Abstract
In our previous studies, colony-forming progenitor cells isolated from murine embryonic stem cell-derived cultures were differentiated into morphologically distinct insulin-expressing colonies. These colonies were small and not light-reflective when observed by phase-contrast microscopy (therefore termed “Dark” colonies). A single progenitor cell capable of giving rise to a Dark colony was termed a Dark colony-forming unit (CFU-Dark). The goal of the current study was to test whether endogenous pancreas, and its developmentally related liver, harbored CFU-Dark. Here we show that dissociated single cells from liver and pancreas of one-week-old mice give rise to Dark colonies in methylcellulose-based semisolid culture media containing either Matrigel or laminin hydrogel (an artificial extracellular matrix protein). CFU-Dark comprise approximately 0.1% and 0.03% of the postnatal hepatic and pancreatic cells, respectively. Adult liver also contains CFU-Dark, but at a much lower frequency (~0.003%). Microfluidic qRT-PCR, immunostaining, and electron microscopy analyses of individually handpicked colonies reveal the expression of insulin in many, but not all, Dark colonies. Most pancreatic insulin-positive Dark colonies also express glucagon, whereas liver colonies do not. Liver CFU-Dark require Matrigel, but not laminin hydrogel, to become insulin-positive. In contrast, laminin hydrogel is sufficient to support the development of pancreatic Dark colonies that express insulin. Postnatal liver CFU-Dark display a cell surface marker CD133+CD49flowCD107blow phenotype, while pancreatic CFU-Dark are CD133-. Together, these results demonstrate that specific progenitor cells in the postnatal liver and pancreas are capable of developing into insulin-expressing colonies, but they differ in frequency, marker expression, and matrix protein requirements for growth.
Keywords: in vitro colony assays, insulin expression, methylcellulose, Matrigel, laminin hydrogel, progenitor cell
Abbreviations: AFP - alpha-fetoprotein; APC - allophycocyanin; BSA - bovine serum albumin; CFU - colony-forming unit; CK - cytokeratin; Cre - cyclization recombinase; Ct - threshold cycle; DAPI - 4',6-diamidino-2-phenylindole; DMEM - Dulbecco’s modified Eagle's medium; DNase - deoxyribonucleic acid nuclease; E - embryonic day; ECM - extracellular matrix; EGFP - enhanced green fluorescent protein; ES - embryonic stem; FACS - fluorescence-activated cell sorting; FITC - fluorescein isothiocyanate; FSC - forward scatter; HMG - high mobility group; IFC - integrated fluidic circuit; Ig - immunoglobulin; Lamp2 - lysosomal associated membrane protein 2; Lox - locus of crossing over; MafA - v-maf musculoaponeurotic fibrosarcoma oncogene homolog A; mESC - mouse embryonic stem cell; MLS - multi-laser sorter; Ngn3 - neurogenin 3; PBS - phosphate buffered saline; Pdx1 - pancreatic and duodenal homeobox 1; PE - phycoerythrin; qRT-PCR - quantitative reverse transcription polymerase chain reaction; R - region; RT-PCR - reverse transcription polymerase chain reaction; SORP - special order research product; Sox17 - Sry-related HMG box 17; SSC - side scatter; SD - standard deviation
1. Introduction
Type 1 diabetes is a chronic disease resulting from autoimmune attack on the endocrine insulin-secreting beta-cells that reside within the islets of Langerhans in the pancreas. Allogeneic islet transplantation is a promising treatment for end-stage patients. However, this procedure is limited by a dearth of cadaveric organ donors. Therefore, attention has been focused on stem and progenitor cells as sources of large numbers of insulin-expressing cells for transplantation.
Pancreas specification is controlled by many transcription factors during development (see review [1]). In short, Sry-related HMG box (Sox) 17, a high-mobility group box-containing transcription factor, specifies definitive endoderm at approximately embryonic day (E) 7.5 [2]. This leads to the formation of gut tube (at ~E8.5), which has the potential to give rise to many internal organs, including pancreas and liver. Next, expression of pancreatic and duodenal homeobox 1 (Pdx-1) at the foregut region instructs pancreas morphogenesis [3, 4]. Lineage tracing experiments have demonstrated that Pdx-1 expressing cells labeled at ~E12.5 or earlier are multipotent, capable of giving rise to all lineages, including ducts, acinar, and endocrine cells [5]. Starting at ~E9.5 and throughout pancreas ontogeny, expression of neurogenin (Ngn) 3, a helix-loop-helix protein, commits Pdx-1+ progenitors to becoming endocrine cells [6, 7]. Mice deficient in Ngn3 fail to form endocrine pancreas [6]. Lineage-tracing experiments confirm that Ngn3+ cells are capable of giving rise only to endocrine cells [5]. Finally, MafA, a basic leucine zipper transcription factor, controls the maturation of beta-cells capable of glucose-responsive insulin secretion [8].
Studies from several laboratories including ours have established differentiation protocols that allow commitment of human [9-16] or murine [17-21] embryonic stem (ES) cells into pancreas-like cells in culture. In our protocol, we find two sets of reagents sufficient to induce the commitment of pancreas-like cells from murine ES cells [17, 21]. In the first step, involving embryoid body formation, high concentrations of monothioglycerol induce Sox17+ definitive endoderm formation by day five in culture, followed by spontaneous differentiation into pancreatic endoderm at day six. Thereafter, pancreas-like cells, grown in attachment culture, are specified over time in the presence of a combination of nicotinamide [22], exendin-4 [23], and activin B [24] added on day thirteen and onwards. Late-stage cultures express various pancreatic markers, such as Pdx-1, Ngn3, C-peptide (surrogate marker for insulin), and amylase 2A (acinar marker), as determined by reverse transcription polymerase chain reaction (RT-PCR), immunostaining, and gene reporter analyses [17, 21]. After five weeks post-transplantation under the kidney capsule of diabetic mice, the murine ES cell-derived grafts further develop into mini-organs, with C-peptide+ cells dispersed among well-organized acinar-like structures [21]. Murine ES cell-derived cultures also behave similarly to embryonic pancreas, because forced expression of Sox17, Ngn3, or MafA at various stages of differentiation increases differentiation of pancreatic, endocrine, or maturing beta-cells (in vitro glucose-responsive insulin secretion), respectively [21].
During the course of our previous studies, a class of progenitor cells was identified in murine ES cell-derived, day-sixteen cultures [25, 26]. These progenitor cells are enriched in cells expressing enhanced green fluorescent protein (EGFP) under the control of Ngn3 promoter, and give rise to morphologically distinct, small, dark colonies that express insulin [25, 26]. We therefore name these colonies "Dark". C-peptide+ cells in some Dark colonies simultaneously express glucagon, another endocrine hormone [25]. Therefore, we speculate that Dark colonies may represent the first-wave [27] development of pancreatic endocrine cells that are poly-hormonal.
Dark colonies are formed in a three-dimensional culture assay devised in our laboratory [25, 26]. In brief, the culture media are semisolid, containing methylcellulose (to enhance viscosity), Matrigel (a rich source of various extracellular matrix (ECM) proteins), and growth factors (nicotinamide, exendin-4, activin B, vascular endothelial growth factor A, and conditioned media from murine ES cell-derived day-sixteen cells). Because the viscosity of the medium restricts the movements of dispersed single cells, the formation of a colony indicates the presence of a progenitor cell at the time of plating. Progenitor cells capable of giving rise to Dark colonies are termed "Dark colony-forming units" (CFU-Dark), similar to the concept used for hematopoietic colony-forming progenitors.
Whether CFU-Dark detected in murine ES cell-derived cultures exist in primary tissues is not known. In this study, we therefore tested the hypothesis that murine endogenous organs contain CFU-Dark. Both the pancreas and its developmentally related liver were examined. The liver was studied because, in normal development, small clusters of insulin-expressing cells are found in liver parenchyma and around extrahepatic bile ducts in late gestation to adults in mice [28] and in humans [29]. In addition to the Matrigel-containing colony assay described above, we also tested the use of a well-defined artificial ECM protein [30] containing an α1 laminin and an elastin sequences (referred as laminin hydrogel) [31]. Laminin hydrogel was shown to promote endocrine cell differentiation from adult pancreatic ductal progenitor-like cells in vitro [31].
Here we report that CFU-Dark are detected in postnatal (one-week old) pancreas and liver. CFU-Dark are also present in the adult liver, but the frequency is at least 30-fold lower compared with the postnatal liver. We found that formation of Dark colonies can be supported by Matrigel or laminin hydrogel. However, postnatal pancreatic and hepatic CFU-Dark display different culture requirements to become insulin-positive. The incidence of CFU-Dark was higher in the postnatal liver compared with postnatal pancreas and adult liver. Expression profiles of other genes, such as cytokeratins, alpha-fetoprotein, and albumin, were different among Dark colonies derived from postnatal liver or pancreas, suggesting distinct origins of these cells. Collectively, these results demonstrate that postnatal liver and pancreas contain progenitor-like cells capable of differentiation into specific insulin-expressing colonies in culture. These results demonstrate that our ES cell-to-pancreas differentiation protocol produces cells similar to those in endogenous tissues. Our results also have clinical implications in generating a significant number of transplantable insulin-expressing cells from liver due to the larger cell mass of this organ compared with pancreas.
2. Material and methods
2.1 Mice
Postnatal mice (1 week old) or adult mice (2-4 months old) of CD1 outbred or C57BL/6 inbred background (Charles River Laboratory, Wilmington, MA) were maintained under specific pathogen-free conditions. The experiments were conducted according to the Institutional Animal Care and Use Committee at City of Hope.
2.2 Dissociation of pancreas and liver
Dissected pancreata (cleaned of fatty tissues) or liver lobes (devoid of gallbladder and extrahepatic billiary ducts) were minced (3 min) with a spring scissor in a dry petri dish on ice, placed in PBS/0.1% (wt/vol) BSA containing collagenase B (2-4 mg/ml) (Roche, Mannheim, Germany) and DNase I (2,000 U/ml) (Calbiochem, Darmstadt, Germany), and incubated (37°C, 20-30 min) to yield a predominately single cell suspension. To accelerate digestion, the tissue was gently pipetted every 5-10 min. The single cell suspension was filtered through 40 μm cell strainers before use.
2.3 In vitro colony assays
Cells were resuspended (typically 2.5×104 cells/0.5ml/well) in methylcellulose-based colony culture medium, as described previously [25, 26, 31]. In short, culture mixture (1 ml) contained DMEM/F12 media, methylcellulose (1%, wt/vol, Sinetsu Chemical, Tokyo, Japan), MatrigelTM (5%, vol/vol) (growth factor reduced and phenol red free; BD Biosciences, Franklin Lakes, NJ), or laminin hydrogel (100 μg/ml) (see below), conditioned media from murine embryonic stem cell-derived pancreatic-like cells (50%, vol/vol), fetal calf serum (5%, vol/vol, FCS), nicotinamide (10 mmol/l, Sigma, St. Louis, MO), human recombinant activin B (10 ng/ml), exendin-4 (0.1 nmol/l), and vascular endothelial growth factor-A (1 ng/ml; R and D Systems, Minneapolis, MN). The cells were plated in 24-well ultralow protein-binding plates (Corning, Corning, NY) and incubated in a humidified 5% CO2 atmosphere. Triplicate wells were routinely plated. Colony numbers were scored after one week in culture.
2.4 Quantitative (q) RT-PCR
Total RNA extraction, reverse transcription and conventional qRT-PCR analysis, using Taqman probes, were performed as described [21]. Microfluidic qRT-PCR was performed using the BioMarkTM 48.48 Dynamic Array system (Fluidigm, South San Francisco, CA). Single colonies were lifted one by one from the methylcellulose medium under direct microscopic visualization by using a 10-μl Eppendorf pipette or a fine glass pipette with an opening of approximately 50 μm, collected in reaction buffer (10 μl), and followed by pre-amplification (14 cycles) according to manufacturer's instructions (Fluidigm).
Amplified cDNA was loaded onto a 48.48 Dynamic Array using the NanoFlex integrated fluidic circuit (IFC) controller (Fluidigm). The threshold cycle (Ct), as a measure of fluorescence intensity, was determined by the BioMark PCR analysis software (Fluidigm) and expressed as a heat map or delta Ct compared to β-actin. All experiments were performed with negative (water) and positive (postnatal pancreatic cell) controls. Taqman probes (Life Technologies, Grand Island, NY) and their catalog numbers are listed in Table 1.
Table 1. List of murine Taqman probes used for microfluidic quantitative RT-PCR analysis.

Legend: * from Applied Biosystems Invitrogen. Abbreviations: CK - cytokeratin; RT-PCR - reverse transcription polymerase chain reaction.
2.5 Expression and purification of laminin hydrogel
Methods for cloning, expression, and purification of the artificial ECM protein were performed as described previously [30]. The amino acid sequence of the laminin hydrogel, comprised of an elastin backbone plus an α1 laminin extracellular matrix protein domain, was as described [31].
2.6 Flow cytometry and cell sorting
The cell suspension was first incubated with anti-mouse CD16/32 (10 μg/ml; 5 min, on ice, BioLegend, San Diego, CA) to diminish nonspecific binding. Biotin-conjugated anti-mouse CD133 (clone 13A4; 5 μg/ml; eBioscience, San Diego, CA), phycoerythrin (PE)-conjugated anti-mouse CD107b (clone M3/84; 2.5 μg/ml; BioLegend), and FITC-conjugated anti-mouse CD49f (clone GoH3; 10 μg/ml; BioLegend) antibodies were added and cells were incubated (20 min, on ice), washed twice, treated with streptavidin-labeled allophycocyanin (APC) (2 μg/ml; 15 min, on ice, BioLegend), washed twice, and resuspended in PBS/BSA/DNase I-containing DAPI (0.2 μg/ml).
Control antibodies used were biotin-conjugated rat immunoglobin (Ig)G1 (5 μg/ml; eBioscience), PE-conjugated rat IgG1 (2.5 μg/ml; BioLegend), and FITC-conjugated rat IgG2a isotypes (10 μg/ml; BioLegend). Cell sorting was performed on a MoFlowTM MLS (Beckman Coulter, Brea, CA) or an Aria-special order research product (SORP) (Becton Dickinson). All analyses included an initial gating of forward (FSC) and side (SSC) scatters to exclude debris. Sorting further excluded doublets by gating out high pulse-width cells, and live cells were selected by DAPI-negative staining. The purity of the sorted population was routinely >95%.
2.7 Whole-mount immunostaining
Colonies were manually picked, pooled, and fixed in 4% paraformaldehyde at 4°C overnight, followed by incubation with blocking buffer containing 5% donkey serum and 0.1% Triton X-100 at 4°C overnight. Primary and secondary antibodies used were as listed in Table 2. Images were captured by a Zeiss LSM510 META NLO Axiovert 200M inverted microscope, and figures prepared with LSM Image Browser software (Carl Zeiss, Germany).
Table 2. List of antibodies used for whole-mount immunostaining analysis.
2.8 Transmission electron microscopy
Single colonies were collected, pooled, and fixed in Karnovsky's fixative at 4°C overnight. The colonies were placed in a round-bottom 96-well plate under direct visualization of a microscope to facilitate rinsing without losing them, and were washed three times with cacodylate buffer [32]. The colonies were then transferred to an Eppendorf tube, incubated with 1% osmium tetroxide in 0.1M cacodylate buffer for 30 min, washed three times, dehydrated, embedded in eponate, and processed for transmission electron microscopy.
2.9 Statistical analysis
All values are shown as mean ± standard deviation. p-values were calculated using Student's two-tailed t-test with p < 0.05 considered significant.
3. Results
3.1 Dark colonies formed from dissociated postnatal liver or pancreas single cell suspension
Consistent with our previous findings [25] and in Matrigel-containing colony assay (Figure 1A), dissociated murine ES cell-derived day sixteen cells gave rise to Dark colonies (Figure 1B). Colonies were small (<100 μm in diameter) and not light-reflective. To test whether Dark colonies could be grown from liver or pancreas, organs were dissociated into a single cell suspension by collagenase B and DNase I digestion, and plated (2.5 x 104 cells/0.5ml/well) into our colony assays containing either Matrigel or laminin hydrogel. Seven days post-plating, Dark colonies were detected in cultures initiated with postnatal (one-week-old) liver in the presence of Matrigel (Figure 1C) or laminin hydrogel (Figure 1D). Dissociated postnatal pancreatic cells also gave rise to Dark colonies in the presence of laminin hydrogel (Figure 1E). When plated in Matrigel assay, a small number of pancreatic Dark colonies were observed. However, these cells were not studied sufficiently because the culture was overwhelmed with numerous cystic colonies. Cystic colonies have been described previously by several laboratories including ours [31, 33-36]. They appear as ductal-like cells and are not the subject of the current study.
Figure 1. "Dark" colonies are formed from dissociated one-week-old murine liver or pancreas in our 3D colony assays.

A: Schematic of an in vitro methylcellulose-based colony assay for progenitor cells. Two different colony assays were used in this report: one contained Matrigel and the other laminin hydrogel. B-E: Morphologically distinct colonies are identified when observed under a phase-contrast light microscope one week after plating. B: A photomicrograph of a Dark colony developed from dissociated murine ES cell-derived day-16 cultures, which confirms our prior finding [25]. Dark colony grown in Matrigel (C) or laminin hydrogel (D) from dissociated postnatal liver. E: Dark colony grown in laminin hydrogel from dissociated postnatal pancreas. Abbreviation: mESC - mouse embryonic stem cells.
The diameter of various day-seven Dark colonies (n = 20) was determined to be 25 ± 8, 15 ± 2, and 28 ± 4 μm for postnatal hepatic Dark colonies grown in Matrigel, laminin hydrogel, and for pancreatic Dark colonies grown in laminin hydrogel, respectively (Figure 2A). The average number of cells per colony ranged between approximately 20 to 30 cells (Figure 2B). Both hepatic and pancreatic Dark colonies started as single cells and developed into morphologically distinct clusters of cells by day seven (Figure 2C). In later section, FACS-sorted single cell suspension, devoid of cell doublets, also formed Dark colonies, suggesting a single cell is sufficient to initiate colony formation. These results demonstrate that Dark colonies observed on day seven were not derived from aggregation of cells at the time of plating.
Figure 2. Dark colonies are small in size and originate from dissociated single cell suspension.

Using one week old mice, colonies were analyzed seven days post-plating (A, B). A: Data represent mean ± SD of diameters from 20 colonies. B: A total of 50 colonies were handpicked, pooled, and dissociated into a single cell suspension by trypsin digestion. Afterwards, the cell number was determined using a hematocytometer. Data represent mean ± SD of triplicate samples. C: Locations of individual cells after plating were noted and the development into Dark colonies was followed over time. N.D. - not done.
Colony-forming efficiency (total colonies formed / number of plated cells) was ~0.1% and 0.03% from postnatal day seven liver and pancreas, respectively (Figure 3). Dark colony formation was not mouse strain-specific; postnatal cells from both B6 and CD1 background gave rise to colonies. Dissociated adult (2-4 months old) liver and pancreas did not give rise to Dark colonies in Matrigel or laminin hydrogel colony assays when plated at the maximum capacity of our assays (2.5 x 104 cells/well). However, this does not rule out the possibility that CFU-Dark are rare in adult liver or pancreas, thus below the detection limits of our assays. Indeed this is the case for adult liver cells, which will be shown in a later section of the results.
Figure 3. Colony-forming efficiency of cells is higher from postnatal liver than pancreas.
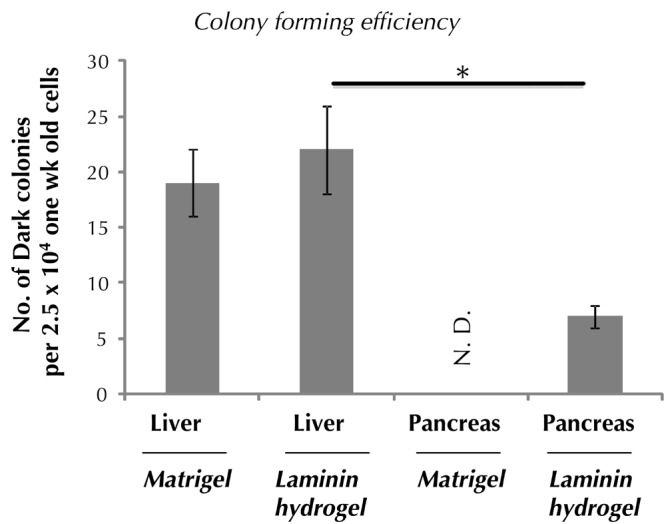
One-week-old mice were used. Colonies were counted seven days post-plating. Data represent mean ± SD in triplicate wells. N.D. denotes not done. * p < 0.05.
3.2 Many individually handpicked Dark colonies express insulin genes
To determine gene expression patterns, seven days post-plating single Dark colonies were individually lifted from the semisolid media under direct microscope visualization. Expression of a panel of genes in individual colonies (n = 10/group) was analyzed by microfluidic qRT-PCR analysis. Microfluidic qRT-PCR is a relatively new technology used to determine gene expression in as little as one colony and in a reaction volume in the nanoliter range [37]. We found that postnatal liver-derived Dark colonies grown in Matrigel (Figure 4A), but not laminin hydrogel (Figure 4B), expressed insulin genes (Insulin1 and Insulin2). This suggests that certain components from Matrigel are important to induce insulin gene expression in liver colonies. Out of the ten liver-derived Dark colonies grown in the presence of Matrigel, five expressed insulin genes. Among those five liver colonies, two (#5 and #6) expressed liver-specific genes (Alpha-fetoprotein and Albumin) (Figure 4A), demonstrating the heterogeneity of Dark colonies from postnatal liver.
Figure 4. Individual Dark colonies from postnatal liver or pancreas express different sets of genes.

One week old mice were used, and day-seven colonies were analyzed. Single-colony microfluidic qRT-PCR analysis demonstrates that Dark colonies can express insulin genes if they are from liver grown in Matrigel (A), or from pancreas grown in laminin hydrogel (C). Some colonies derived from liver express liver-specific genes (alpha-fetoprotein and albumin) (A and B), whereas pancreatic colonies express ductal (CK7 and CK19) and Glucagon genes (C). Each column represents a single colony. D: Data from A to C were converted into relative gene expression compared to β-actin. Each bar represents a single colony. Abbreviations: AFP - alpha-fetoprotein; B2MG - beta-2 microglobulin; CK7 - cytokeratin 7; Ct - threshold cycle; qRT-PCR - quantitative reverse transcription polymerase chain reaction.
Postnatal pancreas-derived Dark colonies did not express Alpha-fetoprotein or Albumin, with the exception of colony #24, which expressed low levels of Albumin (Figure 4C). Nine out of ten pancreatic colonies expressed Insulin genes (Figure 4C). Glucagon, a pancreas-specific alpha cell marker, was expressed in seven out of the nine insulin+ pancreatic Dark colonies, but not in liver-derived colonies. Ductal markers (Cytokeratin (CK) 7 and CK19) were expressed by some pancreatic Dark colonies, but not in liver-derived colonies. These results again reveal the heterogeneity of Dark colonies, and demonstrate that Dark colonies from postnatal liver and pancreas have very different gene expression profiles. When gene expression levels in individual colonies were normalized to β-actin, we found that Insulin2 messages were more abundant than Insulin1 in Dark colonies derived from postnatal hepatic cells grown in Matrigel (red bars in Figure 4D). This is reminiscent of a prior finding demonstrating that Insulin2, compared to Insulin1, is the major insulin gene expressed in rat fetal liver [38].
Whole-mount immunostaining and confocal imaging analyses of pooled colonies further confirmed the presence of C-peptide+Glucagon- cells in liver Dark colonies (Figure 5A) and C-peptide+Glucagon- and C-peptide-Glucagon+ in the pancreatic Dark colonies (Figure 5B). At least one pancreatic cell was clearly double-stained with C-peptide and glucagon (Figure 5B; arrow). Consistently, transmission electron microscopy analysis revealed the presence of different types of granules in one cell in a Dark colony derived from postnatal pancreas (Figure 5C). These results demonstrate that postnatal pancreatic CFU-Dark are able to differentiate into poly-hormonal cells in vitro.
Figure 5. Immunostaining and transmission electron analysis of Dark colonies.
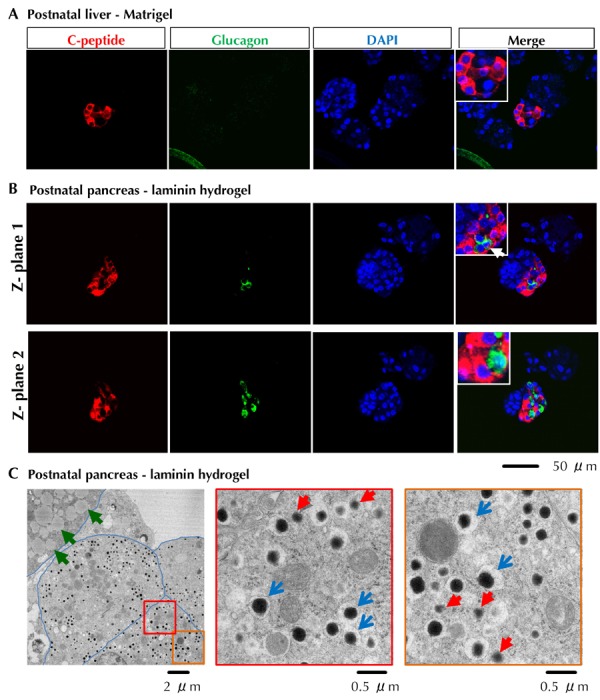
A and B: Whole-mount double immunostaining of pooled colonies with antibodies against C-peptide (red) and glucagon (green). C: Transmission electron microscopy analysis of a seven day old Dark colony derived from postnatal pancreas grown in laminin hydrogel. Blue arrows indicate typical insulin-like granules. Red arrows indicate non-insulin endocrine granules. Green arrows indicate other unidentified granules, possibly lipid droplets. Blue lines mark cell membranes.
3.3 CFU-Dark are enriched in postnatal liver CD133+CD49flowCD107blow cells and pancreatic CD133- cells
Enrichment of CFU-Dark cells is highly desirable because of their low incidence. We tested whether CFU-Dark could be enriched using cell surface markers and fluorescence-activated cell sorting. CD133 and/or CD49f have been used to isolate embryonic or adult progenitor cells from pancreas [39-42] and liver [43]. In our prior studies, genome-wide gene expression analysis showed that sorted murine ES cell-derived Ngn3/EGFP+ cells expressed higher levels of CD107b (a.k.a. Lamp2) [44, 45], compared to Ngn3/EGFP- cells [25], suggesting CFU-Dark may express CD107b. We therefore tested whether the above cell surface markers may enrich for CFU-Dark. Dissociated postnatal liver cells were stained with antibodies against CD133 and analyzed by flow cytometry (Figure 6A). Cell debris was excluded by appropriate forward and side scatters (region (R) 1). Live cells were further gated by DAPI- staining (R2), and single cells were selected by low pulse width readings (R3). Under these gated regions, we found that CD133+ cells comprised ~10% of total one-week-old liver cells (R4). Using qRT-PCR analysis, freshly sorted liver cells from R4, CD133+ region were found to express higher levels of Pdx1 and CK7, but lower levels of Ngn3, compared to the CD133- cells (Figure 6B, bar 2 vs. 3). When plated into Matrigel colony assay (1 x 104 cells/well), only cells in CD133+, but not CD133-, fraction gave rise to Dark colonies seven days after plating (Figure 6C, left panel).
Figure 6. In postnatal liver, Dark colony-forming progenitor cells are most enriched in the CD133+CD49flowCD107blow cell population.
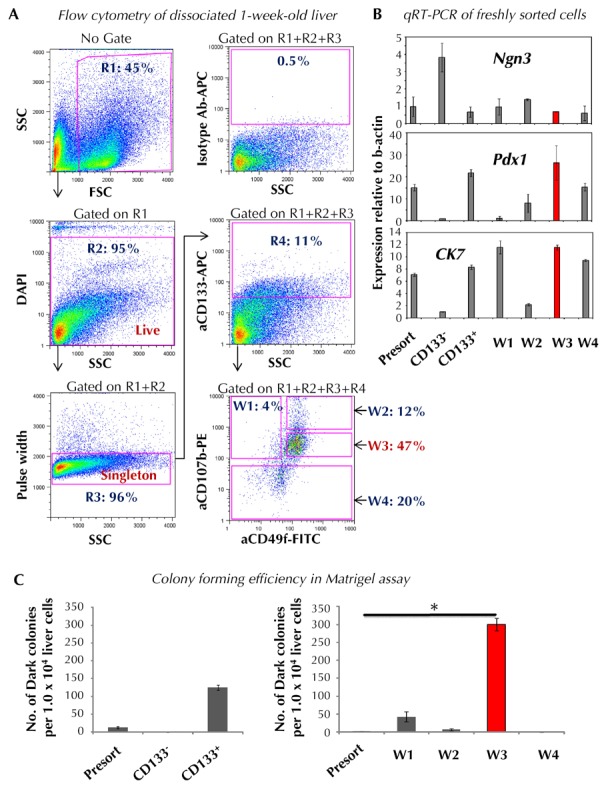
A: Postnatal liver was dissociated into single cell suspension, stained with a cocktail of antibodies against CD133, CD49f, and CD107 which were conjugated with different designated fluorochromes, and analyzed by flow cytometry. Notations: R1: forward (FSC) and side scatter (SSC) gate; R2: DAPI- staining for live cells; R3: cell singletons; R4: CD133+ cells; W1: CD133+CD49f-CD107b+; W2: CD133+CD49flowCD107bhigh; W3: CD133+CD49flowCD107blow; W4: CD133+CD107b-. B: Gene expression analysis of freshly sorted cells. C: Designated cells were sorted and plated into the Matrigel-containing colony assay. Colonies were counted seven days post-plating. Data represent mean ± SD in triplicate wells. Abbreviations: APC - allophycocyanin; CK7 - cytokeratin; DAPI - 4',6-diamidino-2-phenylindole; FITC - fluorescein isothiocyanate; FSC - forward scatter; Ngn3 - neurogenin 3; Pdx1 - pancreatic and duodenal homeobox 1; PE - phycoerythrin; qRT-PCR - quantitative reverse transcription polymerase chain reaction; R - region; SSC - side scatter. * p < 0.05.
Next, we tested whether CFU-Dark can be further enriched using antibodies against CD49f and CD107b in addition to CD133. We found that in the CD133+ region, liver cells can be further divided into CD49f-CD107b+ (window (W) 1 in Figure 6A), CD49flowCD107bhigh (W2), CD49flowCD107blow (W3), and CD107b- (W4) cells. These four populations were subsequently sorted and plated into the Matrigel-containing colony assay (1 x 104 cells/well) and Dark colonies quantitated seven days after plating. We found that CD133+CD49flowCD107blow (W3) postnatal liver cells were most enriched for CFU-Dark (Figure 6C, right panel). Freshly sorted CD133+CD49flowCD107blow cells retained higher expression levels of Pdx1 and CK7 and lower levels of Ngn3, similar to CD133+ cells (Figure 6B). However, it should be noted that the expression levels of Pdx1 and CK7 in the liver CD133+CD49flowCD107blow cells were much lower compared with pancreatic CD133+ cells (Figure 7). Also, only ~3% of postnatal liver CD133+CD49flowCD107blow cells were CFU-Dark (Figure 6C, right panel). Thus, the origin of the postnatal liver CFU-Dark cannot be deduced from these data; lineage-tracing experiments are required for future analysis.
Figure 7. In postnatal pancreas, Dark colony-forming progenitor cells are enriched in the CD133- cell population.

A: Postnatal pancreas was dissociated into single cell suspension, stained with anti-CD133 antibodies and analyzed by flow cytometry. B: Gene expression analysis of freshly sorted cells. C: Designated cells were sorted and plated into the laminin hydrogel-containing colony assay. Colonies were counted seven days post-plating. Data represent mean ± SD in triplicate wells. Abbreviations: Ab - antibody; APC - allophycocyanin; CK7 - cytokeratin; DAPI - 4',6-diamidino-2-phenylindole; FSC - forward scatter; Ngn3 - neurogenin 3; Pdx1 - pancreatic and duodenal homeobox 1; qRT-PCR - quantitative reverse transcription polymerase chain reaction; R - region; SSC - side scatter.
In postnatal day-seven pancreata, CD133+ cells consisted of approximately 30% of total dissociated cells (Figure 7A). Dissimilar to postnatal liver CD133+ cells, pancreatic CD133+ cells expressed higher levels of Ngn3 compared with presort cells, in addition to the higher levels of Pdx1 and CK7 (Figure 7B). Unlike the liver and surprisingly, pancreatic CD133-, but not CD133+, cells gave rise to Dark colonies in the laminin hydrogel assay (Figure 7C). Therefore, we did not further pursue additional antibody staining analysis. Again, the cellular origin of pancreatic CFU-Dark cannot be deduced from these data and needs to be clarified in future lineage-tracing analysis.
3.4 Adult liver contains low incidence of CFU-Dark
Finally, we tested whether adult liver contains CFU-Dark, and whether they may be enriched using cell surface markers. CD133+ cells consisted of approximately 10% of the total dissociated adult hepatic cells (Figure 8A). Unlike postnatal liver, over 90% of the adult hepatic CD133+ cells co-expressed CD49f and CD107b (Figure 8A). Therefore, we sorted adult liver cells based solely on CD133-positive expression. Upon plating into Matrigel and laminin hydrogel colony assays (1 x 104 cells/well), we found that only CD133+, but not CD133-, cell fractions contained detectable CFU-Dark at a low frequency (approximately 0.03%) (Figures 8B and 8C). Considering that the incidence of CD133+ cells was ~10% among total cells, this means that ~0.003% of total adult liver cells were CFU-Dark.
Figure 8. In adult liver, Dark colony-forming cells are enriched in the CD133+ fraction.
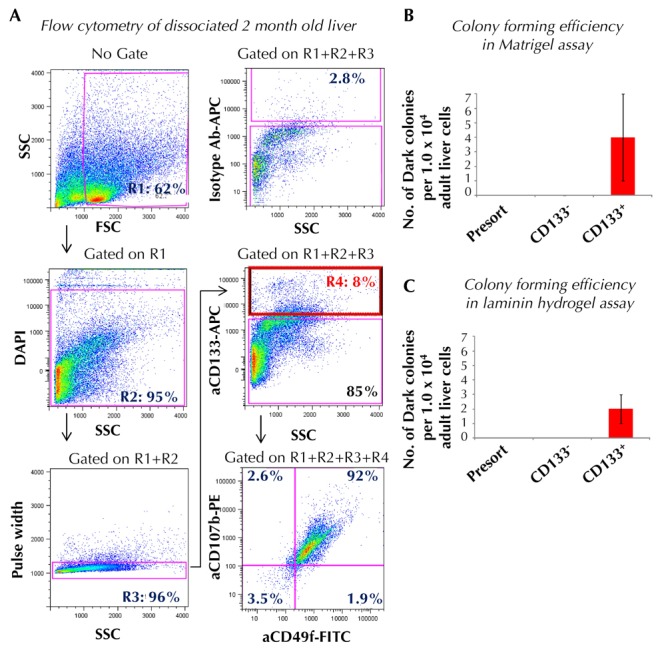
A: Adult (2-4 month old) liver was dissociated into single cell suspension, stained with designated antibodies, and analyzed by flow cytometry. Cells were sorted and plated into Matrigel (B) or laminin hydrogel-containing colony assay (C). Colonies were counted seven days post-plating. Data represent mean ± SD in triplicate wells. Abbreviations: Ab - antibody; APC - allophycocyanin; DAPI - 4',6-diamidino-2-phenylindole; FITC - fluorescein isothiocyanate; FSC - forward scatter; PE - phycoerythrin; R - region; SSC - side scatter.
4. Discussion
In vitro colony assays have played an essential role in deciphering the biology of hematopoietic progenitor cells in the past decades [46]. The basis of the colony assay is that cells in a single cell suspension are mixed in semisolid media and cannot migrate. However, the medium is still soft enough to allow a single cell, if capable of doing so, to proliferate and differentiate into a colony of cells within a three-dimensional space. By analyzing the lineage composition of a colony, the lineage potential of the originating colony-forming cell can be deduced. It is also a quantitative assay, in which the prevalence of CFUs in a given population of cells can be calculated accurately by dividing the number of colonies formed by the total number of plated cells. The use of semisolid media is essential for this quantitative aspect of the colony assay, which is achieved by inclusion of methylcellulose, a biologically inert material derived from wood fibers. The colony assays used in our study employed a concept similar to that of the hematopoietic colony assay, but with different culture components, such as growth factors and ECM proteins pertinent to pancreatic cell growth [25, 26, 31].
Using our colony assays, we find that both postnatal liver and pancreas contain progenitor-like cells that are capable of differentiating into morphologically distinct Dark colonies when observed by phase-contrast microscopy. Individual postnatal liver Dark colonies can express insulin if cultured in the presence of Matrigel, but not in laminin hydrogel. Matrigel is known to contain several types of extracellular matrix proteins, such as type IV collagen, perlecan, nidogen/entactin, and laminin, as well as various growth factors [47]. The laminin hydrogel that we produced and used in this study contains defined peptide (~20 amino acids) domains from elastin and α1 laminin molecules [31]. It remains to be determined as to which of the components derived from Matrigel may be responsible for inducing insulin gene expression in postnatal liver Dark colonies.
The expression pattern of other genes is different among the postnatal liver and pancreas Dark colonies. Glucagon was expressed by pancreatic Dark colonies, but not expressed by any of the colonies derived from postnatal liver (Figure 4 A-D). This is consistent with previous findings, including our own [17, 28], showing the lack of glucagon expression in murine fetal and adult liver. Liver Dark colonies express liver, but not ductal, markers, while pancreatic Dark colonies express ductal, but not liver, genes (Figure 4 A-D). Taken together, our data show that Dark colonies grown from postnatal liver and pancreas have different growth requirements and lineage marker expression, although displaying similar morphology.
Our results confirm our previous data showing that at least some of the murine ES cell-derived Dark colonies were pancreas-like, because glucagon is co-expressed in some of the Dark colonies examined [25]. This study takes our previous findings a step further to demonstrate that liver procured from postnatal (one-week-old) mice also contains CFU-Dark capable of giving rise to insulin-expressing Dark colonies in vitro. Cells from the fetal liver are known to express low levels of insulin genes during development [38]. Moreover, small clusters of insulin+ cells are detected in vivo in liver parenchyma and along the extrahepatic bile ducts in late gestation to adult mice [28] and in humans [29]. Experiments using cyclization recombinase (Cre) and locus of crossing over (Lox) lineage-tracing system demonstrated that the hepatic insulin+ cells are descendants of albumin-expressing liver rudiment from early development, and that they do not emigrate from the pancreas [28]. Hepatic insulin-expressing cells are glucose-responsive in insulin secretion in vitro [28]. It is therefore suggested that these insulin+ cells are evolutionary residues of endocrine cells that occur in the biliary system of the vertebrate ancestors [28]. In our studies, the majority of liver Dark colonies express albumin, with a subgroup also expressing insulin genes (Figure 4A). We speculate that CFU-Dark may be the source of liver insulin+ cells in vivo. However, further studies are needed to confirm this hypothesis.
Cell surface marker expression of CFU-Dark differed in postnatal liver and pancreas. Postnatal liver CFU-Dark were CD133+CD49flowCD107blow, while those from the pancreas were CD133-. CD133, also known as prominin-1, is expressed by progenitor cells in various tissues, such as bone marrow, brain, and liver [48]. We recently demonstrated that in adult murine pancreas, ductal colony-forming progenitors are capable of differentiating into insulin-secreting cells in vitro [31]. These progenitor-like cells, which we termed CFU-Ring/Dense, also express CD133 [31]. It is therefore surprising to find that postnatal pancreatic CFU-Dark is enriched in the CD133- cell fraction (Figure 7C). We speculate that pancreatic CFU-Dark (from current study) and CFU-Ring/Dense [31] may represent two separate classes of progenitor cells, such as those observed for the primitive and definitive waves of hematopoietic progenitor cells [49]. Alternatively, CFU-Ring/Dense and CFU-Dark may originate from the same stem or progenitor cells that are capable of sequentially giving rise to one and then the other cell type. These possibilities need further investigation. In addition, the cellular compartment from which the pancreatic CFU-Dark originate also awaits further studies using lineage-tracing strategy.
Liver and pancreas are developmentally-related organs; both derive from the foregut endoderm [50]. The liver cell mass is much larger than that of the pancreas. Consequently, if reprogramming can be accomplished, the liver holds exciting promise as an alternative source for the generation of insulin-secreting cells for transplantation. It has already been shown that primary liver cells isolated from fetus or adults can be induced to express insulin after overexpressing key pancreatic transcription factors such as Pdx-1 and Ngn3 [51-55]. The origin of cells amenable to transdifferentiation in liver, however, is not entirely clear. In the adult liver, the biliary ducts are the candidate source of reprogrammed cells [56, 57]. It remains to be seen whether CFU-Dark contained in the CD133+CD49flowCD107blow cell fraction of the postnatal liver may originate from the biliary ducts (Figure 6B; CK7 expression on freshly sorted cells), albumin-expressing [28], or other cellular compartments using lineage-tracing strategy. Finally, whether CFU-Dark could be a more efficient and abundant source for liver-to-beta cell trans-differentiation will be addressed in future studies.
Funding: This work is supported in part by National Institutes of Health (NIH) grants R01DK081587 and R01DK099734 to H.T.K., U01DK089533 to A.D.R., and P30 CA33572 to the Analytical Cytometry Core at City of Hope, and by National Science Foundation grant DMR-1206121 and California Institute for Regenerative Medicine grant RB5-07398 to D.A.T.
Disclosures: The authors report no conflict of interests.
Acknowledgments
This work is supported in part by National Institutes of Health (NIH) grants R01DK081587 and R01DK099734 to H.T.K., U01DK089533 to A.D.R., and P30 CA33572 to the Analytical Cytometry Core at City of Hope, and by National Science Foundation grant DMR-1206121 and California Institute for Regenerative Medicine grant RB5-07398 to D.A.T.
References
- 1.Pan FC, Wright C. Pancreas organogenesis: from bud to plexus to gland. Dev Dyn. 2011;240(3):530–565. doi: 10.1002/dvdy.22584. [DOI] [PubMed] [Google Scholar]
- 2.Kanai-Azuma M, Kanai Y, Gad JM, Tajima Y, Taya C, Kurohmaru M, Sanai Y, Yonekawa H, Yazaki K, Tam PP, Hayashi Y. Depletion of definitive gut endoderm in Sox17-null mutant mice. Development. 2002;129:2367–2379. doi: 10.1242/dev.129.10.2367. [DOI] [PubMed] [Google Scholar]
- 3.Jonsson J, Carlsson L, Edlund T, Edlund H. Insulin-promoter-factor 1 is required for pancreas development in mice. Nature. 1994;371(6498):606–609. doi: 10.1038/371606a0. [DOI] [PubMed] [Google Scholar]
- 4.Offield MF, Jetton TL, Labosky PA, Ray M, Stein RW, Magnuson MA, Hogan BL, Wright CV. PDX-1 is required for pancreatic outgrowth and differentiation of the rostral duodenum. Development. 1996;122(3):983–995. doi: 10.1242/dev.122.3.983. [DOI] [PubMed] [Google Scholar]
- 5.Gu G, Dubauskaite J, Melton DA. Direct evidence for the pancreatic lineage: NGN3+ cells are islet progenitors and are distinct from duct progenitors. Development. 2002;129(10):2447–2457. doi: 10.1242/dev.129.10.2447. [DOI] [PubMed] [Google Scholar]
- 6.Gradwohl G, Dierich A, LeMeur M, Guillemot F. neurogenin3 is required for the development of the four endocrine cell lineages of the pancreas. Proc Natl Acad Sci U S A. 2000;97(4):1607–1111. doi: 10.1073/pnas.97.4.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mellitzer G, Martin M, Sidhoum-Jenny M, Orvain C, Barths J, Seymour PA, Sander M, Gradwohl G. Pancreatic islet progenitor cells in neurogenin 3-yellow fluorescent protein knock-add-on mice. Mol Endocrinol. 2004;18(11):2765–2776. doi: 10.1210/me.2004-0243. [DOI] [PubMed] [Google Scholar]
- 8.Zhang C, Moriguchi T, Kajihara M, Esaki R, Harada A, Shimohata H, Oishi H, Hamada M, Morito N, Hasegawa K, Kudo T, Engel JD, Yamamoto M, Takahashi S. MafA is a key regulator of glucose-stimulated insulin secretion. Mol Cellular Biol. 2005;25(12):4969–4976. doi: 10.1128/MCB.25.12.4969-4976.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.D'Amour KA, Agulnick AD, Eliazer S, Kelly OG, Kroon E, Baetge EE. Efficient differentiation of human embryonic stem cells to definitive endoderm. Nat Biotechnol. 2005;23(12):1534–1541. doi: 10.1038/nbt1163. [DOI] [PubMed] [Google Scholar]
- 10.D'Amour KA, Bang AG, Eliazer S, Kelly OG, Agulnick AD, Smart NG, Moorman MA, Kroon E, Carpenter MK, Baetge EE. Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat Biotechnol. 2006;24(11):1392–1401. doi: 10.1038/nbt1259. [DOI] [PubMed] [Google Scholar]
- 11.Kroon E, Martinson LA, Kadoya K, Bang AG, Kelly OG, Eliazer S, Young H, Richardson M, Smart NG, Cunningham J, Agulnick AD, D'Amour KA, Carpenter MK, Baetge EE. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat Biotechnol. 2008;26(4):443–452. doi: 10.1038/nbt1393. [DOI] [PubMed] [Google Scholar]
- 12.Nostro MC, Sarangi F, Ogawa S, Holtzinger A, Corneo B, Li X, Micallef SJ, Park IH, Basford C, Wheeler MB, Daley GQ, Elefanty AG, Stanley EG, Keller G. Stage-specific signaling through TGFbeta family members and WNT regulates patterning and pancreatic specification of human pluripotent stem cells. Development. 2011;138(5):861–871. doi: 10.1242/dev.055236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rezania A, Bruin JE, Riedel MJ, Mojibian M, Asadi A, Xu J, Gauvin R, Narayan K, Karanu F, O'Neil JJ, Ao Z, Warnock GL, Kieffer TJ. Maturation of human embryonic stem cell-derived pancreatic progenitors into functional islets capable of treating pre-existing diabetes in mice. Diabetes. 2012;61(8):2016–2029. doi: 10.2337/db11-1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brolen GK, Heins N, Edsbagge J, Semb H. Signals from the embryonic mouse pancreas induce differentiation of human embryonic stem cells into insulin-producing beta-cell-like cells. Diabetes. 2005;54(10):2867–2874. doi: 10.2337/diabetes.54.10.2867. [DOI] [PubMed] [Google Scholar]
- 15.Jiang J, Au M, Lu K, Eshpeter A, Korbutt G, Fisk G, Majumdar AS. Generation of insulin-producing islet-like clusters from human embryonic stem cells. Stem Cells. 2007;25(8):1940–1953. doi: 10.1634/stemcells.2006-0761. [DOI] [PubMed] [Google Scholar]
- 16.Jiang W, Shi Y, Zhao D, Chen S, Yong J, Zhang J, Qing T, Sun X, Zhang P, Ding M, Li D, Deng H. In vitro derivation of functional insulin-producing cells from human embryonic stem cells. Cell Res. 2007;17(4):333–344. doi: 10.1038/cr.2007.28. [DOI] [PubMed] [Google Scholar]
- 17.Ku HT, Zhang N, Kubo A, O'Connor R, Mao M, Keller G, Bromberg JS. Committing embryonic stem cells to early endocrine pancreas in vitro. Stem Cells. 2004;22(7):1205–1217. doi: 10.1634/stemcells.2004-0027. [DOI] [PubMed] [Google Scholar]
- 18.Moritoh Y, Yamato E, Yasui Y, Miyazaki S, Miyazaki J. Analysis of insulin-producing cells during in vitro differentiation from feeder-free embryonic stem cells. Diabetes. 2003;52:1163–1168. doi: 10.2337/diabetes.52.5.1163. [DOI] [PubMed] [Google Scholar]
- 19.Schroeder IS, Rolletschek A, Blyszczuk P, Kania G, Wobus AM. Differentiation of mouse embryonic stem cells to insulin-producing cells. Nat Protoc. 2006;1(2):495–507. doi: 10.1038/nprot.2006.71. [DOI] [PubMed] [Google Scholar]
- 20.Mfopou JK, De Groote V, Xu X, Heimberg H, Bouwens L. Sonic hedgehog and other soluble factors from differentiating embryoid bodies inhibit pancreas development. Stem Cells. 2007;25(5):1156–1165. doi: 10.1634/stemcells.2006-0720. [DOI] [PubMed] [Google Scholar]
- 21.Chen C, Chai J, Singh L, Kuo CY, Jin L, Feng T, Marzano S, Galeni S, Zhang N, Iacovino M, Qin L, Hara M, Stein R, Bromberg JS, Kyba M, Ku HT. Characterization of an in vitro differentiation assay for pancreatic-like cell development from murine embryonic stem cells: detailed gene expression analysis. Assay Drug Dev Technol. 2011;9(4):403–419. doi: 10.1089/adt.2010.0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Otonkoski T, Beattie GM, Mally MI, Ricordi C, Hayek A. Nicotinamide is a potent inducer of endocrine differentiation in cultured human fetal pancreatic cells. J Clin Invest. 1993;92(3):1459–1166. doi: 10.1172/JCI116723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Movassat J, Beattie GM, Lopez AD, Hayek A. Exendin 4 up-regulates expression of PDX 1 and hastens differentiation and maturation of human fetal pancreatic cells. J Clin Endocrinol Metab. 2002;87:4775–4781. doi: 10.1210/jc.2002-020137. [DOI] [PubMed] [Google Scholar]
- 24.Hebrok M, Kim SK, Melton DA. Notochord repression of endodermal Sonic hedgehog permits pancreas development. Genes Dev. 1998;12(11):1705–1713. doi: 10.1101/gad.12.11.1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ku HT, Chai J, Kim YJ, White P, Purohit-Ghelani S, Kaestner KH, Bromberg JS. Insulin-expressing colonies developed from murine embryonic stem cell-derived progenitors. Diabetes. 2007;56(4):921–929. doi: 10.2337/db06-0468. [DOI] [PubMed] [Google Scholar]
- 26.Winkler M, Trieu N, Feng T, Jin L, Walker S, Singh L, Ku HT. A quantitative assay for insulin-expressing colony-forming progenitors. J Vis Exp. 2011;57:e3148. doi: 10.3791/3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Teitelman G, Alpert S, Polak JM, Martinez A, Hanahan D. Precursor cells of mouse endocrine pancreas coexpress insulin, glucagon and the neuronal proteins tyrosine hydroxylase and neuropeptide Y, but not pancreatic polypeptide. Development. 1993;118(4):1031–1039. doi: 10.1242/dev.118.4.1031. [DOI] [PubMed] [Google Scholar]
- 28.Dutton JR, Chillingworth NL, Eberhard D, Brannon CR, Hornsey MA, Tosh D, Slack JM. Beta cells occur naturally in extrahepatic bile ducts of mice. J Cell Sci. 2007;120(Pt 2):239–245. doi: 10.1242/jcs.03330. [DOI] [PubMed] [Google Scholar]
- 29.Sahu S, Joglekar MV, Dumbre R, Phadnis SM, Tosh D, Hardikar AA. Islet-like cell clusters occur naturally in human gall bladder and are retained in diabetic conditions. J Cell Mol Med. 2009;13(5):999–1000. doi: 10.1111/j.1582-4934.2008.00572.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu JC, Heilshorn SC, Tirrell DA. Comparative cell response to artificial extracellular matrix proteins containing the RGD and CS5 cell-binding domains. Biomacromolecules. 2004;5(2):497–504. doi: 10.1021/bm034340z. [DOI] [PubMed] [Google Scholar]
- 31.Jin L, Feng T, Shih HP, Zerda R, Luo A, Hsu J, Mahdavi A, Sander M, Tirrell DA, Riggs AD, Ku HT. Colony-forming cells in the adult mouse pancreas are expandable in Matrigel and form endocrine/acinar colonies in laminin hydrogel. Proc Natl Acad Sci U S A. 2013;110(10):3907–3912. doi: 10.1073/pnas.1301889110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reddy JK, Rao MS, Qureshi SA, Reddy MK, Scarpelli DG, Lalwani ND. Induction and origin of hepatocytes in rat pancreas. J Cell Biol. 1984;98(6):2082–2090. doi: 10.1083/jcb.98.6.2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bonner-Weir S, Taneja M, Weir GC, Tatarkiewicz K, Song KH, Sharma A, O'Neil JJ. In vitro cultivation of human islets from expanded ductal tissue. Proc Natl Acad Sci U S A. 2000;97(14):7999–8004. doi: 10.1073/pnas.97.14.7999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Githens S. The pancreatic duct cell: proliferative capabilities, specific characteristics, metaplasia, isolation, and culture. J Pediatr Gastroenterol Nutr. 1988;7(4):486–506. [PubMed] [Google Scholar]
- 35.Gao R, Ustinov J, Pulkkinen MA, Lundin K, Korsgren O, Otonkoski T. Characterization of endocrine progenitor cells and critical factors for their differentiation in human adult pancreatic cell culture. Diabetes. 2003;52:2007–2015. doi: 10.2337/diabetes.52.8.2007. [DOI] [PubMed] [Google Scholar]
- 36.Wescott MP, Rovira M, Reichert M, von Burstin J, Means A, Leach SD, Rustgi AK. Pancreatic ductal morphogenesis and the Pdx1 homeodomain transcription factor. Mol Biol Cell. 2009;20(22):4838–4844. doi: 10.1091/mbc.E09-03-0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Narsinh KH, Sun N, Sanchez-Freire V, Lee AS, Almeida P, Hu S, Jan T, Wilson KD, Leong D, Rosenberg J, Yao M, Robbins RC, Wu JC. Single cell transcriptional profiling reveals heterogeneity of human induced pluripotent stem cells. J Clin Invest. 2011;121(3):1217–1221. doi: 10.1172/JCI44635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Devaskar SU, Singh BS, Carnaghi LR, Rajakumar PA, Giddings SJ. Insulin II gene expression in rat central nervous system. Regul Pept. 1993;48:55–63. doi: 10.1016/0167-0115(93)90335-6. [DOI] [PubMed] [Google Scholar]
- 39.Sugiyama T, Rodriguez RT, McLean GW, Kim SK. Conserved markers of fetal pancreatic epithelium permit prospective isolation of islet progenitor cells by FACS. Proc Natl Acad Sci U S A. 2007;104(1):175–180. doi: 10.1073/pnas.0609490104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oshima Y, Suzuki A, Kawashimo K, Ishikawa M, Ohkohchi N, Taniguchi H. Isolation of mouse pancreatic ductal progenitor cells expressing CD133 and c-Met by flow cytometric cell sorting. Gastroenterology. 2007;132(2):720–732. doi: 10.1053/j.gastro.2006.11.027. [DOI] [PubMed] [Google Scholar]
- 41.Lardon J, Corbeil D, Huttner WB, Ling Z, Bouwens L. Stem cell marker prominin-1/AC133 is expressed in duct cells of the adult human pancreas. Pancreas. 2008;36(1):e1–e6. doi: 10.1097/mpa.0b013e318149f2dc. [DOI] [PubMed] [Google Scholar]
- 42.Hori Y, Fukumoto M, Kuroda Y. Enrichment of putative pancreatic progenitor cells from mice by sorting for prominin1 (CD133) and platelet-derived growth factor receptor beta. Stem Cells. 2008;26(11):2912–2920. doi: 10.1634/stemcells.2008-0192. [DOI] [PubMed] [Google Scholar]
- 43.Rountree CB, Barsky L, Ge S, Zhu J, Senadheera S, Crooks GM. A CD133-expressing murine liver oval cell population with bilineage potential. Stem Cells. 2007;25(10):2419–2429. doi: 10.1634/stemcells.2007-0176. [DOI] [PubMed] [Google Scholar]
- 44.Kain R, Matsui K, Exner M, Binder S, Schaffner G, Sommer EM, Kerjaschki D. A novel class of autoantigens of anti-neutrophil cytoplasmic antibodies in necrotizing and crescentic glomerulonephritis: the lysosomal membrane glycoprotein h-lamp-2 in neutrophil granulocytes and a related membrane protein in glomerular endothelial cells. J Exp Med. 1995;181(2):585–597. doi: 10.1084/jem.181.2.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schneede A, Schmidt CK, Holtta-Vuori M, Heeren J, Willenborg M, Blanz J, Domanskyy M, Breiden B, Brodesser S, Landgrebe J, Sandhoff K, Ikonen E, Saftig P, Eskelinen EL. Role for LAMP-2 in endosomal cholesterol transport. J Cell Mol Med. 2011;15(2):280–295. doi: 10.1111/j.1582-4934.2009.00973.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ogawa M. Differentiation and proliferation of hematopoietic stem cells. Blood. 1993;81(11):2844–2253. [PubMed] [Google Scholar]
- 47.Kleinman HK, Martin GR. Matrigel: basement membrane matrix with biological activity. Semin Cancer Biol. 2005;15(5):378–386. doi: 10.1016/j.semcancer.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 48.Mizrak D, Brittan M, Alison M. CD133: molecule of the moment. J Pathol. 2008;214(1):3–9. doi: 10.1002/path.2283. [DOI] [PubMed] [Google Scholar]
- 49.de Jong JL, Zon LI. Use of the zebrafish system to study primitive and definitive hematopoiesis. Annu Rev Genet. 2005;39:481–501. doi: 10.1146/annurev.genet.39.073003.095931. [DOI] [PubMed] [Google Scholar]
- 50.Deutsch G, Jung J, Zheng M, Lora J, Zaret KS. A bipotential precursor population for pancreas and liver within the embryonic endoderm. Development. 2001;128(6):871–881. doi: 10.1242/dev.128.6.871. [DOI] [PubMed] [Google Scholar]
- 51.Kaneto H, Nakatani Y, Miyatsuka T, Matsuoka TA, Matsuhisa M, Hori M, Yamasaki Y. PDX-1/VP16 fusion protein, together with NeuroD or Ngn3, markedly induces insulin gene transcription and ameliorates glucose tolerance. Diabetes. 2005;54(4):1009–1022. doi: 10.2337/diabetes.54.4.1009. [DOI] [PubMed] [Google Scholar]
- 52.Zalzman M, Anker-Kitai L, Efrat S. Differentiation of human liver-derived, insulin-producing cells toward the beta-cell phenotype. Diabetes. 2005;54(9):2568–2575. doi: 10.2337/diabetes.54.9.2568. [DOI] [PubMed] [Google Scholar]
- 53.Sapir T, Shternhall K, Meivar-Levy I, Blumenfeld T, Cohen H, Skutelsky E, Eventov-Friedman S, Barshack I, Goldberg I, Pri-Chen S. et al. Cell-replacement therapy for diabetes: Generating functional insulin-producing tissue from adult human liver cells. Proc Natl Acad Sci U S A. 2005;102(22):7964–7969. doi: 10.1073/pnas.0405277102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang AY, Ehrhardt A, Xu H, Kay MA. Adenovirus transduction is required for the correction of diabetes using Pdx-1 or Neurogenin-3 in the liver. Mol Ther. 2007;15(2):255–263. doi: 10.1038/sj.mt.6300032. [DOI] [PubMed] [Google Scholar]
- 55.Aviv V, Meivar-Levy I, Rachmut IH, Rubinek T, Mor E, Ferber S. Exendin-4 promotes liver cell proliferation and enhances the PDX-1-induced liver to pancreas transdifferentiation process. J Biol Chem. 2009;284(48):33509–33520. doi: 10.1074/jbc.M109.017608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cardinale V, Wang Y, Carpino G, Cui CB, Gatto M, Rossi M, Berloco PB, Cantafora A, Wauthier E, Furth ME. et al. Multipotent stem/progenitor cells in human biliary tree give rise to hepatocytes, cholangiocytes, and pancreatic islets. Hepatology. 2011;54(6):2159–2172. doi: 10.1002/hep.24590. [DOI] [PubMed] [Google Scholar]
- 57.Banga A, Akinci E, Greder LV, Dutton JR, Slack JM. In vivo reprogramming of Sox9+ cells in the liver to insulin-secreting ducts. Proc Natl Acad Sci U S A. 2012;109(38):15336–15341. doi: 10.1073/pnas.1201701109. [DOI] [PMC free article] [PubMed] [Google Scholar]


