Abstract
Osteochondral lesions of the talus are common injuries following acute and chronic ankle sprains. Numerous surgical treatment strategies have been employed for treating these lesions; arthroscopic bone marrow stimulation is recognized as the first-line technique to provide fibrocartilage infill of the defect site. While the short- and medium-term outcomes of this technique are good, the long-term outcomes are not yet known. An increasing number of studies, however, show a cause for concern in employing this technique, including declining outcome scores over time. The current authors have therefore developed a treatment strategy based on previously established guidelines in addition to morphological cartilage-sensitive fast spin echo techniques and quantitative T2 mapping magnetic resonance imaging (MRI). Accordingly, the authors advocate arthroscopic bone marrow stimulation in lesion sizes up to 8 mm in diameter and osteochondral autograft transplant (OATS) in lesion sizes greater than 8 mm in diameter. In the absence of long-term studies, confining the use of arthroscopic bone marrow stimulation to smaller lesions may support prolonged joint life by decreasing the rate at which the fibrocartilage ultimately degenerates over time. Employing the OATS procedure in larger lesions has the advantage of replacing “like with like.” The current review examines the role of arthroscopic bone marrow stimulation techniques of the talus.
Keywords: osteochondral lesion, articular cartilage repair, fibrocartilage, ankle, arthroscopy
Introduction
Osteochondral lesions of the talus are becoming increasingly recognized injuries and are commonly associated with postresidual pain following acute and chronic ankle sprains. These lesions have a poor potential for spontaneous healing and if left untreated may predispose the joint to degenerative arthrosis in the long term.1,2 Numerous surgical treatment strategies have been employed with varied success in treating osteochondral lesions of the talus. Arthroscopic bone marrow stimulation (i.e., microfracture, drilling) is a well-accepted and proven technique to allow fibrocartilage differentiation and thereby provide infill at the site of a cartilage defect in several joints, including the ankle. The short- to medium-term functional outcomes of marrow stimulation techniques are predominantly good. However, while the long-term outcomes of the procedure are not widely known, an increasing number of studies are beginning to show a possible cause for concern, which includes the degradation of the fibrocartilage over time.3 The popularity of marrow stimulation as a means of first-line treatment is attributed to a technically undemanding procedure, cost-effectiveness, low complication rates, and less postoperative pain in contrast to more invasive procedures.4 In the following review, the current authors examine the role of arthroscopic bone marrow stimulation techniques as a means of treatment for osteochondral lesions of the talus.
Biomechanics of Fibrocartilage
Articular hyaline cartilage is a resilient connective tissue that functions to cover the ends of long bones.5 In a synovial environment, this promotes a nearly frictionless surface such that load profiles are distributed evenly over the subchondral bone and thereby prevented in high concentrations over small surface areas. The innate biomechanical properties of articular cartilage are characterized by a high type-II collagen content and the specific composition of an extracellular matrix.6 Moreover, the morphology of articular cartilage can be separated into superficial, middle, deep, and calcified layers. By comparison to articular cartilage, fibrocartilage has a different composition and durability.
Fibrocartilage is both biomechanically and biologically inferior to that of native hyaline cartilage and accordingly lacks the ability to adequately protect the below-lying subchondral bone.7,8 Mesenchymal stem cells in a repair defect display high type-II collagen differentiation 6 to 8 weeks following bone marrow stimulation.9 However, surface fibrillation causes depletion of the type-II collagen content and an increase in the differentiation of type-I collagen.9 Type-I collagen is biomechanically inferior, and fibrocartilage therefore degenerates over time in response to the mechanical loading of the joint.9 In smaller sized lesions, fibrocartilage may act as a “grout” or filler of the articulating surface. In this regard, it may function adequately over the long term, as the mechanical loads imparted to it are small. However, in larger lesions, the durability of this less resilient fibrocartilage may ultimately fail over time.
Operative Technique
The principal objective of arthroscopic bone marrow stimulation is to create multiple openings in the subchondral bone whereby pluripotent mesenchymal stem cells aggregate to the defect site and, in response to growth factors, stimulate the differentiation of fibrocartilaginous tissue (Fig. 1). The procedure commonly includes excision of the damaged fragment and curettage to stabilize the margins of the defect site prior to marrow stimulation. Takao et al. reported significant improvement in healing at second-look arthroscopies when remaining degenerative cartilage is excised from the site of the lesion prior to drilling.10 Following debridement of the lesion, a K-wire or microfracture awl is commonly used to perforate the subchondral bone at 3- to 4-mm intervals to promote vascularization (Fig. 2). Van Bergen et al. have identified a potential drawback in the microfracture technique, in which loose bony particles may be created and, if not removed properly, can act as loose bodies within the joint.11 Chen et al. reported that the microfracture awl causes acute fracturing, bone compaction (preventing the aggregation of adjacent bone marrow), and high levels of osteocyte necrosis.12 As such, they advocated drilling with cooled irrigation, which prevented the bone compaction and thermal necrosis.
Figure 1.
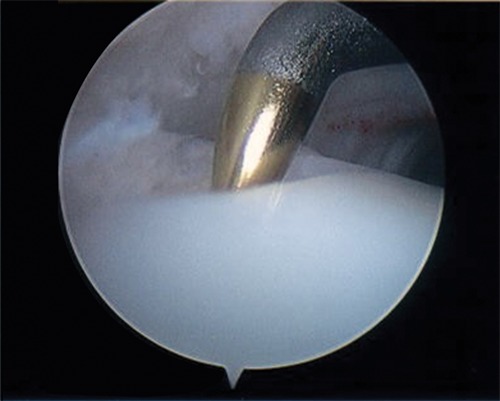
A microfracture awl is used to penetrate the subchondral bone in marrow stimulation.
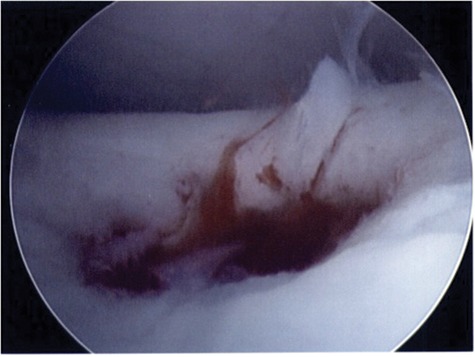
Figure 2.
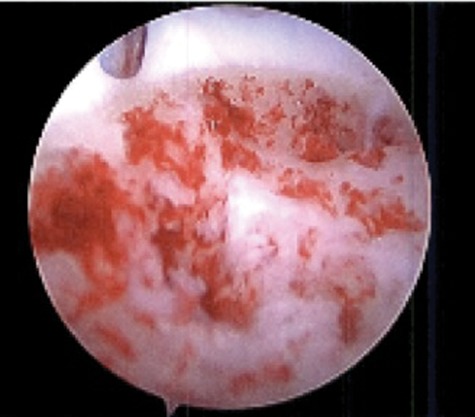
Tourniquet release displays sufficient hemorrhage and vascularization of the subchondral bone of an osteochondral lesion following bone marrow stimulation.
Rehabilitation
Following arthroscopic marrow stimulation procedures, the patient is typically advised to be nonweightbearing for a total of 4 to 6 weeks. Within the initial 72 hours following surgery, the patients are asked to restrain from moving the ankle joint. Thereafter, ankle pumps are encouraged for 20 minutes each day for the next 3.5 weeks. This stimulates the synovial nutrient pathway for the remaining cartilage and can “mold” the newly differentiating fibrocartilage to conform to the articular surface in the absence of the shear-loading forces associated with weightbearing. In addition, dorsi and plantar flexion exercises on a regular basis prevent the cicatrization process about the ankle joint capsule, thereby reducing ankle stiffness. At the 4-week postoperative time point, patients are allowed 10% of their body weight, which is then gradually increased to 100% by the end of the sixth week. At this stage, physical therapy is begun with a concentration on strengthening and proprioceptive exercises over the following 4 weeks. At the 10-week time point, sport-specific therapies can then commence.
Indications for Marrow Stimulation
The present consensus among the available evidence is that arthroscopic debridement followed by bone marrow stimulation is the primary step in treating symptomatic osteochondral lesions of the talus less than or equal to 15 mm in size.13-18 In a current concepts review, Giannini and Vannini15 suggested that marrow stimulation can even be attempted in lesions up to sizes of 2.0 cm2. One recent study by Choi et al.19 reported successful results at a mean 44.5 months following surgery in 89.5% of patients with lesion sizes less than 150 mm2 and increased success (94.8%) with lesion sizes less than 100 mm2. The depth of the lesion was found to only weakly correlate with the overall clinical outcome. Chuckpaiwong et al.14 reported the outcomes of 32 patients with lesion sizes greater than 15 mm. Only 1 patient of the total 32 met the study criteria for a successful outcome, providing a clear caveat to the use of microfracture in treating larger lesions. In addition, the authors noted significantly better improvements in terms of outcome with a younger patient age, lower body mass index (BMI), and a shorter interval of symptoms prior to surgery, while a history of trauma and the presence of osteophytes negatively affected patient outcome. In contrast to Chuckpaiwong et al.,14 no correlation between age or symptom duration was reported by Choi et al.,19 Beecher and Thermann,20 and Robinson et al.21 Also noted in the analysis by Chuckpaiwong et al.14 was better outcomes in those treated with ankle instability (18.9%) and anterolateral scar (56.8%), which the current authors emphasize may confound the results had the osteochondral lesion been treated in isolation. Lesion location has not been seen to significantly affect the overall outcome of the patient.14,19
Short- and Medium-Term Results
The short- and medium-term results of arthroscopic bone marrow stimulation techniques in treating osteochondral lesions of the talus are primarily good. Lee et al. reported 89% good and excellent outcomes following microfracture at a mean follow-up of 33 months and a postoperative American Orthopaedic Foot and Ankle Society (AOFAS) score of 90 (range, 73-100).22 Becher and Thermann prospectively evaluated 30 patients for 2 years following microfracture surgery and reported 83% of patients to have a good or excellent outcome.20 Schuman et al.23 found 82% good to excellent results following drilling at an average 4.8-year follow-up, while Van Buecken et al.24 reported good to excellent results in 87% of patients at an average 26-month follow-up. Gobbi et al.25 also showed good results with microfracture at a mean follow-up of 53 months. In the previously mentioned article by Takao et al., significant 2-year improvements in AOFAS scores were shown in addition to 93.1% improvement of repair infill at 1 year via second-look arthroscopy.10 Saxena and Eakin studied the results of microfracture in high-demand athletes at a mean follow-up of 32 months and showed significant improvement in AOFAS scores of 54.6 preoperatively to 94.4 following surgery (96% good to excellent).26 Return to activity was seen following microfracture at a mean of 15.1 weeks by comparison to a mean of 19.6 weeks needed for the talar bone–grafting group of the same study.
Multiple systematic reviews of the most effective treatment strategies for osteochondral lesions of the talus have been performed.27-29 Verhagen et al.28 showed excision, curettage, and drilling to have an 86% success rate in treating these lesions, while similarly, Tol et al.29 concluded the same with an 85% success rate. A recent review by Zengerink et al. also reported the successful treatment with bone marrow stimulation to be 85%.27
Causes for Concern with Marrow Stimulation
There has been a distinct paucity of literature reporting long-term results following bone marrow stimulation techniques about the talus. While numerous studies displayed good results at shorter-term follow-up for bone marrow stimulation, several studies now show a cause for concern in employing such a technique. The concerns include deteriorating outcome scores, lack of infill at the defect site during second-look arthroscopy, and the inability to return to the same level of sport. Some studies even show poor results at shorter term follow-up. These data are therefore concerning.
Ferkel et al. reported only 64% to 72% good to excellent results at a mean follow-up time of 71 months, with 35% deterioration of outcome scores in patients who had been seen 5 years prior.3 Preoperative to postoperative comparison of plain radiographs showed that 34% decreased by at least one grade of arthritis at follow-up. The authors also noted the possibility of persistent pain in patients with unstable defects at the time of arthroscopy. In addition, Hunt and Sherman found that 54% of their cohort had fair or poor results at a mean of 66 months following arthroscopic drilling, with pain noted in 52% of patients at follow-up examination.30 Similarly, Robinson et al. reported 47.7% fair or poor results at a mean 3.5-year follow-up time period.21 Kumai et al. also reported only 72% good results at a mean 4.6-year follow-up.31 While Schumer, Struijs, and van Dijk reported good functional scores following surgery, only 55% had resumed sporting activities and the remaining 45% were either limited or had not resumed activity at all.23
Recently, Lee et al. were the first to report a case series by second-look arthroscopy for osteochondral lesions of the talus, performed 1 year after the index procedure.32 While 90% of ankles had good to excellent functional outcomes in terms of AOFAS score, 35% of lesions were incompletely healed at the time of arthroscopy. Notably, only 30% of lesions were integrated completely with the surrounding cartilage and 80% had visible cracks and fissuring.
Although knee cartilage differs from talar cartilage in both thickness and biomechanical properties, the biological characteristics and the response to marrow stimulation techniques are similar. In a review of 48 knees at a mean follow-up time point of 41 months following microfracture, Mithoefer et al. found that knee function worsened in patients with a lack of fibrocartilage infill after 24 months.4 Knutsen et al. observed an association between the repair appearance at second-look arthroscopy of the knee at 2 years and the risk for treatment failure at 5 years.33
In the absence of similar studies evaluating bone marrow stimulation techniques of the talus, outcomes following these studies of the knee may provide useful and compelling insight into the limitations of the technique. An extensive systematic review on the clinical efficacy of microfracture in the knee by Mithoefer et al. found the technique effective in providing short-term functional improvement, but insufficient data are available on the longer-term results of the procedure.34 “Normal or nearly normal” cartilage was seen in only 45% to 77% of repair defects at 8 to 24 months following microfracture during second-look arthroscopy. With regard to the use of microfracture among athletes, several studies are concerning.
In a separate study by Mithoefer et al., the authors systematically reported that while an average of 79% good to excellent results were reported in athletes undergoing articular cartilage repair of the knee, only 67% of results were good to excellent with the use of microfracture.35 Furthermore, return to sports participation was seen in only 66% following microfracture. By comparison, 93% good to excellent results were seen with osteochondral autograft transplant (OATS), while 91% returned to sports participation. A third study by Mithoefer et al. studied high-impact athletes following articular cartilage repair of the knee with microfracture.36 Only 66% of the athletes involved in the study reported good to excellent results at the mean follow-up time of 41 months, and while 47% reported an initial increase in activity, a decline was subsequently observed in those patients. Cerynik et al. found that 21% of professional basketball players never returned to competition in a National Basketball Association (NBA) game after microfracture surgery.37
Analysis of Marrow Stimulation Using T2 Magnetic Resonance Imaging (MRI)
Magnetic resonance images are shown in Figures 3 and 4.
Figure 3.
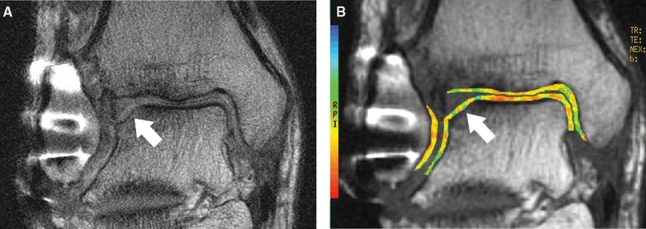
Coronal cartilage-sensitive fast spin magnetic resonance image (A) 25 months following microfracture to the lateral margin of the talar dome demonstrates depression of the subchondral plate and overlying high signal intensity repair cartilage. Corresponding coronal quantitative T2 map (B) demonstrates diffuse prolongation of T2 values and loss of color stratification at the site of cartilage repair, reflecting “immature” repair cartilage.
Figure 4.
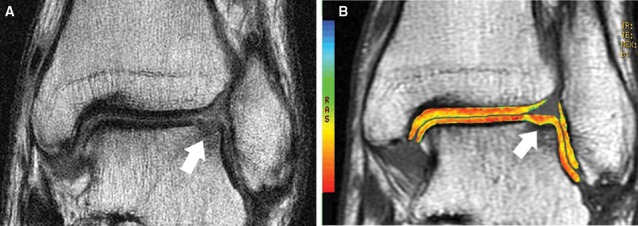
Coronal cartilage-sensitive fast spin magnetic resonance image (A) 13 months following microfracture to the lateral margin of the talar dome demonstrates mild depression of the subchondral plate and good fill by overlying isointense to hypointense repair cartilage. Corresponding coronal quantitative T2 map (B) demonstrates diffuse short T2 values without color stratification at the site of cartilage repair.
Biological Adjuncts to Marrow Stimulation
With increasing concerns over the durability and structure of fibrocartilage following bone marrow stimulation techniques, further research is underway to provide useful biological adjuncts to the healing of repair tissue, including hyaluronic acid (HA) treatment, ultrasound stimulation, platelet-rich plasma (PRP), and autologous pluripotent stem cells.
A recent study by Strauss et al. concluded that 3 weekly intra-articular injections of HA following microfracture surgery in a rabbit had a positive effect on the repair tissue.38 Furthermore, the HA injections provided a chondroprotective effect on the joint, thereby limiting further degenerative change. Szczodry et al. advocated the need for chondroprotective therapy following injury to the articular surface to delay or prevent the onset of posttraumatic arthrosis.39
Ultrasound stimulation following cartilage repair is a progressively researched entity; early results in animal models have shown promising signs. Korstjens et al. reported that ultrasound stimulates chondrocyte proliferation and matrix production.40 A separate study compared bilateral osteochondral lesions in the knee of a rabbit model and the effects of low-intensity pulsed ultrasound.41 Gross appearance, histology, and proteoglycan content were increased by comparison to the contralateral, untreated side.
Despite the scarcity of supporting evidence, PRP therapy is becoming an increasingly utilized adjunct in cartilage repair procedures. Sun et al. found that PRP injections improved healing of an osteochondral defect of a rabbit model.42 While control models showed mainly fibrous repair tissue, the experimental group showed repair tissue similar to that of hyaline cartilage in terms of cell orientation and integration with the native cartilage.
The senior surgeon of the current article routinely utilizes bone marrow aspirate concentrate (BMAC), extracted from the ipsilateral iliac crest, as an adjunct to cartilage repair (Fig. 5). The efficacy of the BMAC adjunct in cartilage repair is clinically not yet known. Ongoing studies at our institution are underway to evaluate this in the use of both arthroscopic marrow stimulation procedures and open OATS procedures.
Figure 5.
A bone marrow aspirate concentrate (BMAC) is injected into an osteochondral lesion following marrow stimulation of the talus.
TGF-β3 therapies have also been shown to enhance the chondrogenic cascade and are emerging as a relatively unstudied isoform of the TGF-β family.9 Mrugala et al. reported an ovine study using a chitosan scaffold with mesenchymal stem cells and 50 ng of TGF-β3.43 This therapy adequately filled lesions of the patella. Histologically, chondrocyte-like cells and a hyaline-like matrix displayed complete integration into the native cartilage tissue at the 2-month time point.
The Role of Osteochondral Autograft Transplant (OATS)
Until the ideal size guidelines for the use of marrow stimulation techniques are established through long-term randomized control trials, the current authors have developed a treatment strategy for osteochondral lesions of the talus. This specific treatment strategy is based on both morphological assessment of repair tissue using previously validated cartilage-sensitive fast spin echo techniques44 and quantitative T2 mapping MRI for the assessment of collagen orientation,45 as well as the previously established guidelines from Scranton.46 Scranton identified the ideal size lesion treated with OATS as 8 to 12 mm in diameter. The OATS procedure provides the advantage of replacing “like with like” at the repair site and may support the prolonged endurance of the joint (Figs. 6 and 7). Zengerink et al. systematically reported 87% good to excellent results for the OATS procedure, with success rates as high as 100%.27 Hangody et al. reported 93% good to excellent results in the talus with a follow-up time of up to 7 years.47 The durability and quality of the autografts were confirmed at the time of second-look arthroscopies. Biopsy specimens were compared to control specimens and were found to be of similar quality in terms of the normal type-II collagen and proteoglycan content of articular cartilage.48 Hangody et al. did not report any long-term donor site morbidity.49
Figure 6.
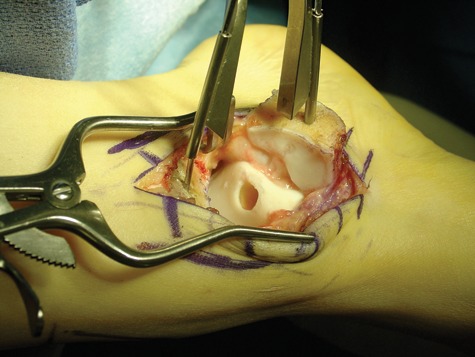
The damaged portion of the cartilage and bone has been removed from the talus prior to autograft transplantation.
Figure 7.
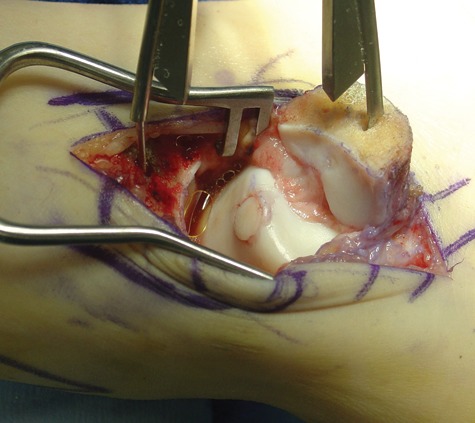
An osteochondral autograft has been transplanted from the ipsilateral knee to the medial talar dome; the graft is seated flush with the surface contour of the talus.
In the absence of long-term follow-up with marrow stimulation techniques, it is impossible to know whether the resultant fibrocartilage is adequate in providing the needed mechanics for ankle joint longevity. The current authors therefore reserve the techniques of bone marrow stimulation for lesions up to 8 mm in diameter or smaller and accordingly employ the OATS procedures in lesions larger than 8 mm in diameter.
Analysis of OATS Using T2 MRI
Magnetic resonance images are shown in Figure 8.
Figure 8.
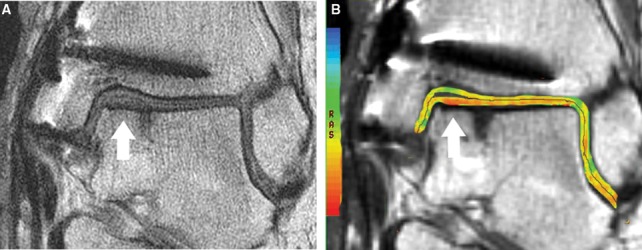
Coronal cartilage-sensitive fast spin magnetic resonance image (A) of autologous osteochondral plug transplanted into the medial margin of the talar dome demonstrates good fill of the cartilage defect and restoration of the radius of curvature of the articular surface by repair cartilage, which maintains gray-scale stratification and which is flush with the adjacent native cartilage. There is good osseous incorporation of the graft. Corresponding coronal quantitative T2 map (B) demonstrates normal color stratification of T2 values at the site of cartilage repair, similar to that of native cartilage.
Conclusion
While the short- to medium-term outcomes in treating osteochondral lesions of the talus with arthroscopic bone marrow stimulation are primarily good, long-term outcomes in larger lesions are not yet known. A significant number of studies now show a cause for concern in employing marrow stimulation as a first-line treatment technique for osteochondral lesions of the talus. Prospective, randomized clinical trials are needed to establish ideal size guidelines with respect to treatment strategy. Using morphological cartilage-sensitive fast spin echo, quantitative T2 mapping MRI and previously established guidelines, the current authors advocate microfracture in lesion sizes 8 mm and smaller and the OATS procedure in larger lesions, with an emphasis on the advantage of replacing “like with like.” Cartilage repair procedures remain controversial. Until ideal treatment guidelines are established through long-term studies, cartilage repair remains the “holy grail” of orthopedic surgery.
Footnotes
All figures appear in color in the online version of this article. The authors specifically emphasize the importance of the color images of the quantitative T2 mapping MRIs.
The authors declared no potential conflicts of interest with respect to the authorship and/or publication of this article.
The authors received no financial support for the research and/or authorship of this article.
References
- 1. Mankin HJ. The response of articular cartilage to mechanical injury. J Bone Joint Surg Am. 1982;64(3):460-6. [PubMed] [Google Scholar]
- 2. Jackson DW, Lalor PA, Aberman HM, Simon TM. Spontaneous repair of full-thickness defects of articular cartilage in a goat model: a preliminary study. J Bone Joint Surg Am. 2001;83-A(1):53-64. [DOI] [PubMed] [Google Scholar]
- 3. Ferkel RD, Zanotti RM, Komenda GA, Sgaglione NA, Cheng MS, Applegate GR, Dopirak RM. Arthroscopic treatment of chronic osteochondral lesions of the talus: long-term results. Am J Sports Med. 2008;36(9):1750-62. [DOI] [PubMed] [Google Scholar]
- 4. Mithoefer K, Williams RJ, 3rd, Warren RF, Potter HG, Spock CR, Jones EC, et al. The microfracture technique for the treatment of articular cartilage lesions in the knee: a prospective cohort study. J Bone Joint Surg Am. 2005;87(9):1911-20. [DOI] [PubMed] [Google Scholar]
- 5. Aigner T, Stove J. Collagens: major component of the physiological cartilage matrix, major target of cartilage degeneration, major tool in cartilage repair. Adv Drug Deliv Rev. 2003;55:1569. [DOI] [PubMed] [Google Scholar]
- 6. Bastiaansen-Jenniskens YM, Koevoet W, de Bart AC, van der Linden JC, Zuurmond AM, Weinans H, et al. Contribution of collagen network features to functional properties of engineered cartilage. Osteoarthritis Cartilage. 2008;16(3):359-66. [DOI] [PubMed] [Google Scholar]
- 7. Ahmed TA, Hincke MT. Strategies for articular cartilage lesion repair and functional restoration. Tissue Eng Part B Rev. 2009. December 21 [Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 8. Nehrer S, Spector M, Minas T. Histologic analysis of tissue after failed cartilage repair procedures. Clin Orthop Relat Res. 1999;365:149-62. [DOI] [PubMed] [Google Scholar]
- 9. Tang QO, Shakib K, Heliotis M, Tsiridis E, Mantalaris A, Ripamonti U, et al. TGF-beta3: a potential biological therapy for enhancing chondrogenesis. Expert Opin Biol Ther. 2009;9(6):689-701. [DOI] [PubMed] [Google Scholar]
- 10. Takao M, Uchio Y, Kakimaru H, Kumahashi N, Ochi M. Arthroscopic drilling with debridement of remaining cartilage for osteochondral lesions of the talar dome in unstable ankles. Am J Sports Med. 2004;32(2):332-6. [DOI] [PubMed] [Google Scholar]
- 11. van Bergen CJ, de Leeuw PA, van Dijk CN. Potential pitfall in the microfracturing technique during the arthroscopic treatment of an osteochondral lesion. Knee Surg Sports Traumatol Arthrosc. 2009;17(2):184-7. [DOI] [PubMed] [Google Scholar]
- 12. Chen H, Sun J, Hoemann CD, Lascau-Coman V, Ouyang W, McKee MD, et al. Drilling and microfracture lead to different bone structure and necrosis during bone-marrow stimulation for cartilage repair. J Orthop Res. 2009;27(11):1432-8. [DOI] [PubMed] [Google Scholar]
- 13. Amendola A, Panarella L. Osteochondral lesions: medial versus lateral, persistent pain, cartilage restoration options and indications. Foot Ankle Clin. 2009;14:215-27. [DOI] [PubMed] [Google Scholar]
- 14. Chuckpaiwong B, Berkson EM, Theodore GH. Microfracture for osteochondral lesions of the ankle: outcome analysis and outcome predictors of 105 cases. Arthroscopy. 2008;24:106-12. [DOI] [PubMed] [Google Scholar]
- 15. Giannini S, Vannini F. Operative treatment of osteochondral lesions of the talar dome: current concepts review. Foot Ankle Int. 2004;25:168-75. [DOI] [PubMed] [Google Scholar]
- 16. van Bergen CJ, de Leeuw PA, van Dijk CN. Treatment of osteochondral defects of the talus. Rev Chir Orthop Reparatrice Appar Mot. 2008;94:398-408. [DOI] [PubMed] [Google Scholar]
- 17. van Dijk CN, van Bergen CJ. Advancements in ankle arthroscopy. J Am Acad Orthop Surg. 2008;16:635-46. [DOI] [PubMed] [Google Scholar]
- 18. Lahm A, Erggelet C, Steinwachs M, Reichelt A. Arthroscopic management of osteochondral lesion of the talus: results of drilling and usefulness of the magnetic resonance imaging before and after treatment. Arthroscopy. 2000;16:299-304. [DOI] [PubMed] [Google Scholar]
- 19. Choi WJ, Park KK, Kim BS, Lee JW. Osteochondral lesion of the talus: is there a critical defect size for poor outcome? Am J Sports Med. 2009;37(10):1974-80. [DOI] [PubMed] [Google Scholar]
- 20. Becher C, Thermann H. Results of microfracture in the treatment of articular cartilage defects of the talus. Foot Ankle Int. 2005;26(8):583-9. [DOI] [PubMed] [Google Scholar]
- 21. Robinson DE, Winson IG, Harries WJ, Kelly AJ. Arthroscopic treatment of osteochondral lesions of the talus. J Bone Joint Surg Br. 2003;85:989-93. [DOI] [PubMed] [Google Scholar]
- 22. Lee KB, Bai LB, Chung JY, Seon JK. Arthroscopic microfracture for osteochondral lesions of the talus. Knee Surg Sports Traumatol Arthrosc. 2009. September 25 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 23. Schuman L, Struijs PA, van Dijk CN. Arthroscopic treatment for osteochondral defects of the talus: results at follow-up at 2 to 11 years. J Bone Joint Surg Br. 2002;84(3):364-8. [DOI] [PubMed] [Google Scholar]
- 24. Van Buecken K, Barrack RL, Alexander AH, Ertl JP. Arthroscopic treatment of transchondral talar dome fractures. Am J Sports Med. 1989;17(3):350-5, discussion 355-6. [DOI] [PubMed] [Google Scholar]
- 25. Gobbi A, Francisco RA, Lubowitz JH, Allegra F, Canata G. Osteochondral lesions of the talus: randomized controlled trial comparing chondroplasty, microfracture, and osteochondral autograft transplantation. Arthroscopy. 2006;22(10):1085-92. [DOI] [PubMed] [Google Scholar]
- 26. Saxena A, Eakin C. Articular talar injuries in athletes: results of microfracture and autogenous bone graft. Am J Sports Med. 2007;35(10):1680-7. [DOI] [PubMed] [Google Scholar]
- 27. Zengerink M, Struijs PA, Tol JL, van Dijk CN. Treatment of osteochondral lesions of the talus: a systematic review. Knee Surg Sports Traumatol Arthrosc. 2009. October 27 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Verhagen RA, Struijs PA, Bossuyt PM, van Dijk CN. Systematic review of treatment strategies for osteochondral defects of the talar dome. Foot Ankle Clin. 2003;8(2):233-42, viii-ix. [DOI] [PubMed] [Google Scholar]
- 29. Tol JL, Struijs PA, Bossuyt PM, Verhagen RA, van Dijk CN. Treatment strategies in osteochondral defects of the talar dome: a systematic review. Foot Ankle Int. 2000;21(2):119-26. [DOI] [PubMed] [Google Scholar]
- 30. Hunt SA, Sherman O. Arthroscopic treatment of osteochondral lesions of the talus with correlation of outcome scoring systems. Arthroscopy. 2003;19(4):360-7. [DOI] [PubMed] [Google Scholar]
- 31. Kumai T, Takakura Y, Higashiyama I, Tamai S. Arthroscopic drilling for the treatment of osteochondral lesions of the talus. J Bone Joint Surg Am. 1999;81(9):1229-35. [DOI] [PubMed] [Google Scholar]
- 32. Lee KB, Bai LB, Yoon TR, Jung ST, Seon JK. Second-look arthroscopic findings and clinical outcomes after microfracture for osteochondral lesions of the talus. Am J Sports Med. 2009;37 Suppl 1:63S-70S. [DOI] [PubMed] [Google Scholar]
- 33. Knutsen G, Drogset JO, Engebretsen L, Grøntvedt T, Isaksen V, Ludvigsen TC, et al. A randomized trial comparing autologous chondrocyte implantation with microfracture: findings at five years. J Bone Joint Surg Am. 2004;86:455-64. [DOI] [PubMed] [Google Scholar]
- 34. Mithoefer K, McAdams T, Williams RJ, Kreuz PC, Mandelbaum BR. Clinical efficacy of the microfracture technique for articular cartilage repair in the knee: an evidence-based systematic analysis. Am J Sports Med. 2009;37(10):2053-63. [DOI] [PubMed] [Google Scholar]
- 35. Mithoefer K, Hambly K, Della Villa S, Silvers H, Mandelbaum BR. Return to sports participation after articular cartilage repair in the knee: scientific evidence. Am J Sports Med. 2009;37 Suppl 1:167S-76S. [DOI] [PubMed] [Google Scholar]
- 36. Mithoefer K, Williams RJ, 3rd, Warren RF, Wickiewicz TL, Marx RG. High-impact athletics after knee articular cartilage repair: a prospective evaluation of the microfracture technique. Am J Sports Med. 2006;34(9):1413-8. [DOI] [PubMed] [Google Scholar]
- 37. Cerynik DL, Lewullis GE, Joves BC, Palmer MP, Tom JA. Outcomes of microfracture in professional basketball players. Knee Surg Sports Traumatol Arthrosc. 2009;17(9):1135-9. [DOI] [PubMed] [Google Scholar]
- 38. Strauss E, Schachter A, Frenkel S, Rosen J. The efficacy of intra-articular hyaluronan injection after the microfracture technique for the treatment of articular cartilage lesions. Am J Sports Med. 2009;37(4):720-6. [DOI] [PubMed] [Google Scholar]
- 39. Szczodry M, Coyle CH, Kramer SJ, Smolinski P, Chu CR. Progressive chondrocyte death after impact injury indicates a need for chondroprotective therapy. Am J Sports Med. 2009;37(12):2318-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Korstjens CM, van der Rijt RH, Albers GH, Semeins CM, Klein-Nulend J. Low-intensity pulsed ultrasound affects human articular chondrocytes in vitro. Med Biol Eng Comput. 2008;46(12):1263-70. [DOI] [PubMed] [Google Scholar]
- 41. Jia XL, Chen WZ, Zhou K, Wang ZB. Effects of low-intensity pulsed ultrasound in repairing injured articular cartilage. Chin J Traumatol. 2005;8(3):175-8. [PubMed] [Google Scholar]
- 42. Sun Y, Feng Y, Zhang CQ, Chen SB, Cheng XG. The regenerative effect of platelet-rich plasma on healing in large osteochondral defects. Int Orthop. 2009. May 12 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mrugala D, Bony C, Neves N, Caillot L, Fabre S, Moukoko D, et al. Phenotypic and functional characterisation of ovine mesenchymal stem cells: application to a cartilage defect model. Ann Rheum Dis. 2008;67(3):288-95. [DOI] [PubMed] [Google Scholar]
- 44. Brown WE, Potter HG, Marx RG, Wickiewicz TL, Warren RF. Magnetic resonance imaging appearance of cartilage repair in the knee. Clin Orthop Rel Res. 2004;422:214-23. [DOI] [PubMed] [Google Scholar]
- 45. Xia Y, Moody JB, Burton-Wurster N, Lust G. Quantitative in situ correlation between microscopic MRI and polarized light microscopy studies of articular cartilage. Osteoarthritis Cartilage. 2001;9:393-406. [DOI] [PubMed] [Google Scholar]
- 46. Scranton PE., Jr Osteochondral lesions of the talus: autograft and allograft replacement. Tech Foot Ankle Surg. 2004;3:25-39. [Google Scholar]
- 47. Hangody L, Vasarhelyi G, Hangody LR, Sukosd Z, Tibay G, Bartha L, Bodo G. Autologous osteochondral grafting: technique and long-term results. Injury. 2008;39(Suppl 1):S32-9. [DOI] [PubMed] [Google Scholar]
- 48. Hangody L, Rathonyi GK. Mosaicplasty in active sportsmen. Sportorthopadie Sporttraumatologie. 2004;20:159-64. [Google Scholar]
- 49. Hangody L, Kish G, Modis L, Szerb I, Gaspar L, Dioszegi Z, et al. Mosaicplasty for the treatment of osteochondritis dissecans of the talus: two to seven year results in 36 patients. Foot Ankle Int. 2001;22(7):552-8. [DOI] [PubMed] [Google Scholar]


