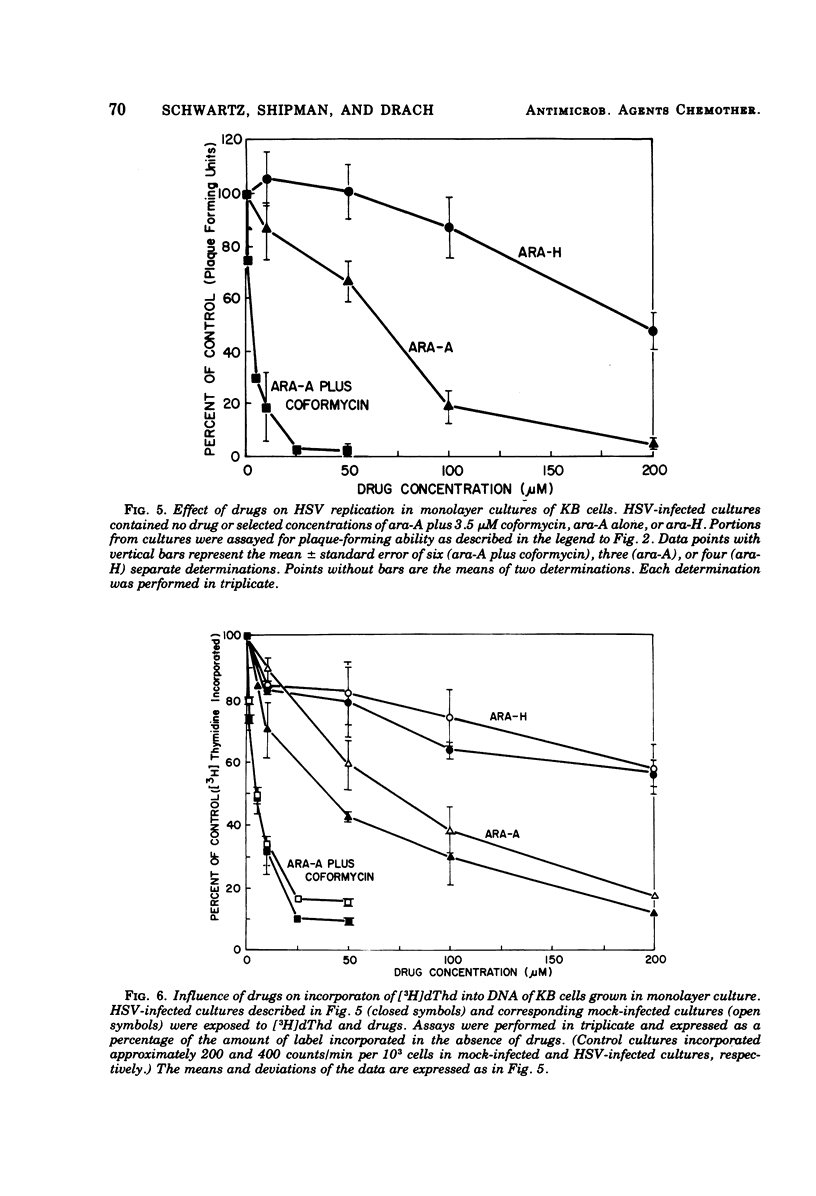
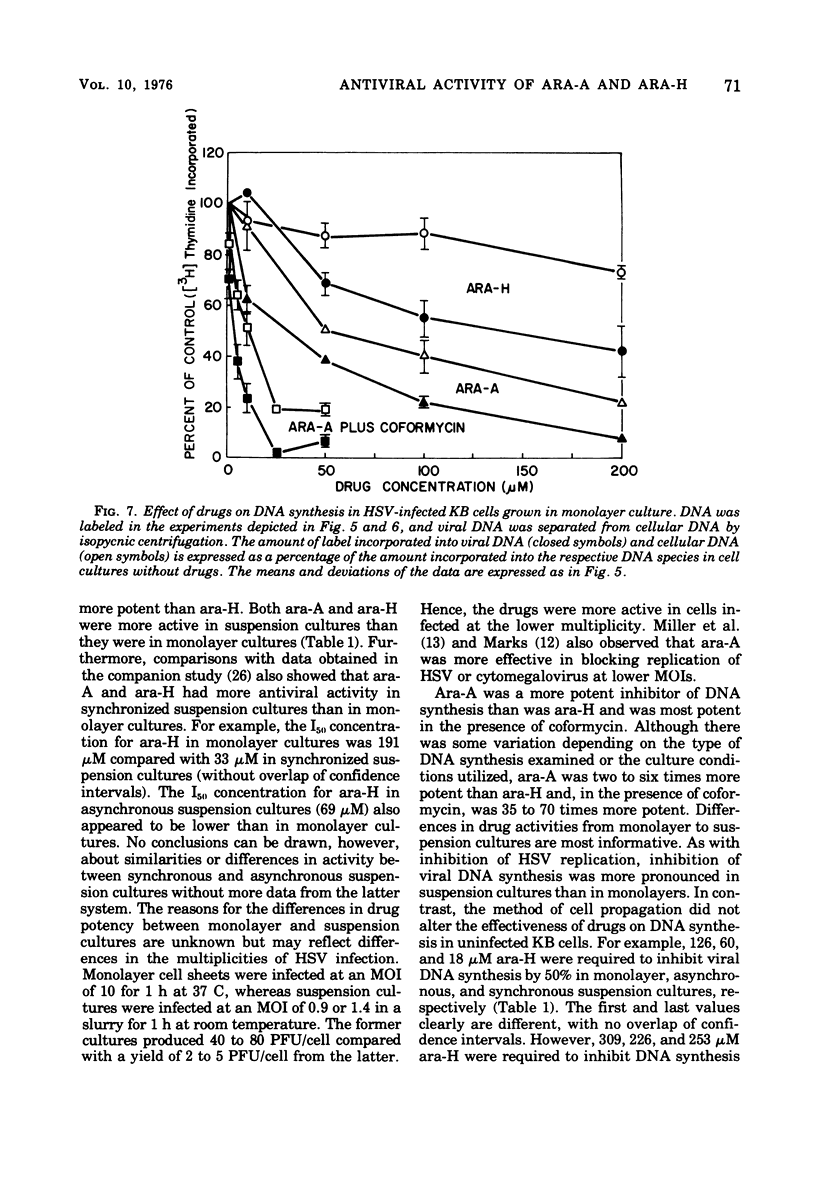
Abstract
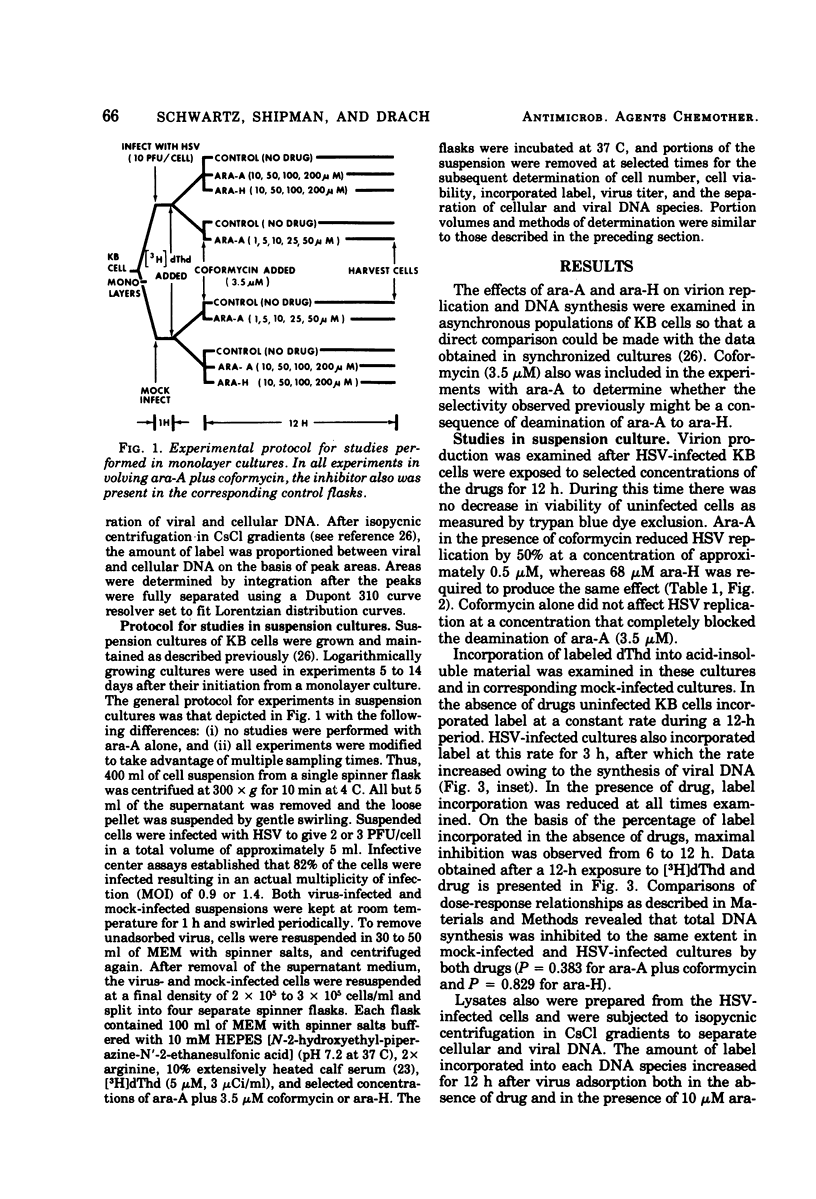
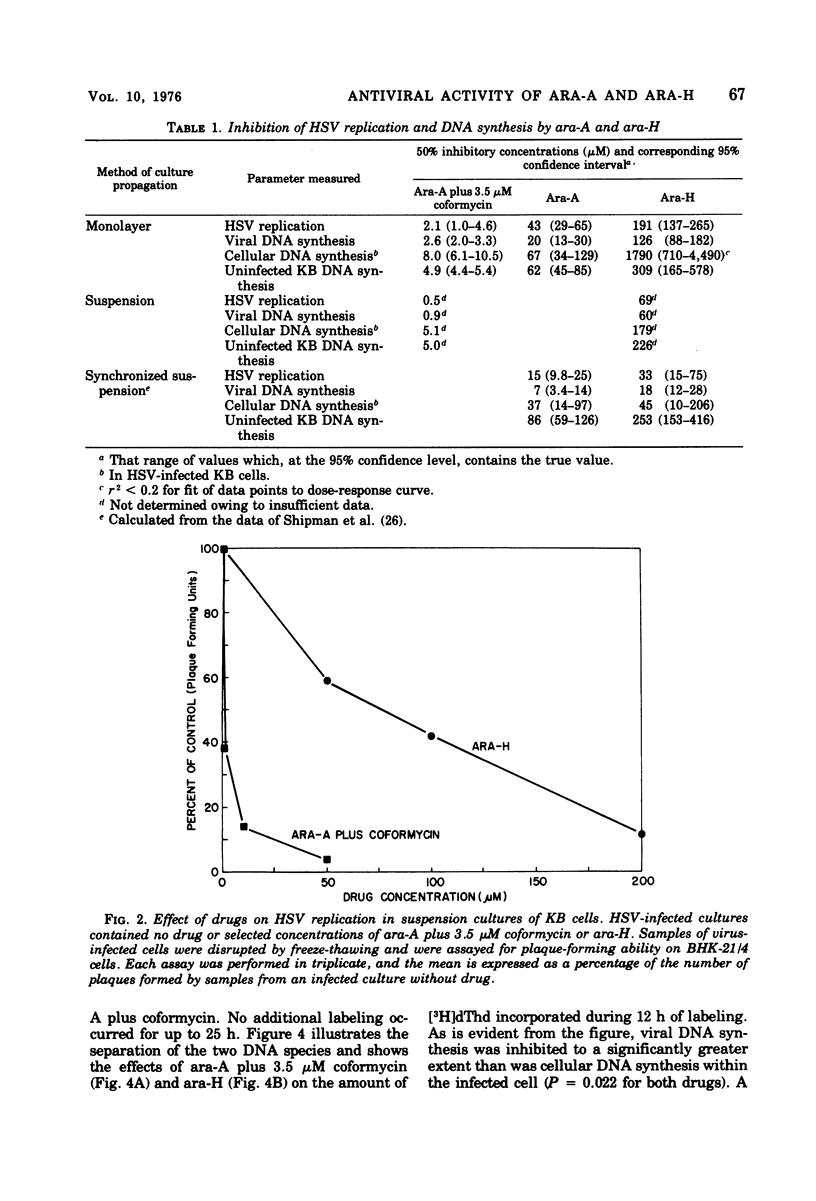
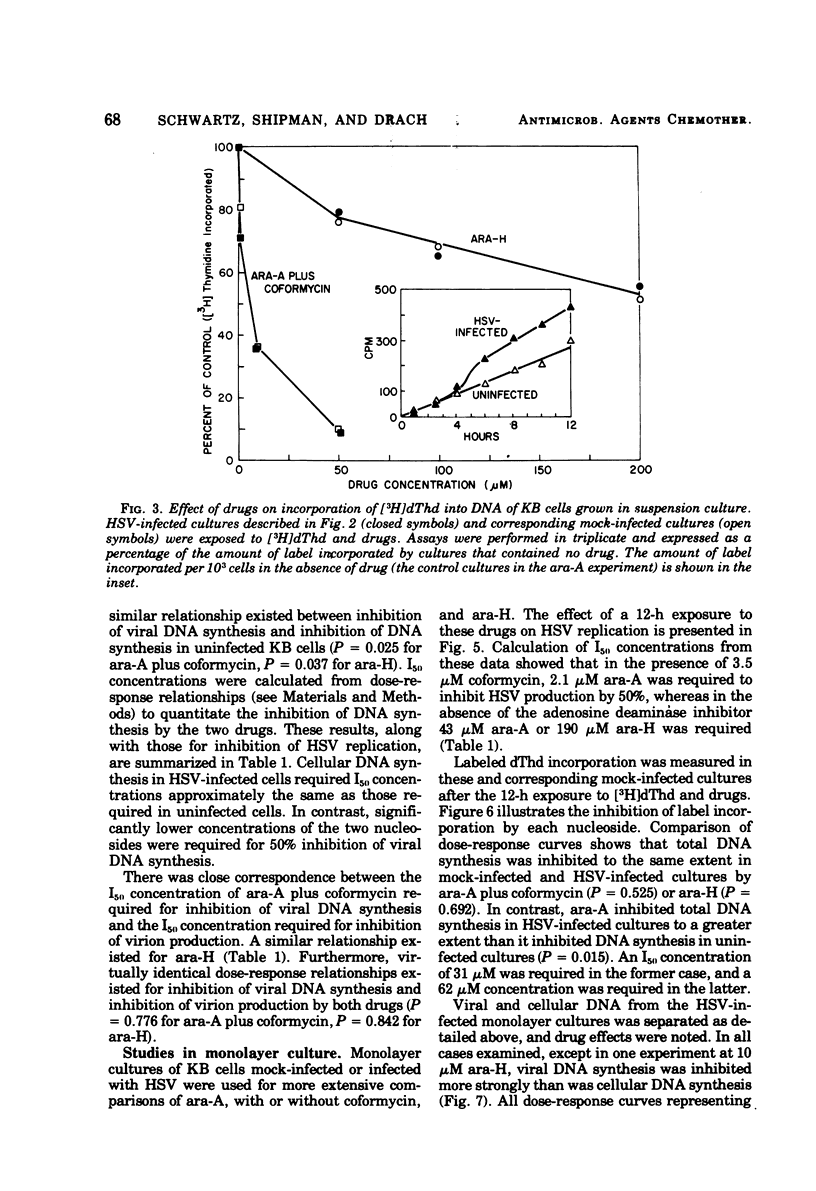
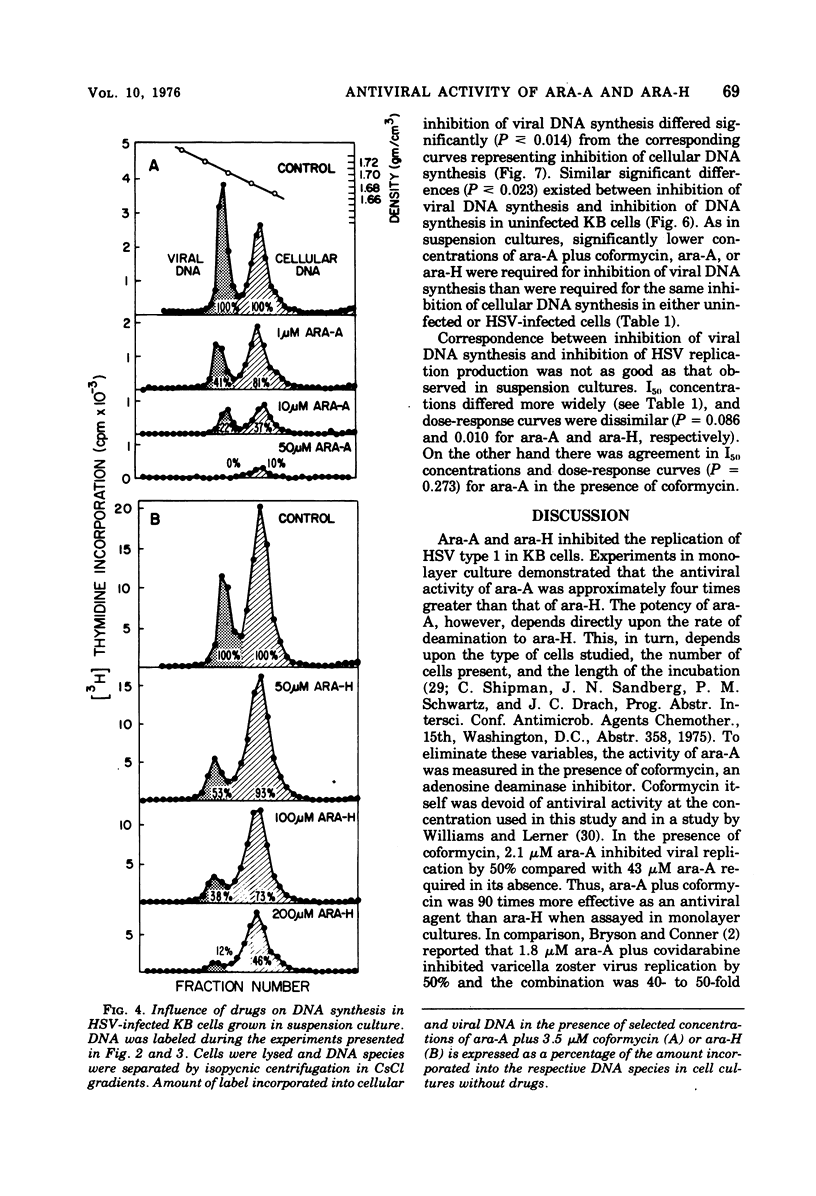
The antiviral activity of the fraudulent nucleoside arabinosyladenine (ara-A) against herpes simplex virus (HSV) type 1 was increased nearly 20-fold by the adenosine deaminase inhibitor, coformycin. The combination of ara-A plus coformycin was 90 times more potent in blocking HSV replication than was arabinosylhypoxanthine (ara-H). In suspension culture both drugs were more active than they were in monolayer culture. Deoxyribonucleic acid (DNA) synthesis also was inhibited by the nucleosides. Depending upon the species of DNA examined, ara-A was 8 to 15 times more active in the presence of coformycin, and the combination was 35 to 70 times more potent than ara-H. Both drugs inhibited total DNA synthesis to the same extent in uninfected and HSV-infected KB cells. In contrast, viral DNA synthesis was three to six times more susceptible to inhibition than was cellular DNA synthesis. Inhibition of viral DNA synthesis was more pronounced in suspension culture than in monolayer culture. However, the method of cell propagation did not alter the degree to which the drugs inhibited DNA synthesis in uninfected KB cells. An index has been derived to quantitate the extent of the selective inhibition of viral or cellular DNA synthesis. Fifty percent inhibitory concentrations of a drug were calculated for uninfected KB DNA synthesis and viral DNA synthesis and expressed as a ratio. The logarithm of this ratio was termed the selective index and was positive if viral DNA synthesis was inhibited preferentially or negative if uninfected KB DNA synthesis was more strongly inhibited. Data from experiments performed in monolayer culture gave positive selective index values of 0.3, 0.5, and 0.4 for ara-A plus coformycin, ara-A, and ara-H, respectively. Values of 0.7 and 0.6 were obtained from suspension culture data for ara-A plus coformycin and ara-H, respectively. Considered collectively, the data presented in this communication establish that coformycin increased the potency of ara-A but did not increase its selectivity.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett L. L., Jr, Shannon W. M., Allan P. W., Arnett G. Studies on the biochemical basis for the antiviral activities of some nucleoside analogs. Ann N Y Acad Sci. 1975 Aug 8;255:342–358. doi: 10.1111/j.1749-6632.1975.tb29242.x. [DOI] [PubMed] [Google Scholar]
- Bryson Y. J., Connor J. D. In vitro susceptibility of varicella zoster virus to adenine arabinoside and hypoxanthine arabinoside. Antimicrob Agents Chemother. 1976 Mar;9(3):540–543. doi: 10.1128/aac.9.3.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y. C., Goz B., Prusoff W. H. Deoxyribonucleotide metabolism in Herpes simplex virus infected HeLa cells. Biochim Biophys Acta. 1975 May 16;390(3):253–263. doi: 10.1016/0005-2787(75)90346-9. [DOI] [PubMed] [Google Scholar]
- Cohen S. S. Introduction to the biochemistry of D-arabinosyl nucleosides. Prog Nucleic Acid Res Mol Biol. 1966;5:1–88. doi: 10.1016/s0079-6603(08)60231-7. [DOI] [PubMed] [Google Scholar]
- Connor J. D., Sweetman L., Carey S., Stuckey M. A., Buchanan R. Effect of adenosine deaminase upon the antiviral activity in vitro of adenine arabinoside for vaccinia virus. Antimicrob Agents Chemother. 1974 Nov;6(5):630–636. doi: 10.1128/aac.6.5.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doering A., Keller J., Cohen S. S. Some effects of D-arabinosyl nucleosides on polymer syntheses in mouse fibroblasts. Cancer Res. 1966 Dec;26(12):2444–2450. [PubMed] [Google Scholar]
- LePage G. A. Alterations in enzyme activity in tumors and the implications for chemotherapy. Adv Enzyme Regul. 1970;8:323–332. doi: 10.1016/0065-2571(70)90027-0. [DOI] [PubMed] [Google Scholar]
- LePage G. A. Manipulation of DNA synthesis in normal and neoplastic tissues with drugs. Adv Enzyme Regul. 1974;12:373–381. doi: 10.1016/0065-2571(74)90022-3. [DOI] [PubMed] [Google Scholar]
- Marks M. I. Variables influencing the in vitro susceptibilities of herpes simplex viruses to antiviral drugs. Antimicrob Agents Chemother. 1974 Jul;6(1):34–38. doi: 10.1128/aac.6.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller W. E., Rohde H. J., Beyer R., Maidhof A., Lachmann M., Taschner H., Kahn R. K. Mode of action of 9-beta-D-arabinofuranosyladenine on the synthesis of DNA, RNA, and protein in vivo and in vitro. Cancer Res. 1975 Aug;35(8):2160–2168. [PubMed] [Google Scholar]
- NEWTON A., DENDY P. P., SMITH C. L., WILDY P. A pool size problem associated with the use of tritiated thymidine. Nature. 1962 Jun 2;194:886–887. doi: 10.1038/194886a0. [DOI] [PubMed] [Google Scholar]
- Nakamura H., Koyama G., Iitaka Y., Ono M., Yagiawa N. Structure of coformycin, an unusual nucleoside of microbial origin. J Am Chem Soc. 1974 Jun 26;96(13):4327–4328. doi: 10.1021/ja00820a049. [DOI] [PubMed] [Google Scholar]
- Ortiz P. J., Manduka M. J., Cohen S. S. The lethality of some D-arabinosyl nucleotides to mouse fibroblasts. Cancer Res. 1972 Jul;32(7):1512–1517. [PubMed] [Google Scholar]
- Plunkett W., Cohen S. S. Two approaches that increase the activity of analogs of adenine nucleosides in animal cells. Cancer Res. 1975 Jun;35(6):1547–1554. [PubMed] [Google Scholar]
- Roller B., Cohen G. H. Deoxyribonucleoside triphosphate pools in synchronized human cells infected with herpes simplex virus types 1 and 2. J Virol. 1976 Apr;18(1):58–64. doi: 10.1128/jvi.18.1.58-64.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schabel F. M., Jr The antiviral activity of 9-beta-D-arabinofuranosyladenine (ARA-A). Chemotherapy. 1968;13(6):321–338. doi: 10.1159/000220567. [DOI] [PubMed] [Google Scholar]
- Schaeffer H. J., Schwender C. F. Enzyme inhibitors. 26. Bridging hydrophobic and hydrophilic regions on adenosine deaminase with some 9-(2-hydroxy-3-alkyl)adenines. J Med Chem. 1974 Jan;17(1):6–8. doi: 10.1021/jm00247a002. [DOI] [PubMed] [Google Scholar]
- Schwartz P. M., Shipman C., Jr, Carlson R. H., Drach J. C. Thermal inactivation as a means of inhibiting the serum-associated deamination of 9-beta-D Arabinofuranosyladenine in tissue culture media. Antimicrob Agents Chemother. 1974 Mar;5(3):337–343. doi: 10.1128/aac.5.3.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon W. M., Westbrook L., Schabel F. M., Jr Antiviral activity of 9-beta-D-arabinofuranosyladenine (ara-A) against Gross murine leukemia virus in vitro. Proc Soc Exp Biol Med. 1974 Feb;145(2):542–545. doi: 10.3181/00379727-145-37848. [DOI] [PubMed] [Google Scholar]
- Shipman C., Jr, Smith S. H., Carlson R. H., Drach J. C. Antiviral activity of arabinosyladenine and arabinosylhypoxanthine in herpes simplex virus-infected KB cells: selective inhibition of viral deoxyribonucleic acid synthesis in synchronized suspension cultures. Antimicrob Agents Chemother. 1976 Jan;9(1):120–127. doi: 10.1128/aac.9.1.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidwell R. W., Huffman J. H. Use of disposable micro tissue culture plates for antiviral and interferon induction studies. Appl Microbiol. 1971 Nov;22(5):797–801. doi: 10.1128/am.22.5.797-801.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams B. B., Lerner A. M. Antiviral activity of an adenosine deaminase inhibitor: decreased replication of herpes simplex virus. J Infect Dis. 1975 Jun;131(6):673–677. doi: 10.1093/infdis/131.6.673. [DOI] [PubMed] [Google Scholar]


