Abstract
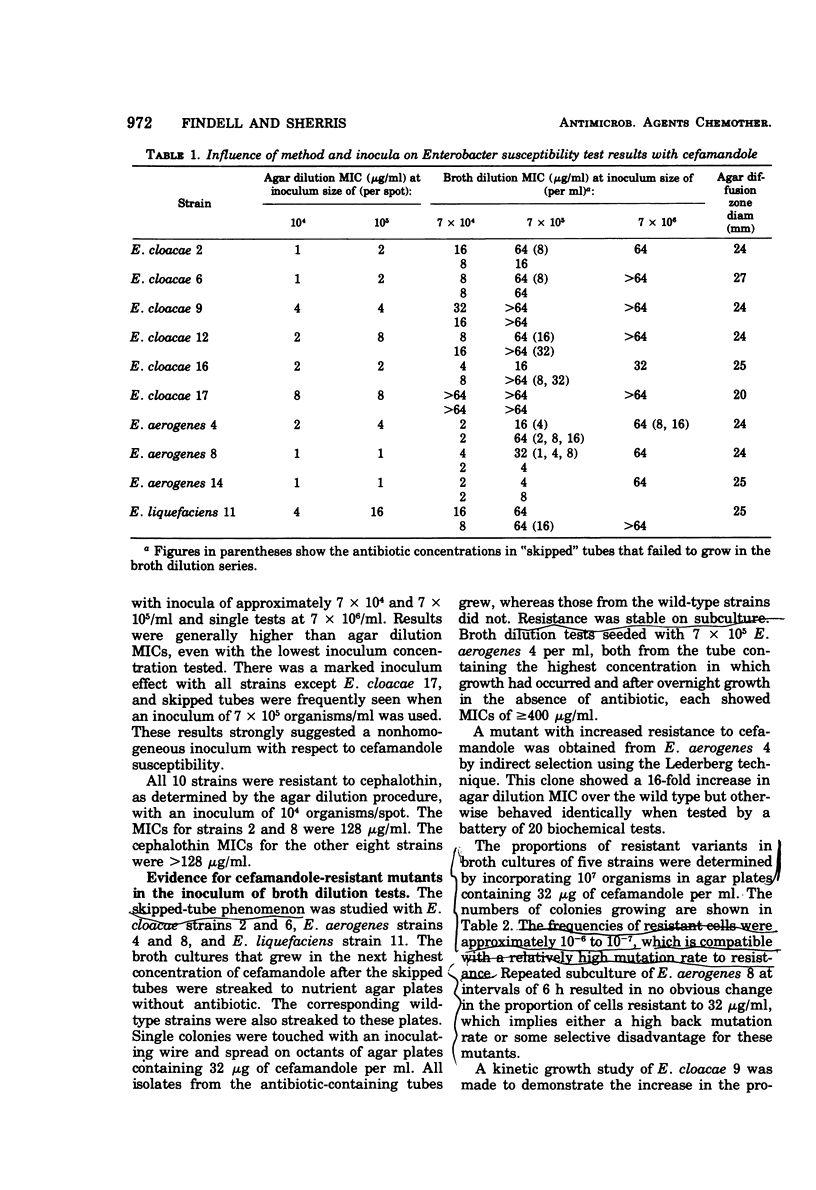
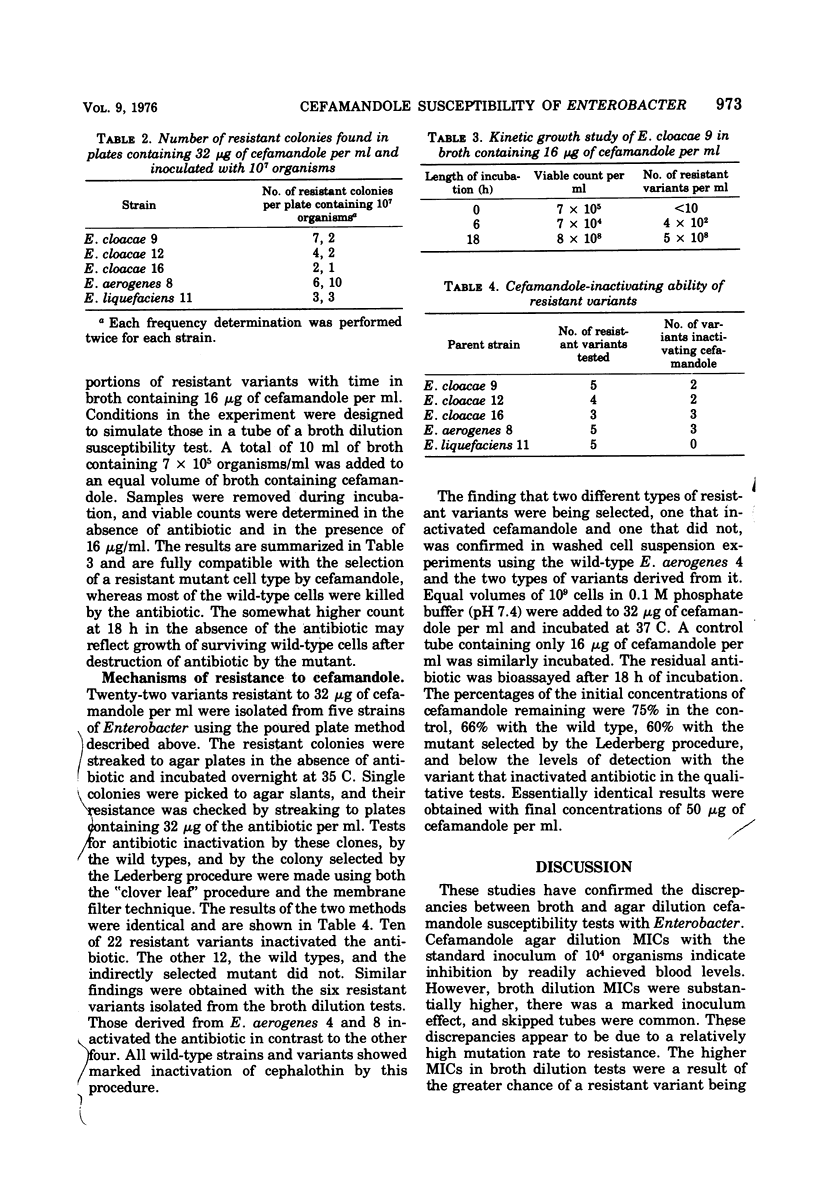
Cefamandole minimum inhibitory concentrations (MICs) of 10 strains of Enterobacter were determined by the ICS agar dilution and broth dilution procedures. Agar dilution MICs ranged from 1 to 8 μg/ml, with an inoculum of 104 organisms/spot. Broth dilution MICs were consistently higher, with an inoculum of approximately 7 × 105 organisms/ml. Seven strains showed MICs of ≥64 μg/ml. There was a marked inoculum effect in broth, and skipped tubes were often observed. Variants resistant to 32 μg/ml or more were isolated by direct selection and were shown to occur at a frequency of approximately 10−6 to 10−7. A mutant showing a 16-fold increase in agar dilution MIC was also isolated by indirect selection. These variants and others isolated from broth in the presence of cefamandole were tested for ability to inactivate the antibiotic, using both a biological and a chemical procedure. Two distinct classes of variants were seen. Twelve of 28 were shown by both methods to inactivate the antibiotic, whereas the others, including the indirectly selected mutant, did not. The wild types were also negative by both tests. The higher cefamandole MICs of Enterobacter in broth, thus, appeared to reflect a high frequency of resistant variants that were not detected with the inoculum and end point criteria usually used in agar dilution methods. The ability of some variants to inactivate cefamandole may have resulted from a mutation that extended the activity of Enterobacter cephalosporinase to include this antibiotic.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BENNER E. J., MICKLEWAIT J. S., BRODIE J. L., KIRBY W. M. NATURAL AND ACQUIRED RESISTANCE OF KLEBSIELLA-AEROBACTER TO CEPHALOTHIN AND CEPHALORIDINE. Proc Soc Exp Biol Med. 1965 Jun;119:536–541. doi: 10.3181/00379727-119-30231. [DOI] [PubMed] [Google Scholar]
- Ericsson H. M., Sherris J. C. Antibiotic sensitivity testing. Report of an international collaborative study. Acta Pathol Microbiol Scand B Microbiol Immunol. 1971;217(Suppl):1+–1+. [PubMed] [Google Scholar]
- Eykyn S., Jenkins C., King A., Phillips I. Antibacterial activity of cefamandole, a new cephalosporin antibiotic, compared with that of cephaloridine, cephalothin, and cephalexin. Antimicrob Agents Chemother. 1973 Jun;3(6):657–661. doi: 10.1128/aac.3.6.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FLEMING P. C., GOLDNER M., GLASS D. G. Observations on the nature, distribution, and significance of cephalosporinase. Lancet. 1963 Jun 29;1(7296):1399–1401. doi: 10.1016/s0140-6736(63)92051-8. [DOI] [PubMed] [Google Scholar]
- Farrar W. E., Krause J. M. Relationship Between beta-Lactamase Activity and Resistance of Enterobacter to Cephalothin. Infect Immun. 1970 Nov;2(5):610–616. doi: 10.1128/iai.2.5.610-616.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldner M., Glass D. G., Fleming P. C. Characteristics of Aerobacter beta-lactamase. Can J Microbiol. 1968 Feb;14(2):139–145. doi: 10.1139/m68-023. [DOI] [PubMed] [Google Scholar]
- Goldner M., Glass D. G., Fleming P. C. Spontaneous mutant with loss of beta-lactamase in Aerobacter cloacae. J Bacteriol. 1969 Feb;97(2):961–961. doi: 10.1128/jb.97.2.961-.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennessey T. D. Inducible beta-lactamase in Enterobacter. J Gen Microbiol. 1967 Nov;49(2):277–285. doi: 10.1099/00221287-49-2-277. [DOI] [PubMed] [Google Scholar]
- KNOX R., SMITH J. T. Use of cellulose acetate membranes for detecting penicillinase-producing organisms. Nature. 1961 Aug 26;191:926–927. doi: 10.1038/191926a0. [DOI] [PubMed] [Google Scholar]
- LEDERBERG J., LEDERBERG E. M. Replica plating and indirect selection of bacterial mutants. J Bacteriol. 1952 Mar;63(3):399–406. doi: 10.1128/jb.63.3.399-406.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu H. C. Cefamandole, a cephalosporin antibiotic with an unusually wide spectrum of activity. Antimicrob Agents Chemother. 1974 Aug;6(2):177–182. doi: 10.1128/aac.6.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu H. C., Winshell E. B. Relation of beta-lactamase activity and cellular location to resistance of Enterobacter to penicillins and cephalosporins. Antimicrob Agents Chemother. 1972 Feb;1(2):107–111. doi: 10.1128/aac.1.2.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabath L. D., Casey J. I., Ruch P. A., Stumpf L. L., Finland M. Rapid microassay of gentamicin, kanamycin, neomycin, streptomycin, and vancomycin in serum or plasma. J Lab Clin Med. 1971 Sep;78(3):457–463. [PubMed] [Google Scholar]
- Wick W. E., Preston D. A. Biological properties of three 3-heterocyclic-thiomethyl cephalosporin antibiotics. Antimicrob Agents Chemother. 1972 Mar;1(3):221–234. doi: 10.1128/aac.1.3.221. [DOI] [PMC free article] [PubMed] [Google Scholar]


