Abstract
Microfracture is a marrow-stimulating technique used in the hip to treat cartilage defects associated with femoro-acetabular impingement, instability, or traumatic hip injury. These defects have a low probability of healing spontaneously and therefore often require surgical intervention. Originally adapted from the knee, microfracture is part of a spectrum of cartilage repair options that include palliative procedures such as debridement and lavage, reparative procedures such as marrow-stimulating techniques (abrasion arthroplasty and microfracture), and restorative procedures such as autologous chondrocyte implantation and osteochondral allograft/autografts. The basic indications for microfracture of the hip include focal and contained lesions typically less than 4 cm in diameter, full-thickness (Outerbridge grade IV) defects in weightbearing areas, unstable lesions with intact subchondral bone, and focal lesions without evidence of surrounding chondromalacia. Although not extensively studied in the hip, there are some small clinical series with promising early outcomes. Although the widespread use of microfracture in the hip is hindered by difficulties in identifying lesions on preoperative imaging and instrumentation to circumvent the femoral head, this technique continues to gain acceptance as an initial treatment for small, focal cartilage defects.
Keywords: marrow stimulation, hip, arthroscopy, cartilage repair
Introduction
Articular cartilage defects of synovial joints generally occur as the result of age-related superficial fibrillation, cartilage degeneration due to osteoarthritis, and focal chondral and osteochondral defects.1 Whether these defects are a result of acute, chronic, or degenerative processes, they all have a low probability of healing spontaneously2 and therefore often require surgical intervention. The experience in the hip is limited at this point, but the spectrum of options for cartilage repair, which has been adapted from the knee, includes palliative procedures such as debridement and lavage, reparative procedures such as marrow-stimulating techniques (abrasion arthroplasty and microfracture), and restorative procedures such as autologous chondrocyte implantation and osteochondral allograft/autografts.
Microfracture is a marrow-stimulating technique that involves perforation of subchondral bone within a chondral defect. The rationale of the technique is to recruit pluripotent mesenchymal stem cells into the cartilage defect to create fibrocartilage. Immediately following microfracture, a marrow clot forms, providing the ideal environment for pluripotent marrow cells and mesenchymal stem cells to differentiate into stable repair tissue.3
Although not extensively studied in the hip, there are some small clinical series after microfracture in the hip with promising early outcomes.4-7 The purpose of the present article is to review the etiology of cartilage injury in the hip; to discuss the pathomechanics of the hip, cartilage biology, and classification of cartilage injury; to describe microfracture technique in the hip; and to discuss clinical outcomes after microfracture in the hip.
Chondral Lesions of the Hip
Chondral injuries may occur in association with various hip conditions, including femoroacetabular impingement (FAI), labral tears, certain pediatric conditions including Legg Calves Perthes disease, slipped capital femoral epiphysis, developmental dysplasia of the dip, loose bodies, and hip dislocation/subluxation.8
There are 2 subtypes of FAI that have been identified and may occur alone or in combination with each other.8,9 Pincer FAI typically presents in women in the 3rd and 4th decade and possesses either areas of focal overcoverage (retroversion) or global overcoverage (coxa profunda, acetabular protrusio). The pincer occurs when there is abnormal contact of the acetabular rim with the femoral head-neck junction and may occur as focal (acetabular retroversion) or global (coxa profunda or protrusio) acetabular overcoverage. In these cases, persistent anterior contact causes labral injury at the periphery as well as chronic leverage of the head in the posterior-inferior aspect of the acetabulum, resulting in a “contra-coup” mechanism of cartilage injury.8,9
Cam FAI is more commonly present in men in their 2nd and 3rd decades, and these lesions are more destructive but can be clinically asymptomatic for a long period of time.8,9 Cam FAI occurs as a result of a bony prominence located on the anterolateral aspect of the femoral head-neck junction that enters the spherical acetabulum, causing intraarticular injury to the articular cartilage and labrum.8,9 The abnormal femoral head-neck junction causes an avulsion of the articular cartilage from the acetabular labrum and eventually the underlying subchondral bone. These patients may have a focal area of cartilage delamination with normal surrounding articular cartilage with or without labral detachment. The repetitive microscopic injury to the acetabular articular cartilage may become extensive enough that the weightbearing portion of the femoral head may migrate into the defect, and long-standing disease will result in joint space narrowing on radiographs. This disease process is thought to initiate the early stages of the extensive, generalized process that ultimately results in hip osteoarthritis.8,9 The morphologic abnormalities associated with FAI are thought to be present in the setting of most, if not all, labral tears, and most surgeons believe that the majority of labral tears are caused by these subtle osseous deformities.10
FAI has been observed to be increased among patients with a history of certain pediatric conditions such as slipped capital femoral epiphysis and Perthes disease.8 Most patients with FAI do not have an underlying pediatric hip abnormality; therefore, the etiology of most FAI abnormalities remains unknown.11,12 Certain posttraumatic and iatrogenic deformities (femoral varus osteotomy, retroversion after pelvic osteotomy13) of the hip have also been shown to have an association with FAI.
Labral tears have been reported as a common form of intraarticular pathology of the hip often identified in athletes during arthroscopy, but recent literature suggests that the overwhelming majority of labral tears occur in the setting of underlying osseous abnormalities.14-16 Biomechanical studies have suggested that labral tears often occur as a result of stresses similar to those that lead to FAI and chondral injuries, particularly bony abnormalities and repetitive stress at extreme ranges of hip motion where the intact labrum contributes most to the maintenance of joint stability.17 In a review of 436 consecutive hip arthroscopies, McCarthy et al.16 identified 241 (55.3%) patients with labral tears and 273 (62.6%) patients with lesions of the acetabular articular cartilage. In all, 477 lesions were found in the anterior quadrant (259 lesions; 54%), posteriorly (112 lesions; 23%), and laterally (106 lesions; 22%). The location of the lesion was also related to the severity of the Outerbridge score, with the most severe lesions found anteriorly (average Outerbridge = 2.88), then posteriorly (2.22, P < 0.0001), then laterally (2.17, P < 0.0001). The labral and chondral injuries were highly associated with one another. Of the patients with labral damage, 73% also had chondral injuries, with 94% of those patients having labral and chondral damage in the same location within the acetabulum. Approximately 37% of patients had extensive cartilage damage consisting of large areas of fissuring (Outerbridge III; 11%) or full-thickness erosion (Outerbridge IV; 26%), which is a significant finding because of the previously reported correlation between high Outerbridge scores and poor outcomes.18-20
Loose bodies are also a cause of hip cartilage injury and generally occur as isolated fragments secondary to posterior dislocations21 or osteochondritis dissicans.22 In a case series by Philippon et al.21 of 14 professional athletes with traumatic hip dislocations (85% posterior, 15% anterior), 100% of them were found to have both labral tears and chondral defects on hip arthroscopy performed an average of 125 d after the initial injury. Two had isolated femoral head chondral defects, and 6 had isolated acetabular chondral defects. Alternatively, there are some cases in which there are multiple loose bodies in the setting of synovial chondromatosis.23,24 These fragments can aggregate to form grapelike clusters that adhere to the synovium and damage the articular cartilage as a result of 3rd-body wear.25 The presence of multiple loose bodies is also associated with collagen diseases, crystalline hip diseases, idiopathic chondrolysis, hypertrophic synovitis, and following total hip arthroplasty.22
Biomechanics of the Hip
Cam Biomechanics
Cam impingement occurs as a result of asphericity of the femoral head, causing abutment of the acetabular rim and femoral neck, thereby functioning as a cam, the eccentric portion of a rotating device designed to turn rotary motion into linear motion.9 This process starts as decreased wasting of the junction between the femoral neck and head, which results in an increased radius of the femoral epiphysis as it joins the neck. These changes are often referred to as the pistol grip26 or tilt27 deformity. Damage from cam impingement tends to occur in the anterosuperior area of the labrum, which has been shown in clinical studies10 and computer simulation.28
A study by Beck et al.10 of patients who had undergone surgical dislocation of the hip for the treatment of intraarticular pathology at a mean age of 32 years demonstrated a relationship between the shape of the hip and damage to the cartilage and/or labrum. Of 302 hips examined, they found 26 hips with a pure pistol grip deformity to represent cam impingement and 16 hips with a pure coxa profunda deformity to represent pincer impingement. The study concluded that the coexistence of cartilage damage and labral tears in the same location in patients with cam impingement suggests that cam impingement leads to extensive damage of the acetabular cartilage and that separation between the labrum and cartilage arises because the cartilage is ripped off of the labrum. They hypothesized that during flexion, the aspherical part of the femoral head is jammed into the acetabulum, compressing the cartilage and pushing it at the same time centrally until shearing it off the subchondral bone creating a chondral lesion, most commonly located in the anterosuperior acetabulum. Alternatively, in pincer impingement, cartilage damage is found circumferentially, and the labrum is crushed between the acetabular rim and the femoral neck, leading to degeneration and ossification of the labrum. Both cam and pincer impingement result in degeneration of the hip and can lead to early osteoarthritis.8
Finite Element Modeling
In finite element modeling (FEM), the intraarticular surface of the hip is divided computationally into many small parts, and equations are created to represent the forces present at each discrete element during activities that would likely be encountered in vivo. The equations are solved simultaneously to determine the effect of these activities on the hip joint. Researchers often use patient-specific anatomy from computed tomography (CT) scans to create the initial geometry and material properties of their models.29
In 2000, Ferguson et al.30,31 published a poroelastic FEM to investigate the relationship between the labrum and cartilage in the hip joint. Their analysis demonstrated that the role of the labrum is to seal a layer of pressurized fluid between the acetabulum and femur, thus preventing contact of the articular surfaces. After removal of the labrum, the contact forces between the acetabulum and femur increased by as much as 92%, increasing friction between the 2 surfaces. The increase in subsurface stress and strain may contribute to fatigue and cartilage damage. Their findings corroborate in vitro studies, which showed that the labrum could prevent fluid flowing in or out of the joint space, improve hip stability through the vacuum effect, and maintain lubrication by means of a pressurized fluid layer in the joint.30-34
Researchers have shown that the hips must support more than 3 times the individual’s body weight during the normal gait cycle.35-37 Russell et al.38 used FEM to show that contact pressures are even higher in dysplastic hips when compared with normal counterparts and that this elevation in contact pressure may be responsible for the increase in cartilage degeneration and osteoarthritis.39-41
Cartilage Biology
Cartilage is a dense, fibrous substance that consists of cells, matrix water, and a matrix macromolecular framework. Articular cartilage lines the large synovial joints, including the hip, knee, and glenohumeral joints, which produce rapid controlled movements required for participation in sports.
Chondrocytes are the mesenchymal cells responsible for manufacturing the extracellular matrix (ECM), which makes up approximately 95% of articular cartilage.42 They live isolated from one another, only rarely forming connections with other cells or dividing. Cartilage tissue lacks blood vessels, nerves, and a lymphatic system; chondrocytes must rely on diffusion through the matrix for their nutrition, and they rely primarily on anaerobic metabolism. In addition to providing nutrients, the synovial fluid also removes waste products of cellular metabolism.43
Throughout life, chondrocytes use amino acids and sugars to manufacture ECM consisting primarily of collagen (type II), proteoglycans, and noncollagenous proteins, which comprise approximately 60%, 25%, and 15% of its dry weight, respectively. The collagen fibrillar meshwork and cross-linking give collagen its form and tensile strength. The proteoglycans and noncollagenous proteins give bind to the meshwork and allow it to fill with water, giving cartilage its stiffness in compression and its resilience.
The macromolecular structure of cartilage consists of 4 zones that blend into one another (named the superficial, transitional, radial, and a calcified zone) and attaches the cartilage to the subchondral bone via an irregular cement line. The superficial zone includes 2 layers: The top is an acellular fine sheet of fibrils with a small amount of polysaccharides, and the bottom consists of ellipsoid chondrocytes that create ECM that has a high-collagen and low-proteoglycan concentration relative to the other zones. The transitional zone has a larger volume than the superficial zone and has a high concentration of synthetic organelles, endoplasmic reticulum, and Golgi membranes. This zone has spheroidal chondrocytes that synthesize large collagen fibrils. The deep zone contains the largest diameter collagen fibrils, the highest concentration of proteoglycans, and the lowest concentration of water. The cartilage in this layer is aligned perpendicularly to the joint line to provide bulk resistance to compressive forces. Beneath the deep zone is the tidemark that delineates the boundary between calcified and uncalcified cartilage. The deepest layer is the zone of calcified cartilage, which forms adjacent to the subchondral bone. It has a smaller volume per cell and thins with age. This remodeling is theorized to be the result of repetitive microtrauma.44
Cartilage Injury Assessment
Many authors have made attempts to grade cartilage lesions.45-51 The Outerbridge classification system, developed in 1961, was originally designed to evaluate chondral lesions in chondromalacia patellae (Tables 1 and 2).51
Table 1.
Outerbridge Grading
| Grade 1 | Softening and swelling of the cartilage |
| Grade 2 | Fragmentation and fissuring in an area ≤½ in. in diameter |
| Grade 3 | Fragmentation and fissuring in an area >½ in. in diameter |
| Grade 4 | Erosion of cartilage down to the bone |
Table 2.
International Cartilage Repair Society Grading
| Grade 0: Normal | |
| Grade 1: Nearly normal | Soft indentation and/or superficial fissures and cracks |
| Grade 2: Abnormal | Lesions extending down to <50% of cartilage depth |
| Grade 3: Severely abnormal | Cartilage defects extending down >50% of cartilage depth as well as down to calcified layer and down to but not through the subchondral bone; blisters are included in this grade |
| Grade 4: Severely abnormal | Lesions extending through the subchondral bone plate and deeper defects through the trabecular bone |
One disadvantage of this method is that grades I and IV are classified completely by size and grades II and III are classified completely by appearance. Although it has been shown that the severity of hip dysplasia correlates well with the Outerbridge classification, the fact that this classification is done only on visual inspection makes it difficult to use this grading system alone to determine the appropriate treatment. Although no studies exist that evaluate the reliability of the Outerbridge classification for hip cartilage, there are numerous studies that show a moderate to high interrater agreement when used to evaluate knee cartilage.52-54 In their arthroscopic evaluation of 31 knee articular cartilage lesions (with grades II and III combined to improve statistical validity), Marx et al.53 observed a confirmed agreement rate between 81% and 94%, with kappa ranging between 0.34 and 0.87. Cameron et al.52 also investigated the reliability of the Outerbridge classification in cadaveric knees using a postarthrotomy evaluation as the gold standard. This study found an interobserver kappa of 0.52, which increased to 0.70 for more experienced surgeons and decreased to 0.50 for less experienced surgeons. A recent survey of 105 German orthopedic surgeons by Spahn et al.54 reported using the Outerbridge (n = 87, 82.9%) grading system most often followed by the International Cartilage Repair Society (ICRS; n = 8, 7.6%) and Insall (n = 5, 4.8%). Most surgeons (61%) who participated in this survey preferred Outerbridge because they felt that the differentiation between healthy cartilage and low-grade cartilage was simple.
To supplement the arthroscopic evaluation of chondral lesions, the ICRS developed a histological method of evaluation of cartilage lesions in 2003,55 which was revised in 2008.56 The ICRS I Visual Histology Score uses visual patterns to evaluate many parameters to assess the extent of cartilage damage, including cell morphology, surface regularity, clustering/distribution, mineral content, subchondral bone, and viability of cell population. The ICRS II Visual Analog Scale (VAS) evaluates the similar parameters, except for viability of cell population, while also including matrix staining, structural integrity, osteochondral junction, basal integration, blood vessels, and inflammation.57 The ICRS II uses a larger number of categories on a 100-mm VAS scale, which facilitates statistical comparisons of individual cartilage characteristics.58 This method was applied clinically in a trial comparing both the clinical and histological results of microfracture and autologous chondrocyte implantation for the treatment of chondral lesions of the knee.56
Treatment of Chondral Lesions
Any patient with FAI may have an unstable area of focal cartilage delamination. These patients may present with insidious onset of hip and groin pain. In some cases, these patients may recall an acute traumatic event with ongoing symptoms. Patients typically complain of deep groin pain that is worse with prolonged sitting, stair climbing, rotational maneuvers, and recreational activities that involved pivoting. Imaging studies with plain radiographs, magnetic resonance imaging or magnetic resonance arthrography, and CT scans are important for visualizing the 3-dimensional hip morphology as well as areas of cartilage or labral injury. The initial course of treatment should be nonsurgical with nonsteroidal anti-inflammatory medications and physical therapy. Intraarticular steroid injections can be useful from a diagnostic and therapeutic standpoint. If the patient has symptoms refractory to conservative treatment, the next step in treatment is hip arthroscopy to evaluate the cartilage and labrum.
If a focal chondral lesion is visualized at the time of arthroscopy, then the surgeon must decide whether this lesion fits the criteria for use of the microfracture technique. Surgeons who perform this procedure on locations other than the knee may consider using guidelines for treating chondral defects of the knee to determine the appropriate treatment. The basic indications for microfracture of the hip include focal and contained lesions typically less than 4 cm in diameter, full-thickness (Outerbridge grade IV) defects in weightbearing areas, unstable lesions with intact subchondral bone, and focal lesions without evidence of surrounding chondromalacia. A patient’s age, level of activity, and ability to comply with the rehabilitation protocol should also be considered prior to performing the procedure.
Steadman et al.59 followed 68 patients (71 knees) who underwent microfracture treatment for an average of 11 years. Using a multivariable regression model, they reported a negative correlation (correlation coefficient = −0.146, P = 0.225) between lesions >400 mm and Lysholm score, although the association was not statistically significant. This study showed only 1 independent predictor of Lysholm scores: age (correlation coefficient = −0.299, P = 0.011), which was negatively correlated.
Contraindications to microfracture include partial-thickness defect, chondral lesions associated with bony defects, and patients who are unwilling or unable to comply with the rehabilitation protocol, including patients who are unable to use their nonoperative leg for weightbearing. Some authors recommend using the age of 60 years as a relative contraindication for situations with rehabilitation because some patients of advanced age may experience difficulty with crutches during the nonweightbearing period.60,61
Compliance is an important factor in recovery from microfracture because rehabilitation begins immediately following surgery and may involve restricted weightbearing coupled with 6 to 8 hours per day of continuous passive motion for up to 8 weeks to maximize fibrocartilaginous healing.3 Other contraindications are immune-mediated disease and systemic disease–induced arthritis or cartilage injury.59,62,63 Morbid obesity is also considered a relative contraindication to hip arthroscopy because of the difficulty in creating the necessary hip distraction22 and the limitations of the strength and reach of the arthroscopic equipment.64
Microfracture Technique
The authors preferred microfracture technique, initially described by Crawford et al.,6 which begins with the patient anesthetized on a standard fracture table in the supine position. The hip is placed in 10° of flexion, 15° of internal rotation, 10° of lateral tilt, and neutral abduction. A foot stirrup is used to place adequate traction of 25 to 50 lb of force on the operative limb, thereby creating 7 mm to 15 mm of joint distraction for adequate visualization and instrumentation.65 Once the fluoroscopy confirms adequate distraction of the hip, the anterolateral portal is created 1 cm proximal and 1 cm anterior to the anterior border of the greater trochanter under fluoroscopic guidance. The anterior portal is established at the intersection of a vertical line from the anterior superior iliac spine and a horizontal line from the tip of the greater trochanter.66 Some authors prefer to use a midanterior portal, which is the midpoint between the traditional anterior and anterolateral portals and 5 to 7 cm inferior. The anterosuperior labrum and femoral head are visualized from the anterolateral portal, and the anterior portal is established under direct arthroscopic visualization.66-70
A peripheral portal, also referred to as the distal anterolateral accessory portal,71 is crucial to the evaluation of FAI because it enables the surgeon to visualize the femoral head-neck junction and see the femoral head entering the acetabulum. This also allows the surgeon to look for evidence of osteophytes, loose bodies, and synovitis.72 The peripheral compartment is examined after the intraarticular region of the hip has been evaluated. Flexing the hip 45° to relax the anterior capsule enables access to this compartment.
After portal placement, the surgeon does a complete diagnostic evaluation of the hip joint and characterization of the chondral lesion visually using the Outerbridge or ICRS systems. Any unstable cartilage is removed from the subchondral bone (Figs. 1 and 2) using a full-radius mechanical shaver and a curette. A ring curette is used to create a border of cartilage perpendicular to the adjacent healthy cartilage to help the marrow clot form. The curette is used to remove the calcified cartilage layer at the base of the cartilage lesion (Fig. 3). For lesions of the femoral head where the cartilage is thinner, an adequate border must be prepared to maintain the clot.6
Figure 1.
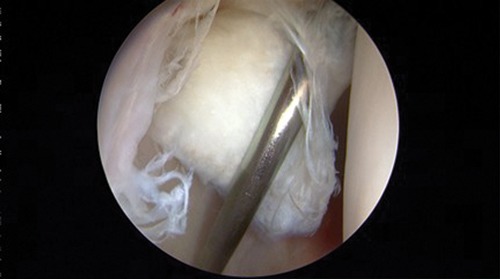
Cartilage delamination.
Figure 2.
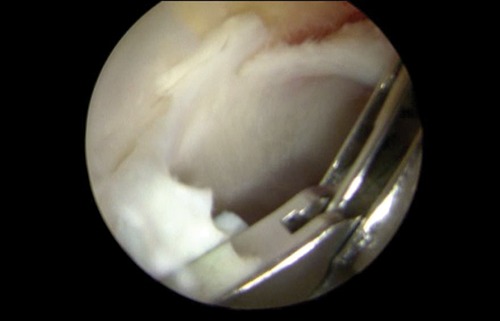
Removal of unstable cartilage.
Figure 3.
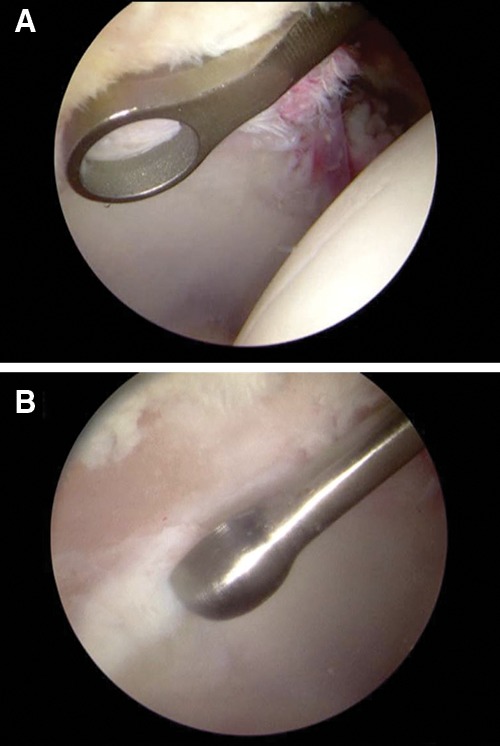
Defect preparation with (A) ring curette and (B) curved curette.
Special awls are then used to perforate the subchondral bone in the periphery of the chondral defect adjacent to the rim of healthy cartilage. The awls can be difficult to position perpendicularly to the acetabulum subchondral bone because the femoral head may obstruct proper positioning. There are awls that have been specially designed for the hip with concave curves to accommodate the femoral head (Fig. 4). In general, the awls placed through the anterior or midanterior portals, but the anterolateral or posterolateral portals can also be used depending on the location of the cartilage defect. Holes are made 3- to 4-mm apart and 2- to 4-mm deep to access marrow elements. When the irrigation pressure is decreased, the marrow elements, including blood and fat droplets, should be observed protruding from the microfracture holes (Fig. 5). If the defect cannot be accessed appropriately with the awls, the surgeon can elect to perform an abrasion arthroplasty with an arthroscopic burr to stimulate bleeding from the subchondral bone at the base of the cartilage defect. Surgeons can discharge the patient following recovery from anesthesia or may elect to have the patient stay overnight for monitoring and to facilitate the initiation of rehabilitation.22,61,63
Figure 4.
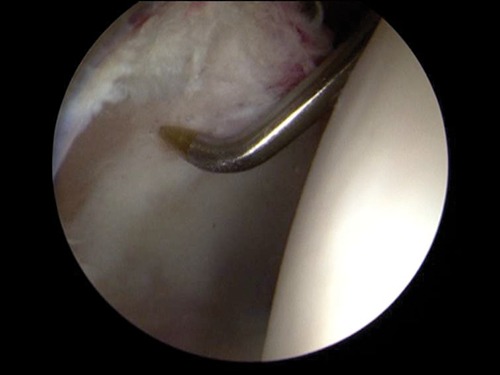
Microfracture awl perforates subchondral bone.
Figure 5.
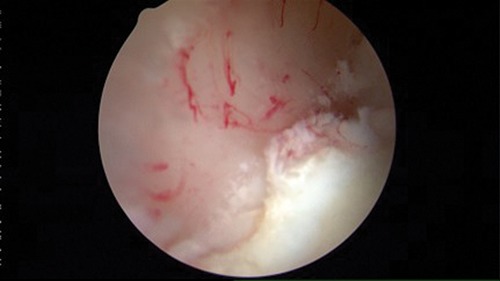
Bleeding at the microfracture site.
The protocol of rehabilitation for hip microfracture parallels that of the knee. CPM is initiated soon after surgery using a stationary bicycle, to increase the passive range of motion. Over the next 8 weeks, a gradual shift actives the range of motion with an emphasis on restoration of internal rotation. The patient is on crutches for 8 weeks, 20 lb of flat-foot weightbearing for the first 6 weeks and gradually returns to full weightbearing at 8 weeks. Cryotherapy is also used postoperatively to decrease pain and inflammation. The patient may engage in contact sports 4 to 6 months after surgery following restoration of motion, strength, and functional agility.59,62
Complications specific to microfracture are not well documented in the literature. In a study of 1,054 hip arthroscopy patients with an average age of 37 years, Clarke et al.67 reported an overall complication rate of 1.4% (95% upper limit confidence intervals, 2.4%). These patients underwent arthroscopy for a variety of indications, including undiagnosed hip pain (41%), osteoarthritis (21%), labral tears (18%), removal of loose bodies (7%), and other miscellaneous conditions (13%). The most common complications after hip arthroscopy included neuropraxia, portal wound bleeding, portal hematoma, trochanteric bursitis, and instrument breakage.
Related Procedures
Various alternative procedures are currently used to repair chondral lesions, including autologous chondrocyte implantation (ACI) and osteochondral grafting. Although there have been no comprehensive studies of these procedures in the hip, ACI has shown similar outcomes to microfracture in the knee.56,73 Microfracture may still be the preferred procedure; however, there are experimental studies on cell-based therapies using a 3-dimensional matrix that is being implanted in the hip.
Osteochondral grafting of the femoral head, used for larger lesions of the hip, has shown poor results. In 2005, Rittmeister et al.74 reported a series of 5 patients who underwent osteochondral autograft with transplants between 9 and 13 mm in diameter. The patients were followed for 57 months, and 80% of the hips failed, having to undergo total hip replacement an average of 49 months following the initial transplant. Some authors recommend total hip replacement for any patients with cartilage lesions on both the acetabulum and femoral head.75
Clinical Outcomes
There are few studies on arthroscopic microfracture for the treatment of chondral defects of the hip. In 2008, Phillipon et al.76 published a study of 9 patients undergoing revision hip arthroscopy who initially were treated an average of 20 months prior to the revision for various diagnoses including chondral lesions requiring arthroscopic microfracture. During the revision, it was noted that 8 of 9 patients had 95% to 100% coverage of an isolated acetabular chondral lesion or an acetabular lesion associated with femoral head lesion, with a grade I or II appearance (using the grading system described by Blevins et al.77) of the repair product. This cohort had an average age of 37.2 years at time of the index procedure and an average lesion size of 163 mm2, with all lesions located in the superior acetabular quadrant. The 1 patient who failed had diffuse osteoarthritis and only 25% coverage with a grade IV appearance of the repair product observed 10 months after the original procedure.
J. W. Byrd (unpublished data, 2005) reported a complication rate of 0% after 2-year follow-up of 21 hip microfracture patients with an average age of 35 years and average lesion size of 12.2 mm2.
Byrd and Jones78 recently published a 10 year follow-up study of 15 athletes with hip pathology requiring unspecified arthroscopic intervention, 8 of whom had chondral lesions. The median improvement on the Harris Hip Score was 45 points (from 51 to 96 points), with 13 patients (87%) returning to their sport.
Although there are no published long-term prospective or randomized controlled studies on microfracture of the hip, these types of studies do exist for microfracture of the knee,56,59,73 and these studies have shown good long-term results. In 2003, Steadman et al.59 published an outcome study of 72 patients treated with arthroscopic microfracture of the knee and followed for an average of 11 years (range, 7 to 17 years). Patients reported significant improvement (P < 0.05) using the Lysholm (59 to 89, best = 100) and Tegner (3 to 6, best = 10) scores and good to excellent results on the WOMAC and SF-36 assessments. After 7 years, 80% of patients considered themselves as improved.
Conclusion
Cartilage defects are commonly seen in association with FAI, instability, or traumatic hip injury, and preliminary results appear to indicate that microfracture of the hip is a safe and effective treatment option. Some of the obstacles to improving treatment are identifying cartilage lesions through preoperative imaging and instrumentation to circumvent the femoral head.79,80 Future development in cell-based therapy using a 3-dimensional matrix may be able to provide an alternative treatment that may have the potential to provide hyaline or hyaline-like cartilage.
Footnotes
The authors declared that they had no conflicts of interest in their authorship and publication of this contribution.
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1. Mankin HJ, Buckwalter JA. Restoration of the osteoarthrotic joint. J Bone Joint Surg Am. 1996;78(1):1-2. [DOI] [PubMed] [Google Scholar]
- 2. Buckwalter JA. Articular cartilage: injuries and potential for healing. J Orthop Sports Phys Ther. 1998;28(4):192-202. [DOI] [PubMed] [Google Scholar]
- 3. Frisbie DD, Oxford JT, Southwood L, Trotter GW, Rodkey WG, Steadman JR, et al. Early events in cartilage repair after subchondral bone microfracture. Clin Orthop Relat Res. 2003;407:215-27. [DOI] [PubMed] [Google Scholar]
- 4. Byrd JW, Jones KS. Microfracture for grade IV chondral lesions of the hip. Arthroscopy. 2004;20(e41). [Google Scholar]
- 5. Byrd JW, Jones KS. Osteoarthritis caused by an inverted acetabular labrum: radiographic diagnosis and arthroscopic treatment. Arthroscopy. 2002;18(7):741-7. [DOI] [PubMed] [Google Scholar]
- 6. Crawford K, Philippon MJ, Sekiya JK, Rodkey WG, Steadman JR. Microfracture of the hip in athletes. Clin Sports Med. 2006;25(2):327-35. [DOI] [PubMed] [Google Scholar]
- 7. Philippon MJ, Schenker ML, Briggs KK, Maxwell RB. Can microfracture produce repair tissue in acetabular chondral defects? Arthroscopy. 2008;24(1):46-50. [DOI] [PubMed] [Google Scholar]
- 8. Ganz R, Leunig M, Leunig-Ganz K, Harris WH. The etiology of osteoarthritis of the hip: an integrated mechanical concept. Clin Orthop Relat Res. 2008;466(2):264-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ganz R, Parvizi J, Beck M, Leunig M, Notzli H, Siebenrock KA. Femoroacetabular impingement: a cause for osteoarthritis of the hip. Clin Orthop Relat Res. 2003;417:112-20. [DOI] [PubMed] [Google Scholar]
- 10. Beck M, Kalhor M, Leunig M, Ganz R. Hip morphology influences the pattern of damage to the acetabular cartilage: femoroacetabular impingement as a cause of early osteoarthritis of the hip. J Bone Joint Surg Br. 2005;87(7):1012-8. [DOI] [PubMed] [Google Scholar]
- 11. Siebenrock KA, Wahab KH, Werlen S, Kalhor M, Leunig M, Ganz R. Abnormal extension of the femoral head epiphysis as a cause of cam impingement. Clin Orthop Relat Res. 2004;418:54-60. [DOI] [PubMed] [Google Scholar]
- 12. Leunig M, Beck M, Woo A, Dora C, Kerboull M, Ganz R. Acetabular rim degeneration: a constant finding in the aged hip. Clin Orthop Relat Res. 2003;413:201-7. [DOI] [PubMed] [Google Scholar]
- 13. Dora C, Mascard E, Mladenov K, Seringe R. Retroversion of the acetabular dome after Salter and triple pelvic osteotomy for congenital dislocation of the hip. J Pediatr Orthop. 2002;11(1):34-40. [DOI] [PubMed] [Google Scholar]
- 14. Zaragoza EJ, Beaule PE. Imaging of the painful non-arthritic hip: a practical approach to surgical relevancy. Oper Tech Orthop. 2004;14:42-8. [Google Scholar]
- 15. McCarthy JC, Busconi B. The role of hip arthroscopy in the diagnosis and treatment of hip disease. Orthopedics. 1995;18(8):753-6. [DOI] [PubMed] [Google Scholar]
- 16. McCarthy JC, Noble PC, Schuck MR, Wright J, Lee J. The Otto E. Aufranc Award: the role of labral lesions to development of early degenerative hip disease. Clin Orthop Relat Res. 2001;393:25-37. [DOI] [PubMed] [Google Scholar]
- 17. Crawford MJ, Dy CJ, Alexander JW, Thompson M, Schroder SJ, Vega CE, et al. The 2007 Frank Stinchfield Award. The biomechanics of the hip labrum and the stability of the hip. Clin Orthop Relat Res. 2007;465:16-22. [DOI] [PubMed] [Google Scholar]
- 18. Byrd JW, Jones KS. Prospective analysis of hip arthroscopy with 2-year follow-up. Arthroscopy. 2000;16(6):578-87. [DOI] [PubMed] [Google Scholar]
- 19. Farjo LA, Glick JM, Sampson TG. Hip arthroscopy for acetabular labral tears. Arthroscopy. 1999;15(2):132-7. [DOI] [PubMed] [Google Scholar]
- 20. McCarthy JC, Lee JA. Acetabular dysplasia: a paradigm of arthroscopic examination of chondral injuries. Clin Orthop Relat Res. 2002;405:122-8. [DOI] [PubMed] [Google Scholar]
- 21. Philippon MJ, Kuppersmith DA, Wolff AB, Briggs KK. Arthroscopic findings following traumatic hip dislocation in 14 professional athletes. Arthroscopy. 2009;25(2):169-74. [DOI] [PubMed] [Google Scholar]
- 22. McCarthy JC, Lee JA. Arthroscopic intervention in early hip disease. Clin Orthop Relat Res. 2004;429:157-62. [DOI] [PubMed] [Google Scholar]
- 23. Krebs VE. The role of hip arthroscopy in the treatment of synovial disorders and loose bodies. Clin Orthop Relat Res. 2003;406:48-59. [DOI] [PubMed] [Google Scholar]
- 24. Maurice H, Crone M, Watt I. Synovial chondromatosis. J Bone Joint Surg Br. 1988;70(5):807-11. [DOI] [PubMed] [Google Scholar]
- 25. Shah A, Busconi BD. Chapter 21: Hip, pelvis, and thigh. In: DeLee JC, Drez D, Miller MD, editors. DeLee and Drez’s Orthopaedic Sports Medicine. Cambridge, MA: Saunders; 2009. [Google Scholar]
- 26. Stulberg SD, Cordell LD, Harris WH, Ramsey PL, MacEwen GD. Unrecognized childhood hip disease: a major cause of idiopathic osteoarthritis of the hip. In: The Hip: Proc of the Third Open Scientific Meeting of The Hip Society. St. Louis, MO: CV Mosby; 1975. p. 212-28. [Google Scholar]
- 27. Murray RO. The aetiology of primary osteoarthritis of the hip. Br J Radiol. 1965;38:810-24. [DOI] [PubMed] [Google Scholar]
- 28. Tannast M, Goricki D, Beck M, Murphy SB, Siebenrock KA. Hip damage occurs at the zone of femoroacetabular impingement. Clin Orthop Relat Res. 2008;466(2):273-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shim VB, Pitto RP, Streicher RM, Hunter PJ, Anderson IA. The use of sparse CT datasets for auto-generating accurate FE models of the femur and pelvis. J Biomech. 2007;40(1):26-35. [DOI] [PubMed] [Google Scholar]
- 30. Ferguson SJ, Bryant JT, Ganz R, Ito K. The acetabular labrum seal: a poroelastic finite element model. Clin Biomech. 2000;15(6):463-8. [DOI] [PubMed] [Google Scholar]
- 31. Ferguson SJ, Bryant JT, Ganz R, Ito K. The influence of the acetabular labrum on hip joint cartilage consolidation: a poroelastic finite element model. J Biomech. 2000;33(8):953-60. [DOI] [PubMed] [Google Scholar]
- 32. Takechi H, Nagashima H, Ito S. Intra-articular pressure of the hip joint outside and inside the limbus. Nippon Seikeigeka Gakkai Zasshi. 1982;56(6):529-36. [PubMed] [Google Scholar]
- 33. Terayama K, Takei T, Nakada K. Joint space of the human knee and hip joint under a static load. Eng Med. 1980;9:67-74. [Google Scholar]
- 34. Weber W, Weber E. Uber die mechanik der menschlichten gehwerkzeuge nebst der beschreibung eines versuches uber das herausfallen des schenkelkopfes aus der pfanne im luftverdennten raum. Annalen Physik und Chemie. 1837;40:1-13. [Google Scholar]
- 35. Brand RA, Pedersen DR, Davy DT, Kotzar GM, Heiple KG, Goldberg VM. Comparison of hip force calculations and measurements in the same patient. J Arthroplast. 1994;9(1):45-51. [DOI] [PubMed] [Google Scholar]
- 36. Michaeli DA, Murphy SB, Hipp JA. Comparison of predicted and measured contact pressures in normal and dysplastic hips. Med Eng Phys. 1997;19(2):180-6. [DOI] [PubMed] [Google Scholar]
- 37. Pedersen DR, Brand RA, Davy DT. Pelvic muscle and acetabular contact forces during gait. J Biomech. 1997;30(9):959-65. [DOI] [PubMed] [Google Scholar]
- 38. Russell ME, Shivanna KH, Grosland NM, Pedersen DR. Cartilage contact pressure elevations in dysplastic hips: a chronic overload model. J Orthop Surg Res. 2006;1:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hadley NA, Brown TD, Weinstein SL. The effects of contact pressure elevations and aseptic necrosis on the long-term outcome of congenital hip dislocation. J Orthop Res. 1990;8(4):504-13. [DOI] [PubMed] [Google Scholar]
- 40. Konrath GA, Hamel AJ, Guerin J, Olson SA, Bay B, Sharkey NA. Biomechanical evaluation of impaction fractures of the femoral head. J Orthop Trauma. 1999;13(6):407-13. [DOI] [PubMed] [Google Scholar]
- 41. von Eisenhart-Rothe R, Eckstein F, Muller-Gerbl M, Landgraf J, Rock C, Putz R. Direct comparison of contact areas, contact stress and subchondral mineralization in human hip joint specimens. Anat Embryol. 1997;195(3):279-88. [DOI] [PubMed] [Google Scholar]
- 42. Brinker MR, O’Connor DP, Almekinders LC, Best TM, Buckwalter JA, Garrett WE, et al. Volume 1: Part 1: Basics: Chapter 1: Basic science and injury of muscle, tendon, and ligament: Section A: Physiology of injury to musculoskeletal structures: 3. Articular cartilage injury. In: DeLee JC, Drez D, Miller MD, editors. DeLee and Drez’s orthopaedic sports medicine. 3rd ed. Cambridge, MA: Saunders; 2009. [Google Scholar]
- 43. Bhosale AM, Richardson JB. Articular cartilage: structure, injuries and review of management. Br Med Bull. 2008;87:77-95. [DOI] [PubMed] [Google Scholar]
- 44. Donohue JM, Buss D, Oegema TR, Jr, Thompson RC., Jr The effects of indirect blunt trauma on adult canine articular cartilage. J Bone Joint Surg Am. 1983;65(7):948-57. [PubMed] [Google Scholar]
- 45. Bentley G, Dowd G. Current concepts of etiology and treatment of chondromalacia patellae. Clin Orthop Relat Res. 1984;189:209-28. [PubMed] [Google Scholar]
- 46. Casscells SW. Gross pathological changes in the knee joint of the aged individual: a study of 300 cases. Clin Orthop Relat Res. 1978;132:225-32. [PubMed] [Google Scholar]
- 47. Ficat RP, Philippe J, Hungerford DS. Chondromalacia patellae: a system of classification. Clin Orthop Relat Res. 1979;144:55-62. [PubMed] [Google Scholar]
- 48. Goodfellow J, Hungerford DS, Woods C. Patello-femoral joint mechanics and pathology. 2. Chondromalacia patellae. J Bone Joint Surg Br. 1976;58(3):291-9. [DOI] [PubMed] [Google Scholar]
- 49. Insall J, Falvo KA, Wise DW. Chondromalacia patellae: a prospective study. J Bone Joint Surg Am. 1976;58(1):1-8. [PubMed] [Google Scholar]
- 50. Noyes FR, Stabler CL. A system for grading articular cartilage lesions at arthroscopy. Am J Sports Med. 1989;17(4):505-13. [DOI] [PubMed] [Google Scholar]
- 51. Outerbridge RE. The etiology of chondromalacia patellae. J Bone Joint Surg Br. 1961;43-B:752-7. [DOI] [PubMed] [Google Scholar]
- 52. Cameron ML, Briggs KK, Steadman JR. Reproducibility and reliability of the outerbridge classification for grading chondral lesions of the knee arthroscopically. Am J Sports Med. 2003;31(1):83-6. [DOI] [PubMed] [Google Scholar]
- 53. Marx RG, Connor J, Lyman S, Amendola A, Andrish JT, Kaeding C, et al. Multirater agreement of arthroscopic grading of knee articular cartilage. Am J Sports Med. 2005;33(11):1654-7. [DOI] [PubMed] [Google Scholar]
- 54. Spahn G, Klinger HM, Hofmann GO. How valid is the arthroscopic diagnosis of cartilage lesions? Results of an opinion survey among highly experienced arthroscopic surgeons. Arch Orthop Trauma Surg. 2009;129(8):1117-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Mainil-Varlet P, Aigner T, Brittberg M, Bullough P, Hollander A, Hunziker E, et al. Histological assessment of cartilage repair: a report by the Histology Endpoint Committee of the International Cartilage Repair Society (ICRS). J Bone Joint Surg Am. 2003;85-A(suppl 2):45-57. [PubMed] [Google Scholar]
- 56. Saris DB, Vanlauwe J, Victor J, Almqvist KF, Verdonk R, Bellemans J, et al. Characterized chondrocyte implantation results in better structural repair when treating symptomatic cartilage defects of the knee in a randomized controlled trial versus microfracture. Am J Sports Med. 2008;36(2):235-46. [DOI] [PubMed] [Google Scholar]
- 57. Rutgers M, van Pelt MJ, Dhert WJ, Creemers LB, Saris DB. Evaluation of histological scoring systems for tissue-engineered, repaired and osteoarthritic cartilage. Osteoarthritis Cartilage. 2010. January;18(1):12-23. [DOI] [PubMed] [Google Scholar]
- 58. Price DD, McGrath PA, Rafii A, Buckingham B. The validation of visual analogue scales as ratio scale measures for chronic and experimental pain. Pain. 1983;17(1):45-56. [DOI] [PubMed] [Google Scholar]
- 59. Steadman JR, Briggs KK, Rodrigo JJ, Kocher MS, Gill TJ, Rodkey WG. Outcomes of microfracture for traumatic chondral defects of the knee: average 11-year follow-up. Arthroscopy. 2003;19(5):477-84. [DOI] [PubMed] [Google Scholar]
- 60. Steadman JR, Rodkey WG, Briggs KK, Rodrigo JJ. The microfracture technic in the management of complete cartilage defects in the knee joint. Orthopade. 1999;28(1):26-32. [DOI] [PubMed] [Google Scholar]
- 61. Steadman JR, Rodkey WG, Rodrigo JJ. Microfracture: surgical technique and rehabilitation to treat chondral defects. Clin Orthop Relat Res. 2001;391(suppl):S362-9. [DOI] [PubMed] [Google Scholar]
- 62. McCarthy JC. The diagnosis and treatment of labral and chondral injuries. Instructional Course Lectures. 2004;53:573-7. [PubMed] [Google Scholar]
- 63. Steadman JR, Miller BS, Karas SG, Schlegel TF, Briggs KK, Hawkins RJ. The microfracture technique in the treatment of full-thickness chondral lesions of the knee in National Football League players. J Knee Surg. 2003;16(2):83-6. [PubMed] [Google Scholar]
- 64. Smart LR, Oetgen M, Noonan B, Medvecky M. Beginning hip arthroscopy: indications, positioning, portals, basic techniques, and complications. Arthroscopy. 2007;23(12):1348-53. [DOI] [PubMed] [Google Scholar]
- 65. Byrd JW. Hip arthroscopy: the supine position. Clin Sports Med. 2001;20(4):703-31. [PubMed] [Google Scholar]
- 66. Enseki KR, Martin RL, Draovitch P, Kelly BT, Philippon MJ, Schenker ML. The hip joint: arthroscopic procedures and postoperative rehabilitation. J Orthop Sports Phys Ther. 2006;36(7):516-25. [DOI] [PubMed] [Google Scholar]
- 67. Clarke MT, Arora A, Villar RN. Hip arthroscopy: complications in 1054 cases. Clin Orthop Relat Res. 2003;406:84-8. [DOI] [PubMed] [Google Scholar]
- 68. Mason JB, McCarthy JC, O’Donnell J, Barsoum W, Mayor MB, Busconi BD, et al. Hip arthroscopy: surgical approach, positioning, and distraction. Clin Orthop Relat Res. 2003;406:29-37. [DOI] [PubMed] [Google Scholar]
- 69. Monllau JC, Solano A, Leon A, Hinarejos P, Ballester J. Tomographic study of the arthroscopic approaches to the hip joint. Arthroscopy. 2003;19(4):368-72. [DOI] [PubMed] [Google Scholar]
- 70. Sampson TG. Arthroscopic treatment of femoroacetabular impingement: a proposed technique with clinical experience. Instructional Course Lectures. 2006;55:337-46. [PubMed] [Google Scholar]
- 71. Robertson WJ, Kelly BT. The safe zone for hip arthroscopy: a cadaveric assessment of central, peripheral, and lateral compartment portal placement. Arthroscopy. 2008;24(9):1019-26. [DOI] [PubMed] [Google Scholar]
- 72. Yusaf MA, Hame SL. Arthroscopy of the hip. Curr Sports Med Rep. 2008;7(5):269-74. [DOI] [PubMed] [Google Scholar]
- 73. Knutsen G, Drogset JO, Engebretsen L, Grøntvedt T, Isaksen V, Ludvigsen TC, et al. A randomized trial comparing autologous chondrocyte implantation with microfracture: findings at five years. J Bone Joint Surg Am. 2007;89(10):2105-12. [DOI] [PubMed] [Google Scholar]
- 74. Rittmeister M, Hochmuth K, Kriener S, Richolt J. Five-year results following autogenous osteochondral transplantation to the femoral head. Orthopade. 2005;34(4):320, 322-6. [DOI] [PubMed] [Google Scholar]
- 75. von Stechow D, Drees P. Surgical treatment concepts for femoral head necrosis. Orthopade. 2007;36(5):451-7. [DOI] [PubMed] [Google Scholar]
- 76. Philippon MJ, Schenker ML, Briggs KK, Kuppersmith DA, Maxwell RB, Stubbs AJ. Revision hip arthroscopy. Am J Sports Med. 2007;35(11):1918-21. [DOI] [PubMed] [Google Scholar]
- 77. Blevins FT, Steadman JR, Rodrigo JJ, Silliman J. Treatment of articular cartilage defects in athletes: an analysis of functional outcome and lesion appearance. Orthopedics. 1998;21(7):761-7. [DOI] [PubMed] [Google Scholar]
- 78. Byrd JW, Jones KS. Hip arthroscopy in athletes: 10-year follow-up. Am J Sports Med. 2009;37(11):2140-3. [DOI] [PubMed] [Google Scholar]
- 79. Keeney JA, Peelle MW, Jackson J, Rubin D, Maloney WJ, Clohisy JC. Magnetic resonance arthrography versus arthroscopy in the evaluation of articular hip pathology. Clin Orthop Relat Res. 2004;429:163-9. [DOI] [PubMed] [Google Scholar]
- 80. Schmid MR, Notzli HP, Zanetti M, Wyss TF, Hodler J. Cartilage lesions in the hip: diagnostic effectiveness of MR arthrography. Radiology. 2003;226(2):382-6. [DOI] [PubMed] [Google Scholar]


