Abstract
Objective:
We investigated the effects of freeze-thawing on the properties of articular cartilage.
Design:
The reproducibility of repeated biomechanical assay of the same osteochondral sample was first verified with 11 patellar plugs from 3 animals. Then, 4 osteochondral samples from 15 bovine patellae were divided into 4 groups. The reference samples were immersed in phosphate-buffered saline (PBS) containing proteolysis inhibitors and biomechanically tested before storage for further analyses. Samples of group 1 were biomechanically tested before and after freeze-thawing in PBS in the absence and those of group 2 in the presence of inhibitors. Samples of the group 3 were biomechanically tested in PBS-containing inhibitors, but frozen in 30% dimethyl sulfoxide/PBS and subsequently tested in PBS supplemented with the inhibitors. Glycosaminoglycan contents of the samples and immersion solutions were analyzed, and proteoglycan structures examined with SDS-agarose gel electrophoresis.
Results:
Freeze-thawing decreased slightly dynamic moduli in all 3 groups. The glycosaminoglycan contents and proteoglycan structures of the cartilage were similar in all experimental groups. Occasionally, the diffused proteoglycans were partly degraded in group 1. Digital densitometry revealed similar staining intensities for the glycosaminoglycans in all groups. Use of cryopreservant had no marked effect on the glycosaminoglycan loss during freeze-thawing.
Conclusion:
The freeze-thawed cartilage samples appear suitable for the biochemical and biomechanical studies.
Keywords: cryopreservation, cartilage, proteoglycans, biomechanics, dimethyl sulfoxide
Introduction
Proteoglycans (PGs) consist of a protein core with one or more sulfated glycosaminoglycan (GAG).1-4 In addition to collagens, the PGs are major components of articular cartilage. Loss of PGs is a characteristic feature of cartilage degradation in osteoarthritis, which besides collagen degradation has significant effects on the cartilage functional properties. Previously, it has been reported that extraction of PGs would reduce compressive,5 but not tensile,6 stiffness, whereas collagen degradation decreases tensile stiffness and strength.7 In addition to collagen content, the organization of collagen fibrils affects the biomechanical properties of the articular cartilage.8 As PGs affect the cartilage permeability and equilibrium modulus,9 a significant loss of PGs would lead to wrong measuring data and, thus, misleading interpretations of the articular cartilage functional properties.
Cartilage tissue used for experimentation is often stored frozen prior to use. Long incubations in aqueous solutions (even overnight) are occasionally required for cartilage analyses, such as diffusion studies of contrast agents used for delayed gadolinium-enhanced magnetic resonance imaging (MRI) or computer or contrast agent–enhanced computed tomography.10 Thus, it is important to determine whether there are any structural changes that could occur in the cartilage tissue due to freezing or as a result of the long incubations during testing.
Dimethyl sulfoxide (DMSO) is a commonly used cryoprotectant for cell storage and cartilage tissue preservation11-15 and has been shown to reduce the loss of the GAGs from the cartilage during freeze-thawing.16 Although the freezing causes degenerative cellular alterations, no marked changes in extracellular matrix (ECM) structure were detected with electron microscopy.17,18 In contrast, a recent study with MRI suggested that a significant decrease in the PG content of porcine cartilage occurred during 36 to 72 hours of incubation in phosphate-buffered saline (PBS).14 A single freeze–thaw cycle did not cause changes on the cartilage T2 or km (magnetization transfer rate) parameters of MRI measurement, whereas additional cycles resulted in significant alterations.14 The conflicting results published in literature14,16-20 warranted us to investigate the influence of the freeze–thaw cycle on the biochemical and biomechanical properties of the articular cartilage. Biomechanical stress-relaxation tests were conducted before the freezing for 21 to 24 hours, and again following 18-hour storage at room temperature after thawing. The content, the structure, and the zonal distribution of the PGs in cartilage tissue were analyzed before and after various storage protocols.
Materials and Methods
Sample Preparation
Eighteen intact, mature bovine knee joints (age 18-24 months) were obtained from a local abattoir (Atria Corporation, Kuopio, Finland) 4 to 5 hours after the cows were slaughtered. Eleven osteochondral samples from 3 animals were first used for reproducibility analysis of repeated biomechanical measurements, whereas the samples from 15 other animals were then collected to study freeze–thaw effects on the articular cartilage. An osteochondral disk (d = 25.4 mm) was prepared from each patella within 4 to 5 hours of death. The osteochondral plugs (d = 6 mm) were detached from each disk using a biopsy punch (Fig. 1). The patella was chosen for sampling as it has a wide area of flat surface, rather constant thickness,21,22 and its stiffness is on average level compared with other anatomical sites of the bovine knee joint.21,22 Therefore, we believe that the results obtained using these samples are rather representative also to the other cartilage areas.
Figure 1.
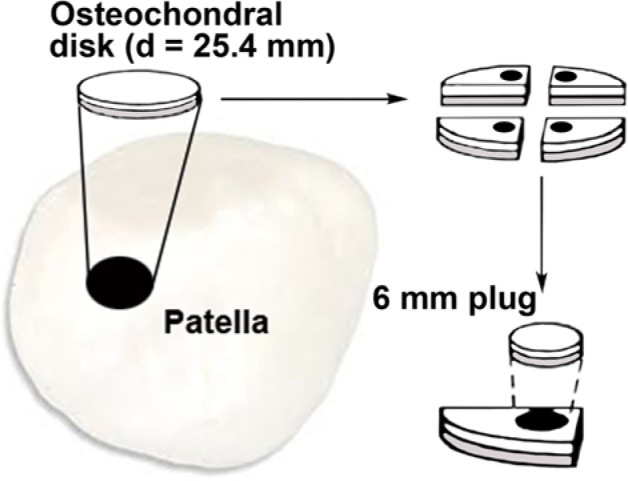
Schematic presentation of the sample preparation procedure.
For freeze-thawing experiments, the samples were randomly divided into either the reference group or 1 of 3 experimental groups. The samples of group 1 were stored in 5 mL of PBS (pH 7.4, Euroclone, Pero, Italy) during all testing and storage, whereas those in group 2 were stored in 5 mL of PBS containing inhibitors of proteolytic enzymes (5 mM benzamide-HCl and 5 mM EDTA; Sigma-Aldrich, St. Louis, MO) and antibiotics (100 U/mL penicillin and 100 mg/mL streptomycin; Euroclone) during all testing and storage. The specimens in group 3 were stored in 5 mL of PBS-inhibitor solution during the first biomechanical testing. Before freezing they were immersed for 90 minutes in 30% DMSO–PBS solution bath, containing the same concentrations of the enzyme inhibitors and antibiotics as the PBS solution, to protect the cartilage from possible damages during freezing.23 The reference group was representative of intact in vivo cartilage, which was stored in 5 mL of PBS-inhibitor solution during the biomechanical testing, and then immediately processed for histological and biochemical assessments after testing. Fifteen plugs in each group were used in this study.
As the samples in the reference group were processed to represent the intact tissue as closely as possible, they were kept immersed in the PBS-inhibitor solution only for the period of the biomechanical testing and were not taken through the freeze–thaw treatment. Each plug in group 1 was always immersed in 5 mL of PBS, representing the worst scenario for the PG loss. Subsequently, the thickness of the articular cartilage layer in each plug was measured using a stereomicroscope (Nikon SMZ-10, Nikon Co, Tokyo, Japan).
Biomechanical Testing of Cartilage Samples
For biomechanical testing,24 the osteochondral samples were mounted on the bottom of the measuring chamber with ethyl cyanoacrylate (Loctite, Westlake, OH). Using the motion controller (resolution 0.1 µm) of the test apparatus, the cartilage surface in the middle part of the sample was detected based on the non-zero force signal (resolution of the load cell 1 mN) with a plane-ended indenter (d = 1.01 mm). This was followed by stepwise stress relaxation tests (3 × 5% steps, 2 mm/s ramp velocity, and 900 seconds relaxation time after each step). Equilibrium elastic modulus of the cartilage was calculated from the linear region of the equilibrium stress–strain curves by assuming the Poisson’s ratio of 0.1 for cartilage.25,26 Dynamic elastic modulus as a function of strain was calculated using the peak stresses of the stress–relaxation curves. To determine the dynamic modulus, the cartilage was assumed to be incompressible during instantaneous loading (Poisson’s ratio = 0.5).
The reproducibility of the biomechanical measurements was determined by measuring the equilibrium and the dynamic modulus of the fresh cartilage samples (n = 11) twice.27 The indenter was placed as close as possible to the center of the samples. After the initial measurement, the samples were detached from the sample holder, allowed to re-swell in the supplemented PBS mixtures at room temperature for 30 minutes, and reattached to the sample holder for repeated biomechanical testing.
The biomechanical properties of the osteochondral samples in groups 1 to 3 were measured before and after the freeze–thaw cycle described above. The reference samples were measured only once, after which histological samples were immediately fixed and embedded in paraffin, whereas the samples reserved for the biochemical analyses were frozen immediately and analyzed at the same time as the samples taken through the freeze–thaw cycle. This compromise was chosen to be able to perform the biochemical analyses together with the samples taken though the freeze–thaw cycle.
Freeze–Thaw Cycle
After biomechanical testing, samples in groups 1 to 3 were frozen in the storage solutions described above for 21 to 24 hours at −21°C. Although cartilage samples are often stored for a rather long time frozen, we chose a relatively short storage time, as the freezing and the subsequent thawing are most likely the steps most harmful for the stored tissue in comparison to the more prolonged storage as frozen in stable temperature. For the second biomechanical testing, the samples were allowed to thaw for 1 hour at room temperature. The samples in group 1 were placed back into PBS, and those in groups 2 and 3 into the PBS-inhibitor solution, and stored at room temperature for 18 hours. This time period was chosen to simulate the challenging conditions needed for, for example, MRI28 or quantitative computed tomography29 contrast agent diffusion studies in order to reach the equilibrium state. After room temperature storage, the biomechanical testing was performed again, and all the samples were processed for histological and compositional analyses. The samples were always placed in the same individual bathing solutions and were stored at −21°C for further GAG and PG analyses. The DMSO solutions used during freezing in group 3 samples were also collected.
Water Content Analysis
Prior to the analysis of the PG content, the water contents of the samples were measured. Briefly, the cartilage samples were cut vertically into 2 halves, and the subchondral bone was removed with a razor blade. One half was fixed and processed for histology, whereas the other half was used for biochemical analyses. The wet weight of each biochemical sample (half of the cartilage plug) was quantified by weighing them in PBS solution to ensure full hydration. The samples were then lyophilized for 18 to 68 hours until a constant weight was reached to determine the dry weight of the samples, and the water content was calculated from the wet and dry weights of the samples.
Proteoglycan Extraction and Quantification
The frozen cartilage tissues were thawed at room temperature, and the PGs were extracted with 4 M guanidine-HCl containing 50 mM sodium acetate, 10 mM sodium EDTA, pH 5.8, and 5 mM benzamidine-HCl. The extraction was performed for 48 hours at 4°C. The extracted PGs and the washing solutions were dissolved in sterilized water after precipitation with 75% alcohol and stored frozen at −70°C for further analysis. The nonextractable PGs were solubilized by papain digestion at 60°C overnight. The content of the PGs was measured with a dimethylmethylene blue (DMMB) assay.30 Chondroitin sulfate (CS; Sigma-Aldrich) from shark was used to create the standard solutions for the DMMB assay.
Proteoglycan Separation with Agarose Gel Electrophoresis
The extracted PGs were separated with 1.2% agarose gels as in previous studies31 after a small modification. Briefly, based on the DMMB assay 5 µg of the extracted PGs were dissolved in 10 µL of sample buffer (1% SDS in 40 mM Tris-acetate and 1 mM sodium sulfate buffer, pH 67.8) and boiled for 5 minutes. After cooling, 5 µL of color solution (60% sucrose and 0.05% bromphenol blue) was added to the samples. Electrophoresis was performed for 3 hours at a constant current and voltage of 50 mA and 35 V, respectively. Methanol-fixed gel was stained with 1% toluidine blue in 3% acetic acid and destained with 3% acetic acid until the background was uniformly light blue. Subsequently, the gel was scanned and photographed. The scanned images were analyzed using Image J software (version 1.36b, National Institutes of Health, Bethesda, MD). Each lane area was selected separately to obtain a corresponding densitogram, and the background of the sample-free areas from both sides of the gel was averaged and subtracted from the sample densitograms. The mobility of CS was used to standardize the mobility data, giving CS a mobility of 1. The percentual amount of staining intensity in each pixel line of the densitogram was calculated to draw the background-corrected densitograms. The relative mobility of the slower-migrating large PG band was estimated from the first peak of the densitogram.
Digital Densitometry of Proteoglycans
The cartilage samples were embedded in paraffin, and three 3-µm-thick sections were cut and stained with Safranin O (ICN Biomedicals Inc., Aurora, OH). The optical density of the stain was quantified with a digital densitometric assay to estimate the tissue fixed charge content and the distribution. Grayscale images of the Safranin O–stained sections were captured and converted into absorbance units.32,33 The optical density measurements were conducted using a computer-controlled CCD camera (SenSys, Photometrics Inc., Tucson, AZ) attached to a light microscope. To obtain the optical density values for statistical evaluation, the average optical density values were calculated for superficial, intermediate, middle, and deep zones. First, the whole depth of cartilage in each sample was divided into 12 depth-wise sectors. The average values from the first sector represented the superficial zone, and the next one the intermediate zone. The next 5 sectors (3 to 7) were used to calculate the average optical density of the middle zone, and the last 5 sectors the deep zone. The selection of the sectors used for each zone was based on a previous study.32 Although the differences in the cartilage thickness may cause some inaccuracy in the discrimination of identical 4 zones, a rather small variance in the patellar cartilage thickness21,22 was considered to justify the chosen division. All the measurements were performed at the same session to minimize the methodological variation.
Statistical Analyses
Bland–Altman method was used to analyze the agreement of 2 measurements on the equilibrium and dynamic modulus, because it is considered by most statisticians the most appropriate method to test whether the measurements agree.34 The normality of distributions was confirmed with the Shapiro–Wilk test (P ≥ 0.122). Therefore, parametric tests were used in the analysis. Statistical significance of the differences in the defined parameters was determined prior and after the freeze–thaw cycles, and the differences between the control and experimental groups were analyzed by paired-sample and independent sample t-test with Bonferroni–Holm correction. A difference was considered statistically significant when the P-value was less than 0.05. IBM SPSS statistics 19 (SPSS, Chicago, IL) was used for the statistical analysis.
Results
In this study, repeated biomechanical measurements were performed to evaluate the effects of the freeze-thawing on the cartilage properties. The Bland–Altman analysis showed that the 2 measurements of the equilibrium and the dynamic modulus agreed well. The limits of agreement for the equilibrium modulus are the following: mean difference ± 2 SD = −0.0009 ± 0.07872 MPa (Fig. 2); for the dynamic modulus: mean difference ± 2 SD = −0.0164 ± 0.94446 MPa (Fig. 2). The differences between the measurements lie within the limits of agreement in more than 95% of the measurements, showing the repeatability of the measurements.
Figure 2.
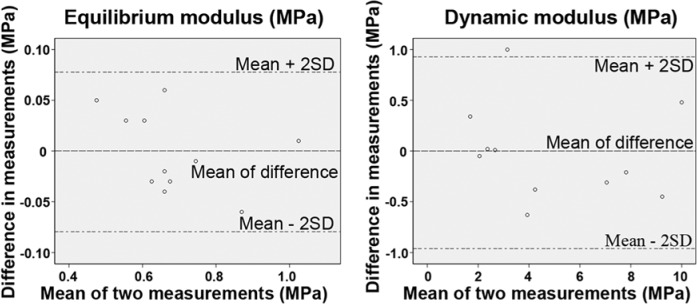
The Bland–Altman limits of agreement analysis for the equilibrium and the dynamic modulus (n = 11).
Biomechanical testing was then performed for the samples collected from 15 animals before and after the freeze-thawing. We observed that the freeze–thaw cycle did not change the equilibrium moduli in 3 groups, and the water contents did not differ between the 4 groups (Table 1). However, a small statistically significant decrease in the dynamic moduli appeared after the freeze–thaw cycle in the samples in groups 1 to 3 (P < 0.05, Table 1).
Table 1.
The Biomechanical Parameters (Mean, MPa), Water Content, and Relative Mobility of Major Large PG (Mean, %) of the Samples Analyzed Before and After the Freeze–Thaw Cycle.
| Equilibrium modulus |
Dynamic modulus |
Water content |
PG mobility |
|||
|---|---|---|---|---|---|---|
| Group | Before Mean (95% CI) | After, Mean (95% CI) | Before, Mean (95% CI) | After, Mean (95% CI) | Mean (95% CI) | Mean (95% CI) |
| Ref | 0.81 (0.71-0.91) | 4.70 (3.37-6.03) | 77.1 (75.9-78.4) | 43.6 (41.5-45.7) | ||
| 1 | 0.96 (0.85-1.06) | 0.93 (0.83-1.04) | 6.42 (4.94-7.89) | 6.10 (4.79-7.41)* | 77.7 (76.5-78.8) | 44.6 (42.6-46.6)* |
| 2 | 0.80 (0.65-0.96) | 0.83 (0.68-0.97) | 4.56 (3.68-5.45) | 4.41 (3.56-5.26)* | 78.0 (76.8-79.2) | 44.7 (43.5-46.0)* |
| 3 | 0.87 (0.76-0.98) | 0.87 (0.77-0.97) | 4.89 (3.68-6.08) | 4.57 (3.55-5.59)* | 77.8 (76.4-79.1) | 44.4 (43.0-45.7) |
PG = proteoglycan; CI = confidence interval; Ref = reference group; 1 = samples in PBS bath; 2 = samples in PBS bath containing enzyme inhibitors and antibiotics; 3 = samples in PBS with enzyme inhibitors and antibiotics, freezing in 30% DMSO; *P < 0.05, paired-sample t test (equilibrium and dynamic modulus) and independent-sample t test with Bonferroni–Holm correction (water content and PG mobility) statistical analyses were used (n = 15).
We analyzed the GAG content and the PG structures to check whether the freeze-thawing affected the PG content and the PG subpopulation sizes. The cryoprotected group had slightly higher content of the tissue GAGs (the sum of the GAGs present in the extract and the extraction tissue residue) than the other groups. It was even higher than in the reference sample, which was reserved for the analyses immediately after the first biomechanical testing and did not go through the freeze–thaw cycle. However, the differences between the groups were not statistically significant (Fig. 3). The GAG contents, which diffused into the bathing solutions, were the following: group 1, 3.1 ± 1.2%; group 2, 4.2 ± 0.8%; and group 3, 3.1 ± 0.7% (mean ± SD). Besides showing no significant differences, these values are only approximate, because the diffused GAGs were derived from the whole cartilage disk, whereas the tissue analyses were performed for half of the disk. The loss of the GAGs to the bathing media in the reference group was 1.4 ± 0.6%, which is not comparable to the other groups because of the much shorter time the samples were in the aqueous solution. The amounts of GAGs in the DMSO bathing solution were below the detection level.
Figure 3.
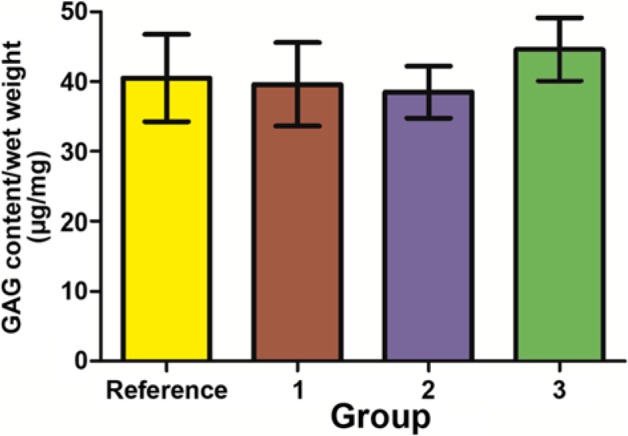
The contents of the tissue GAGs (sum of the extractable and the residual ones, mean ± 95% confidence intervals). The groups did not differ significantly from each other. Numbers 1 to 3 represent groups 1 to 3, respectively. Reference denotes the reference group, n = 15.
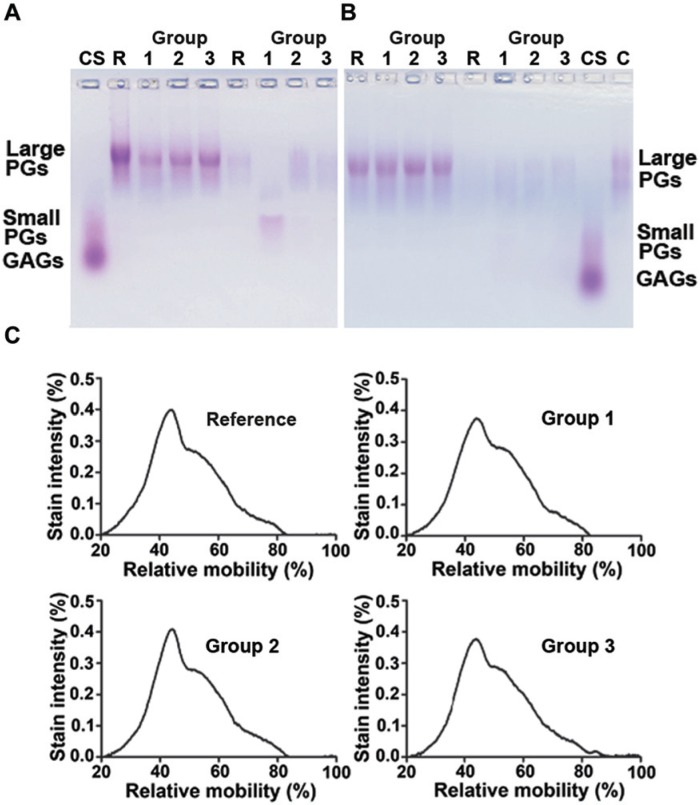
In agarose gel electrophoresis, all the samples had one major, slowly migrating band of the large PGs (Fig. 4A and B). The mobility of the small PGs decorin and biglycan has been previously shown to be 0.80 to 0.85 in relation to CS.35 The band for the small PGs was only barely detectable in the tissue-extracted samples. The sample sets in Figure 4A and B are from 2 different animals. A weakly stained band of the diffused large PGs could be seen in all the sample groups (Fig. 4A and B). However, an obvious degradation of the diffused large PGs could be seen in group 1 (which contained no inhibitors of proteolysis) in 10 out of 15 animals (Fig. 4A).
Figure 4.
Agarose gel electrophoresis of the extracted proteoglycans (PGs) shows the presence of the slowly migrating bands, representing the large PGs, and the faster the migrating smaller PGs (lanes 1-4). Sample sets from 2 different animals are shown in the subfigures (A) and (B). The diffused PGs (lanes 5-8) often revealed a fast mobility band in group 2, indicating that the PGs occasionally degraded in the absence of proteolysis inhibitors (A, lane 6). The representative densitometry plot profiles of the extracted PGs showed that the structures of PGs in groups 1 to 3 and reference group were comparable with each other (C). C = bovine native cartilage PGs; CS = chondroitin sulfate; lanes 2-4 = PGs from cartilage in groups 1 to 3; lanes 6-8 = PGs diffused into bathing solutions in groups 1 to 3; and lanes 1 and 5 are reference samples. Reference denotes reference group.
The structure of the PGs was investigated from the agarose gel densitographs, using the migration position of the CS as a reference. As the partially cleaved PGs should be smaller and faster migrating than the native ones, we located the relative migration positions for the peak maxima of densitographs for the largest PGs. The relative mobility of the large PGs appeared slightly different between the reference and the group 1 samples (Table 1, p = 0.051). All in all, the densitometry plot profiles of the PGs indicate that the structures of the PGs in all the groups were highly comparable (Fig. 4C).
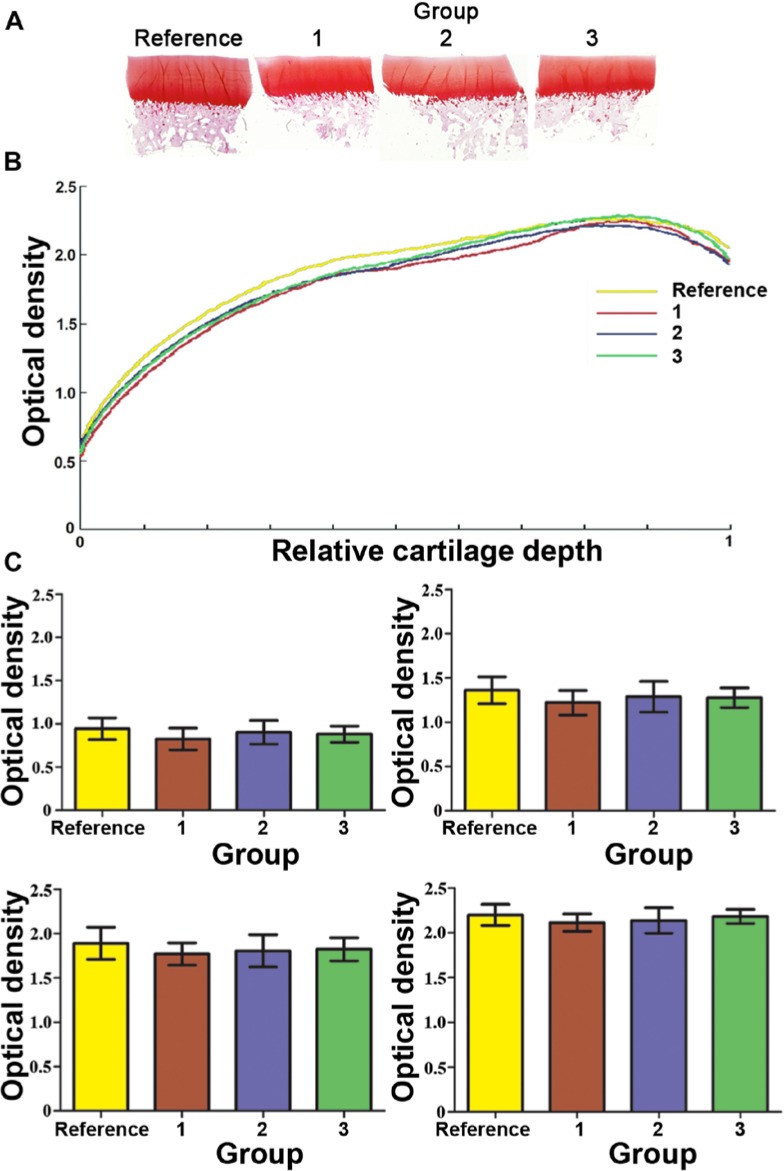
Safranin O has been shown to be useful for quantification of the GAGs both for in vitro36 and in vivo37 samples. The digital densitometry of Safranin O–stained sections (Fig. 5A) was used to estimate the amount of fixed charge density of the samples. The comparison of the plots showed that the reference group appeared to have the highest PG content, whereas group 1 had the lowest one (Fig. 5B). The average zonal staining intensity was also calculated. In the reference group, the Safranin O staining intensity appeared slightly higher in every zone. However, no significant differences could be found between the groups in any cartilage zone (Fig. 5C).
Figure 5.
Representative Safranin O stained histological sections (A) and the plots of optical density of Safranin O–stained histological sections, measured by means of the digital densitometry (B). Quantitatively, no obvious differences in Safranin O staining could be seen between any group. For the relative cartilage depth, “0” indicates the superficial cartilage and “1” the full depth in the deep zone. The control group had the highest PG content throughout the cartilage depth, whereas group 2 had the lowest one. The average values of the optical density were determined for the superficial, the intermediate, the middle, and the deep zones (mean ± 95% confidence intervals) (C). No significant differences could be found between the groups at any cartilage zone. Numbers 1 to 3 in A, B, and C represent groups 1 to 3, respectively, and Reference denotes the reference group (n = 15).
Discussion
The maintenance of the ECM structure of the articular cartilage during storage is important, because very often all the desired analyses cannot be done immediately after the collection of the tissue. Long incubation times are also necessary, for example, to reach the diffusion equilibrium of the contrast agents used for MRI or computed or contrast agent–enhanced computed tomography. The biomechanical measurements of the bovine articular cartilage after chondroitinase ABC or collagenase enzyme treatments suggested that the collagens would be responsible for the dynamic properties, whereas the PGs give the static (or equilibrium) properties.21 This study indicates that the freeze-thawing did not affect the equilibrium moduli of the samples. However, the dynamic moduli were 4.5% to 6.1% lower in all 3 groups, which were taken through the freeze–thaw cycle and the subsequent storage at room temperature. Thus, our results may indicate that some damage of the collagen network has occurred during the process. On the other hand, there were no differences between the groups in the water contents. As loosened network due to a partial collagen degradation would likely result in the higher water content of the tissue, this result suggests that only minor changes in the collagen structure may have happened.
The contents of the tissue GAGs were slightly higher in the cryoprotected freeze–thaw samples than in the reference samples. The reference samples were collected for the biochemical analysis immediately after the biomechanical testing and, as the least processed samples, were predicted to have the highest GAG content. The higher level of the GAGs in the cryoprotected cartilage samples may derive from a normal variation between the individuals and the sampling sites. However, DMSO may also interfere with the DMMB assay. This seems possible, because in a previous study the DMSO-treated freeze–thaw samples also had 6.9% to 29.8% higher GAG concentrations than the fresh ones.16 Additional attention was paid to this possibility in the densitometric analysis of Safranin O–stained sections. In the processed thin histologic sections, the possible interference caused by DMSO should be minimal. In fact, digital densitometry showed that the reference group had slightly higher staining intensity for the GAGs than the other 3 groups, as would be expected.
It has been suggested that the formation of ice crystals during the freezing procedure interrupts the cartilage ECM. However, the degradation caused by the freeze-thawing did not appear to be very significant, because the biochemical and the biophysical properties of the frozen cartilage were very close to those in the control samples. Also, the structures of the extracted PGs were very similar in all the groups, showing no apparent degradation of the PGs. These results are consistent with some other studies, which either showed only minor changes in the PG contents or the biomechanical/electromechanical properties after the freeze–thaw of cartilage.17,18,20 However, conflicting reports are also present, reporting rather dramatic changes in the GAG contents14,16 due to the freeze–thaw procedure.
There are some differences in the experimental set-ups in the conflicting studies. In the report by Laouar et al.,14 the samples that were stored for up 2 weeks at −80°C were measured both immediately after thawing and after 36 to 72 hours of incubation in PBS at 37°C. Under these conditions, a loss of CS (or in fact PGs), shown by the MRI analyses performed after the 12 to 24 hours of incubation in the antibiotics-supplemented PBS at 37°C, was statistically significant in the samples either slowly cooled in PBS or snap-frozen before the freezing for 2 weeks, whereas the samples slowly cooled in DMSO had values rather close to the control ones.14 However, the biochemical data they collected after the 36 to 72 hours of incubation in PBS showed statistically significant loss of GAGs in the samples snap-frozen or those slowly cooled in DMSO (78% and 83% loss, respectively), whereas the samples slowly cooled in PBS did not differ significantly from the control ones.14 Thus, the conflicting data on the PG loss are evident also in that study.
On the other hand, one previous study stated that in all the sample groups there was 36% to 52% increase in hydroxyproline (equivalent to collagen) content per dry weight during the 36 to 72 hours of incubation in PBS after the thawing.14 The authors proposed that the result may reflect the time-sensitive nature of the biochemical assay and illustrate the up-regulation of the injured chondrocytes that occurs during the degradation processes.14 However, the increase in the total collagen content is very high, especially for the samples that were frozen in PBS without cryoprotectant. Such treatments would apparently kill most, if not all, of the frozen chondrocytes in the cartilage tissue. Thus, it is obscure how the cartilage tissue could produce so much new collagen.
In the study by Zheng et al.,16 one major difference in comparison to our study is the species used for the study (canine vs. bovine cartilage). Therefore, the thickness and the architecture are somewhat different, which can affect the tendency of PG diffusion from the cartilage. The duration of the freezing can also be different. We used 21 to 24 hours, whereas Zheng et al.16 stated that the freezing time for their samples was 4 days. However, their duration of the freezing given in the Methods section is 24 hours.16 Although we may have a shorter duration for the freezing, we assume that the freezing and thawing are processes more harmful for the maintenance of the cartilage structure and the integrity than the freezing time at constant temperature, and we do not believe that the difference in the freezing time can cause the major differences found in our results.
The sample size can be assumed to have importance for the loss of PGs. Zheng et al.16 used a smaller sample size (1.75 × 2 × 6 mm), whereas we used cylindrical samples with a diameter of 6 mm. Faster diffusion of the PGs from the samples with smaller dimensions can partly explain the variation in the results. This is a fact that is relevant for human samples, in case only small biopsies can be collected. The use of proteinase inhibitors was also different in our study compared with the others.14,16 They may protect PG loss by preventing their proteolytic cleavage, although we did not notice a big difference between the groups incubated with or without inhibitors.
Dimethyl sulfoxide, a common cryoprotectant, is used to protect the cartilage against damage during storage by preventing the formation of ice crystals. Our present study showed that DMSO may have a minor role in protection against GAG loss. Only about 3% to 4% of the GAGs from total were diffused to the bathing solution when the freeze-thawed samples were kept there for up to 18 hours. These results are not consistent with the previous study showing that more than 50% of the GAGs can be lost from the cartilage tissue by immersing the frozen–thawed tissue in PBS.16
There are certain limitations in this study that have to be considered when interpreting the generalization of the results. The samples were collected only from the bovine patellae, and thus, they may not fully represent the conditions of the other anatomical sites of the articular cartilages. The comparison with small biopsy samples is probably not relevant, because the diffusion of the PGs from the smaller sized samples is relatively much easier. Also the duration of the freezing was short compared to the likely storage time of the collected experimental sample series. Because of the experimental design, the reference samples had a slightly different treatment compared with the biomechanically tested samples.
Conclusion
The freeze–thaw cycle caused minor changes only in the dynamic modulus of the articular cartilage, but otherwise no significant differences could be observed between the different freeze–thaw treatments. The frozen samples were very similar to the minimally processed reference samples. Thus, a single freeze-thawed (frozen at −21°C for 21-24 hours, thawed at room temperature for up to 18 hours) cartilage samples appear suitable for biochemical and biomechanical studies.
Footnotes
Acknowledgments and Funding: The author(s) are grateful to Mrs. Eija Rahunen and Elina Reinikainen for their excellent assistance. This work was supported by the Academy of Finland (Project Nos. 128117 and 132367) and strategic funding of the University of Eastern Finland.
Declaration of Conflicting Interest: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1. Kuettner KE, Kimura JH. Proteoglycans: an overview. J Cell Biochem. 1985;27:327-36. [DOI] [PubMed] [Google Scholar]
- 2. Lohmander S. Proteoglycans of joint cartilage. Structure, function, turnover and role as markers of joint disease. Baillieres Clin Rheumatol. 1988;2:37-62. [DOI] [PubMed] [Google Scholar]
- 3. Heinegård D. Proteoglycans and more—from molecules to biology. Int J Exp Pathol. 2009;90:575-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Becerra J, Andrades JA, Guerado E, Zamora-Navas P, Lopez-Puertas JM, Reddi AH. Articular cartilage: structure and regeneration. Tissue Eng Part B Rev. 2010;16:617-27. [DOI] [PubMed] [Google Scholar]
- 5. Bader DL, Kempson GE, Egan J, Gilbey W, Barrett AJ. The effects of selective matrix degradation on the short-term compressive properties of adult human articular cartilage. Biochim Biophys Acta. 1992;116:147-54. [DOI] [PubMed] [Google Scholar]
- 6. Schmidt MB, Mow VC, Chun LE, Eyre DR. Effects of proteoglycan extraction on the tensile behavior of articular cartilage. J Orthop Res. 1990;8:353-63. [DOI] [PubMed] [Google Scholar]
- 7. Bader DL, Kempson GE, Barrett AJ, Webb W. The effects of leucocyte elastase on the mechanical properties of adult human articular cartilage in tension. Biochim Biophys Acta. 1981;677:103-8. [DOI] [PubMed] [Google Scholar]
- 8. Mäkelä JTA, Huttu MRJ, Korhonen RK. Structure-function relationship in osteorthritic human hip joint articular cartilage. Osteoarthritis Cartilage. 2012;20:1268-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Korhonen RK, Laasanen MS, Töyräs J, Lappalainen R, Helminen HJ, Jurvelin JS. Fibril reinforced poroelastic model predicts specifically mechanical behavior of normal, proteoglycan depleted and collagen degraded articular cartilage. J Biomech. 2003;36:1373-9. [DOI] [PubMed] [Google Scholar]
- 10. Kallioniemi AS, Jurvelin JS, Nieminen MT, Lammi MJ, Töyräs J. Contrast agent enhanced pQCT of articular cartilage. Phys Med Biol. 2007;52:1209-19. [DOI] [PubMed] [Google Scholar]
- 11. Lubke C, Sittinger M, Burmester GR, Paulitschke M. Cryopreservation of artificial cartilage: viability and functional examination after thawing. Cells Tissues Organs. 2001;169:368-76. [DOI] [PubMed] [Google Scholar]
- 12. Jomha NM, Anoop PC, McGann LE. Chondrocyte recovery in cryopreserved porcine articular cartilage after bone carrier alteration. Cryo Letters. 2002;23:263-8. [PubMed] [Google Scholar]
- 13. Jomha NM, Anoop PC, McGann LE. Intramatrix events during cryopreservation of porcine articular cartilage using rapid cooling. J Orthop Res. 2004;22:152-7. [DOI] [PubMed] [Google Scholar]
- 14. Laouar L, Fishbein K, McGann LE, Horton WE, Spencer RG, Jomha NM. Cryopreservation of porcine articular cartilage: MRI and biochemical results after different freezing protocols. Cryobiology. 2007;54:36-43. [DOI] [PubMed] [Google Scholar]
- 15. Ock SA, Rho GJ. Effect of dimethyl sulfoxide (DMSO) on cryopreservation of porcine mesenchymal stem cells (pMSCs). Cell Transplant. 2011;20:1231-9. [DOI] [PubMed] [Google Scholar]
- 16. Zheng S, Xia Y, Bidthanapally A, Badar F, Ilsar I, Duvoisin N. Damages to the extracellular matrix in articular cartilage due to cryopreservation by microscopic magnetic resonance imaging and biochemistry. Magn Reson Imaging. 2009;27:648-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tavakol K, Miller RG, Bazett-Jones DP, Hwang WS, McGann LE, Schachar NS. Ultrastructural changes of articular cartilage chondrocytes associated with freeze-thawing. J Orthop Res. 1993;11:1-9. [DOI] [PubMed] [Google Scholar]
- 18. Guan J, Urban JP, Li ZH, Ferguson DJ, Gong CY, Cui ZF. Effects of rapid cooling on articular cartilage. Cryobiology. 2006;52:430-9. [DOI] [PubMed] [Google Scholar]
- 19. Fishbein KW, Canuto HC, Bajaj P, Camacho NP, Spencer RG. Optimal methods for the preservation of cartilage samples in MRI and correlative biochemical studies. Magn Reson Med. 2007;57:866-73. [DOI] [PubMed] [Google Scholar]
- 20. Changoor A, Fereydoonzad L, Yaroshinsky A, Buschmann MD. Effects of refrigeration and freezing on the electromechanical and biomechanical properties of articular cartilage. J Biomech Eng. 2010;132:064502. [DOI] [PubMed] [Google Scholar]
- 21. Laasanen MS, Töyräs J, Korhonen RK, Rieppo J, Saarakkala S, Nieminen MT, et al. Biomechanical properties of knee articular cartilage. Biorheology. 2003;40:133-40. [PubMed] [Google Scholar]
- 22. Töyräs J, Laasanen MS, Saarakkala S, Lammi MJ, Rieppo J, Kurkijärvi Jet al. Speed of sound in normal and degenerated bovine articular cartilage. Ultrasound Med Biol. 2003:29:447-54. [DOI] [PubMed] [Google Scholar]
- 23. Mukherjee IN, Li Y, Song YC, Long RC Jr, Sambanis A. Cryoprotectant transport through articular cartilage for long-term storage: experimental and modeling studies. Osteoarthritis Cartilage. 2008;16:1379-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Töyräs J, Rieppo J, Nieminen MT, Helminen HJ, Jurvelin JS. Characterization of enzymatically induced degradation of articular cartilage using high frequency ultrasound. Phys Med Biol. 1999;44:2723-33. [DOI] [PubMed] [Google Scholar]
- 25. Hayes WC, Keer LM, Herrmann G, Mockros LF. A mathematical analysis for indentation tests of articular cartilage. J Biomech. 1972;5:541-51. [DOI] [PubMed] [Google Scholar]
- 26. Korhonen RK, Laasanen MS, Töyräs J, Rieppo J, Hirvonen J, Helminen HJ, et al. Comparison of the equilibrium response of articular cartilage in unconfined compression, confined compression and indentation. J Biomech. 2002;35:903-9. [DOI] [PubMed] [Google Scholar]
- 27. Glüer CC, Blake G, Lu Y, Blunt BA, Jergas M, Genant HK. Accurate assessment of precision errors: how to measure the reproducibility of bone densitometry techniques. Osteoporos Int. 1995;5:262-70. [DOI] [PubMed] [Google Scholar]
- 28. Salo EN, Nissi MJ, Kulmala KA, Tiitu V, Töyräs J, Nieminen MT. Diffusion of Gd-DTPA(2) into articular cartilage. Osteoarthritis Cartilage. 2012;20:117-26. [DOI] [PubMed] [Google Scholar]
- 29. Silvast TS, Jurvelin JS, Aula AS, Lammi MJ, Töyräs J. Contrast agent-enhanced computed tomography of articular cartilage: association with tissue composition and properties. Acta Radiol. 2009;50:78-85. [DOI] [PubMed] [Google Scholar]
- 30. Farndale RW, Buttle DJ, Barrett AJ. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim Biophys Acta. 1986;883:173-7. [DOI] [PubMed] [Google Scholar]
- 31. Qu C, Lindeberg H, Ylärinne JH, Lammi MJ. Five percent oxygen tension is not beneficial for neocartilage formation in scaffold-free cell cultures. Cell Tissue Res. 2012;348:109-17. [DOI] [PubMed] [Google Scholar]
- 32. Panula HE, Hyttinen MM, Arokoski JP, Långsjö TK, Pelttari A, Kiviranta I, et al. Articular cartilage superficial zone collagen birefringence reduced and cartilage thickness increased before surface fibrillation in experimental osteoarthritis. Ann Rheum Dis. 1998;57:237-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rieppo L, Saarakkala S, Närhi T, Holopainen J, Lammi M, Helminen HJ, et al. Quantitative analysis of spatial proteoglycan content in articular cartilage with Fourier transform infrared imaging spectroscopy: critical evaluation of analysis methods and specificity of the parameters. Microsc Res Tech. 2010;73:503-12. [DOI] [PubMed] [Google Scholar]
- 34. Zaki R, Bulgiba A, Ismail R, Ismail NA. Statistical methods used to test for agreement of medical instruments measuring continuous variables in method comparison studies: a systematic review. PLoS One. 2012;7:e37908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Inkinen RI, Lammi MJ, Lehmonen S, Puustjärvi K, Kääpä E, Tammi MI. Relative increase of biglycan and decorin and altered chondroitin sulfate epitopes in the degenerating human intervertebral disc. J Rheumatol. 1998;25:506-14. [PubMed] [Google Scholar]
- 36. Lammi M, Tammi M. Densitometric assay of nanogram quantities of proteoglycans precipitated on nitrocellulose membrane with Safranin O. Anal Biochem. 1988;168:352-7. [DOI] [PubMed] [Google Scholar]
- 37. Kiviranta I, Jurvelin J, Tammi M, Säämänen AM, Helminen HJ. Microspectrophotometric quantitation of glycosaminoglycans in articular cartilage sections stained with Safranin O. Histochemistry. 1985;82:249-55. [DOI] [PubMed] [Google Scholar]