stoffels WW, Kroesen GMW. Plasma needle: a non-destructive atmospheric plasma source for fine surface treatment of (bio) materials. Plasma Sources Sci T. 2002;11:383–388. [Google Scholar]
- Stoodley P, Sauer K, Davies DG, Costerton JW. Biofilms as complex differentiated communities. Annu Rev Microbiol. 2002;56:187–209. doi: 10.1146/annurev.micro.56.012302.160705. [DOI] [PubMed] [Google Scholar]
- Sun P, Pan J, Tian Y, Bai N, Wu H, Wang L, Yu C, Zhang J, Zhu W, Becker KH, Fang J. Tooth whitening with hydrogen peroxide assisted by a direct-currect cold atmospheric-pressure air plasma microjet. Plasma Sience, IEEE Transactions. 2010;38:1892–1896. [Google Scholar]
- Traba C, Liang JF. Susceptibility of Staphylococcus aureus biofilms to reactive discharge gases. Biofouling. 2011;27:763–772. doi: 10.1080/08927014.2011.602188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traba C, Chen L, Azzam R, Liang JF. Insights into discharge argon mediated inactivation of biofilms. Biofouling. 2013;29:1205–13. doi: 10.1080/08927014.2013.832222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traba C, Chen L, Liang JF. Low power gas discharge plasma mediated inactivation and removal of biofilms formed on biomaterials. Current Applied Physics. 2013;13:S12–S18. doi: 10.1016/j.cap.2012.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertes A, Hitchins V, Phillips SK. Analytical challenges of microbial biofilms on medical devices. Anal Chem. 2012;84:3858–3866. doi: 10.1021/ac2029997. [DOI] [PubMed] [Google Scholar]
- von Eiff C, Peters G, Heilmann C. Pathogenesis of infections due to coagulase-negative staphylococci. Lancet Infect Dis. 2002;2:677–685. doi: 10.1016/s1473-3099(02)00438-3. [DOI] [PubMed] [Google Scholar]
- Walsh JL, Iza F, Kong MG. Atmospheric glow discharges from the high-frequency to very high-frequency bands. Appl Phys Lett. 2008;93:2515–2552. [Google Scholar]
- Zelaya A, Vandervoort K, Brelles-Mariño G. NATO Science for Peace and Security Series. 2012. Battling bacterial biofilms with gas discharge plasma; pp. 135–148. [Google Scholar]


