Abstract
Autosomal dominant polycystic kidney disease (ADPKD) is an inherited genetic disorder that results in progressive renal cyst formation with ultimate loss of renal function and other systemic disorders. These systemic disorders include abnormalities in cardiovascular, portal, pancreatic and gastrointestinal systems. ADPKD is considered to be among the ciliopathy diseases due to the association with abnormal primary cilia function. In order to understand the full course of primary cilia and its association with ADPKD, the structure, functions and role of primary cilia have been meticulously investigated. As a result, the focus on primary cilia has emerged to support the vital roles of primary cilia in ADPKD. The primary cilia have been shown to have not only a mechanosensory function but also a chemosensory function. Both structural and functional defects in primary cilia result in cystic kidney disease and vascular hypertension. Thus, the mechanosenory and chemosensory functions will be analyzed in regards to ADPKD.
Keywords: aneurysm, chemosensory, hypertension, renal epithelia, vascular endothelia
1. Introduction
Autosomal dominant polycystic kidney disease (ADPKD) is the most common inherited cystic renal disease and is considered the most common single gene disorder of the kidneys. ADPKD affects approximately 600,000 people in the United States and 1:400 to 1:1000 people worldwide [1,2]. ADPKD is a systemic disorder that includes a variety of renal and extra-renal abnormalities that ultimately result in cystic and non-cystic features. The main clinical characteristic of the disease, however, is the progressive increase in the number and size of renal cysts, with secondary destruction of renal parenchyma.
In a prospective clinical study, Grantham JJ et al showed that the total kidney volume in ADPKD patients increased from 204 ml to 218 ml over 3 years, which estimates the total cyst volume increase as 5.27% per year [3]. On the other hand, renal function deterioration was estimated as a decline in glomerular filtration rate of 4.3 ± 8.1 mL/min per year. This ultimately leads to end stage renal disease (ESRD) in about 43–48% of patients by 58–73 years of age [4].
In addition to renal cysts, renal manifestations include urinary tract infection, flank pain, hematuria, nephrolithiasis and renal failure [5,6,7]. Extra-renal cystic features are also developed in organs including the liver, pancreas, ovaries and choroid plexus. Cardiovascular abnormalities include vascular hypertension, left ventricular hypertrophy, intracranial aneurysms, aortic aneurysms, arachnoid aneurysms, cerebral artery dolichoectasia, mitral valve prolapse, mitral regurgitation, aortic insufficiency, and tricuspid regurgitation. Although renal characteristics are prominent features, the cardiovascular abnormalities are responsible for 80% more deaths in ADPKD than ESRD. Furthermore, intracranial aneurysms affect 4–12% of ADPKD patients, with a risk of rupture about five-fold more than in the general population. Thus, aneurysm rupture is considered a serious complication threatening the lives of ADPKD patients [6,8].
Most importantly, ADPKD is a pathology associated with cilia dysfunction, also known as ciliopathy [9,10]. The primary cilium is a solitary “9 + 0” microtubule-based, hair-like organelle anchored to the mother centriole and projecting from the surface of almost all mammalian cells. In addition to the wide range of sensory functions, primary cilia are also critical for developmental and physiological functions. Historically, the story of this cellular antenna is really interesting; it was first described by Zimmermann as early as 1898 [11]. Since that time, primary cilia were regarded as nonfunctional remnants from evolution. As a result, the research on primary cilia was relatively limited until the last decade, when extensive research has been focused on this organelle. Moreover, an assessment of the research done on primary cilia in the last five decades, using PubMed search, revealed that the research comprised only about 10% of the total research performed on primary cilia from 1960 to 2000. This means that primary cilia research increased nine-fold in the last decade compared to the previous four decades. Therefore, not surprisingly, the rapidly growing focus on primary cilia since the year of 2000 has attracted researchers’ interest to uncover many unknown entities and relate them to diseases associated with defective cilia structure/function.
Structurally, the primary cilium composed of five main compartments [12] (Figure 1). Theaxoneme is composed of nine parallel pairs of microtubules posttranslationally acetylated to support the long structure. These microtubules are arranged circumferentially, without a central pair like the one that is always seen in motile cilia. The ciliary membrane houses many receptors, ion channels, transporters and sensory proteins that serve definitive functions. Many of these proteins are not yet completely established. Some of those receptors are localized to the ciliary membrane only at a certain time to perform a defined function and then translocated out of the cilia. Cilioplasm is constituted of the soluble compartment of the cilia. It has been recently proposed that cilioplasm acts as a calcium signaling compartment in response to mechanical and chemical stimuli [13]. Cilioplasm is also enriched with many other signaling proteins. Thus, this dynamic compartment includes mainly two types of proteins, signaling and transport (such as intraflagellar transport) proteins (like IFT proteins). Both signaling and transport proteins are required to coordinate a key role in cilia assembly and function. The basal body is a mother centriole to which the ciliary axoneme is rooted. In addition to its vital structural role, the basal body houses many signaling proteins that serve various functions. The transition zone region composes of transition zone and fibers. The region connects basal body and ciliary axoneme and plays critical roles in ciliogenesis and ciliary access [14].
Figure 1.
The primary cilium is composed of ciliary membrane, cilioplasm, axoneme and basal body. Basal bodies are composed of transition fiber (orange), centrioles (red), basal foot and cap (black) and basal body anchorage (blue). The ciliary membrane and axoneme make up the upper part of the primary cilia.
2. Roles of Primary Cilia
Owing to the unique localization of a variety of receptors, ion channels, transport proteins and signaling proteins, primary cilia serve a broad range of functions. Recent ciliary genomics and proteomics data sets have estimated that the vertebrate cilium function might involve about 1000 different polypeptides [15]. Working as a cellular antenna, primary cilia sense and conduct a range of signaling pathways from mechanical and chemical stimuli [16,17,18]. The ciliary pathways studied include signaling through calcium, sonic hedgehog, Wnt, mTOR, JAK/STAT, and MAPK, among a growing list. These signaling pathways play a key role in various vital cellular processes like development, differentiation, cell cycle, apoptosis, tissue homeostasis and planar cell polarity [19].
Apart from playing a chemosensory role manifested as receiving extracellular information, primary cilia may also perform the opposite “chemosecretory” function, manifested as releasing information to the extracellular environment [20]. This new area of research is supported by a study revealing that polyductin, a ciliary membrane protein, undergoes proteolytic cleavage with the release of extracellular domain into the lumen [21]. In addition, polycystin-1 is shown to undergo cleavage with the secretion of a small amount of N-terminal domain to the extracellular environment [22]. Furthermore, membrane-sheathed objects carry Shh and retinoic acid secreted from the ciliated cells of the embryonic node in response to fluid flow, critical for left-right determination [23]. Equally important, many PKD-associated proteins form exosome-like vesicles, which are shed in the urine [24]. Exosomes are produced by the cell and released from the cell membrane. Because the exosomes emerge from an intracellular vesicle near the base of the cilium, the authors suggest that some exosomes proteins are derived from cilia. Furthermore, exosomes interact with and adhere to ciliary membrane. Although the shedding of these proteins has an unknown function, the idea of ciliary chemosensory function is interesting and worth further investigation.
3. Mechanosensory and Chemosensory Cilia Functions
Functioning as cellular antennae, primary cilia receive a complex pool of external stimuli and transduce them into intracellular signaling to control an expanding list of cellular functions. These external stimuli may consist of physical stresses like flow and pressure, or chemical substances like ligand, growth factor and morphogen. One of the most studied ciliary functions is mechanosensation, which is a flow sensing ability of the primary cilium to sense the overpassing fluid. Genetically manipulated non-ciliated cells or chemically ablated cilia from ciliated cells are found to be mechano-insensitive to fluid flow, supporting ciliary mechanosensory function [19,25]. It is generally accepted that polycystin-1 and -2 are two of many ciliary proteins responsible for the mechanosensing function attributed to the primary cilia [26,27,28]. Furthermore, the ciliary bending model in response to fluid dynamics, hypothesized by Schwartz et al [29], has gained more support through recent studies with different experimental designs [30,31]. In this model, the flexural rigidity of primary cilia is calculated to predict the cilium bending behavior, where a “heavy elastica” model is validated to interpret the mechanosensory function as a result of cilium bending. Recently, our laboratory further confirmed cilia bending-induced calcium signaling [13]. Our data show that cilium bending causes cytoskeletal deformation, and there is a lag time between bending, which is fast, and the delayed cytosolic calcium increase. Cilium bending results in stress building up on the cell membrane, caused by the stretching property of the membrane, which is delayed compared to bending. It is postulated that stress by bending is localized at the base of ciliary membrane, and the delay in calcium response upon cilia bending is caused by mechanical properties of the cell membrane [32]. Our laboratory recently shows that polycystin-2 channel opens to let calcium ions enter into the cilioplasm [13].
Looking at the substantial heterogeneity in flow chamber design, shear stress forces, cell types and other experimental varieties involved in the studies of ciliary mechanosensation, the data strongly conclude that the polycystin-1 and -2 complex localizes to the mechanosensory compartment of primary cilia [33]. Collectively, the ciliary bending model [29], bending-induced membrane stretching at the base of primary cilia [32], or any other hypothetical model to interpret mechanosensation [31] indicate that fluid flow can cause a conformational change within the ciliary membrane.
Another type of external stimuli received by the primary cilia is a chemical signal. The interaction of any chemical mediator or ligand to its specific receptor with the subsequent signaling cascade housed in the primary cilium renders this organelle as a chemosensor. Dopamine receptor type-5 [34], 5-HT6 receptor [35], somatostatin receptor-3 [36], purinergic P2Y12 receptor [37], melanin concentrating hormone receptor-1 [38], patched and smoothened receptors of hedgehog [39,40], Wnt signaling network [41], PDGFRα [42], and vasoactive intestinal receptor-2 [43], among others, are examples of receptors and their associated signaling cascades localized to the primary cilia [12]. An outstanding study from Christensen laboratory further shows that PDGFRα dimerizes and is phosphorylated in the cilium [44]. Our laboratory and others have further confirm the ciliary function in the process of wound healing [44,45,46]. Thus, the unique localization of these signaling pathways proposes the primary cilium as a chemosensor and a key coordinator of various cellular signaling and functions.
A recent study also elegantly shows that a functional ciliary complex composed of polycystin-2, adenylyl cyclase-5/6, phosphodiesterase-4C and A-kinase anchoring protein-150 are cross-talked in the primary cilia to regulate cAMP level [47]. In addition, another study suggests that Mchr1 and Sstr3 form heteromers in the primary cilia membrane, a process that modulates ligand binding properties as well as downstream signaling [48]. More recently, it was shown that ciliary localization of GRP88 protein plays an important role in negatively regulating ciliary D1 dopamine receptor function, while asserting its inhibitory effect on non-ciliary β2 adrenergic receptor [49]. These studies provide evidence of the functional cilia receptor interaction and open the way to further formulate the idea of a cilium as a centerpiece of receptors homing.
4. Renal Epithelial Function of Cilia
4.1 Mechanosensory primary cilia
Polycystin-1 is a large transmembrane protein composed of 4302 amino acids and 11 membrane spanning domains (Figure 2). Polycystin-1 has a long extracellular N terminal domain to mediate mechanosensory function and a short intracellular C-terminus involved in intracellular signaling and interaction with polycystin-2 [51]. Polycystin-1 is expressed in the primary cilia as well as in cell-cell adhesion sites at the basolateral locations like desmosomal junctions and adherence junctions [52,53]. As a signaling entity, ciliary polycystin-1 undergoes several critical functional cleavages, the first of which occurs at a G protein-coupled receptor proteolytic site located at the extracellular N-terminal domain [22]. This cleavage is vital for normal kidney development and polycystin-1 mechanosensory function and signaling [22,54]. The other cleavage site is located at the intracellular C-terminal tail liberating polypeptide fragments that transmit messages to the nucleus and mediate STAT6/P100 [55], AP-1 [56] and canonical Wnt [57] signaling pathways. Fluid-flow is considered an important regulator of these cleavages and contributes to normal function of polycystin-1 [55,56].
Figure 2.
Both polycystin-1 and polycystin-2 form a mechanosensory complex protein through their COOH termini. Polycystin-1 is an eleven-transmembrane protein with a huge extracellular domain, and polycystin-2 is a six-transmembrane calcium channel. There are many other proteins that interact with the intracellular domains of the polycystin complex. This illustration was modified from the original [50].
Beyond the cleavage of polycystin-1, other signaling pathways of polycystin-1 include polycystin-1 interaction with G-proteins, where polycystin-1may act as atypical GPCR [58,59]. Interestingly, polycystin-1 can activate AP-1 transcription factor and JNK through several heterotrimeric G-proteins [60,61]. AP-1 signaling components are important regulators of cell proliferation and differentiation, which have been implicated in the pathogenesis of ADPKD [62,63]. In addition, polycystin-1 regulates JNK/Bcl-2 apoptosis pathway via Gα12 stimulation, an important factor for cyst development [64]. Polycystin-1 also mediates Gαq-activated pathway through calcineurin/NFAT, an important regulator of cell growth, differentiation and adaptation [65]. Another signaling pathway regulated by polycystin-1is mTOR, an important regulator of cell growth. The C-terminal domain of polycystin-1 inhibits the mTOR cascade through the TSC1-TSC2 complex, retarding cell growth [66,67].
Polycystin-2 is a nonselective Ca2+ permeable transient receptor potential channel composed of 968 amino acids (Figure 2). Polycystin-2 is an integral protein with six membrane-spanning domains and intracellular C- and N-terminal domains [68]. In addition to its unique subcellular localization in the primary cilia membrane, polycystin-2 is also expressed in the endoplasmic reticulum membrane [69]. Polycystin-2 is involved in calcium signaling through its physical interaction with polycystin-1 [70,71]. It is thus believed that localization of the polycystin-1 and -2 complex in the cilia is required for proper mechanosensory cilia function [72].
The primary cilium in the renal epithelia senses shear-stress resulting from tubular fluid flow, where this mechanical stimulation is processed by the polycystin complex. This complex is essential for mechanosensory function, as revealed in studies using a mutated form of polycystin-1 and blocking antibodies for polycystin-1 [27]. In addition, the presence of primary cilium is essential for the mechanosensory function of the renal epithelium, as revealed in studies utilizing mutated abnormal cilia structure from Tg737 orpk/orpk cells and chemical ablation of cilia from ciliated cells [25,73]. In MDCK cells, Praetorius and Spring showed that calcium signal was initiated by a calcium influx, followed by calcium release from IP3 sensitive stores [74]. However, Nauli et al found that shear stress-induced calcium signal is independent from phospholipase C or IP3, instead depending upon ryanodine sensitive stores in embryonic mouse collecting duct epithelial cells [13,27]. To address this discrepancy, Xu et al shows that fluid-shear induced cilia activation can also release ATP in renal epithelia [75]. The ATP will then activate the purinergic signaling pathway, which requires phospholipase C or IP3. In addition, the IP3 receptor also physically interacts with and is regulated by polycystin-2 in the endoplasmic reticulum membrane, boosting IP3- mediated calcium release [76]. On the other hand, polycystin-1 negatively regulates the IP3 receptor in the endoplasmic reticulum membrane, creating an opposing effect of polycystin-2 [77].
4.2 Chemosensory primary cilia
In IMCD3 cells derived from a kidney collecting duct, an orphan G protein-coupled receptor (GPR88) has been shown to localize to primary cilia [49]. This orphan GPCR plays a modifying role on dopamine-1 and β2 receptors signaling through cAMP. In the proposed model, ciliary GPR88 negatively regulates the human dopamine receptor that is coexpressed and targeted to primary cilia. On the other hand, ciliary targeting of GPR88 protects β2 receptor mediated cAMP activation from its inhibitory effect. As cAMP is known to have an important role in renal pathogenesis, GPR88 might be a therapeutic target.
It is yet unknown whether a purinergic receptor (P2R) is localized to the renal primary cilium, although it is known to localize to the primary cilium of cholangiocyte [37]. It was also shown that only ciliated cells can releases ATP [75,78]. Furthermore, endogenously released or exogenously added ATP enhanced flow-induced calcium signaling, suggesting a chemosensory role of primary cilia. To confirm this observation, ATP scavengers, as well as antagonists for both P2X and P2Y, weaken this cilium-dependent calcium signal. This implies a chemosensory function for renal primary cilia to ATP.
Another proposed chemosensory role of renal primary cilia is attributed to integrins, the extracellular matrix receptors that play an important role in cell adhesion, differentiation and mechanotransduction. Praetorius et al showed that β1, α3 and α5 integrins were colocalized to renal primary cilia in MDCK cells [79]. These cells respond to the β1 integrin agonist, fibronectin, through eliciting intracellular calcium fluxes. Interestingly, primary cilia potentiate the fibronectin-activated β1 integrin-induced calcium signal; however, this pathway is independent of ciliary-mediated flow-induced calcium signaling. This clearly leads to the conclusion of a chemosensory function of the renal primary cilia.
Several lines of evidence revealed a key role of primary cilia in regulating hedgehog (Hh) signaling (Figure 3). In renal cells, among other mammalian cells, Hh signaling function through Smoothened (Smo) and Patched (Ptc) receptors was reported to be essential for cell proliferation, morphogenesis, organogenesis, tissue differentiation and embryonic development. Mutations in IFT proteins, which are essential for cilia structure and function, led to disruptions of Hh pathways and developmental disorders [80]. In the absence of Hh ligands, Ptc is localized to the primary cilia membrane and negatively regulates Hh signaling by repressing Smo [39]. This allows primary cilia to function as chemosensors in response to the Hh ligand. Upon binding to its ligand, Ptc moves out of the cilium, permitting Smo to accumulate in the primary cilium [39,40] and activating the downstream Hh signaling network, mainly through Gli transcription factors [81].
Figure 3.
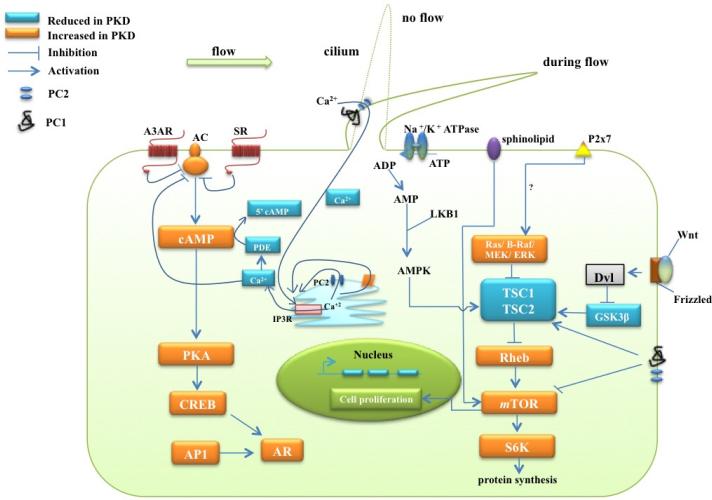
The diagram illustrates the mechanism that polycystin-1 (PC1), polycystin-2 (PC2), signaling proteins, molecules and other receptors exert on signaling pathways leading to cyst formation. The blue box indicates the reduced molecules and signaling proteins in ADKPD. The orange box indicates the increased signaling proteins in ADPKD, which are thought to be responsible for an increase in cell proliferation including cAMP, Ras/Raf/ERK, AC, and mTOR activity. In addition, EGFR activation is also enhanced by amphiregulin (AR) that is abnormally expressed in cystic cells through cAMP, CREB and AP1 signaling (not shown). The sphonigolipid, Na+/K+ ATPase, Wnt and P2x7 purinergic receptors are also involved in the regulation of mTOR and TSC1/TSC2 complex activity. Other receptors that are involved in ADPKD include adenosine receptor-3A (A3AR) and somatostatin receptor (SR), which regulate activity of adenylate cyclase (AC). This illustration was modified from the original [82].
5. Vascular Endothelial Function of Cilia
5.1 Mechanosensory primary cilia
Primary cilia can also be observed in vascular endothelial cells in vitro and in vivo. Endothelial primary cilia are relatively shorter than the renal cilia; however, the mechanosensory function of vascular endothelial cells largely resembles that of renal epithelial cells. The ciliary polycystin complex is the most upstream component of the signaling cascade. This complex mediates the translation of extracellular mechanical signals into intracellular biochemical downstream signals, where intracellular calcium is used as an indicator [46].
In addition to the many other vital functions, blood flowing over the endothelia produces an important drag force, also known as shear-stress. Being one of the most important cell-linings within the cardiovascular system, vascular endothelia mechanically sense shear stress and convert it into an array of biochemical signals [16,83]. Endothelial cells can precisely distinguish shear-stress from other types of physical forces imposed on them by blood flow [84]. The significance of blood flowing within the vasculatures is not a new idea. For over 120 years, it has been known that blood vessels develop branches in fast blood flow areas, while branches are not formed in the slow flowing blood vessels of chick embryos. This observation indicates that blood flow velocity and shear stress regulate branching angiogenesis [85,86]. Furthermore, shear-stress has been confirmed to regulate blood vessel diameter, revealing the importance of shear-induced events in vascular growth and remodeling [87,88,89]. Angiogenesis, vessel diameter, vascular growth and remodeling have important implications in health and disease; therefore, it is fundamental to understand the mechanisms and signaling pathways that govern these physiological and pathophysiological processes. Owing to the unique structure, location, length and localization of various functional proteins, primary cilia can be a promising model to illustrate various physiological and pathological processes, in addition to becoming a novel therapeutic target for a mechano-therapy [90].
Endothelial cells detect shear stress via the polycystin-1 and -2 mechanosensory complex localized to primary cilia [28,91]. Primary cilia and polycystin-1 are essential to the mechasosensing capability of an endothelium, as confirmed by Nauli et al, who used embryonic aortic endothelial cells with genetic models without polycystin-1 or cilia. Endothelial cells lacking polycystin-1 or cilia are not able to sense fluid-shear stress [91]. AbouAlaiwi et al further showed that ciliary polycystin-2 is essential for endothelial mechanosensory function. Endothelial cells lacking polycystin-2 are insensitive to fluid shear-stress [28]. Collectively, ciliary polycystin-1 first detects mechanical force imposed by blood flow and transfers the signal to polycystin-2 through their C-terminal domain interaction (Figure 2). Polycystin-2 will allow calcium entry into the cell and activate intracellular stores to further release intraorganellar calcium. Extracellular calcium entry is an important event and a prerequisite for the downstream signaling, as confirmed by the inability of endothelial cells to convert mechanical force into intracellular signaling when removing extracellular calcium from the medium [28]. Uprising intracellular calcium ultimately stimulates eNOS, with the resultant production of vasoactive NO. Production of endothelial NO is reported to be dependent upon calcium, calmodulin, PKC and Akt (Figure 4). Endothelial cells with defective cilia structure or function are thus unable to generate NO in response to fluid shear-stress. It is believed that endothelia with defective NO production, in response to shear stress, would result in pathophysiological consequences.
Figure 4.
Nitric oxide (NO) synthesis is dependent on the function of endothelial cilia in the vasculature. Primary cilia are sensory organelles that house sensory proteins and function as calcium signaling compartments. The bending of cilia by fluid-shear stress activates the mechanosensory polycystin complex and initiates biochemical synthesis and the release of NO. This biochemical cascade involves extracellular calcium influx, followed by the activation of various calcium-dependent proteins, including calmodulin (CaM), protein kinase C (PKC) and Akt/PKB. This illustration was modified from the original [106].
Recently, Hierck et al interestingly observed that endothelial primary cilia are essential for shear stress-induced activation of Krüppel-Like Factor-2 (KLF2) transcription factor [92]. KLF2 can be induced by high levels of shear-stress and repressed by low and disturbed flow. Thus, KLF2 is considered a shear-stress marker [93]. Being an important regulator of vasculature status at the transcription level, KLF2 can induce eNOS and thrombomodulin, while it represses endothelin, angiotensin converting enzyme, and proinflammatory and anti-fibrinolytic genes transcription [94,95,96]. Hence, KLF2 plays an important protective role and is a hemodynamic regulator required for proper cardiovascular development and function. Thus, endothelial primary cilia, through the KLF2 pathway, might have promising therapeutic implications.
The mechanosensory endothelial primary cilia also play a key role in autoregulating their own structure and function, as well as in regulating cellular structure and function integrity. As an autoregulatory organelle, the structure and function of the mechanosensory endothelial primary cilia are regulated by the dopamine receptor, which is localized to the primary cilium in vitro and in vivo [34,97]. Abdul-Majeed and Nauli found that activation of the ciliary dopamine receptor results in elongation of cilia, with the concomitant enhancement of ciliary mechanosensory function [34]. Enhanced mechanosensory function is reported to be mediated through actin differentiation and cofilin dephosphorylation in wild type cells, while it is distressed in cilia mutant endothelial cells. Interestingly, defective mechanosensory function in mutant cells can be restored by ciliary dopamine receptor activation [34]. Most recently, it was further shown that PDGFRα signaling in the primary cilium regulates NHE1-dependent fibroblast migration via coordinated differential activity of MEK1/2-ERK1/2-p90RSK and AKT signaling pathways [98].
The role of the primary cilium in regulating whole cell integrity and function further reveals cytoskeleton orientation as an indicator [34]. Jones et al also reported that an intact functional cilium is required for actin cytoskeleton organization, directional migration and barrier permeability in the endothelium [45]. Endothelial cells with defective structure or function of cilia exhibit reduced actin stress fibers and focal adhesions, resulting in impaired directional migration and high apico-basal permeability. These events are proposed to be mediated in part through hsp27, which was found suppressed in the mutant endothelial cells. Collectively, these results verify the importance of primary cilia and sensory polycystins complex in cytoskeleton organization.
The significance of functional mechanosensory endothelial primary cilia on cell division has also been studied. AbouAlaiwi et al show that structurally and functionally intact endothelial primary cilia are essential for proper cell division [99,100]. Defective primary cilia structure or function shows multipolar spindle formation, mitotic abnormality, centrosomal amplification and cell polyploidy. These cell division abnormalities in the mutant cells are reported to be mediated through abnormally suppressed expression of survivin, a chromosomal passenger. These findings might provide some hints about the pathophysiological pathways of aberrant cell proliferation in cystic kidneys as well as in blood vessel aneurysms associated with ADPKD and support the importance of the role of primary cilia in the disease [101].
The relationship between mechanosensory endothelial primary cilia and atherosclerosis is a vital perspective with direct clinical consequences. In relation to shear stress, atherosclerosis is generated in areas where endothelial cells are exposed to turbulent blood flow and are found at bifurcations of blood vessels. On the other hand, high shear-stress and laminar blood flow can retard atherosclerosis formation in these areas, proposing a vital role for shear-stress in this pathological process [102]. In studies using adult mouse aortic arch and common carotid arteries, primary cilia are found at the atherosclerotic predilection sites [103]. Furthermore, in various experimentally induced flow patterns, primary cilia are found expressed in areas of low or turbulent shear stress. Supporting these findings, primary cilia are expressed in atheromatous plaques of adult human aortic endothelial cells more than in the non-affected areas or fibrous plaque areas [104]. It was also reported that primary cilia disassembled after 2 hours of continuous laminar high shear stress with the termination of IFT in cultured human umbilical vein endothelial cells [105]. These studies suggest a role for endothelial primary cilia as mechanosensors in endothelial dysfunction and consequently in atherogenesis at various vascular sites.
5.2 Chemosensory primary cilia
In addition to its mechanosensory function, the primary cilium gains some attention as a chemosensor. To be a chemosensor, endothelial primary cilium should be a host for a functional receptor that mediates distinct downstream signaling cascades when binding to a ligand. Dopamine receptor type-5 (DR5) is a D1-like dopaminergic receptor. Abdul-Majeed and Nauli found that DR5 localizes to primary cilia of cultured mouse embryonic aortic endothelial cells in vitro and mouse femoral arteries in vivo [34]. By binding to its ligand, ciliary DR5 triggers downstream signaling manifested by increasing intracellular calcium. In addition to calcium, dopamine and many other chemical activators and inhibitors also evoke endothelial cilia.
Teilmann and Christensen reported the presence of primary cilia in ovarian and extraovarian tissues, including endothelial cells of the female mouse reproductive system [107]. They found that angiopoietin receptors Tie1 and Tie2, receptor tyrosine kinases, are localized to the primary cilia of ovarian endothelial cells in mice. Upon binding their ligands, angiopoietins, these receptors play a vital role in vascularization through VEGF [108] and endothelial apoptosis through the PI3K and Akt pathways [109]. These studies provide another attribute to endothelial primary cilia as chemosensory organelles.
6. Conclusion
More evidence has emerged to support the important roles of primary cilia play in disease and development. It is now known that primary cilia function as mechanosensory and chemosensory organelles. However, any defect in primary cilia can trigger a wide range of complications, such as in the kidney, vasculatures and many other organs. Yet, many more ciliary proteins involved in either mechanosensory or chemosensory function are still to be sorted out. Only through thorough understanding of individual molecules within the sensory cilia can we appreciate the complexity of the primary cilium in its very diverse roles.
Acknowledgement
This work was funded by awards from NIH (DK080640 and DK096870). Authors would like to thank Charisse Montgomery for editing assistance.
Footnotes
Conflict of Interest
The authors declare that there are no conflicts of interest related to this study.
@2013, Surya M. Nauli, licensee AIMS Press.
References
- 1.Lentine KL, Xiao H, Machnicki G, et al. Renal function and healthcare costs in patients with polycystic kidney disease. Clin J Am Soc Nephrol. 2010;5:1471–1479. doi: 10.2215/CJN.00780110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Torres VE, Harris PC. Autosomal dominant polycystic kidney disease: the last 3 years. Kidney Int. 2009;76:149–168. doi: 10.1038/ki.2009.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grantham JJ, Torres VE, Chapman AB, et al. Volume progression in polycystic kidney disease. N Engl J Med. 2006;354:2122–2130. doi: 10.1056/NEJMoa054341. [DOI] [PubMed] [Google Scholar]
- 4.Churchill DN, Bear JC, Morgan J, et al. Prognosis of adult onset polycystic kidney disease re-evaluated. Kidney Int. 1984;26:190–193. doi: 10.1038/ki.1984.154. [DOI] [PubMed] [Google Scholar]
- 5.Dell KM. The spectrum of polycystic kidney disease in children. Adv Chronic Kidney Dis. 2011;18:339–347. doi: 10.1053/j.ackd.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Romao EA, Moyses Neto M, Teixeira SR, et al. Renal and extrarenal manifestations of autosomal dominant polycystic kidney disease. Braz J Med Biol Res. 2006;39:533–538. doi: 10.1590/s0100-879x2006000400014. [DOI] [PubMed] [Google Scholar]
- 7.Gabow PA. Autosomal dominant polycystic kidney disease. N Engl J Med. 1993;329:332–342. doi: 10.1056/NEJM199307293290508. [DOI] [PubMed] [Google Scholar]
- 8.Huston J, 3rd, Torres VE, Sulivan PP, et al. Value of magnetic resonance angiography for the detection of intracranial aneurysms in autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 1993;3:1871–1877. doi: 10.1681/ASN.V3121871. [DOI] [PubMed] [Google Scholar]
- 9.Abdul-Majeed S, Nauli SM. Polycystic diseases in visceral organs. Obstet Gynecol Int. 2011;2011:609370. doi: 10.1155/2011/609370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ratnam S, Nauli SM. Hypertension in Autosomal Dominant Polycystic Kidney Disease: A Clinical and Basic Science Perspective. Int J Nephrol Urol. 2010;2:294–308. [PMC free article] [PubMed] [Google Scholar]
- 11.Zimmermann K. Beiträge zur Kenntnis einiger Drüsen und Epithelien. Arch MikroskopAnat. 1898;52:552–706. [Google Scholar]
- 12.Nauli SM, Haymour HS, AbouAlaiwi WA, et al. Chapter 14: Primary Cilia are Mechanosensory Organelles in Vestibular Tissues. Mechanosensitivity and Mechanotransduction. 2011 ISBN: 978-990-481-9880-9881. [Google Scholar]
- 13.Jin X, Mohieldin AM, Muntean BS, et al. Cilioplasm is a cellular compartment for calcium signaling in response to mechanical and chemical stimuli. Cell Mol Life Sci. 2013 doi: 10.1007/s00018-013-1483-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garcia-Gonzalo FR, Reiter JF. Scoring a backstage pass: mechanisms of ciliogenesis and ciliary access. J Cell Biol. 2012;197:697–709. doi: 10.1083/jcb.201111146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gherman A, Davis EE, Katsanis N. The ciliary proteome database: an integrated community resource for the genetic and functional dissection of cilia. Nat Genet. 2006;38:961–962. doi: 10.1038/ng0906-961. [DOI] [PubMed] [Google Scholar]
- 16.Nauli SM, Jin X, Hierck BP. The mechanosensory role of primary cilia in vascular hypertension. Int J Vasc Med. 2011;2011:376281. doi: 10.1155/2011/376281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abdul-Majeed S, Nauli SM. Calcium-mediated mechanisms of cystic expansion. Biochim Biophys Acta. 2011;1812:1281–1290. doi: 10.1016/j.bbadis.2010.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nauli SM, Zhou J. Polycystins and mechanosensation in renal and nodal cilia. Bioessays. 2004;26:844–856. doi: 10.1002/bies.20069. [DOI] [PubMed] [Google Scholar]
- 19.Satir P, Pedersen LB, Christensen ST. The primary cilium at a glance. J Cell Sci. 2010;123:499–503. doi: 10.1242/jcs.050377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wood CR, Huang K, Diener DR, et al. The cilium secretes bioactive ectosomes. Curr Biol. 2013;23:906–911. doi: 10.1016/j.cub.2013.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaimori JY, Nagasawa Y, Menezes LF, et al. Polyductin undergoes notch-like processing and regulated release from primary cilia. Hum Mol Genet. 2007;16:942–956. doi: 10.1093/hmg/ddm039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qian F, Boletta A, Bhunia AK, et al. Cleavage of polycystin-1 requires the receptor for egg jelly domain and is disrupted by human autosomal-dominant polycystic kidney disease 1-associated mutations. Proc Natl Acad Sci U S A. 2002;99:16981–16986. doi: 10.1073/pnas.252484899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tanaka Y, Okada Y, Hirokawa N. FGF-induced vesicular release of Sonic hedgehog and retinoic acid in leftward nodal flow is critical for left-right determination. Nature. 2005;435:172–177. doi: 10.1038/nature03494. [DOI] [PubMed] [Google Scholar]
- 24.Hogan MC, Manganelli L, Woollard JR, et al. Characterization of PKD protein-positive exosome-like vesicles. J Am Soc Nephrol. 2009;20:278–288. doi: 10.1681/ASN.2008060564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Praetorius HA, Spring KR. Removal of the MDCK cell primary cilium abolishes flow sensing. J Membr Biol. 2003;191:69–76. doi: 10.1007/s00232-002-1042-4. [DOI] [PubMed] [Google Scholar]
- 26.Nauli SM, Rossetti S, Kolb RJ, et al. Loss of polycystin-1 in human cyst-lining epithelia leads to ciliary dysfunction. J Am Soc Nephrol. 2006;17:1015–1025. doi: 10.1681/ASN.2005080830. [DOI] [PubMed] [Google Scholar]
- 27.Nauli SM, Alenghat FJ, Luo Y, et al. Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat Genet. 2003;33:129–137. doi: 10.1038/ng1076. [DOI] [PubMed] [Google Scholar]
- 28.AbouAlaiwi WA, Takahashi M, Mell BR, et al. Ciliary polycystin-2 is a mechanosensitive calcium channel involved in nitric oxide signaling cascades. Circ Res. 2009;104:860–869. doi: 10.1161/CIRCRESAHA.108.192765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schwartz EA, Leonard ML, Bizios R, et al. Analysis and modeling of the primary cilium bending response to fluid shear. Am J Physiol. 1997;272:F132–138. doi: 10.1152/ajprenal.1997.272.1.F132. [DOI] [PubMed] [Google Scholar]
- 30.Downs ME, Nguyen AM, Herzog FA, et al. An experimental and computational analysis of primary cilia deflection under fluid flow. Comput Methods Biomech Biomed Engin. 2012 doi: 10.1080/10255842.2011.653784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Resnick A, Hopfer U. Mechanical stimulation of primary cilia. Front Biosci. 2008;13:1665–1680. doi: 10.2741/2790. [DOI] [PubMed] [Google Scholar]
- 32.Rydholm S, Zwartz G, Kowalewski JM, et al. Mechanical properties of primary cilia regulate the response to fluid flow. Am J Physiol Renal Physiol. 2010;298:F1096–1102. doi: 10.1152/ajprenal.00657.2009. [DOI] [PubMed] [Google Scholar]
- 33.Forman JR, Qamar S, Paci E, et al. The remarkable mechanical strength of polycystin-1 supports a direct role in mechanotransduction. J Mol Biol. 2005;349:861–871. doi: 10.1016/j.jmb.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 34.Abdul-Majeed S, Nauli SM. Dopamine receptor type 5 in the primary cilia has dual chemo- and mechano-sensory roles. Hypertension. 2011;58:325–331. doi: 10.1161/HYPERTENSIONAHA.111.172080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hamon M, Doucet E, Lefevre K, et al. Antibodies and antisense oligonucleotide for probing the distribution and putative functions of central 5-HT6 receptors. Neuropsychopharmacology. 1999;21:68S–76S. doi: 10.1016/S0893-133X(99)00044-5. [DOI] [PubMed] [Google Scholar]
- 36.Handel M, Schulz S, Stanarius A, et al. Selective targeting of somatostatin receptor 3 to neuronal cilia. Neuroscience. 1999;89:909–926. doi: 10.1016/s0306-4522(98)00354-6. [DOI] [PubMed] [Google Scholar]
- 37.Masyuk AI, Gradilone SA, Banales JM, et al. Cholangiocyte primary cilia are chemosensory organelles that detect biliary nucleotides via P2Y12 purinergic receptors. Am J Physiol Gastrointest Liver Physiol. 2008;295:G725–734. doi: 10.1152/ajpgi.90265.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Berbari NF, Johnson AD, Lewis JS, et al. Identification of ciliary localization sequences within the third intracellular loop of G protein-coupled receptors. Mol Biol Cell. 2008;19:1540–1547. doi: 10.1091/mbc.E07-09-0942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rohatgi R, Milenkovic L, Scott MP. Patched1 regulates hedgehog signaling at the primary cilium. Science. 2007;317:372–376. doi: 10.1126/science.1139740. [DOI] [PubMed] [Google Scholar]
- 40.Corbit KC, Aanstad P, Singla V, et al. Vertebrate Smoothened functions at the primary cilium. Nature. 2005;437:1018–1021. doi: 10.1038/nature04117. [DOI] [PubMed] [Google Scholar]
- 41.Kim E, Arnould T, Sellin LK, et al. The polycystic kidney disease 1 gene product modulates Wnt signaling. J Biol Chem. 1999;274:4947–4953. doi: 10.1074/jbc.274.8.4947. [DOI] [PubMed] [Google Scholar]
- 42.Schneider L, Clement CA, Teilmann SC, et al. PDGFRalphaalpha signaling is regulated through the primary cilium in fibroblasts. Curr Biol. 2005;15:1861–1866. doi: 10.1016/j.cub.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 43.Soetedjo L, Glover DA, Jin H. Targeting of vasoactive intestinal peptide receptor 2, VPAC2, a secretin family G-protein coupled receptor, to primary cilia. Biol Open. 2013;2:686–694. doi: 10.1242/bio.20134747. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 44.Schneider L, Cammer M, Lehman J, et al. Directional cell migration and chemotaxis in wound healing response to PDGF-AA are coordinated by the primary cilium in fibroblasts. Cell Physiol Biochem. 2010;25:279–292. doi: 10.1159/000276562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jones TJ, Adapala RK, Geldenhuys WJ, et al. Primary cilia regulates the directional migration and barrier integrity of endothelial cells through the modulation of hsp27 dependent actin cytoskeletal organization. J Cell Physiol. 2012;227:70–76. doi: 10.1002/jcp.22704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jones TJ, Nauli SM. Mechanosensory calcium signaling. Adv Exp Med Biol. 2012;740:1001–1015. doi: 10.1007/978-94-007-2888-2_46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Choi YH, Suzuki A, Hajarnis S, et al. Polycystin-2 and phosphodiesterase 4C are components of a ciliary A-kinase anchoring protein complex that is disrupted in cystic kidney diseases. Proc Natl Acad Sci U S A. 2011;108:10679–10684. doi: 10.1073/pnas.1016214108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Green JA, Gu C, Mykytyn K. Heteromerization of ciliary G protein-coupled receptors in the mouse brain. PLoS One. 2012;7:e46304. doi: 10.1371/journal.pone.0046304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Marley A, Choy RW, von Zastrow M. GPR88 reveals a discrete function of primary cilia as selective insulators of GPCR cross-talk. PLoS One. 2013;8:e70857. doi: 10.1371/journal.pone.0070857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kolb RJ, Nauli SM. Ciliary dysfunction in polycystic kidney disease: an emerging model with polarizing potential. Front Biosci. 2008;13:4451–4466. doi: 10.2741/3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nims N, Vassmer D, Maser RL. Transmembrane domain analysis of polycystin-1, the product of the polycystic kidney disease-1 (PKD1) gene: evidence for 11 membrane-spanning domains. Biochemistry. 2003;42:13035–13048. doi: 10.1021/bi035074c. [DOI] [PubMed] [Google Scholar]
- 52.Scheffers MS, van der Bent P, Prins F, et al. Polycystin-1, the product of the polycystic kidney disease 1 gene, co-localizes with desmosomes in MDCK cells. Hum Mol Genet. 2000;9:2743–2750. doi: 10.1093/hmg/9.18.2743. [DOI] [PubMed] [Google Scholar]
- 53.Huan Y, van Adelsberg J. Polycystin-1, the PKD1 gene product, is in a complex containing E-cadherin and the catenins. J Clin Invest. 1999;104:1459–1468. doi: 10.1172/JCI5111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yu S, Hackmann K, Gao J, et al. Essential role of cleavage of Polycystin-1 at G protein-coupled receptor proteolytic site for kidney tubular structure. Proc Natl Acad Sci U S A. 2007;104:18688–18693. doi: 10.1073/pnas.0708217104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Low SH, Vasanth S, Larson CH, et al. Polycystin-1, STAT6, and P100 function in a pathway that transduces ciliary mechanosensation and is activated in polycystic kidney disease. Dev Cell. 2006;10:57–69. doi: 10.1016/j.devcel.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 56.Chauvet V, Tian X, Husson H, et al. Mechanical stimuli induce cleavage and nuclear translocation of the polycystin-1 C terminus. J Clin Invest. 2004;114:1433–1443. doi: 10.1172/JCI21753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lal M, Song X, Pluznick JL, et al. Polycystin-1 C-terminal tail associates with beta-catenin and inhibits canonical Wnt signaling. Hum Mol Genet. 2008;17:3105–3117. doi: 10.1093/hmg/ddn208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Delmas P, Nauli SM, Li X, et al. Gating of the polycystin ion channel signaling complex in neurons and kidney cells. FASEB J. 2004;18:740–742. doi: 10.1096/fj.03-0319fje. [DOI] [PubMed] [Google Scholar]
- 59.Parnell SC, Magenheimer BS, Maser RL, et al. The polycystic kidney disease-1 protein, polycystin-1, binds and activates heterotrimeric G-proteins in vitro. Biochem Biophys Res Commun. 1998;251:625–631. doi: 10.1006/bbrc.1998.9514. [DOI] [PubMed] [Google Scholar]
- 60.Parnell SC, Magenheimer BS, Maser RL, et al. Polycystin-1 activation of c-Jun N-terminal kinase and AP-1 is mediated by heterotrimeric G proteins. J Biol Chem. 2002;277:19566–19572. doi: 10.1074/jbc.M201875200. [DOI] [PubMed] [Google Scholar]
- 61.Arnould T, Kim E, Tsiokas L, et al. The polycystic kidney disease 1 gene product mediates protein kinase C alpha-dependent and c-Jun N-terminal kinase-dependent activation of the transcription factor AP-1. J Biol Chem. 1998;273:6013–6018. doi: 10.1074/jbc.273.11.6013. [DOI] [PubMed] [Google Scholar]
- 62.Le NH, van der Wal A, van der Bent P, et al. Increased activity of activator protein-1 transcription factor components ATF2, c-Jun, and c-Fos in human and mouse autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 2005;16:2724–2731. doi: 10.1681/ASN.2004110913. [DOI] [PubMed] [Google Scholar]
- 63.Shaulian E, Karin M. AP-1 as a regulator of cell life and death. Nat Cell Biol. 2002;4:E131–136. doi: 10.1038/ncb0502-e131. [DOI] [PubMed] [Google Scholar]
- 64.Yu W, Kong T, Beaudry S, et al. Polycystin-1 protein level determines activity of the Galpha12/JNK apoptosis pathway. J Biol Chem. 2010;285:10243–10251. doi: 10.1074/jbc.M109.070821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Puri S, Magenheimer BS, Maser RL, et al. Polycystin-1 activates the calcineurin/NFAT (nuclear factor of activated T-cells) signaling pathway. J Biol Chem. 2004;279:55455–55464. doi: 10.1074/jbc.M402905200. [DOI] [PubMed] [Google Scholar]
- 66.Distefano G, Boca M, Rowe I, et al. Polycystin-1 regulates extracellular signal-regulated kinase-dependent phosphorylation of tuberin to control cell size through mTOR and its downstream effectors S6K and 4EBP1. Mol Cell Biol. 2009;29:2359–2371. doi: 10.1128/MCB.01259-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shillingford JM, Murcia NS, Larson CH, et al. The mTOR pathway is regulated by polycystin-1, and its inhibition reverses renal cystogenesis in polycystic kidney disease. Proc Natl Acad Sci U S A. 2006;103:5466–5471. doi: 10.1073/pnas.0509694103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mochizuki T, Wu G, Hayashi T, et al. PKD2, a gene for polycystic kidney disease that encodes an integral membrane protein. Science. 1996;272:1339–1342. doi: 10.1126/science.272.5266.1339. [DOI] [PubMed] [Google Scholar]
- 69.Cai Y, Maeda Y, Cedzich A, et al. Identification and characterization of polycystin-2, the PKD2 gene product. J Biol Chem. 1999;274:28557–28565. doi: 10.1074/jbc.274.40.28557. [DOI] [PubMed] [Google Scholar]
- 70.Hanaoka K, Qian F, Boletta A, et al. Co-assembly of polycystin-1 and -2 produces unique cation-permeable currents. Nature. 2000;408:990–994. doi: 10.1038/35050128. [DOI] [PubMed] [Google Scholar]
- 71.Qian F, Germino FJ, Cai Y, et al. PKD1 interacts with PKD2 through a probable coiled-coil domain. Nat Genet. 1997;16:179–183. doi: 10.1038/ng0697-179. [DOI] [PubMed] [Google Scholar]
- 72.Nauli SM, Jin X, AbouAlaiwi WA, et al. Non-motile primary cilia as fluid shear stress mechanosensors. Methods Enzymol. 2013;525:1–20. doi: 10.1016/B978-0-12-397944-5.00001-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liu W, Murcia NS, Duan Y, et al. Mechanoregulation of intracellular Ca2+ concentration is attenuated in collecting duct of monocilium-impaired orpk mice. Am J Physiol Renal Physiol. 2005;289:F978–988. doi: 10.1152/ajprenal.00260.2004. [DOI] [PubMed] [Google Scholar]
- 74.Praetorius HA, Spring KR. Bending the MDCK cell primary cilium increases intracellular calcium. J Membr Biol. 2001;184:71–79. doi: 10.1007/s00232-001-0075-4. [DOI] [PubMed] [Google Scholar]
- 75.Xu C, Shmukler BE, Nishimura K, et al. Attenuated, flow-induced ATP release contributes to absence of flow-sensitive, purinergic Cai2+ signaling in human ADPKD cyst epithelial cells. Am J Physiol Renal Physiol. 2009;296:F1464–1476. doi: 10.1152/ajprenal.90542.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li Y, Wright JM, Qian F, et al. Polycystin 2 interacts with type I inositol 1,4,5-trisphosphate receptor to modulate intracellular Ca2+ signaling. J Biol Chem. 2005;280:41298–41306. doi: 10.1074/jbc.M510082200. [DOI] [PubMed] [Google Scholar]
- 77.Li Y, Santoso NG, Yu S, et al. Polycystin-1 interacts with inositol 1,4,5-trisphosphate receptor to modulate intracellular Ca2+ signaling with implications for polycystic kidney disease. J Biol Chem. 2009;284:36431–36441. doi: 10.1074/jbc.M109.068916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Praetorius HA, Leipziger J. Released nucleotides amplify the cilium-dependent, flow-induced [Ca2+]i response in MDCK cells. Acta Physiol (Oxf) 2009;197:241–251. doi: 10.1111/j.1748-1716.2009.02002.x. [DOI] [PubMed] [Google Scholar]
- 79.Praetorius HA, Praetorius J, Nielsen S, et al. Beta1-integrins in the primary cilium of MDCK cells potentiate fibronectin-induced Ca2+ signaling. Am J Physiol Renal Physiol. 2004;287:F969–978. doi: 10.1152/ajprenal.00096.2004. [DOI] [PubMed] [Google Scholar]
- 80.Eggenschwiler JT, Anderson KV. Cilia and developmental signaling. Annu Rev Cell Dev Biol. 2007;23:345–373. doi: 10.1146/annurev.cellbio.23.090506.123249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lum L, Beachy PA. The Hedgehog response network: sensors, switches, and routers. Science. 2004;304:1755–1759. doi: 10.1126/science.1098020. [DOI] [PubMed] [Google Scholar]
- 82.Mohieldin AM, Upadhyay VS, Ong ACM, et al. Autosomal Dominant Polycystic Kidney Disease: Pathophysiology and Treatment. Autosomal Dominant Disorders: New Research. 2013 ISBN: 978-1-62808-761-1: 6x9 - (NBC-R) [Google Scholar]
- 83.Nauli SM. An ACE inhibitor improves vascular outcomes in a PKD model. Am J Physiol Renal Physiol. 2011;301:F958. doi: 10.1152/ajprenal.00489.2011. [DOI] [PubMed] [Google Scholar]
- 84.Andersson M, Karlsson L, Svensson PA, et al. Differential global gene expression response patterns of human endothelium exposed to shear stress and intraluminal pressure. J Vasc Res. 2005;42:441–452. doi: 10.1159/000087983. [DOI] [PubMed] [Google Scholar]
- 85.Masuda H, Zhuang YJ, Singh TM, et al. Adaptive remodeling of internal elastic lamina and endothelial lining during flow-induced arterial enlargement. Arterioscler Thromb Vasc Biol. 1999;19:2298–2307. doi: 10.1161/01.atv.19.10.2298. [DOI] [PubMed] [Google Scholar]
- 86.Pries AR, Secomb TW, Gaehtgens P. Design principles of vascular beds. Circ Res. 1995;77:1017–1023. doi: 10.1161/01.res.77.5.1017. [DOI] [PubMed] [Google Scholar]
- 87.Ohura N, Yamamoto K, Ichioka S, et al. Global analysis of shear stress-responsive genes in vascular endothelial cells. J Atheroscler Thromb. 2003;10:304–313. doi: 10.5551/jat.10.304. [DOI] [PubMed] [Google Scholar]
- 88.Langille BL, Bendeck MP, Keeley FW. Adaptations of carotid arteries of young and mature rabbits to reduced carotid blood flow. Am J Physiol. 1989;256:H931–939. doi: 10.1152/ajpheart.1989.256.4.H931. [DOI] [PubMed] [Google Scholar]
- 89.Langille BL, O'Donnell F. Reductions in arterial diameter produced by chronic decreases in blood flow are endothelium-dependent. Science. 1986;231:405–407. doi: 10.1126/science.3941904. [DOI] [PubMed] [Google Scholar]
- 90.Huang C, Holfeld J, Schaden W, et al. Mechanotherapy: revisiting physical therapy and recruiting mechanobiology for a new era in medicine. Trends Mol Med. 2013;19:555–564. doi: 10.1016/j.molmed.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 91.Nauli SM, Kawanabe Y, Kaminski JJ, et al. Endothelial cilia are fluid shear sensors that regulate calcium signaling and nitric oxide production through polycystin-1. Circulation. 2008;117:1161–1171. doi: 10.1161/CIRCULATIONAHA.107.710111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hierck BP, Van der Heiden K, Alkemade FE, et al. Primary cilia sensitize endothelial cells for fluid shear stress. Dev Dyn. 2008;237:725–735. doi: 10.1002/dvdy.21472. [DOI] [PubMed] [Google Scholar]
- 93.Wang N, Miao H, Li YS, et al. Shear stress regulation of Kruppel-like factor 2 expression is flow pattern-specific. Biochem Biophys Res Commun. 2006;341:1244–1251. doi: 10.1016/j.bbrc.2006.01.089. [DOI] [PubMed] [Google Scholar]
- 94.Boon RA, Horrevoets AJ. Key transcriptional regulators of the vasoprotective effects of shear stress. Hamostaseologie. 2009;29:39–40. 41–33. [PubMed] [Google Scholar]
- 95.Dekker RJ, van Thienen JV, Rohlena J, et al. Endothelial KLF2 links local arterial shear stress levels to the expression of vascular tone-regulating genes. Am J Pathol. 2005;167:609–618. doi: 10.1016/S0002-9440(10)63002-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.SenBanerjee S, Lin Z, Atkins GB, et al. KLF2 Is a novel transcriptional regulator of endothelial proinflammatory activation. J Exp Med. 2004;199:1305–1315. doi: 10.1084/jem.20031132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Abdul-Majeed S, Moloney BC, Nauli SM. Mechanisms regulating cilia growth and cilia function in endothelial cells. Cell Mol Life Sci. 2012;69:165–173. doi: 10.1007/s00018-011-0744-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Clement DL, Mally S, Stock C, et al. PDGFRalpha signaling in the primary cilium regulates NHE1-dependent fibroblast migration via coordinated differential activity of MEK1/2-ERK1/2-p90RSK and AKT signaling pathways. J Cell Sci. 2013;126:953–965. doi: 10.1242/jcs.116426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.AbouAlaiwi WA, Ratnam S, Booth RL, et al. Endothelial cells from humans and mice with polycystic kidney disease are characterized by polyploidy and chromosome segregation defects through survivin down-regulation. Hum Mol Genet. 2011;20:354–367. doi: 10.1093/hmg/ddq470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.AbouAlaiwi WA, Rodriguez I, Nauli SM. Spectral karyotyping to study chromosome abnormalities in humans and mice with polycystic kidney disease. J Vis Exp. 2012 doi: 10.3791/3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Aboualaiwi WA, Muntean BS, Ratnam S, et al. Survivin-Induced Abnormal Ploidy Contributes to Cystic Kidney and Aneurysm Formation. Circulation. 2013 doi: 10.1161/CIRCULATIONAHA.113.005746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zaragoza C, Marquez S, Saura M. Endothelial mechanosensors of shear stress as regulators of atherogenesis. Curr Opin Lipidol. 2012;23:446–452. doi: 10.1097/MOL.0b013e328357e837. [DOI] [PubMed] [Google Scholar]
- 103.Van der Heiden K, Hierck BP, Krams R, et al. Endothelial primary cilia in areas of disturbed flow are at the base of atherosclerosis. Atherosclerosis. 2008;196:542–550. doi: 10.1016/j.atherosclerosis.2007.05.030. [DOI] [PubMed] [Google Scholar]
- 104.Bystrevskaya VB, Lichkun VV, Antonov AS, et al. An ultrastructural study of centriolar complexes in adult and embryonic human aortic endothelial cells. Tissue Cell. 1988;20:493–503. doi: 10.1016/0040-8166(88)90052-3. [DOI] [PubMed] [Google Scholar]
- 105.Iomini C, Tejada K, Mo W, et al. Primary cilia of human endothelial cells disassemble under laminar shear stress. J Cell Biol. 2004;164:811–817. doi: 10.1083/jcb.200312133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Abou Alaiwi WA, Lo ST, Nauli SM. Primary cilia: highly sophisticated biological sensors. Sensors (Basel) 2009;9:7003–7020. doi: 10.3390/s90907003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Teilmann SC, Christensen ST. Localization of the angiopoietin receptors Tie-1 and Tie-2 on the primary cilia in the female reproductive organs. Cell Biol Int. 2005;29:340–346. doi: 10.1016/j.cellbi.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 108.Hazzard TM, Molskness TA, Chaffin CL, et al. Vascular endothelial growth factor (VEGF) and angiopoietin regulation by gonadotrophin and steroids in macaque granulosa cells during the peri-ovulatory interval. Mol Hum Reprod. 1999;5:1115–1121. doi: 10.1093/molehr/5.12.1115. [DOI] [PubMed] [Google Scholar]
- 109.Kim I, Kim HG, So JN, et al. Angiopoietin-1 regulates endothelial cell survival through the phosphatidylinositol 3′-Kinase/Akt signal transduction pathway. Circ Res. 2000;86:24–29. doi: 10.1161/01.res.86.1.24. [DOI] [PubMed] [Google Scholar]