Abstract
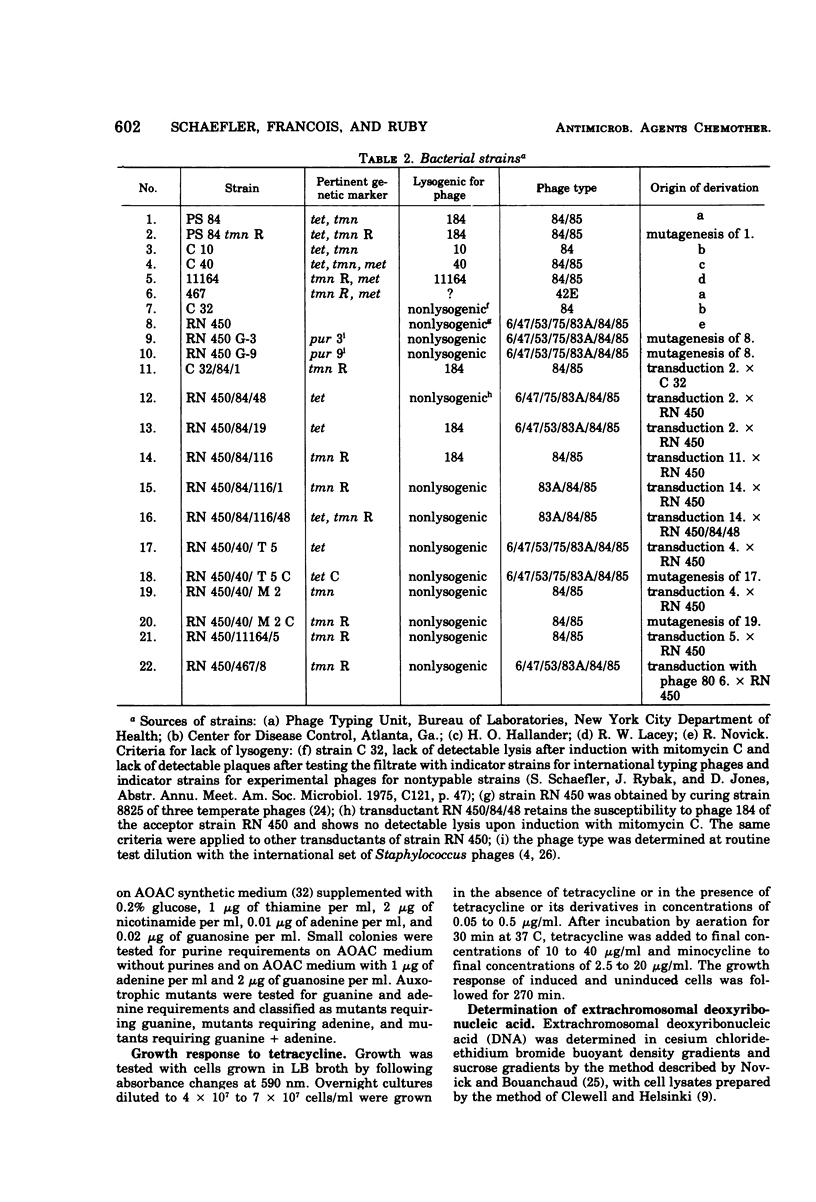
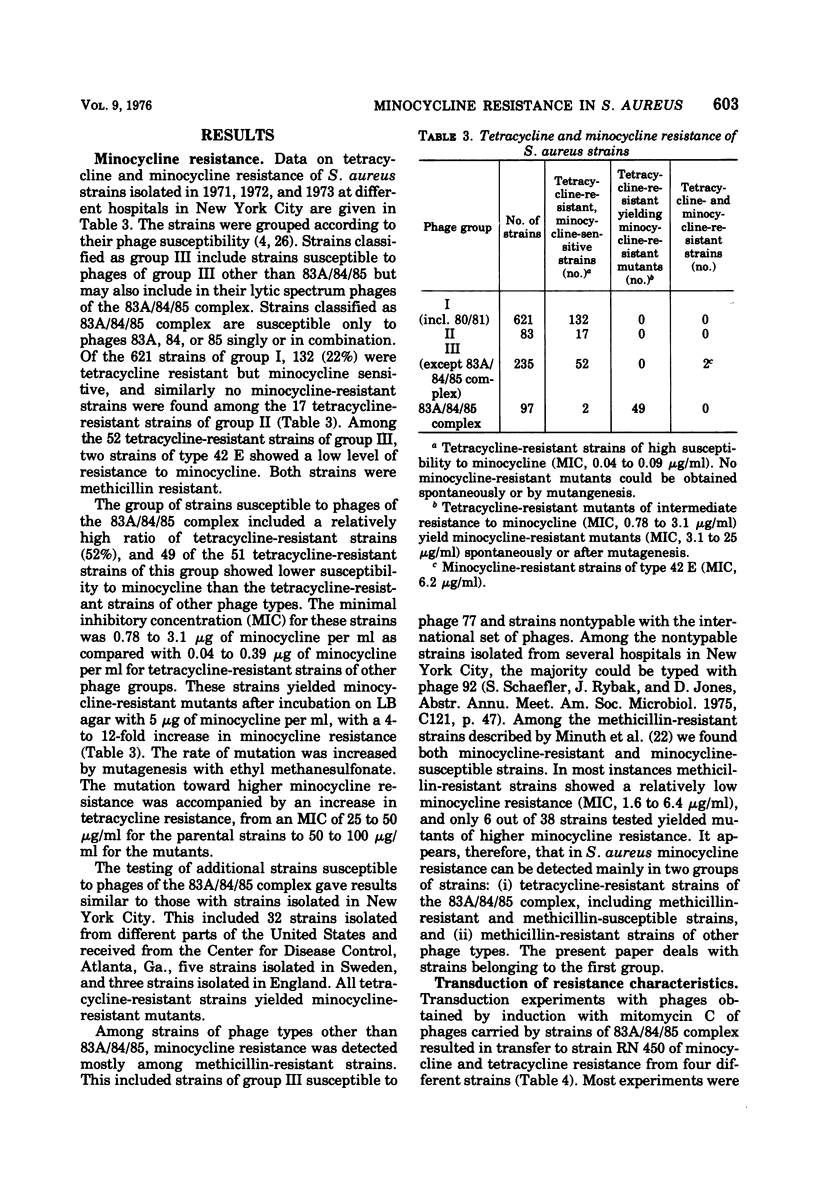
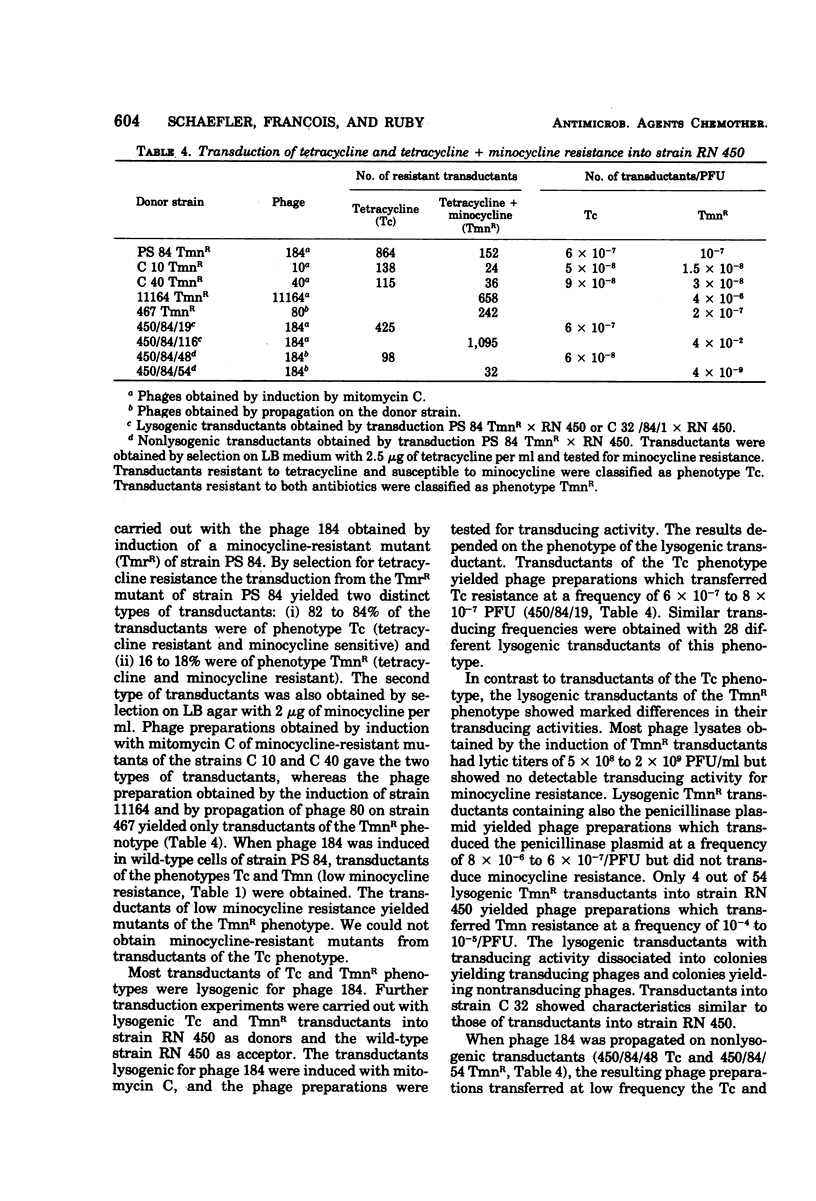
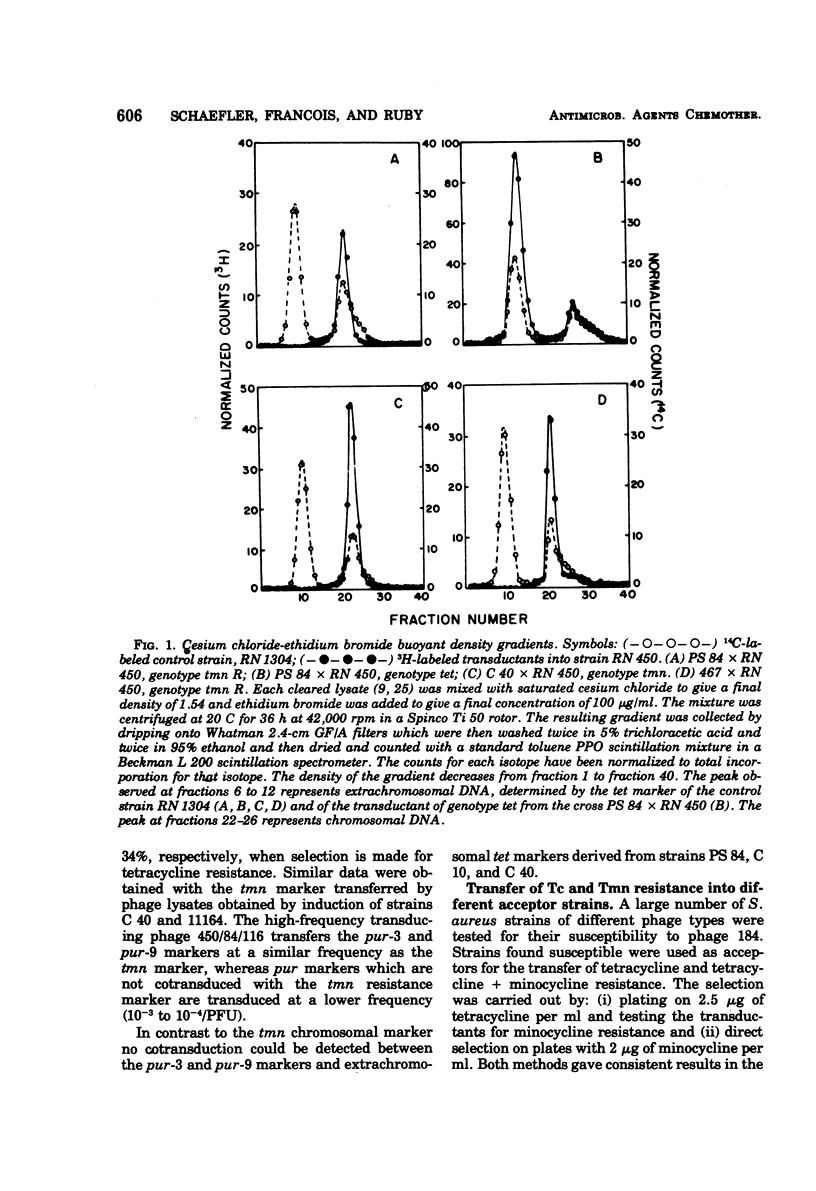
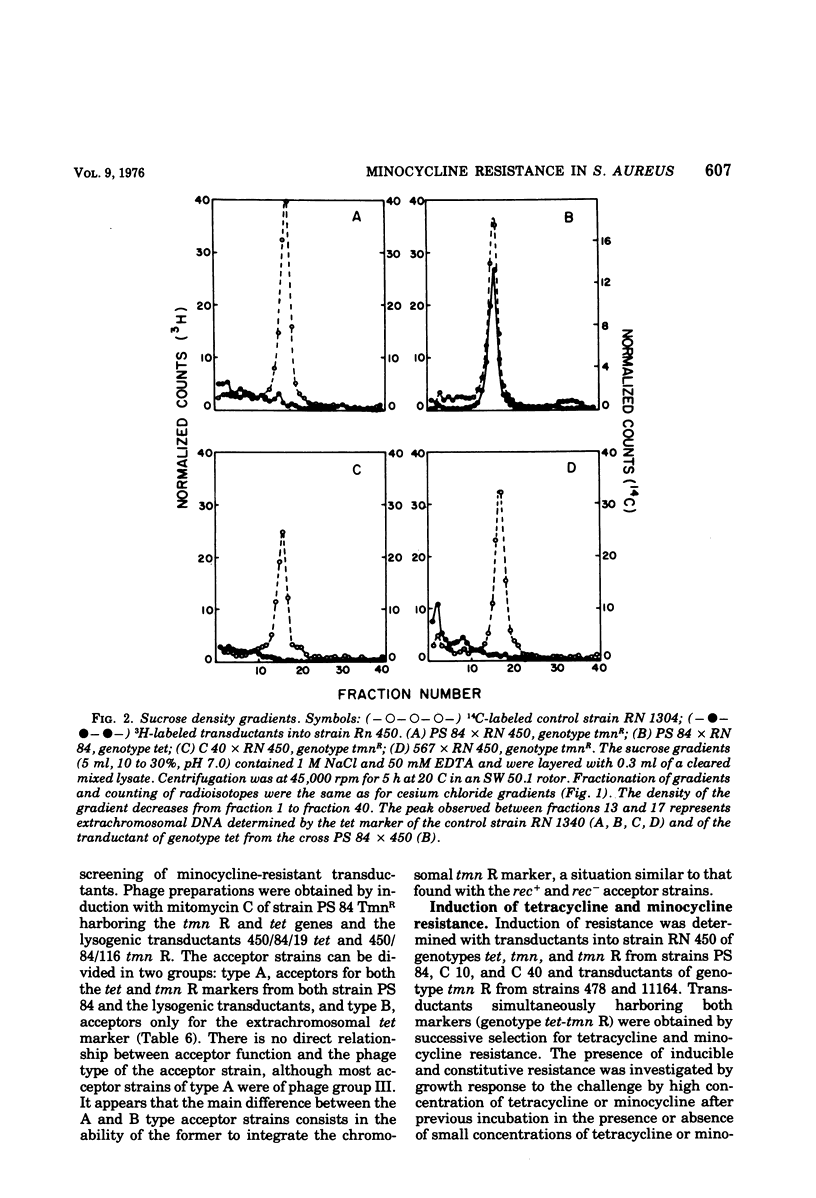
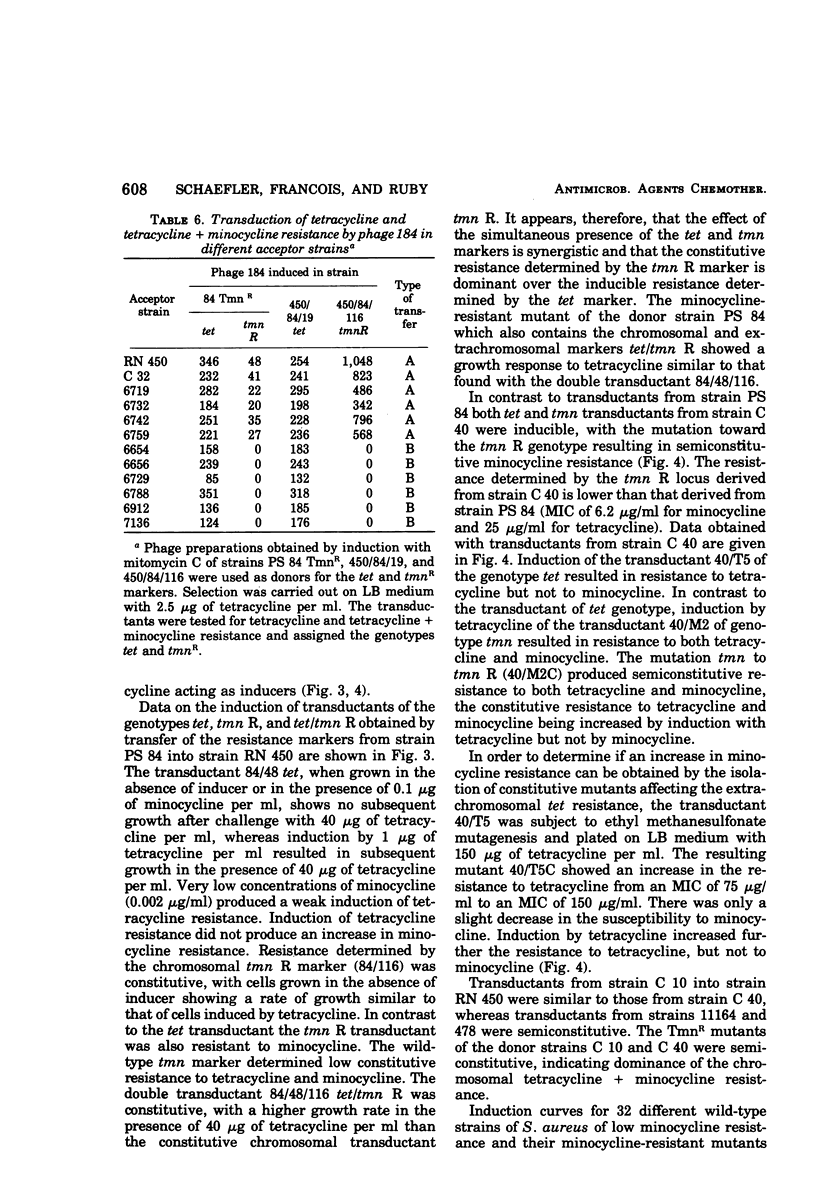
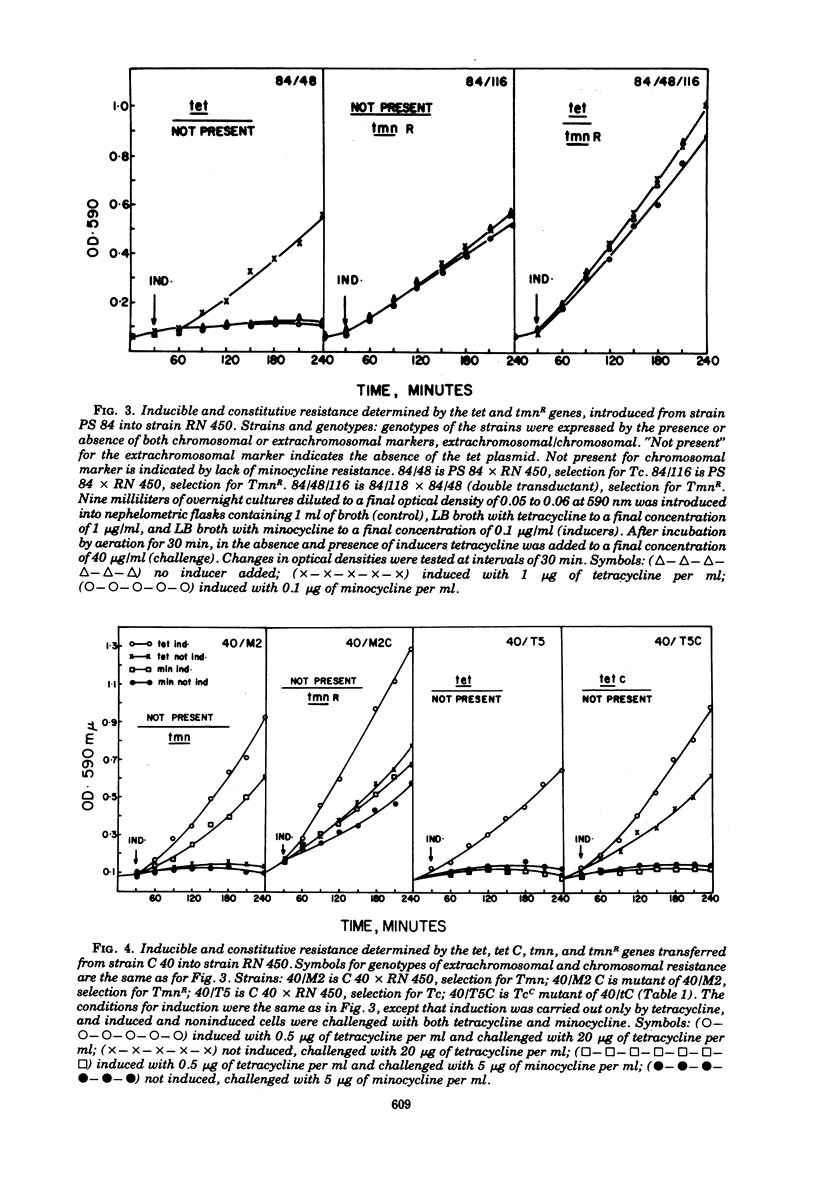
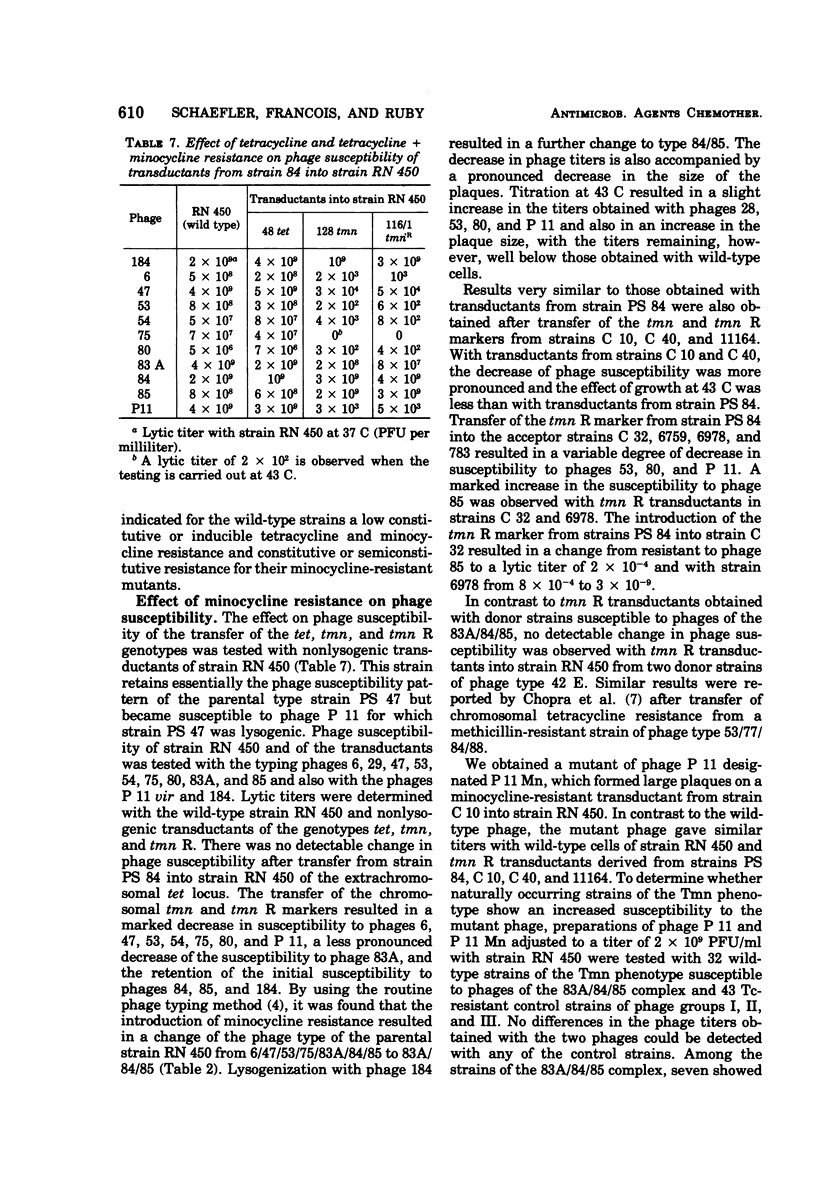
Tetracycline-resistant strains of Staphylococcus aureus are minocycline sensitive, with the exception of strains susceptible to phages of the 83A/84/85 complex and some methicillin-resistant strains of other phage types. Strains of the 83A/84/85 complex yield mutants with increased minocycline resistance. Transduction of minocycline resistance into the susceptible strain RN 450 was obtained with donor strains possessing either markers for both extrachromosomal tetracycline resistance (tet) and chromosomal tetracycline + minocycline resistance (tmn R), or only for chromosomal tmn R resistance. The chromosomal marker was differentiated from the extrachromosomal marker by the lack of detectable extrachromosomal deoxyribonucleic acid after transfer into strain RN 450, transfer into a rec+ strain, lack of transfer into rec− acceptor strain, and cotransduction with chromosomal determinants for guanine biosynthesis. Both chromosomal and extrachromosomal tetracycline resistance can be induced by tetracycline. Induction by tetracycline of chromosomal tetracycline resistance resulted in simultaneous induction of minocycline resistance. The mutation toward increased minocycline resistance (tmn → tmn R) is a regulatory mutation toward constitutivity or semiconstitutivity. Constitutive resistance is dominant in tmn R/tet diploids. Transfer of the tet marker does not affect the phage susceptibility of the acceptor strain. The tmn R marker, originating from donor strains of the 83A/84/85 complex, renders strain RN 450 resistant to several typing phages, with the exception of phages of the 83A/84/85 complex. This could possibly account for the phage typing patterns of minocycline-resistant staphylococci.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asheshov E. H. The genetics of tetracycline resistance in Staphylococcus aureus. J Gen Microbiol. 1975 May;88(1):132–140. doi: 10.1099/00221287-88-1-132. [DOI] [PubMed] [Google Scholar]
- Avtalion R. R., Ziegler-Schlomowitz R., Pearl M., Wojdani A., Sompolinsky D. Depressed resistance to tetracycline in Staphylococcus aureus. Microbios. 1971 Mar;3(10):165–180. [PubMed] [Google Scholar]
- Bauer A. W., Kirby W. M., Sherris J. C., Turck M. Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol. 1966 Apr;45(4):493–496. [PubMed] [Google Scholar]
- Boldur I., Sompolinsky D. Antigen specific for bacteria resistant to tetracycline. Antimicrob Agents Chemother. 1974 Aug;6(2):117–120. doi: 10.1128/aac.6.2.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bülow P. Prevalence of extrachromosomal drug resistance. Staphylococci in Danish hospitals during the last decade: factors influencing some properties of predominant epidemic strains. Ann N Y Acad Sci. 1971 Jun 11;182:21–39. doi: 10.1111/j.1749-6632.1971.tb30640.x. [DOI] [PubMed] [Google Scholar]
- Chopra I., Lacey R. W., Connolly J. Biochemical and genetic basis of tetracycline resistance in Staphylococcus aureus. Antimicrob Agents Chemother. 1974 Oct;6(4):397–404. doi: 10.1128/aac.6.4.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuit C. F., Pitton J. S. Non-transferable tetracycline resistance in Escherichia coli K 12. Chemotherapy. 1969;14(4):253–257. doi: 10.1159/000220634. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Supercoiled circular DNA-protein complex in Escherichia coli: purification and induced conversion to an opern circular DNA form. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1159–1166. doi: 10.1073/pnas.62.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorko J., Katz S., Allnoch H. In vitro activity of minocycline, a new tetracycline. Am J Med Sci. 1968 Apr;255:252–258. doi: 10.1097/00000441-196804000-00006. [DOI] [PubMed] [Google Scholar]
- Franklin T. J., Cook J. M. R factor with a mutation in the tetracycline resistance marker. Nature. 1971 Jan 22;229(5282):273–274. doi: 10.1038/229273a0. [DOI] [PubMed] [Google Scholar]
- Franklin T. J., Higginson B. Active accumulation of tetracycline by Escherichia coli. Biochem J. 1970 Jan;116(2):287–297. doi: 10.1042/bj1160287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin T. J. Resistance of Escherichia coli to tetracyclines. Changes in permeability to tetracyclines in Escherichia coli bearing transferable resistance factors. Biochem J. 1967 Oct;105(1):371–378. doi: 10.1042/bj1050371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessen O., Rosendal K., Bülow P., Faber V., Eriksen K. R. Changing staphylococci and staphylococcal infections. A ten-year study of bacteria and cases of bacteremia. N Engl J Med. 1969 Sep 18;281(12):627–635. doi: 10.1056/NEJM196909182811201. [DOI] [PubMed] [Google Scholar]
- Kayser F. H., Wüst J., Corrodi P. Transduction and elimination of resistance determinants in methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 1972 Sep;2(3):217–223. doi: 10.1128/aac.2.3.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klastersky J., Daneau D. Bacteriological evaluation of minocycline, a new tetracycline. Chemotherapy. 1972;17(1):51–58. doi: 10.1159/000220838. [DOI] [PubMed] [Google Scholar]
- Kuck N. A., Forbes M. Uptake of minocycline and tetracycline by tetracycline-susceptible and -resistant bacteria. Antimicrob Agents Chemother. 1973 Jun;3(6):662–664. doi: 10.1128/aac.3.6.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacey R. W. Genetic control in methicillin-resistant strains of Staphylococcus aureus. J Med Microbiol. 1972 Nov;5(4):497–508. doi: 10.1099/00222615-5-4-497. [DOI] [PubMed] [Google Scholar]
- Lacey R. W., Grinsted J. Genetic analysis of methicillin-resistant strains of Staphylococcus aureus; evidence for their evolution from a single clone. J Med Microbiol. 1973 Nov;6(4):511–526. doi: 10.1099/00222615-6-4-511. [DOI] [PubMed] [Google Scholar]
- Levy S. B., McMurry L. Detection of an inducible membrane protein associated with R-factor-mediated tetracycline resistance. Biochem Biophys Res Commun. 1974 Feb 27;56(4):1060–1068. doi: 10.1016/s0006-291x(74)80296-2. [DOI] [PubMed] [Google Scholar]
- MAY J. W., HOUGHTON R. H., PERRET C. J. THE EFFECT OF GROWTH AT ELEVATED TEMPERATURES ON SOME HERITABLE PROPERTIES OF STAPHYLOCOCCUS AUREUS. J Gen Microbiol. 1964 Nov;37:157–169. doi: 10.1099/00221287-37-2-157. [DOI] [PubMed] [Google Scholar]
- Minuth J. N., Holmes T. M., Musher D. M. Activity of tetracycline, doxycycline, and minocycline against methicillin-susceptible and -resistant staphylococci. Antimicrob Agents Chemother. 1974 Oct;6(4):411–414. doi: 10.1128/aac.6.4.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOVICK R. P. ANALYSIS BY TRANSDUCTION OF MUTATIONS AFFECTING PENICILLINASE FORMATION IN STAPHYLOCOCCUS AUREUS. J Gen Microbiol. 1963 Oct;33:121–136. doi: 10.1099/00221287-33-1-121. [DOI] [PubMed] [Google Scholar]
- Novick R. P., Bouanchaud D. The problems of drug-resistant pathogenic bacteria. Extrachromosomal nature of drug resistance in Staphylococcus aureus. Ann N Y Acad Sci. 1971 Jun 11;182:279–294. doi: 10.1111/j.1749-6632.1971.tb30664.x. [DOI] [PubMed] [Google Scholar]
- Novick R. Properties of a cryptic high-frequency transducing phage in Staphylococcus aureus. Virology. 1967 Sep;33(1):155–166. doi: 10.1016/0042-6822(67)90105-5. [DOI] [PubMed] [Google Scholar]
- Phair J. P., Carleton J. Susceptibility of Staphylococci to Minocycline In Vitro: Identification of Group III Bacteriophage Types by Characteristic Inhibition of Growth. Infect Immun. 1970 Nov;2(5):669–671. doi: 10.1128/iai.2.5.669-671.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeve E. C., Robertson J. M. The characteristics of eleven mutants of R-factor R57 constitutive for tetracycline resistance, selected and tested in Escherichia coli K12. Genet Res. 1975 Jun;25(3):297–312. doi: 10.1017/s001667230001572x. [DOI] [PubMed] [Google Scholar]
- Robertson J. M., Reeve E. C. Analysis of the resistance mediated by several R-factors to tetracycline and minocycline. Genet Res. 1972 Oct;20(2):239–252. doi: 10.1017/s0016672300013744. [DOI] [PubMed] [Google Scholar]
- Schaefler S. Staphylococcus epidermidis BV: antibiotic resistance patterns, physiological characteristics, and bacteriophage susceptibility. Appl Microbiol. 1971 Oct;22(4):693–699. doi: 10.1128/am.22.4.693-699.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sompolinsky D., Krausz J. Action of 12 tetracyclines on susceptible and resistant strains of Staphylococcus aureus. Antimicrob Agents Chemother. 1973 Sep;4(3):237–247. doi: 10.1128/aac.4.3.237. [DOI] [PMC free article] [PubMed] [Google Scholar]


