Abstract
Purpose
the aim of the present study was to evaluate the clinical outcome of the treatment of osteochondritis dissecans (OCD) of the knee with a type-I collagen-hydroxyapatite nanostructural biomimetic osteochondral scaffold.
Methods
twenty-three patients affected by symptomatic knee OCD of the femoral condyles, grade 3 or 4 of the International Cartilage Repair Society (ICRS) scale, underwent biomimetic scaffold implantation. The site of the defect was the medial femoral condyle in 14 patients, whereas in 9 patients the lateral femoral condyle was involved. The average size of the defects was 3.5±1.43 cm2. All patients were clinically evaluated using the ICRS subjective score, the IKDC objective score, the EQ-VAS and the Tegner Activity Score. Minimum follow-up was two years. MRI was performed at 12 and 24 months after surgery and then every 12 months thereafter.
Results
the ICRS subjective score improved from the baseline value of 50.93±20.6 to 76.44±18.03 at the 12 months (p<0.0005) and 82.23± 17.36 at the two-year follow-up (p<0.0005). The IKDC objective score confirmed the results. The EQ-VAS showed a significant improvement from 3.15±1.09 to 8.15±1.04 (p<0.0005) at two years of follow-up. The Tegner Activity Score improvement was statistically significant (p<0.0005).
Conclusions
biomimetic scaffold implantation was a good procedure for treating grade 3 and 4 OCD, in which other classic techniques are burdened by different limitations. This open one-step surgery gave promising stable results at short-term follow-up.
Level of evidence
Level IV, therapeutic case series.
Keywords: cartilage, knee, osteochondral lesions, ostechondritis dissecans, scaffold
Introduction
Chondral and osteochondral lesions are relatively common and are often caused by traumatic injury (1). In the past 20 years, there have been many studies dealing with cartilage restoration and, even though the quality of restored cartilage is now debated, a series of satisfying results have been reported in the literature (2–13). The treatment becomes increasingly challenging in the presence of larger defects and when there is involvement of the subchondral bone (8,14–18). Osteochondritis dissecans (OCD) is an acquired lesion of the subchondral bone that may result in separation and instability of the overlying articular cartilage (19,20). Among the multiple causes hypothesized, repetitive microtrauma correlated with a possible vascular insufficiency is the most credited theory (19). Whereas juvenile patients with small or stable lesions have a much better prognosis and the potential for healing by non-operative management, unstable lesions or OCD in skeletally mature individuals often do not respond to non-operative measures and require surgical intervention (19).
In large lesions of this kind, the use of autologous osteochondral transplantation and mosaic plasty is limited by donor site availability (21). Autologous chondrocyte implantation (ACI), on the other hand, is not limited by the size of the lesion and has been used successfully in the treatment of osteochondral lesions, adding the autogenous bone implant before the implantation of autologous chondrocytes (14,15, 22–24). However, the technique involves two surgical procedures, performed several months apart, which implies long functional recovery times. Efforts to overcome these limitations have led to increasing use, with excellent results, of fresh allograft transplantation (25, 26). However, the management of this procedure is not straight forward and sometimes impossible, due to high costs, lack of donors and, in particular, the need to implant the allograft within 14 days.
To overcome these limitations, a cellular biphasic or triphasic scaffolds have recently been developed with the aim of promoting and inducing both subchondral bone and cartilage regeneration (16, 17, 27–32). Currently, only a few scaffolds have obtained the European Conformity (CE) certification and are available for clinical application in Europe (16, 17, 28, 29, 33, 34).
The aim of the present study was to evaluate the clinical outcome of the treatment of OCD of the knee with a type-I collagen-hydroxyapatite nanostructural biomimetic osteochondral scaffold.
Methods
Twenty-three consecutive patients affected by symptomatic knee OCD of the femoral condyles, grade 3 or 4 of the International Cartilage Repair Society (ICRS) scale, underwent implantation of a biomimetic ostechondral scaffold (MaioRegen; Fin-Ceramica Faenza SpA, Faenza, Italy) between 2009 and 2011 at three different centers highly specialized in the treatment of knee cartilage disorders.
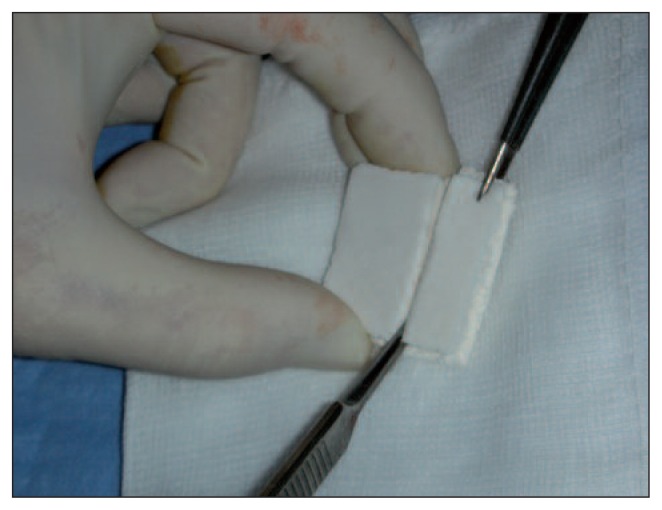
MaioRegen is a three-dimensional matrix that mimics osteocartilaginous tissue. The scaffold consists of hydroxyapatite nanocrystals nucleated on type 1 collagen fibers present, in different concentrations, in three layers: the first layer consists entirely of type I collagen and has a smooth surface; the intermediate layer, which is thicker, is a combination of type I collagen (60%) and hydroxyapatite (40%); the third layer, which is thinner, consists of a mineralized blend of type I collagen (30%) and hydroxyapatite (70%) and it mimics the subchondral bone (Fig. 1).
Fig. 1.
MaioRegen scaffold.
All the patients gave their informed consent prior to their inclusion in the study, which had ethics committee approval and was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its subsequent amendments. Exclusion criteria were: non-corrected axial malalignment (considered as above 5° from the normal axis), evaluated clinically and via radiographic examination, and the presence of co-morbidities such as: infectious, neoplastic, metabolic, and systemic inflammatory disorders.
The patients included 19 men and 4 women, with a mean age of 25.5±7.7 years. The site of the defects was the medial femoral condyle in 14 patients, whereas in 9 patients the lateral femoral condyle was involved. The average size of the defects was 3.5±1.43 cm2.
Surgical procedure
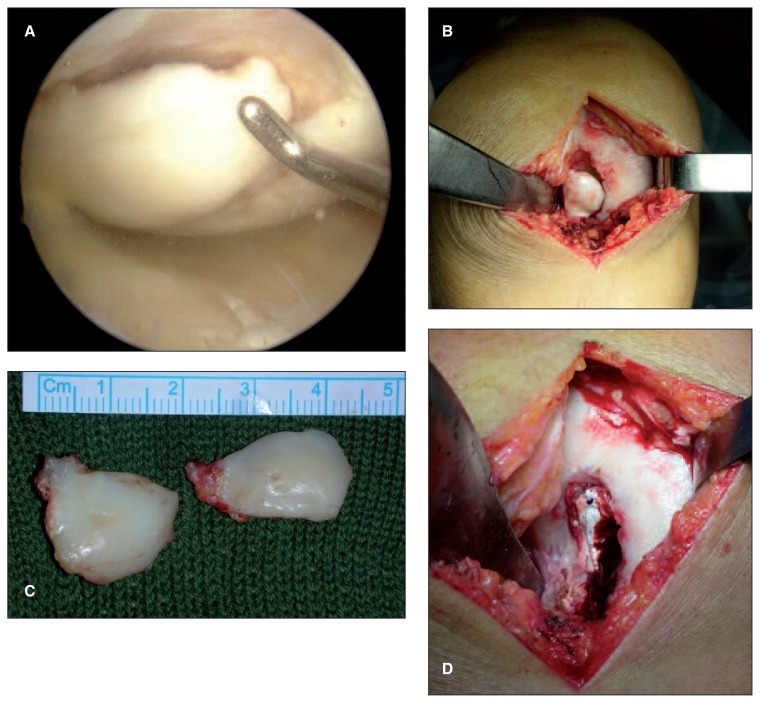
The surgery was performed under spinal or limb anesthesia. A tourniquet was positioned on the thigh with the patient in a supine position. After an arthroscopic evaluation of the knee, the lesion was exposed through a lateral or medial parapatellar approach and was prepared with a drill specific for this purpose. The sclerotic subchondral bone was eliminated and an area approximately 9 mm in depth with a stable base was created to house the scaffold, which was then implanted press fit into the area (Fig. 2). The stability of the transplant was tested by cyclic flexion-extension of the knee while the graft was visualized, both before and after tourniquet removal. On the second postoperative day, mobilization of the knee was started. Early isometric and isotonic exercises and controlled mechanical compression were performed. Muscular voluntary contraction and neuromuscular electrical stimulation were prescribed and could be started at patient discharge. In the third or fourth week, weight touchdown with crutches was allowed, and the patient could then move progressively toward full weight-bearing, which was achieved within six to eight weeks of the surgery.
Fig. 2.
Surgical technique of MaioRegen implantation. A: Arthroscopic appearance of an osteochondritis dissecans of the medial femoral condyle. B: The lesion is exposed through a medial parapatellar incision. C: Resected osteochondral fragment. D: MaioRegen scaffold implanted in the osteochondral defect.
Outcome measurement
The clinical outcome in all the patients was assessed using the ICRS subjective score and the International Knee Documentation Committee (IKDC) score at 12 and at 24 months after the operation, and every 12 months thereafter.
Improvement in quality of life was measured using the EQ-VAS score and resumption of sporting activity using the Tegner Activity Score. The patients underwent magnetic resonance imaging (MRI) preoperatively, at 12 and at 24 months after the operation, and every 12 months thereafter.
Statistical analysis
All the statistical analyses were carried out using the SPSS (Statistical Package of Social Sciences, Chicago, IL, USA) for Windows software program version 13.0. A p value of less than 0.05 was considered statistically significant. The results were expressed as mean values ± SD. The Kolmogorov-Smirnov test was performed to assess the normal data distribution. The paired T test for normally distributed data, or the Wilcoxon test for non-normally distributed data, were used to test for significant differences between the baseline and various follow-up measurements.
Results
No patients were lost at follow-up. Statistically significant increases were recorded in all the assessment scores at the 12-month and 24-month follow-ups. In two cases the treatment failed, one on the lateral femoral condyle and one on the medial femoral condyle, with these patients continuing to experience pain and functional limitation of the joint.
Improvements were recorded in the ICRS subjective score, which increased from the baseline value of 50.93±20.6 to 76.44±18.03 at the 12-month follow-up (p<0.0005) and 82.23±17.36 at the 2-year follow-up.
Before surgery, more than 50% of the patients perceived abnormal or very abnormal knee sensations (IKDC objective C and D grade), due to moderate or severe swelling of the knee.
Assessment of the patients at the first follow-up (one year after surgery) revealed an improvement, and at two years the following scores were recorded: A, 11 patients; B, 7 patients; C, 2 patients, D, only 1 patient.
The EQ-VAS score also showed a significant increase, from 3.15±1.09 to 8.15±1.04 (p<0.0005) at the 2-year follow-up, with satisfaction recorded in 85% of the patients.
The Tegner Activity Score showed a significant increase (from 2.34±0.64 to 5.60±1.72 at the two-year follow-up), even though it did not reach the pre-injury level (6.04±1.89).
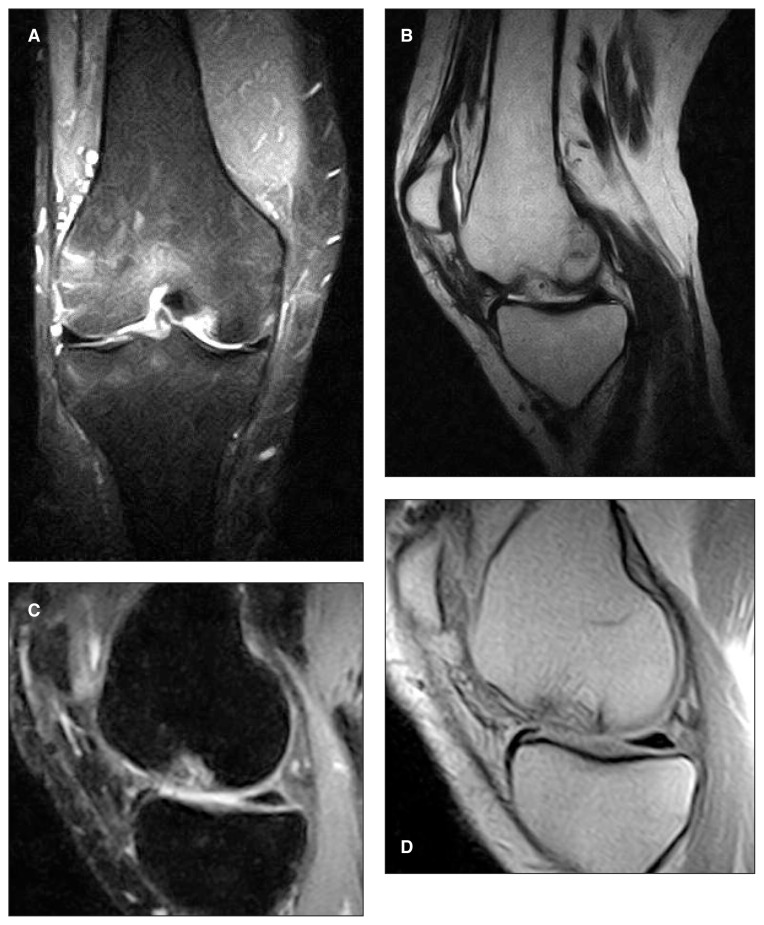
High-resolution MRI scans were performed in all the patients and showed complete filling of the defect in 80% of the lesions; in 70% of cases the repair tissue was isointense in relation to the adjacent cartilage. In all cases, the scaffold was still detectable at the level of the subchondral bone at the two-year follow, producing a different signal with respect to the adjacent subchondral bone.
Nevertheless, no case showed signs of subchondral bone impairment (such as subchondral edema) (Fig. 3).
Fig. 3.
Postoperative imaging of MaioRegen implantation. A–B: MRI follow-up at one year. It is possible to note the scaffold in situ and the ongoing differentiation process. C–D: MRI follow-up at two years. The scaffold is still recognizable at subchondral level, but the cartilage layer appears continuous and the subchondral bone shows no signs of edema.
Discussion
The present study suggests that MaioRegen collagen-hydroxyapatite osteochondral scaffold can be used to treat knee OCD with good clinical results at 2-year follow-up.
Osteochondral lesions are particularly difficult to treat as they involve two types of tissue that show different biomechanical features and healing capacity (19, 20). One of the approaches proposed for the treatment for deep osteochondral defects of the knee is staged open or arthroscopic bone grafting followed by ACI 4–9 months later (10, 11). The clinical outcome of such a procedure has not yet been reported; however, the need for three surgical procedures after the diagnosis poses a significant burden to the patient.
Minas and Peterson (24) and Peterson et al. (35, 36) have advocated the ACI “sandwich technique” as an alternative. Unfortunately, the outcome has been reported in only 7 patients. With the advent of the so-called “second-generation” ACI to overcome the limitations and the problems related to the “first-generation” ACI, a two-step procedure was proposed by different authors for the treatment for osteochondral lesions (22, 23). Bartlett et al. (2), Ochs et al. (12) and recently Filardo et al. (7) analyzed the clinical outcome in patients affected by OCD with the use of second-generation ACI associated with bone grafting harvested from different site (iliac crest, tibial cancellous bone), obtaining satisfactory results.
Despite the good results reported in the literature, these techniques present a number of problems: first of all, the bone grafting site (proximal tibia, iliac crest) is a source of post-operative pain and morbidity, which are directly proportional to the surgical exposure; in addition, the need for two surgical steps makes for longer recovery times.
In the United States, a different treatment option has been developed; fresh osteochondral allografting has been used to treat large osteochondral lesions of the knee (post-traumatic, vascular or idiopathic) and given excellent results in cases of OCD of the femoral condyle (25,26). However, this technique is burdened by high costs and difficult management due to the need to implant the graft within 14 days. For these reasons, many authors have turned their attention to the use of biphasic biomaterials with the capacity to promote joint surface regeneration by exploiting the differentiation (induced by the properties of the scaffold itself) of bone marrow stem cells (31, 32). Various experimental studies, both in vitro and in vivo, have been published (27,30), but in our experience only two scaffolds are available in clinical practice for the treatment of osteochondral lesions. The first is a biopolymercomposite of poly (D,L-lactide-co-glycol-ide), polyglycolic acid and calcium sulfate (TruFit; Smith&Nephew, Andover, Ma). The results obtained following implantation of this osteochondral substitute are discordant (33,34).
The second is MaioRegen. This has given promising results in animals and patients (16–18, 20, 28, 29). Our study confirmed these results. The ICRS subjective score was found to be significantly increased, both at the 12-month and the 24-month follow-up. The Tegner Activity Score increased significantly and almost reached the pre-injury level. We had 13 patients who were athletes before symptom onset and 11 of them returned to the previous level of sports activity between one and two years after the operation. In conclusion, the MaioRegen biomimetic scaffold is an off-the-shelf, cell-free and cost-effective implant that can regenerate cartilage and osteochondral defects. Its implantation is a one-step procedure that can be used for difficult pathologies such as ostechondral lesions and OCD, in which other regenerative treatments have proved to be burdened by several limitations. However, the results are certainly to be confirmed at longer term follow-up.
References
- 1.Hjelle K, Solheim E, Strand T, Muri R, Brittberg M. Articular cartilage defects in 1,000 knee arthroscopies. Arthroscopy. 2002;18:730–734. doi: 10.1053/jars.2002.32839. [DOI] [PubMed] [Google Scholar]
- 2.Bartlett W, Gooding CR, Carrington RW, Skinner JA, Briggs TW, Bentley G. Autologous chondrocyte implantation at the knee using a bilayer collagen membrane with bone graft. A preliminary report. J Bone Joint Surg Br. 2005;87:330–332. doi: 10.1302/0301-620x.87b3.15552. [DOI] [PubMed] [Google Scholar]
- 3.Benthien JP, Behrens P. The treatment of chondral and osteochondral defects of the knee with autologous matrix-induced chondrogenesis (AMIC): method description and recent developments. Knee Surg Sports Traumatol Arthrosc. 2011;19:1316–1319. doi: 10.1007/s00167-010-1356-1. [DOI] [PubMed] [Google Scholar]
- 4.Buda R, Vannini F, Cavallo M, Grigolo B, Cenacchi A, Giannini S. Osteochondral lesions of the knee: a new one step repair technique with bone marrow derived cells. J Bone Joint Surg Am. 2010;92( Suppl 2):2–11. doi: 10.2106/JBJS.J.00813. [DOI] [PubMed] [Google Scholar]
- 5.Dhollander AA, Verdonk PC, Lambrecht S, et al. Midterm results of the treatment of cartilage defects in the knee using alginate beads containing human mature allogenic chondrocytes. Am J Sports Med. 2012;40:75–82. doi: 10.1177/0363546511423013. [DOI] [PubMed] [Google Scholar]
- 6.Dhollander AM, Verdonk P, Lambrecht S, et al. Short-term outcome of the second generation characterized chondrocyte implantation for the treatment of cartilage lesions in the knee. Knee Surg Sports Traumatol Arthrosc. 2012;20:1118–1127. doi: 10.1007/s00167-011-1759-7. [DOI] [PubMed] [Google Scholar]
- 7.Filardo G, Kon E, Berruto M, et al. Arthroscopic second generation autologous chondrocytes implantation associated with bone grafting for the treatment of knee osteochondritis dissecans: results at 6 years. Knee. 2012;19:658–663. doi: 10.1016/j.knee.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 8.Filardo G, Kon E, Roffi A, Di Martino A, Marcacci M. Scaffold-based repair for cartilage healing: a systematic review and technical note. Arthroscopy. 2013;29:174–186. doi: 10.1016/j.arthro.2012.05.891. [DOI] [PubMed] [Google Scholar]
- 9.Gillogly SD, Myers TH. Treatment of full-thickness chondral defects with autologous chondrocyte implantation. Orthop Clin North Am. 2005;36:433–446. doi: 10.1016/j.ocl.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 10.Martin I, Miot S, Barbero A, Jakob M, Wend D. Osteochondral tissue engineering. J Biomech. 2007;40:750–765. doi: 10.1016/j.jbiomech.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 11.Nesic D, Whiteside R, Brittberg M, Wendt D, Martin I, Mainil-Varlet P. Cartilage tissue engineering for degenerative joint disease. Adv Drug Deliv Rev. 2006;58:300–322. doi: 10.1016/j.addr.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 12.Ochs BG, Müller-Horvat C, Albrecht D, et al. Remodeling of articular cartilage and subchondral bone after bone grafting and matrix-associated autologous chondrocyte implantation for osteochondritis dissecans of the knee. Am J Sports Med. 2011;39:764–773. doi: 10.1177/0363546510388896. [DOI] [PubMed] [Google Scholar]
- 13.Pallante AL, Bae WC, Chen AC, Görtz S, Bugbee WD, Sah RL. Chondrocyte viability is higher after prolonged storage at 37° C than at 4° C for osteochondral grafts. Am J Sports Med. 2009;37( Suppl 1):24S–32S. doi: 10.1177/0363546509351496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gomoll AH, Filardo G, Kon E, et al. Surgical treatment for early osteoarthritis. Part I: cartilage repair procedures. Knee Surg Sports Tramatol Arthrosc. 2012;20:450–466. doi: 10.1007/s00167-011-1780-x. [DOI] [PubMed] [Google Scholar]
- 15.Gomoll AH, Filardo G, Marcacci M, et al. Surgical treatment for early osteoarthritis. Part II: allografts and cuncurrent procedures. Knee Surg Sports Tramatol Arthrosc. 2012;20:468–486. doi: 10.1007/s00167-011-1714-7. [DOI] [PubMed] [Google Scholar]
- 16.Kon E, Delcogliano M, Filardo G, Busacca M, Di Martino A, Marcacci M. Novel composite multilayered biomaterial for osteochondral regeneration: a pilot clinical trial. Am J Sports Med. 2011;39:1180–1190. doi: 10.1177/0363546510392711. [DOI] [PubMed] [Google Scholar]
- 17.Kon E, Delcogliano M, Filardo G, et al. A novel nano-composite multi-layered biomaterial for treatment of osteochondral lesions: technique note and an early stability pilot clinical trial. Injury. 2010;41:693–701. doi: 10.1016/j.injury.2009.11.014. [DOI] [PubMed] [Google Scholar]
- 18.Marcacci M, Zaffagnini S, Kon E, et al. Unicompartmental osteoarthritis: an integrated biomechanical and biological approach as alternative to metal resurfacing. Knee Surg Sports Traumatol Arthrosc. 2013 doi: 10.1007/s00167-013-2388-0. [DOI] [PubMed] [Google Scholar]
- 19.Filardo G, Kon E, Di Martino A, Busacca M, Altadonna G, Marcacci M. Treatment of knee osteochondritis dissecans with a cell-free biomimetic osteochondral scaffold: clinical and imaging evaluation at 2-year follow-up. Am J Sports Med. 2013;41:1786–1793. doi: 10.1177/0363546513490658. [DOI] [PubMed] [Google Scholar]
- 20.Kon E, Vannini F, Buda R, et al. How to treat osteochondritis dissecans of the knee: surgical techniques and new trends: AAOS exhibit selection. J Bone Joint Surg Am. 2012;94:e1(1–8). doi: 10.2106/JBJS.K.00748. [DOI] [PubMed] [Google Scholar]
- 21.Hangody L, Dobos J, Baló E, Pánics G, Hangody LR, Berkes I. Clinical experiences with autologous osteochondral mosaic-plasty in an athletic population: a 17-year prospective multi-center study. Am J Sports Med. 2010;38:1125–1133. doi: 10.1177/0363546509360405. [DOI] [PubMed] [Google Scholar]
- 22.Könst YE, Benink RJ, Veldstra R, van der Krieke TJ, Helder MN, van Royen BJ. Treatment of severe osteochondral defects of the knee by combined autologous bone grafting and autologous chondrocyte implantation using fibrin gel. Knee Surg Sports Traumatol Arthrosc. 2012;20:2263–2269. doi: 10.1007/s00167-012-1891-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krishnan SP, Skinner JA, Carrington RW, Flanagan AM, Briggs TW, Bentley G. Collagen-covered autologous chondrocyte implantation for soteochondritis dissecans of the knee: two-to seven-year results. J Bone Joint Surg Br. 2006;88:203–205. doi: 10.1302/0301-620X.88B2.17009. [DOI] [PubMed] [Google Scholar]
- 24.Minas T, Peterson L. Advanced techniques in autologous chondrocyte transplantation. Clin Sports Med. 1999;18:13–44. v–vi. doi: 10.1016/s0278-5919(05)70128-9. [DOI] [PubMed] [Google Scholar]
- 25.Emmerson BC, Görtz S, Jamali AA, Chung C, Amiel D, Bugbee WD. Fresh osteochondral allografting in the treatment of oasteochondritis dissecans of the femoral condyle. Am J Sports Med. 2007;35:907–914. doi: 10.1177/0363546507299932. [DOI] [PubMed] [Google Scholar]
- 26.Görtz S, De Young AJ, Bugbee WD. Fresh osteochondral allografting for steroid-associated osteonecrosis of the femoral condyles. Clin Orthop Relat Res. 2010;468:1269–1278. doi: 10.1007/s11999-010-1250-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deponti D, Di Giancamillo A, Mangiavini L, et al. Fibrin-based model for cartilage regeneration: tissue maturation from in vitro to in vivo. Tissue Eng Part A. 2012;18:1109–1122. doi: 10.1089/ten.TEA.2011.0272. [DOI] [PubMed] [Google Scholar]
- 28.Kon E, Delcogliano M, Filardo G, et al. Orderly osteochondral regeneration in sheep model using a novel nano-composite multilayered biomaterial. J Orthop Res. 2010;28:1116–1124. doi: 10.1002/jor.20958. [DOI] [PubMed] [Google Scholar]
- 29.Kon E, Mutini A, Arcangeli E, Delcogliano M, Filardo G, Nicoli Aldini N, Pressato D, Quarto R, Zaffagnini S, Marcacci M. Novel nanostructured scaffold for osteochondral regeneration: a pilot study in horses. J Tissue Eng Regen Med. 2010;4:300–308. doi: 10.1002/term.243. [DOI] [PubMed] [Google Scholar]
- 30.Marlovits S, Striessnig G, Resinger CT, et al. Definition of pertinent parameters for the evaluation of articular cartilage repair tissue with high- resolution magnetic resonance imaging. Eur J Radiol. 2004;52:310–319. doi: 10.1016/j.ejrad.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 31.Marmotti A, Bruzzone M, Bonasia DE, et al. One-step osteochondral repair with cartilage fragments in a composite scaffold. Knee Surg Sports Traumatol Arthrosc. 2012;20:2590–2601. doi: 10.1007/s00167-012-1920-y. [DOI] [PubMed] [Google Scholar]
- 32.Peterson L. Chondrocyte transplantation. In: Jackson DW, editor. Master techniques in orthopaedic surgery: reconstructive knee surgery. Philadelphia: Lippincott, Williams & Wilkins; 2003. pp. 353–373. [Google Scholar]
- 33.Carmont MR, Carey-Smith R, Saithna A, Dhillon M, Thompson P, Spalding T. Delayed incorporation of a TruFit plug: perseverance is recommended. Arthroscopy. 2009;25:810–814. doi: 10.1016/j.arthro.2009.01.023. [DOI] [PubMed] [Google Scholar]
- 34.Dhollander AA, Liekens K, Almqvist KF, et al. A pilot study of the use of an osteochondral scaffold plug for cartilage repair in the knee and how to deal with early clinical failures. Arthroscopy. 2012;28:225–233. doi: 10.1016/j.arthro.2011.07.017. [DOI] [PubMed] [Google Scholar]
- 35.Peterson L, Minas T, Brittberg M, Nilsson A, Sjögren-Jansson E, Lindahl A. Two- to 9-year outcome after autologous chondrocyte transplantation of the knee. Clin Orthop Relat Res. 2000;374:212–234. doi: 10.1097/00003086-200005000-00020. [DOI] [PubMed] [Google Scholar]
- 36.Peterson L, Vasiliadis HS, Brittberg M, Lindhal A. Autologous chondrocyte implantation: a long-term follow-up. Am J Sports Med. 2010;38:1117–1124. doi: 10.1177/0363546509357915. [DOI] [PubMed] [Google Scholar]